Abstract
This study examines the duration of antibody response to SARS-CoV-2 in health care personnel over a 60-day period in Nashville, Tennessee.
Declines in immunoglobulin antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among patients with symptomatic or asymptomatic infections have been documented.1,2 We assessed the duration of antibody response to SARS-CoV-2 infection in health care personnel, who may be at particular risk if antibody levels decline.
Methods
We evaluated anti–SARS-CoV-2 antibodies at baseline and approximately 60 days later in a convenience sample of health care personnel at Vanderbilt University Medical Center who regularly had direct contact with adult patients with coronavirus disease 2019.3 Staff were informed about the study through emails and meetings and volunteered to participate. Participants completed a survey for symptoms of viral illness since February 1, 2020, and underwent phlebotomy for serology testing between April 3 and April 13, 2020 (baseline visit), and between June 2 and June 27 (60-day visit). The project was determined to be nonresearch public health surveillance by Vanderbilt University Medical Center and the Centers for Disease Control and Prevention. Each participant agreed to join the study.
Serum samples were tested for anti–SARS-CoV-2 antibodies using a validated enzyme-linked immunosorbent assay against the prefusion-stabilized extracellular domain of the SARS-CoV-2 spike protein.3 A specimen was considered reactive if the signal-to-threshold ratio at a serum dilution of 1:100 with background correction was greater than 1.0, with higher ratios indicating higher antibody titers. At this cutoff, assay specificity and sensitivity were 99% and 96%, respectively.4 We describe the change in seropositivity in the overall study cohort, stratified by presence or absence of symptoms (fever, cough, dyspnea, myalgias, sore throat, vomiting, diarrhea, dysgeusia, or anosmia). We evaluated the change in mean and median signal-to-threshold ratios at baseline and 60 days in those who were seropositive at baseline and those who were seropositive vs seronegative at 60 days. Data were analyzed with Stata version 16.
Results
Approximately 600 health care personnel were eligible; serum samples were collected at baseline from the first 249 volunteers (64.5% female; 91.6% White; median age, 33 years; range, 21-70 years), and 230 (92%) returned for a second blood draw. Participants included 42.2% nurses, 34.5% physicians and advanced practice clinicians, 6.8% radiology technicians, and 16.5% other health care personnel. Nineteen (7.6%) had anti–SARS-CoV-2 antibodies detected at baseline. Of these, 8 participants (42%) had antibodies that persisted above the seropositivity threshold at 60 days, whereas 11 (58%) became seronegative. Thus, overall seropositivity changed from 7.6% at baseline (19/249) to 3.2% (8/249) at 60 days. Six of 8 participants (75%) who remained seropositive reported symptoms prior to the baseline visit and 2 (25%) were asymptomatic. Five of 11 participants (45%) in whom antibodies decreased below the seropositivity threshold reported symptoms prior to the baseline visit, whereas 6 (55%) were asymptomatic.
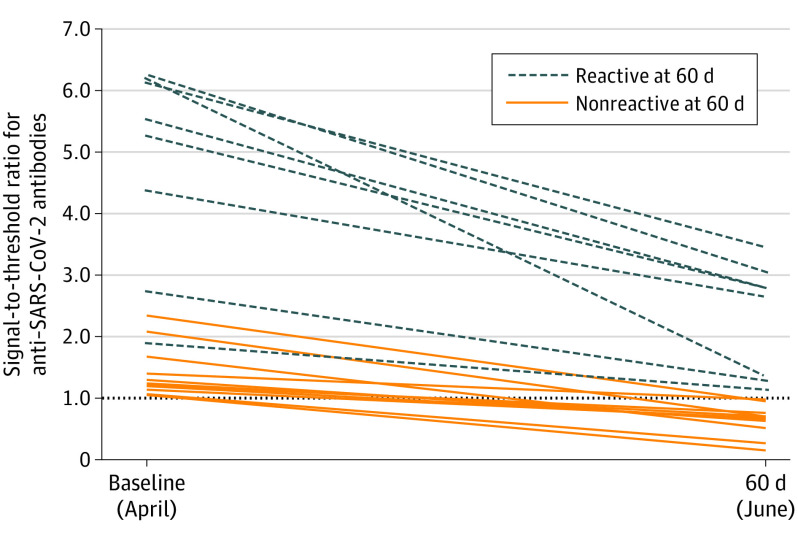
All 19 participants who were seropositive at baseline had antibody decreases at 60 days (Figure). Participants who remained seropositive at 60 days had higher signal-to-threshold ratios at baseline (mean, 4.8; range, 1.9-6.2) compared with participants whose ratios decreased below threshold at 60 days (mean, 1.4; range, 1.1-2.3) (Table). Antibodies declined from a mean signal-to-threshold ratio of 4.8 at baseline to 2.3 at 60 days in participants who remained seropositive and from 1.4 at baseline to 0.6 at 60 days in those whose antibody levels decreased below the threshold.
Figure. Anti–SARS-CoV-2 Signal-to-Threshold Ratios at Baseline and 60 Days in Health Care Personnel Seropositive at Baseline.
SARS-CoV-2 indicates severe acute respiratory syndrome coronavirus 2. The dotted line at y = 1.0 indicates the threshold for seropositivity.
Table. Seropositivity at 60 Days, Symptom Prevalence, and Mean Signal-to-Threshold Values of Anti–SARS-CoV-2 Immunoglobulin Antibodies Among 19 Health Care Personnel Seropositive at Baseline.
| No. (%) | Signal-to-threshold value, mean (median)a | ||||
|---|---|---|---|---|---|
| SARS-CoV-2 ELISA results | Symptomaticb | Asymptomaticb | 0 d | 60 d | |
| Total reactive at baseline | 19 (100) | 11/19 (58) | 8/19 (42) | 2.8 (1.9) | 1.3 (1.0) |
| Total at 60 days | |||||
| Reactivea | 8/19 (42) | 6/8 (75) | 2/8 (25) | 4.8 (5.4) | 2.3 (2.7) |
| Nonreactive | 11/19 (58) | 5/11 (45) | 6/11 (55) | 1.4 (1.2) | 0.6 (0.7) |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
A specimen was considered reactive if, on confirmatory testing, at a background corrected optical density above the threshold at a serum dilution of 1:100, it had a signal-to-threshold ratio greater than 1, which indicated anti–SARS-CoV-2 antibody presence.4
Symptomatic denotes those with symptoms of a viral respiratory illness, including fever, cough, shortness of breath, myalgias, sore throat, vomiting, diarrhea, dysgeusia, or anosmia, between February 1, 2020, and the baseline visit in April 2020. Others were classified as asymptomatic.
Discussion
Anti–SARS-CoV-2 antibodies to the spike protein, which have correlated with neutralizing antibodies,5 decreased over 60 days in health care personnel, with 58% of seropositive individuals becoming seronegative. The consistency in decline in the signal-to-threshold ratio regardless of the baseline ratio and a higher proportion of asymptomatic participants becoming seronegative support the interpretation as a true decline over a 2-month period rather than an artifact of assay performance. If replicated, these results suggest that cross-sectional seroprevalence studies to evaluate population immunity may underestimate rates of prior infections because antibodies may only be transiently detectable following infection.
The window after recovering from SARS-CoV-2 infection when people could donate serum that has sufficiently high antibody levels may be limited. Implications for health care personnel with antibodies assigned to care for infected patients depend on whether decline in these antibodies increases risk of reinfection and disease, which remains unknown, especially given the lack of data on memory B-cell and T-cell responses.6 Limitations of this study include its single-center setting, small sample size, convenience sampling, and lack of information on timing of infection to evaluate antibody kinetics.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. Published online July 21, 2020. doi: 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 3.Stubblefield WB, Talbot HK, Feldstein L, et al. ; Influenza Vaccine Effectiveness in the Critically Ill (IVY) Investigators . Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients—Nashville, Tennessee. Clin Infect Dis. 2020;ciaa936. doi: 10.1093/cid/ciaa936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. doi: 10.1001/jamainternmed.2020.4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413. doi: 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489-1501.e15. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]