This randomized clinical trial uses data from existing patients at a tertiary care facility to assess the efficacy of intranasal thermosensitive timolol gel in treating moderate-to-severe recurrent hereditary hemorrhagic telangiectasia–associated epistaxis.
Key Points
Question
Is an intranasal thermosensitive timolol gel safe and efficacious in treating moderate-to-severe recurrent hereditary hemorrhagic telangiectasia (HHT)–associated epistaxis?
Findings
This randomized clinical trial with 27 patients found that thermosensitive gel, regardless of the presence of timolol, was highly effective and safe for HHT-associated epistaxis. Timolol thermosensitive gel may be more efficacious than thermosensitive gel alone, but larger studies are needed.
Meaning
Physicians treating patients with HHT-associated epistaxis should consider a thermosensitive gel (with or without timolol) as a safe and effective topical nasal therapy.
Abstract
Importance
Other than nasal moisturizers, no standard-of-care medical therapy exists for epistaxis in hereditary hemorrhagic telangiectasia (HHT). With epistaxis as the greatest cause of morbidity in patients with HHT, there is a need to identify effective topical therapies.
Objective
To determine the efficacy and safety of an intranasal timolol thermosensitive gel vs placebo thermosensitive gel in treating HHT-associated epistaxis.
Design, Setting, and Participants
This double-blind, placebo-controlled randomized clinical trial was conducted from October 29, 2019, to May 20, 2020, at a tertiary care center. A total of 27 patients with HHT and moderate-to-severe epistaxis were recruited and included in this prespecified analysis: 14 in the timolol group and 13 in the placebo group. Inclusion criteria included (1) age 18 years or older, (2) clinical or genetic diagnosis of HHT, (3) screening Epistaxis Severity Score (ESS) of 4 or greater and 2 or more nosebleeds cumulatively lasting at least 5 minutes per week, (4) stable epistaxis pattern over the preceding 3 months, and (5) no change in epistaxis treatment or nasal hygiene regimen in the preceding month. Exclusion criteria included (1) contraindications to systemic β-blocker administration, (2) use of medications interacting with timolol, (3) use of antiangiogenic medications in the last month before recruitment, and (4) use of anticoagulants, antiplatelets, or fibrinolytic therapies within the last month.
Interventions
Novel thermosensitive intranasal timolol (0.1%) gel vs placebo thermosensitive gel applied twice daily to each nostril for 8 weeks.
Main Outcomes and Measures
The primary outcome was the median change in ESS and percentage of participants reaching the minimal clinically important difference in ESS. Secondary outcomes were changes in Clinical Global Impression–Severity and Clinical Global Impression–Improvement scores, Nasal Outcome Score for Epistaxis in Hereditary Hemorrhagic Telangiectasia, and hemoglobin level.
Results
Of 27 participants randomized (median [range] age, 55 [20-76] years; 14 women [52%]; 25 White [93%]), a total of 23 patients with HHT completed the primary outcome measure. Within the timolol gel and placebo gel groups, respectively, the median change (range) in ESS was 2.32 (0.22 to 5.97) vs 1.96 (−0.91 to 5.98), and 9 of 11 (82%) vs 9 of 12 (75%) participants experienced a clinically meaningful improvement in ESS. Twenty-two of the 23 participants (96%) reported improvement via the Clinical Global Impression–Improvement score, with 81% vs 58% of participants reporting reduced severity of epistaxis in the timolol vs placebo group, respectively. Of participants completing the Nasal Outcome Score for Epistaxis in HHT at follow-up visit, 7 of 10 (70%) in the timolol group achieved a clinically important difference vs 5 of 10 (50%) in the placebo group. There was no change in hemoglobin level between or within groups. Zero participants in the placebo group and 2 of 13 (15%) in the timolol group withdrew because of adverse events.
Conclusions and Relevance
Thermosensitive gel, alone or in combination with timolol, was highly effective in reducing HHT-associated epistaxis. The timolol group had greater improvement in epistaxis and quality of life than the placebo group, but effect estimates were imprecise, and no definitive conclusions on the superiority of timolol can be drawn. Physicians treating patients with HHT-associated epistaxis should consider a thermosensitive gel (with or without timolol) for their patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT04139018
Introduction
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disorder that affects greater than 50 000 people in the United States.1 It is characterized by abnormal blood vessel formation, commonly manifesting as mucocutaneous bleeding in the nose and gastrointestinal tract. Epistaxis occurs in greater than 90% of patients with HHT and is the leading cause of morbidity.2 Other than nasal moisturizers, no standard-of-care medical therapy exists for HHT-associated epistaxis (HAE) (M.E. Faughnan, MD, personal communication, 2020).
Medical management of HAE is complex, and a multitude of drugs have been evaluated. Topical medications are of particular interest, as oral medications place patients at risk of systemic adverse effects. The landmark North American Study of Epistaxis (NOSE) trial randomized 121 patients with HHT to 4 different topical spray formulations: bevacizumab, tranexamic acid, estrogen, or placebo.3 The study found that none of the active medications were superior to placebo spray and highlighted the need to identify novel topical therapies for HAE.
Topical and systemic β-blockers have been investigated in small studies as treatment for HAE.4,5 The nonselective β-blocker propranolol is used both topically and systemically to treat infantile hemangiomas, a disease thought to have a similar pathophysiologic etiology as HHT.6 The effects of propranolol are likely a combination of vasoconstriction, antiangiogenesis, and a reduction in migration of endothelial cells.7,8 However, intranasal administration of propranolol may irreversibly inhibit cilia beat frequency and thus be toxic for long-term use in the nasal cavity.9 Timolol, also a nonselective β-blocker, has been investigated in small studies in the ophthalmic aqueous form used intranasally for HAE.10,11,12 The randomized clinical trial Efficacy of a Timolol Nasal Spray as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia (TEMPO) investigated an intranasal timolol spray vs placebo spray and showed no difference in epistaxis among the groups.13 The nasal anatomy, crusting, and inflammation characteristics of the nose of a patient with HHT may prevent intranasal sprays from releasing the medication directly on the telangiectasias; in fact, the authors of both the TEMPO and NOSE trials concluded that the efficacy of intranasal treatments for HAE needs to be further evaluated in a gel formulation to allow for greater medication absorption.
Thermosensitive intranasal gels are viscous at room temperature and harden within the warmth of the nasal cavity.14,15 Unlike propranolol, timolol has not been shown to cause histologic changes of the nasal mucosa characteristic of cilia toxicity.16,17,18 We hypothesized that a thermosensitive gel formulation will provide a safe delivery system for the proper absorption of timolol.
Our objective was to assess the efficacy and safety of intranasal timolol delivered in a thermosensitive gel form vs thermosensitive gel alone in the treatment of HAE.
Methods
Study Design
This study was a prospective, randomized, double-blinded, parallel-group, phase 2 clinical trial of a timolol thermosensitive gel vs placebo thermosensitive gel for recurrent moderate-to-severe HAE. Recruitment and follow-up took place from October 29, 2019, to May 20, 2020. Both the study team and participants were blinded to the treatment group until analysis was complete, and only the research pharmacist (J.J.) was unblinded to the treatment group. The study period was 8 weeks, with outcomes assessed at the beginning and end of the 8-week period. The trial protocol (Supplement 1) was reviewed and approved by the institutional review board at the Washington University School of Medicine, and the present study was prespecified in the protocol. All patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Pharmacologic Preparation
Timolol nasal gel, 0.1%, or placebo gel (no active ingredients) was prepared by the research pharmacist with poloxamers (combination of poloxamer 188 and 407; pH adjusted to 4.5-6.5), and participants applied 0.5 mL to each nostril twice daily. The total daily timolol dose amounted to 2 mg, consistent with the doses used in small studies of the ophthalmic form.10,11,12,13 The gel hardens upon application and dissolves in approximately 12 hours, providing justification for a twice-daily application. Participants were instructed to apply the gel using disposable syringes and to target telangiectasias identified via nasal endoscopy.
Participant Selection
Participants were screened and contacted for recruitment through 2 means: (1) existing patients seen at the Washington University HHT Center of Excellence, and (2) online advertisements from Cure HHT, a patient advocacy group. Participants were enrolled by the study authors (A.M.P., J.J.L., and J.F.P.) after receiving written informed consent. All data were collected at in-person visits before the coronavirus disease 2019 (COVID-19) pandemic (March 2020); follow-up data during the pandemic (through May 2020) were collected via telehealth. Eligible participants had to meet all inclusion criteria: (1) age 18 years or older, (2) clinical (meeting at least 3 out of 4 Curaçao Criteria19) or genetic diagnosis of HHT, (3) screening Epistaxis Severity Score20 of 4 or greater and 2 or more nosebleeds cumulatively lasting at least 5 minutes per week, (4) stable epistaxis pattern over the preceding 3 months, and (5) no change in epistaxis treatment or nasal hygiene regimen in the preceding month. Major exclusion criteria included (1) contraindications to systemic β-blocker administration; (2) use of medications interacting with timolol; (3) use of antiangiogenic medications in the last month before recruitment (eg, bevacizumab); and (4) use of anticoagulants, antiplatelets, or fibrinolytic therapies within the last month. See the trial protocol in Supplement 1 for full inclusion and exclusion criteria.
For safety precautions, participants received an electrocardiogram at baseline and had their blood pressure and heart rate taken at baseline and 8 weeks. Electrocardiography results were read by a board-certified pulmonologist (M.M.C.).
Randomization
The randomization scheme used permuted blocks of varying sizes and was generated by the research pharmacist. Participants and all members of the research team were blinded to treatment assignment. Participants received their medication package from Advanced Rx Pharmacy by courier delivery services.
Baseline Assessments and Outcomes
Demographic information, past epistaxis treatments, smoking status, and overall burden of comorbidity as measured by the Adult Comorbidity Evaluation–2721 were collected at baseline. Race/ethnicity was assessed by the investigator and the patient’s self-described race/ethnicity from their medical record, as HHT is found in various racial and ethnic groups (M.E. Faughnan, MD, personal communication, 2020).
The primary outcome measure was the change in Epistaxis Severity Score (ESS) between baseline and follow-up. The ESS is a validated, patient-reported outcome measure concerning the frequency, duration, and intensity of epistaxis, anemia, need for medical attention, and blood transfusions.20 The ESS is scored from 0 to 10 on a continuous scale, with mild epistaxis considered 0 to 4; moderate, 4 to 7; and severe, 7 to 10. The minimal clinically important difference (MCID) of the ESS is 0.71.22 We modified the ESS for the follow-up visit to use a reference time frame of the previous 2 months (rather than 3 months) to match the duration of follow-up of the study.
The secondary outcome measures were changes in Clinical Global Impression,23 NOSE HHT (A.M. Peterson, MSCI, personal communication, 2020), 36-Item Short Form Health Survey (SF-36),24 and hemoglobin level.
The Clinical Global Impression–Severity (CGI-S) score is a modified 5-point Likert scale assessing the current severity of epistaxis at baseline and at 8 weeks. Answer choices are as follows: no problem, mild problem, moderate problem, severe problem, and problem as bad as it can be. The Clinical Global Impression–Improvement (CGI-I) score is a modified 5-point Likert scale that asks at the 8-week follow-up visit how a patient’s epistaxis has changed since the beginning of the study. Answer choices are as follows: much improved, slightly improved, no change, slightly worse, and much worse.
The NOSE HHT is a validated patient-reported outcome measure concerning physical problems, functional limitations, and emotional consequences of epistaxis. It is scored continuously from 0 (epistaxis least burdensome) to 4 (epistaxis most burdensome) and has an MCID of 0.46.
The SF-36 is a widely used general health questionnaire with each health domain scored from 0 (least healthy) to 100 (most healthy).24 The SF-36 measures health in 8 separate domains.
Sample Size
The sample size for the trial was derived from the study of within-subject change in the severity of epistaxis associated with timolol ophthalmic solution used intranasally.10 In that study, 9 of 11 participants (82%) experienced a reduction in the severity of nosebleeds as measured on a 5-point Likert scale. We hypothesized a similar response (82%) in our proposed study among participants randomized to receive timolol gel and a 20% response rate among participants randomized to placebo gel, corresponding to a difference of 62 percentage points. The estimated 20% placebo response rate was based on the TEMPO trial, in which 6 of the 29 participants (21%) in the placebo group met the predetermined outcome cutoff value.
Power analysis estimated that a total sample size of 24 patients (12 in each arm) was needed to allow us to detect with 80% power at the 2-sided α level of .05 a difference of 62 percentage points or more in the proportion of participants who experience reduction of severity of nosebleeds between treatment groups. Considering a 20% dropout rate, we planned to enroll up to 30 participants in the study.
Statistical Analysis
Descriptive statistics were used to describe the study population. Data were analyzed from May 27, 2020, through June 6, 2020, using intention-to-treat principles. For every participant who completed baseline and follow-up assessments, we calculated the difference between ESS at baseline and ESS at the 8-week follow-up visit. Participants with a change in ESS equal to or greater than the MCID were coded as responders. The rate of responders in each study group was calculated by dividing the number of responders in each group by the total number of participants randomized to each group. Proportion differences and 95% CIs were calculated. Mixed-model analysis was used to assess the change in ESS measured continuously from baseline to end of treatment and to compare that value between the 2 study groups. We used an unrestricted covariance structure, and participants were treated as random effects in the model. The mixed-model analysis allows for inclusion in the analysis of all participants following intention-to-treat principles. The Hodges-Lehmann method was used for calculation of CIs for the difference between 2 medians.
Results
Study Population
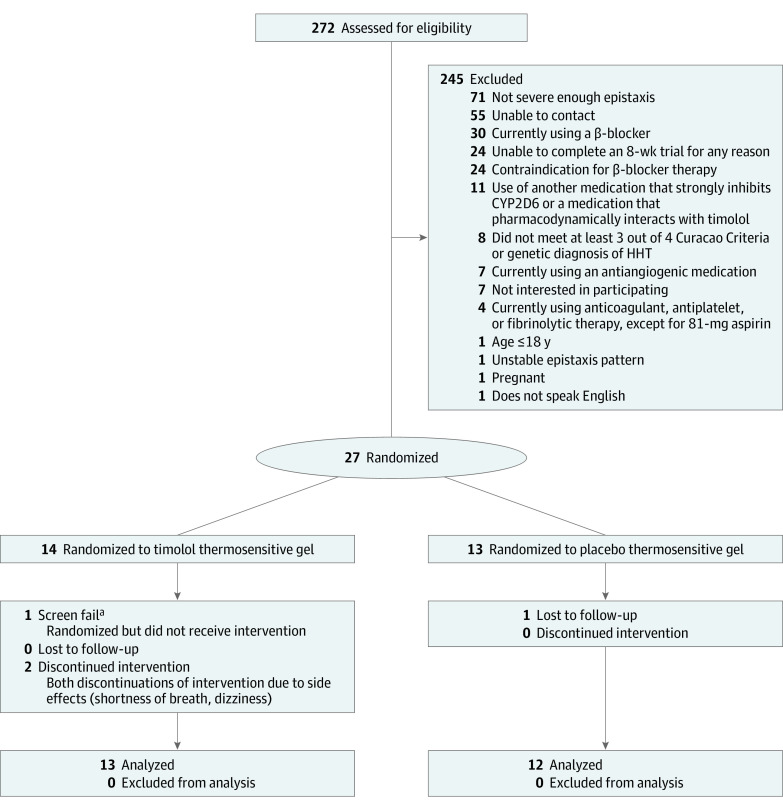
A total of 272 patients with HHT were contacted and screened for eligibility (Figure 1). The 27 willing participants who met the inclusion criteria traveled for their baseline visit from 9 different states; 14 were randomized to timolol thermosensitive gel but only 13 received the gel, and 13 were randomized to placebo thermosensitive gel. Of the 27 participants randomized (median [range] age, 55 [20-76] years), 14 (52%) were women and 13 (48%) were men, and 25 (93%) were White (Table 1). One ESS inclusion criterion exception was made for a participant who had a screening ESS of 2.93 but fulfilled the frequency and duration criteria. Recruitment was ceased on March 17, 2020, after enrollment of the 27th participant owing to the COVID-19 pandemic.
Figure 1. Consort Diagram of Participant Randomization, Allocation, Follow-up, and Analysis.
CYP2D6 indicates cytochrome P450 2D6 isozyme; HHT, hereditary hemorrhagic telangiectasia.
aThe liver enzyme that metabolizes timolol, CYP2D6, was tested in the first 5 patients. This participant had the low metabolizing phenotype and was removed from the study for safety reasons. After multiple ambiguous genetic test reports, the CYP2D6 testing was eliminated from the protocol.
Table 1. Baseline Demographic Characteristics, Comorbidities, and Disease Characteristics.
| Characteristic | No. (%) | Difference (95% CI)a | ||
|---|---|---|---|---|
| All participants (N = 27) | Timolol thermosensitive gel (n = 14) | Placebo thermosensitive gel (n = 13) | ||
| Age, median (range), y | 55 (20 to 76) | 58 (20 to 76) | 52 (30 to 65) | −6 (−17 to 2) |
| Sex | ||||
| Men | 13 (48) | 4 (29) | 9 (69) | 41 (3 to 65) |
| Women | 14 (52) | 10 (71) | 4 (31) | |
| Race/ethnicity | ||||
| White | 25 (93) | 14 (100) | 11 (85) | −15 (−42 to 9) |
| Black | 2 (7) | 0 | 2 (15) | |
| Comorbidities | ||||
| Hypertension | 6 (22) | 4 (31) | 2 (18) | −13 (−42 to 22) |
| Seasonal allergies | 18 (67) | 9 (69) | 9 (82) | 13 (−22 to 42) |
| Comorbidity status | ||||
| None | 17 (63) | 9 (64) | 8 (62) | −3 (−35 to 30) |
| Mild | 7 (26) | 3 (21) | 4 (31) | 9 (−22 to 40) |
| Moderate | 1 (4) | 1 (7) | 0 | −7 (−31 to 16) |
| Severe | 2 (7) | 1 (7) | 1 (8) | 0 (−25 to 27) |
| Current smoker | 4 (15) | 2 (14) | 2 (15) | 1 (−27 to 30) |
| Current nasal perforation | 5 (19) | 4 (29) | 1 (8) | −21 (−48 to 10) |
| Past epistaxis treatments | ||||
| Cautery | 19 (70) | 11 (79) | 8 (62) | −17 (−47 to 16) |
| Sclerotherapy | 16 (59) | 8 (57) | 8 (62) | 4 (−29 to 37) |
| Doxycycline | 1 (4) | 1 (7) | 0 | −7 (−31 to 16) |
| Septodermoplasty | 1 (4) | 1 (7) | 0 | −7 (−31 to 16) |
| Other nose/sinus surgery | 7 (26) | 3 (21) | 4 (31) | −21 (−22 to 40) |
| Baseline Clinical Global Impression–Severity Score | ||||
| No problem | 0 | 0 | 0 | 0 (−23 to 23) |
| Mild problem | 7 (27) | 3 (23) | 4 (31) | 8 (−25 to 38) |
| Moderate/severe problem | 19 (73) | 10 (77) | 5 (69) | −21 (−54 to 16) |
| Problem as bad as it could be | 0 | 0 | 0 | 0 (−23 to 23) |
Proportion difference for categorical data and median difference for continuous data.
Primary Outcome
Epistaxis Severity Score
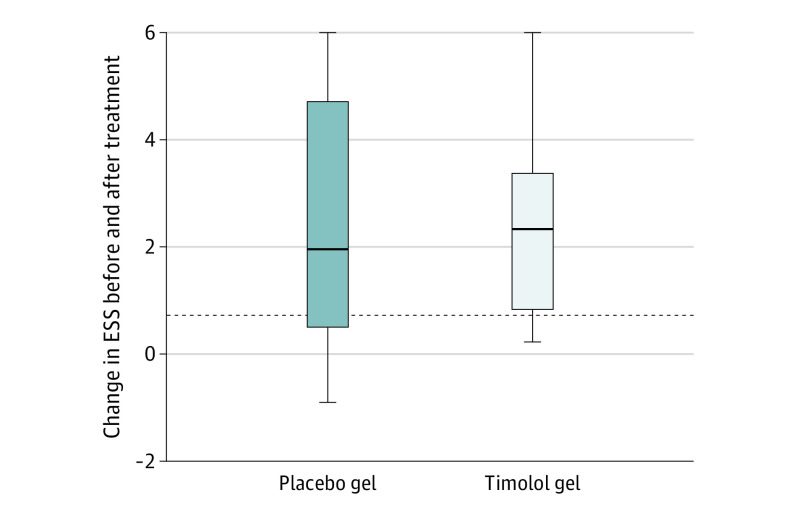
Distribution of ESS before and after intervention is summarized in Table 2. Median (range) ESS scores for timolol gel and placebo gel, respectively, were 4.50 (2.93-7.46) and 5.65 (2.43-8.88) before intervention and 2.03 (0.55-6.56) and 3.04 (1.01-7.07) after intervention. A total of 23 participants completed the ESS at the final assessment, and they experienced a median decrease in ESS of 2.21 points (range, –0.91 to 5.98). Overall, 18 of the participants (78%) experienced a clinically important decrease in ESS at the final visit as compared with baseline. Regarding the change within each group, participants in the timolol group (n = 11) reported a median decrease in ESS of 2.32 points (range, 0.22-5.97), whereas participants in the placebo group (n = 12) reported a median decrease in ESS of 1.96 (range, –0.91 to 5.98) (Figure 2). Nine of 11 participants (82%) in the timolol group and 9 of 12 participants (75%) in the placebo group reported a clinically meaningful improvement in ESS. Mixed-model analysis following intention-to-treat principles revealed that there was a statistically significant and clinically important improvement in ESS from baseline to follow-up visit (least squares mean difference, 2.4 [95% CI, 1.54-3.30]), but the change did not differ by study group.
Table 2. Comparison of Outcome Measures Between the Study Groups Before and After Intervention.
| Variable | Timolol gela | Placebo gela | Difference (95% CI) |
|---|---|---|---|
| ESS | |||
| Before | 4.50 (2.93 to 7.46) | 5.65 (2.43 to 8.88) | 0.80 (−0.6 to 2.2) |
| After | 2.03 (0.55 to 6.56) | 3.04 (1.01 to 7.07) | 0.90 (−0.4 to 2.2) |
| Total NOSE HHT score | |||
| Before | 1.21 (0.69 to 2.21) | 1.24 (0.55 to 2.55) | 0.03 (−0.45 to 0.45) |
| After | 0.60 (0.07 to 1.93) | 0.64 (0.31 to 1.55) | 0.05 (−0.48 to 0.55) |
| Hemoglobin level, g/dL | |||
| Before | 13.3 (9.5 to 15.4) | 11.8 (8.4 to 16.8) | −1 (−3.1 to 1.4) |
| After | 13.3 (8.3 to 15.9) | 12.6 (7.0 to 16.1) | −1.2 (−3.7 to 1.7) |
| SF-36 domains | |||
| PF | |||
| Before | 78 (35 to 100) | 80 (5 to 100) | 0 (−30 to 15) |
| After | 90 (35 to 100) | 85 (15 to 100) | 0 (−40 to 10) |
| RFP | |||
| Before | 80 (5 to 100) | 75 (0 to 100) | 0 (−25 to 50) |
| After | 100 (0 to 100) | 100 (0 to 100) | 0 (−25 to 50) |
| RFE | |||
| Before | 100 (0 to 100) | 100 (0 to 100) | 0 (0 to 33) |
| After | 100 (0 to 100) | 100 (0 to 100) | 0 (0 to 0) |
| EF | |||
| Before | 48 (10 to 80) | 50 (0 to 80) | 0 (−15 to 25) |
| After | 70 (30 to 90) | 60 (30 to 80) | −5 (−25 to 15) |
| EW | |||
| Before | 82 (48 to 96) | 84 (36 to 100) | 4 (−8 to 12) |
| After | 86 (60 to 100) | 86 (76 to 92) | 0 (−8 to 12) |
| SF | |||
| Before | 100 (25 to 100) | 88 (25 to 100) | 0 (−33 to 13) |
| After | 100 (50 to 100) | 88 (38 to 100) | −13 (−25 to 0) |
| P | |||
| Before | 78 (23 to 100) | 78 (23 to 100) | 0 (−33 to 10) |
| After | 85 (68 to 100) | 85 (23 to 100) | 0 (−23 to 13) |
| GH | |||
| Before | 73 (30 to 100) | 60 (10 to 100) | −10 (−30 to 10) |
| After | 80 (35 to 100) | 60 (30 to 90) | −20 (−35 to 0) |
Abbreviations: EF, energy/fatigue; ESS, Epistaxis Severity Score; EW, emotional well-being; GH, general health; HHT, hereditary hemorrhagic telangiectasia; NOSE HHT, Nasal Outcome Score for Epistaxis in HHT; P, pain; PF, physical functioning; RFE, role limitations due to emotional health; RFP, role limitations due to physical health; SF, social functioning; SF-36, 36-Item Short Form Health Survey.
Values expressed as median (range) scores.
Figure 2. Change in Epistaxis Severity Score in the 2 Treatment Groups.
Dashed line represents the MCID of the ESS (0.71). Whiskers represent 95% CIs. ESS indicates Epistaxis Severity Score; MCID, minimal clinically important difference.
Secondary Outcomes
CGI-S and CGI-I Scores
Baseline CGI-S scores are shown in Table 1. Zero patients reported no problem, 7 patients (27%) reported a mild problem, 19 patients (73%) reported a moderate or severe problem, and no patients reported a problem as bad as it can be. In the timolol group, 9 participants (81%) reported improvement by 1 point (n = 6) or 2 points (n = 3). In the placebo group, 7 patients (58%) reported an improvement in disease severity by 1 point (n = 4) or 2 points (n = 3), with an absolute difference of 23% in favor of the timolol group (95% CI, –14% to 53%). No participants in the timolol group and 2 participants in the placebo group reported worsened epistaxis severity assessed by the CGI-S.
Clinical Global Impression–Improvement scoring assessed at 8 weeks revealed that 15 participants (65%) reported symptoms as much improved, 7 participants (30%) reported symptoms as slightly improved, and 1 participant (4%) reported no change in symptoms. Seven of 11 participants (64%) in the timolol group and 8 of 12 participants (67%) in the placebo group reported symptoms as much improved (absolute difference, –3%; 95% CI, –42% to 36%).
NOSE HHT
The distribution of NOSE HHT scores before and after intervention is summarized in Table 2. Median (range) total NOSE HHT scores for timolol gel and placebo gel, respectively, were 1.21 (0.69-2.21) and 1.24 (0.55-2.55) before intervention and 0.60 (0.07-1.93) and 0.64 (0.31-1.55) after intervention. Mixed-model analysis revealed that there was a statistically significant and clinically important improvement in NOSE HHT score from baseline to follow-up visit (least squares mean difference, 0.61 [95% CI, 0.29-0.79]), but this conclusion was not definitive, because the lower bound of the 95% CI was below the established MCID of 0.46 points. The change in NOSE HHT between study visits did not differ by study group. Seven of 10 patients (70%) in the timolol group and 5 of 10 patients (50%) in the placebo group who completed the postintervention NOSE HHT score had a change greater than the MCID for an absolute difference of 20% in favor of the timolol group (95% CI, –20% to 53%).
SF-36
Distribution of scores of the 8 SF-36 domains assessed at baseline and 8 weeks is summarized in Table 2. Mixed-model analysis revealed that there was no difference between the 2 study groups in the change estimated for each domain.
Hemoglobin Level
There was no clinically meaningful change in hemoglobin level between study visits and between groups (Table 2). Median (range) hemoglobin levels for timolol gel and placebo gel, respectively, were 13.3 g/dL (9.5-15.4 g/dL) and 11.8 g/dL (8.4-16.8 g/dL) before intervention and 13.3 g/dL (8.3-15.9 g/dL) and 12.6 (7.0-16.1 g/dL) after intervention.
Safety
Three patients in the placebo group (23%) reported mild and transient potential adverse events. Two patients in the timolol group (14%) withdrew owing to adverse events: 1 case each of shortness of breath and dizziness. The symptoms occurred within 1 week of starting the medication, and both participants recovered fully after discontinuation of the medication without seeking medical care. See Table 3 for the complete list of adverse events.
Table 3. Adverse Events.
| Symptom | No. | |
|---|---|---|
| Timolol gela | Placebo gel | |
| Shortness of breath | 1 | 0 |
| Dizziness | 1 | 0 |
| Nasal congestion | 3 | 0 |
| Headache | 1 | 1 |
| Rhinorrhea | 1 | 0 |
| Nausea | 1 | 0 |
| Bad taste of the medication | 1 | 0 |
| Sore throat | 0 | 1 |
| Irritation upon applying gel | 0 | 1 |
Seven of 13 participants in the timolol gel group experienced any adverse events, because 2 patients experienced 2 adverse events.
Systolic Blood Pressure, Diastolic Blood Pressure, and Heart Rate
Hemodynamic measures were obtained from 6 participants in the placebo group and 5 in the timolol group (not collected during the COVID-19 pandemic). The participants in the timolol group had a median decrease of 5 mm Hg (range, –22 to 26 mm Hg) in systolic blood pressure and a median decrease of 11 mm Hg (range, –9 to 24 mm Hg) in diastolic blood pressure. The participants in the placebo group had a median change of 0 mm Hg (range, –28 to 14 mm Hg) in systolic blood pressure and a median decrease of 4.5 mm Hg (range, –14 to 11 mm Hg) in diastolic blood pressure. When compared between groups, the median decreases in systolic and diastolic blood pressure were 5.5 mm Hg (95% CI, –22 to 34 mm Hg) and 7 mm Hg (95% CI, –17 to 32 mm Hg), respectively, in favor of the timolol group.
The heart rate of participants in the timolol group was reduced at 8 weeks compared with baseline, with a median (range) decrease of 3 beats per minute (–21 to 16 beats per minute), and that in the placebo group was increased, with a median (range) increase of 3.5 beats per minute (range, –19 to 11 beats per minute). The difference of the medians in heart rate change was 6 beats per minute (95% CI, –18 to 21 beats per minute).
Compliance and Satisfaction With Treatment
Of the 23 participants who completed follow-up, 22 (96%) reported that they missed 3 or fewer of the 14 possible doses per week. At the follow-up visit, 22 participants (96%) reported that they would use the gel they received regularly if it was commercially available.
Assessment of Blinding
Participants were asked which group they thought they were assigned to after 1 week of the intervention. Nine timolol recipients (69%) and 5 placebo recipients (46%) guessed their treatment group correctly. After follow-up evaluation, the study team guessed which treatment group each participant was assigned to. The study team guessed 6 timolol recipients (55%) correctly and 4 placebo recipients (33%) correctly. These results indicate adequate blinding both of the participants and the study team.
Discussion
In the present study, we found that regardless of the presence of timolol, thermosensitive gel used for 8 weeks was highly effective and safe in decreasing epistaxis severity and improving quality of life. Compared with the placebo gel group, the timolol gel group had a greater percentage of responders measured by ESS (82% vs 75%) and CGI-S (81% vs 58%) and a greater number of responders in epistaxis-specific quality of life, measured by the NOSE HHT (70% vs 50%). These estimates are imprecise owing to the relatively small sample size of the study and cannot confirm timolol thermosensitive gel superiority over placebo thermosensitive gel.
The novel thermosensitive gel used in this study appears to be safe, and the most common adverse event was nasal congestion. Tolerability of the timolol thermosensitive gel was apparent within the first week of treatment. The placebo thermosensitive gel seems to be particularly safe, with only 3 of the 13 participants (23%) reporting mild and transient potential adverse events. However, 2 of the 13 participants (15%) in the timolol gel group experienced adverse events severe enough to cause their withdrawal, and optimization of who would best benefit from the timolol gel vs those most likely to experience adverse events would require further study.
New Medication Delivery System for Topical Drugs
With 22 (96%) of the participants in the study reporting that they would use the novel thermosensitive gel if it was commercially available, there is a clear need for an improved nasal moisturizing agent for patients with HHT. The high degree of compliance and satisfaction with the thermosensitive gel offers promise that this novel medication delivery system may be beneficial not only alone but also for delivering other topical medications (eg, doxycycline, pomalidomide) for HAE. Furthermore, this gel may be an effective medication delivery medium for other rhinologic conditions, such as chronic rhinosinusitis.
Strengths and Limitations
The greatest limitation of the study is the relatively small sample size, contributing to the imprecise point estimates of the outcome effect sizes. However, the purpose of the study was to obtain pilot data on the efficacy, safety, and feasibility of timolol in a thermosensitive gel in the treatment of HAE. We also did not have participants record their epistaxis for a period of time (termed run-in time) before starting the intervention, which would have given us better data to determine the efficacy of the gel alone without timolol. Omitting a run-in period reduced the required time of the study. However, we propose that future trials should include a run-in period with the thermosensitive gel followed by randomization, as this approach will allow standardization of background management among the groups. We cannot be certain that the improvement in epistaxis seen by the trial participants is not regression to the mean. However, by having an inclusion criterion of a subjectively stable epistaxis pattern within the last 3 months before recruitment, regression to the mean is less likely. Compared with the placebo responder rate of 20.7% within the TEMPO study, it is also unlikely that the significant response rates seen in this study could be solely explained by the placebo effect. Nevertheless, the best way to study the true effect of the thermosensitive gel alone would be to compare the thermosensitive gel with the standard of care or timolol ophthalmic solution, incorporate a run-in period, or use a crossover or N-of-1 trial design.
Conclusions
This study found that application of a novel thermosensitive gel twice daily caused a clinically meaningful improvement in epistaxis among patients with HHT and moderate-to-severe recurrent epistaxis, regardless of the presence of timolol. The timolol gel group had a greater improvement in epistaxis, measured in a variety of ways; however, the imprecision of the estimates due to a relatively small sample size cannot confirm the superiority of timolol gel over placebo gel. A larger trial is required to definitively assess the incremental benefit of timolol over placebo. Nevertheless, we believe health care professionals may consider using the novel thermosensitive gel (with or without timolol) for HAE in addition to or instead of current nasal moisturizers.
Trial Protocol
Data Sharing Statement
References
- 1.Grosse SD, Boulet SL, Grant AM, Hulihan MM, Faughnan ME. The use of US health insurance data for surveillance of rare disorders: hereditary hemorrhagic telangiectasia. Genet Med. 2014;16(1):33-39. doi: 10.1038/gim.2013.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarrabeitia R, Fariñas-Álvarez C, Santibáñez M, et al. Quality of life in patients with hereditary haemorrhagic telangiectasia (HHT). Health Qual Life Outcomes. 2017;15(1):19. doi: 10.1186/s12955-017-0586-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehead KJ, Sautter NB, McWilliams JP, et al. Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: a randomized clinical trial. JAMA. 2016;316(9):943-951. doi: 10.1001/jama.2016.11724 [DOI] [PubMed] [Google Scholar]
- 5.Mei-Zahav M, Blau H, Bruckheimer E, Zur E, Goldschmidt N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—a preliminary report. J Otolaryngol Head Neck Surg. 2017;46(1):58. doi: 10.1186/s40463-017-0235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban-Casado S, Martín de Rosales Cabrera AM, Usarralde Pérez A, et al. Sclerotherapy and topical nasal propranolol: an effective and safe therapy for HHT-epistaxis. Laryngoscope. 2019;129(10):2216-2223. doi: 10.1002/lary.27930 [DOI] [PubMed] [Google Scholar]
- 7.Price A, Rai S, Mcleod RWJ, Birchall JC, Elhassan HA. Topical propranolol for infantile haemangiomas: a systematic review. J Eur Acad Dermatol Venereol. 2018;32(12):2083-2089. doi: 10.1111/jdv.14963 [DOI] [PubMed] [Google Scholar]
- 8.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163(2):269-274. doi: 10.1111/j.1365-2133.2010.09848.x [DOI] [PubMed] [Google Scholar]
- 9.Albiñana V, Recio-Poveda L, Zarrabeitia R, Bernabéu C, Botella LM. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb Haemost. 2012;108(1):41-53. doi: 10.1160/TH11-11-0809 [DOI] [PubMed] [Google Scholar]
- 10.van de Donk HJM, Merkus FWHM. Decreases in ciliary beat frequency due to intranasal administration of propranolol. J Pharm Sci. 1982;71(5):595-596. doi: 10.1002/jps.2600710530 [DOI] [PubMed] [Google Scholar]
- 11.Ichimura K, Kikuchi H, Imayoshi S, Dias MS. Topical application of timolol decreases the severity and frequency of epistaxis in patients who have previously undergone nasal dermoplasty for hereditary hemorrhagic telangiectasia. Auris Nasus Larynx. 2016;43(4):429-432. doi: 10.1016/j.anl.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Olitsky SE. Topical timolol for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. Am J Otolaryngol. 2012;33(3):375-376. doi: 10.1016/j.amjoto.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 13.Epperla N, Brilliant MH, Vidaillet H Topical timolol for treatment of epistaxis in hereditary haemorrhagic telangiectasia associated with bradycardia: a look at CYP2D6 metabolising variants. BMJ Case Rep. 2014;2014:bcr2013203056. doi: 10.1136/bcr-2013-203056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuis-Girod S, Pitiot V, Bergerot C, et al. Efficacy of timolol nasal spray as a treatment for epistaxis in hereditary hemorrhagic telangiectasia. A double-blind, randomized, placebo-controlled trial. Sci Rep. 2019;9(1):11986. doi: 10.1038/s41598-019-48502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalerao AV, Lonkar SL, Deshkar SS, Shirolkar SV, Deshpande AD. Nasal mucoadhesive in situ gel of ondansetron hydrochloride. Indian J Pharm Sci. 2009;71(6):711-713. [Google Scholar]
- 16.Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67. doi: 10.1208/pt070367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieminen T, Lehtimäki T, Mäenpää J, Ropo A, Uusitalo H, Kähönen M. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67(2):237-245. doi: 10.1080/00365510601034736 [DOI] [PubMed] [Google Scholar]
- 18.Volotinen M, Hakkola J, Pelkonen O, Vapaatalo H, Mäenpää J. Metabolism of ophthalmic timolol: new aspects of an old drug. Basic Clin Pharmacol Toxicol. 2011;108(5):297-303. doi: 10.1111/j.1742-7843.2011.00694.x [DOI] [PubMed] [Google Scholar]
- 19.Jagdale S, Shewale N, Kuchekar BS. Optimization of thermoreversible in situ nasal gel of timolol maleate. Scientifica (Cairo). 2016;2016:6401267.doi: 10.1155/2016/6401267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91(1):66-67. doi: [DOI] [PubMed] [Google Scholar]
- 21.Hoag JB, Terry P, Mitchell S, Reh D, Merlo CA. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope. 2010;120(4):838-843. doi: 10.1002/lary.20818 [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. doi: 10.1001/jama.291.20.2441 [DOI] [PubMed] [Google Scholar]
- 23.Yin LX, Reh DD, Hoag JB, et al. The minimal important difference of the epistaxis severity score in hereditary hemorrhagic telangiectasia. Laryngoscope. 2016;126(5):1029-1032. doi: 10.1002/lary.25669 [DOI] [PubMed] [Google Scholar]
- 24.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement