This cohort study evaluates the association of coffee consumption with disease progression and death in patients with advanced or metastatic colorectal cancer.
Key Points
Question
Is increased coffee consumption associated with improved survival in patients with advanced or metastatic colorectal cancer?
Findings
In this cohort study of 1171 patients with advanced or metastatic colorectal cancer, increased coffee consumption at the time of study enrollment was associated with lower risk of disease progression and death. Significant associations were noted for both caffeinated and decaffeinated coffee.
Meaning
Among patients with advanced or metastatic colorectal cancer, this study found increased coffee intake to be associated with lower risk of disease progression and death.
Abstract
Importance
Several compounds found in coffee possess antioxidant, anti-inflammatory, and insulin-sensitizing effects, which may contribute to anticancer activity. Epidemiological studies have identified associations between increased coffee consumption and decreased recurrence and mortality of colorectal cancer. The association between coffee consumption and survival in patients with advanced or metastatic colorectal cancer is unknown.
Objective
To evaluate the association of coffee consumption with disease progression and death in patients with advanced or metastatic colorectal cancer.
Design, Setting, and Participants
This prospective observational cohort study included 1171 patients with previously untreated locally advanced or metastatic colorectal cancer who were enrolled in Cancer and Leukemia Group B (Alliance)/SWOG 80405, a completed phase 3 clinical trial comparing the addition of cetuximab and/or bevacizumab to standard chemotherapy. Patients reported dietary intake using a semiquantitative food frequency questionnaire at the time of enrollment. Data were collected from October 27, 2005, to January 18, 2018, and analyzed from May 1 to August 31, 2018.
Exposures
Consumption of total, decaffeinated, and caffeinated coffee measured in cups per day.
Main Outcomes and Measures
Overall survival (OS) and progression-free survival (PFS).
Results
Among the 1171 patients included in the analysis (694 men [59%]; median age, 59 [interquartile range, 51-67] years). The median follow-up time among living patients was 5.4 years (10th percentile, 1.3 years; IQR, 3.2-6.3 years). A total of 1092 patients (93%) had died or had disease progression. Increased consumption of coffee was associated with decreased risk of cancer progression (hazard ratio [HR] for 1-cup/d increment, 0.95; 95% CI, 0.91-1.00; P = .04 for trend) and death (HR for 1-cup/d increment, 0.93; 95% CI, 0.89-0.98; P = .004 for trend). Participants who consumed 2 to 3 cups of coffee per day had a multivariable HR for OS of 0.82 (95% CI, 0.67-1.00) and for PFS of 0.82 (95% CI, 0.68-0.99), compared with those who did not drink coffee. Participants who consumed at least 4 cups of coffee per day had a multivariable HR for OS of 0.64 (95% CI, 0.46-0.87) and for PFS of 0.78 (95% CI, 0.59-1.05). Significant associations were noted for both caffeinated and decaffeinated coffee.
Conclusions and Relevance
Coffee consumption may be associated with reduced risk of disease progression and death in patients with advanced or metastatic colorectal cancer. Further research is warranted to elucidate underlying biological mechanisms.
Introduction
Despite advances in treatment, colorectal cancer (CRC) remains a deadly disease in the United States.1,2 Abundant experimental and epidemiological data support a link between dietary and other lifestyle factors and the incidence and mortality of this disease.3,4 One such factor that has garnered increasing interest is the consumption of coffee, which possesses antineoplastic properties in the laboratory and may play a role in CRC development and progression. The potential for coffee to slow the growth of cancer may be related to coffee’s ability to decrease blood insulin levels by sensitizing tissues to the effects of insulin, because insulin-resistant states have been associated with worse CRC outcomes.5,6,7 Alternatively, coffee’s anticancer effects may be related to biologically active constituents of coffee that have been shown to have antioxidant, anti-inflammatory, and antiangiogenic effects.8,9,10,11
Recent epidemiological studies found that higher coffee intake was associated with improved survival in patients with stage III CRC.12,13 However, the association between coffee consumption and survival of patients with metastatic CRC is unknown. A significant number of patients with CRC may ultimately develop metastatic disease, and treatment for this group is palliative, with a 5-year survival of 14%.1,2 Therefore, identifying novel treatment strategies to improve the outcomes of patients with metastatic CRC is of particular research and clinical importance. We conducted a prospective study evaluating the association of coffee consumption with overall survival (OS) and progression-free survival (PFS) in patients with advanced or metastatic CRC who were enrolled in a National Cancer Institute–sponsored, multi-institutional phase 3 randomized clinical trial of combined cytotoxic and biologic therapy. We hypothesized that increased coffee consumption is associated with longer survival in patients who were starting first-line chemotherapy for CRC.
Methods
Study Participants
The patients in this analysis were drawn from a cohort of participants enrolled in the completed phase 3 randomized clinical trial, Cancer and Leukemia Group B (CALGB; now a part of the Alliance for Clinical Trials in Oncology)/SWOG 80405. Eligible patients had pathologically confirmed, unresectable, locally advanced or metastatic CRC. The primary objective of the original clinical trial was to determine the optimal biologic therapy (cetuximab and/or bevacizumab) with a concurrent standard chemotherapy backbone, either leucovorin calcium, fluorouracil, and irinotecan hydrochloride (FOLFIRI) or leucovorin, fluorouracil, and oxaliplatin (mFOLFOX6) (investigator’s choice). Patients were required to have had no previous treatment for advanced or metastatic disease, no known concurrent cancers, adequate blood counts and organ function, good performance status, and no contraindications to bevacizumab or cetuximab treatment. When the trial was initiated, molecularly unselected participants were randomized in nonblinded fashion to cetuximab, bevacizumab, or both antibodies. Based on emerging clinical data on the detrimental effect of double antibodies in other trials14,15 and the effect of KRAS mutation status on anti–epidermal growth factor receptor antibody efficacy,16 the study was subsequently amended such that only patients with KRAS wild-type tumors were included, and, in a subsequent amendment, enrollment to the dual antibody arm was halted. Informed consent was obtained from all participants, and the trial was approved by the institutional review board of all participating sites. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
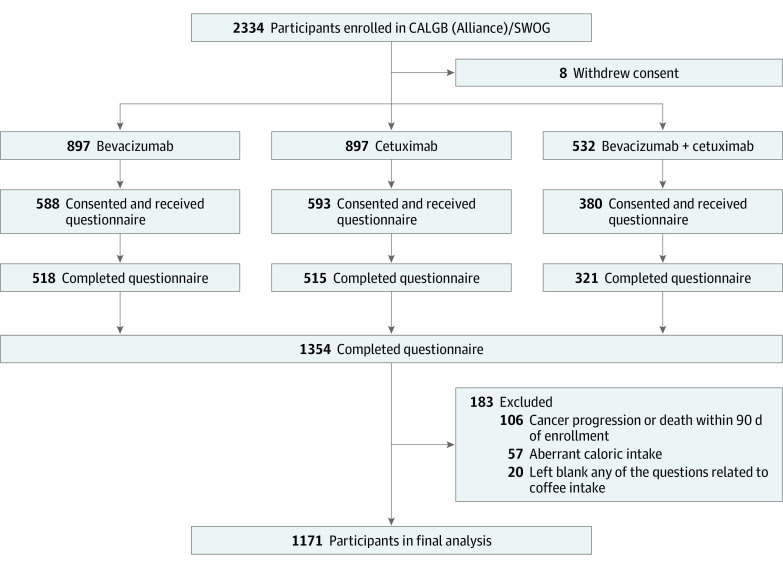
At the time of enrollment in the clinical trial, patients were given the option of inclusion in the diet and lifestyle companion study. Patients who consented to participate completed a diet and lifestyle survey within the first month of enrollment. Ultimately, 1561 (67%) patients consented to the companion study, of whom 1354 (87%) completed the questionnaire. Compared with others in the trial, patients who completed the questionnaire were more likely to be white, had better performance status, and were less likely to have indeterminate or missing KRAS status, but did not differ in other baseline characteristics.17
Figure 1 shows the cohort’s derivation. Because the results of the CALGB (Alliance)/SWOG 80405 trial did not show a significant difference in OS or PFS between patients treated with cetuximab vs bevacizumab,18 all patients who completed the diet questionnaire were pooled into a prospective cohort for analysis of coffee intake. Participants were excluded if they reported aberrant caloric intake (<600 or >4200 kcal/d for men; <500 or >3500 kcal/d for women) or left blank any of the questions related to coffee intake. Participants were also excluded if they had cancer progression or death within 90 days of enrollment to avoid dietary assessment bias related to a decline in general health. Data were collected from October 27, 2005, to January 18, 2018.
Figure 1. Derivation of the Study Population.
Assessment of Coffee Consumption
Patients who consented completed a diet and lifestyle survey within the first month of enrollment, including a semiquantitative food frequency questionnaire that asked participants how often during the previous 3 months they consumed a specific food portion for 131 food items and vitamin/mineral supplements.19,20 The consumption of caffeinated coffee and decaffeinated coffee was asked separately, with 10 possible responses ranging from never to at least 6 cups/d. The frequency of consumption was then converted into a continuous variable in cups per day using the following values for reported frequencies: 0 indicates never; 0.03, less than 1 cup/mo; 0.07, 1 to 3 cups/mo; 0.14, 1 cup/wk; 0.43, 2 to 4 cups/wk; 0.79, 5 to 6 cups/wk; 1.00, 1 cup/d; 2.50, 2 to 3 cups/d; 4.50, 4 to 5 cups/d; and 6.00, at least 6 cups/d. Total coffee consumption was calculated as the sum of caffeinated and decaffeinated coffee. Prespecified cutoffs were applied for total coffee intake (never or <1, 1, 2-3, or ≥4 cups/d), caffeinated coffee intake (never or <1, 1, 2-3, or ≥4 cups/d), and decaffeinated coffee intake (never or <1, 1, or ≥2 cups/d).
Study End Points
The primary end point was OS, defined as time from randomization to death due to any cause. Patients who were still alive were censored for OS at their last known follow-up. The secondary end point was PFS, defined as time from randomization until first documented disease progression or death per Response Evaluation Criteria in Solid Tumors, version 1.0. Patients alive without documented progression were censored for PFS at the most recent disease assessment.
Assessment of Covariates
Body mass index (BMI) was calculated from weight in kilograms divided by and height in meters squared, measured at study entry. A physical activity score was calculated by summing the weekly expenditure of metabolic equivalent for a list of leisure-time activities in the diet and lifestyle questionnaire.17 Current use of medication, including aspirin intake, was assessed in the questionnaire. Diabetes status was ascertained from the medical record at enrollment and supplemented by the self-report on the questionnaire.21 Caffeine intake was computed by multiplying the frequency of consumption of each food by its caffeine content and summing contributions from all foods. Caffeine and alcohol intake were adjusted for total energy intake.22 The RAS wild-type tumors were defined as those lacking mutations in both KRAS and NRAS, and the RAS mutant tumors were those with either a KRAS or an NRAS mutation (eMethods in the Supplement).
Statistical Analysis
Data were analyzed from May 1 to August 31, 2018. The primary exposure was total consumption of coffee, whereas the specific type of coffee (caffeinated vs decaffeinated) was considered in secondary analyses. Baseline characteristics by frequency of total coffee consumption were compared using the Kruskal-Wallis test, χ2 test, or Fisher exact test. Survival curves were generated using the Kaplan-Meier method,23 with statistical significance measured by the log-rank test.24 Cox proportional hazards regression25 was used to examine the association of coffee consumption with OS and PFS while controlling for other factors. The assumption of proportional hazards was tested and satisfied by evaluating a time-dependent variable, which was the product of total coffee intake and time. In both the log-rank test and Cox proportional hazards regression, we tested for a linear trend using frequency of consumption as a continuous variable.
We created a baseline model that was adjusted for age at enrollment and total energy intake,22 as well as a multivariable model to control for additional factors known or suspected to affect survival in patients with CRC, including sex, Eastern Cooperative Oncology Group performance status, prior adjuvant chemotherapy, chemotherapy backbone, assigned treatment arm, BMI, and physical activity. Missing covariates were replaced with the median (1 patient missing BMI and 3 patients missing physical activity).
We analyzed the association of total coffee consumption with OS and PFS across strata of other potential covariates associated with patient outcome, including age, sex, race, Eastern Cooperative Oncology Group performance status, prior adjuvant chemotherapy, chemotherapy backbone, assigned treatment arm, BMI, physical activity, smoking status, and alcohol intake. Interactions were assessed by entering in the model the cross product of coffee consumption as a continuous variable and the stratification variable, evaluated by the likelihood ratio test. Data collection was conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center following Alliance policies. Statistical analysis was performed based on the study database frozen on January 18, 2018, using SAS software, version 9.4 (SAS Institute, Inc). Two-sided P ≤ .05 was considered statistically significant.
Results
Baseline Characteristics
After the exclusions, a total of 1171 patients with advanced or metastatic CRC were included in the analysis (Figure 1). The median patient age was 59 (interquartile range [IQR], 51-67) years, with 694 men (59%) and 477 women (41%). Most of the patients were White (1007 [86%]). Baseline characteristics by frequency of total coffee consumption are displayed in the eTable in the Supplement. Frequent coffee drinkers were more likely to be White (≥4 cups/d, 61 of 63 [97%]; never, 223 of 280 [80%]; P < .001) and male (≥4 cups/d, 52 of 63 [83%]; never, 142 of 280 [51%]; P < .001). They were also more likely to be current or former smokers (≥4 cups/d, 50 of 62 [81%]; never, 107 of 277 [39%]; P < .001), to have a higher median daily energy intake (≥4 cups/d, 2237 [range, 791-4122] kcal/d; never, 1729 [range, 601-4038] kcal/d; P < .001), and to have a higher median consumption of alcohol (≥4 cups/d, 1 [range, 0-29] g/d; never, 0 [range, 0-9]; P < .001).
Association of Coffee Intake With Cancer Progression and Death
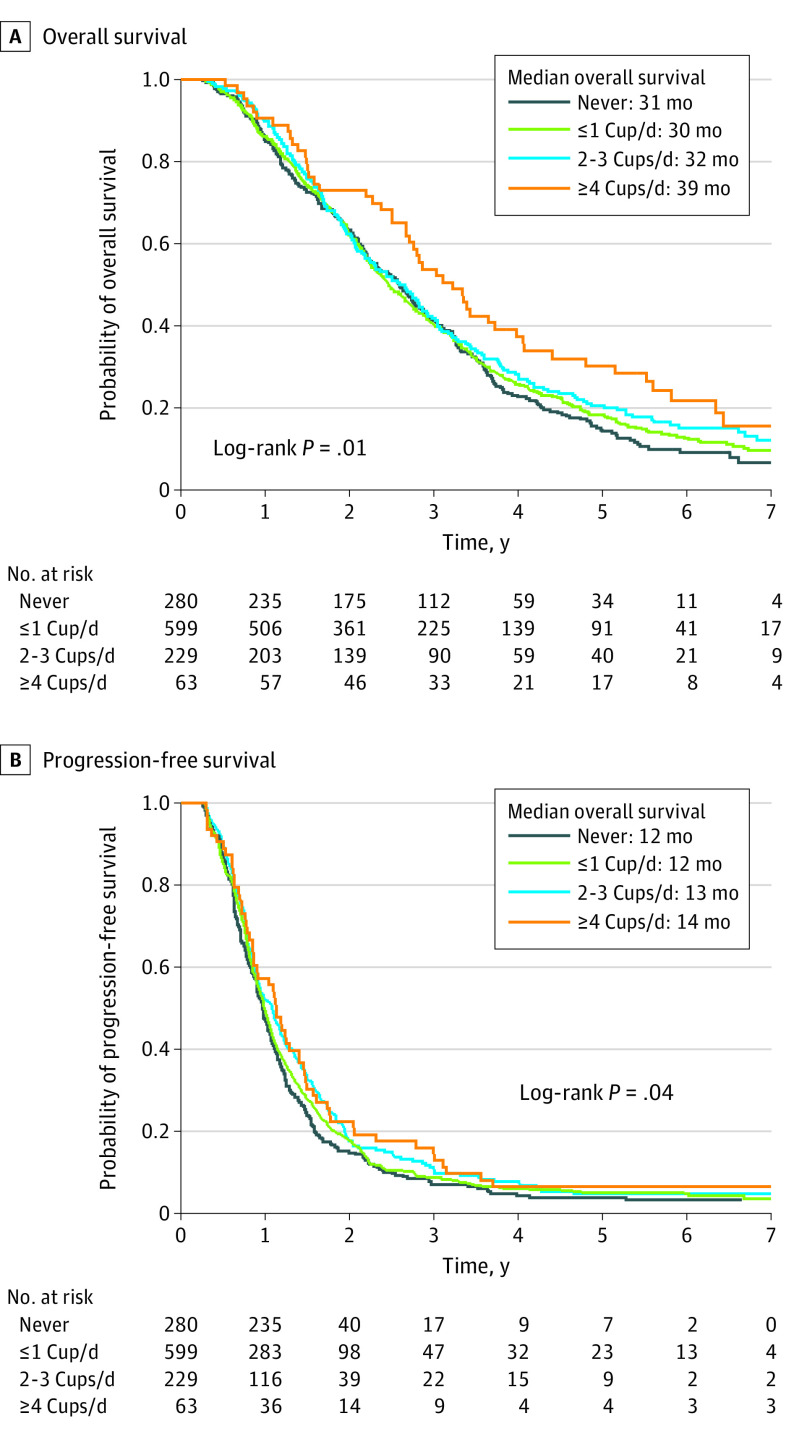
The median follow-up time among living patients was 5.4 years (10th percentile, 1.3 years; IQR, 3.2-6.3 years). A total of 1092 patients (93%) had died or had disease progression. Survival curves by frequency of total coffee consumption are shown in Figure 2 for OS (log-rank P = .01 for trend) and PFS (P = .04 for trend). In multivariable analyses (Table), higher total coffee intake was associated with a significant improvement in OS (hazard ratio [HR] for 1-cup/d increment, 0.93; 95% CI, 0.89-0.98; P = .004 for trend). Compared with never-drinkers, participants who consumed 2 to 3 cups/d had a multivariable HR for OS of 0.82 (95% CI, 0.67-1.00); those who consumed at least 4 cups of coffee per day, 0.64 (95% CI, 0.46-0.87). When total coffee intake and total caffeine intake were included in the model simultaneously, total coffee intake remained significantly associated with OS (HR for 1-cup/d increment, 0.90; 95% CI, 0.83-0.97; P = .01 for trend), whereas no association was noted for total caffeine intake (HR for 100-mg/d increment, 1.04; 95% CI, 0.97-1.11; P = .29 for trend). Higher total coffee intake was also associated with improved PFS (HR for 1-cup/d increment, 0.95; 95% CI, 0.91-1.00; P = .04 for trend). The multivariable HRs for PFS were 0.82 (95% CI, 0.68-0.99) for 2 to 3 cups/d and 0.78 (95% CI, 0.59-1.05) for at least 4 cups/d, compared with abstainers. Additional adjustment for race/ethnicity, smoking status, alcohol intake, aspirin use, diabetes status, RAS status, and intakes of milk, nondairy creamer, and sweeteners did not materially alter the association with OS or PFS.
Figure 2. Overall (OS) and Progression-Free (PFS) Survival by Frequency of Total Coffee Consumption.
Patients who consumed 1 cup/d or less were grouped into a single category for ease of graphic viewing. A, Median OS for patients who never consumed coffee was 31 (interquartile range [IQR], 16-46) months; 1 or fewer cups/d, 30 (IQR, 17-49) months; 2 to 3 cups/d, 32 (IQR, 19-50) months; and 4 or more cups/d, 39 (IQR, 19-67) months. B, Median PFS for patients who never consumed coffee was 12 (IQR, 8-18) months; 1 or fewer cups/d, 12 (IQR, 8-19) months; 2 to 3 cups/d, 13 (IQR, 9-21) months; and 4 or more cups/d, 14 (IQR, 9-21) months. The log-rank P value for trend was calculated by using frequency of consumption as a continuous variable.
Table. Associations of Total, Caffeinated, and Decaffeinated Coffee Consumption With Overall and Progression-Free Survival.
| Variable | Frequency of consumption | 1-Cup/d increment | P value for trenda | ||||
|---|---|---|---|---|---|---|---|
| Never | <1 Cup/d | 1 Cup/d | 2-3 Cups/d | ≥4 Cups/d | |||
| Total coffee consumption | |||||||
| Overall survival | |||||||
| No. of events/No. of patients | 246/280 | 248/301 | 253/298 | 191/229 | 49/63 | NA | NA |
| Adjusted HR (95% CI)b | 1 [Reference] | 0.88 (0.74-1.06) | 0.89 (0.74-1.07) | 0.82 (0.67-0.99) | 0.64 (0.47-0.88) | 0.93 (0.89-0.98) | .004 |
| Multivariable HR (95% CI)c | 1 [Reference] | 0.89 (0.75-1.07) | 0.91 (0.76-1.09) | 0.82 (0.67-1.00) | 0.64 (0.46-0.87) | 0.93 (0.89-0.98) | .004 |
| Progression-free survival | |||||||
| No. of events/No. of patients | 266/280 | 274/301 | 281/298 | 212/229 | 59/63 | NA | NA |
| Adjusted HR (95% CI)b | 1 [Reference] | 0.85 (0.72-1.01) | 0.95 (0.80-1.12) | 0.81 (0.68-0.98) | 0.78 (0.58-1.04) | 0.95 (0.91-1.00) | .04 |
| Multivariable HR (95% CI)c | 1 [Reference] | 0.86 (0.72-1.02) | 0.96 (0.81-1.14) | 0.82 (0.68-0.99) | 0.78 (0.59-1.05) | 0.95 (0.91-1.00) | .04 |
| Caffeinated coffee consumption | |||||||
| Overall survival | |||||||
| No. of events/No. of patients | 326/381 | 303/361 | 151/179 | 169/200 | 38/50 | NA | NA |
| Adjusted HR (95% CI)b | 1 [Reference] | 0.98 (0.84-1.15) | 1.05 (0.86-1.27) | 0.92 (0.76-1.11) | 0.68 (0.49-0.96) | 0.95 (0.90-1.00) | .04 |
| Multivariable HR (95% CI)c | 1 [Reference] | 0.98 (0.84-1.15) | 1.09 (0.89-1.33) | 0.93 (0.77-1.12) | 0.66 (0.47-0.94) | 0.95 (0.90-1.00) | .04 |
| Progression-free survival | |||||||
| No. of events/No. of patients | 355/381 | 335/361 | 168/179 | 187/200 | 47/50 | NA | NA |
| Adjusted HR (95% CI)b | 1 [Reference] | 0.95 (0.82-1.11) | 1.07 (0.89-1.28) | 0.86 (0.72-1.03) | 0.86 (0.63-1.17) | 0.96 (0.92-1.01) | .14 |
| Multivariable HR (95% CI)c | 1 [Reference] | 0.95 (0.82-1.11) | 1.09 (0.91-1.32) | 0.87 (0.72-1.04) | 0.85 (0.62-1.17) | 0.96 (0.92-1.01) | .15 |
| Decaffeinated coffee consumptiond | |||||||
| Overall survival | |||||||
| No. of events/No. of patients | 700/828 | 226/265 | 35/44 | 26/34 | NA | NA | |
| Adjusted HR (95% CI)b | 1 [Reference] | 0.99 (0.85-1.16) | 0.69 (0.49-0.98) | 0.63 (0.42-0.93) | 0.81 (0.71-0.93) | .002 | |
| Multivariable HR (95% CI)c | 1 [Reference] | 0.97 (0.83-1.13) | 0.68 (0.48-0.96) | 0.64 (0.43-0.95) | 0.81 (0.71-0.93) | .003 | |
| Progression-free survival | |||||||
| No. of events/No. of patients | 775/828 | 250/265 | 38/44 | 29/34 | NA | NA | |
| Adjusted HR (95% CI)b | 1 [Reference] | 1.01 (0.88-1.17) | 0.85 (0.61-1.19) | 0.74 (0.51-1.08) | 0.88 (0.78-1.00) | .05 | |
| Multivariable HR (95% CI)c | 1 [Reference] | 1.02 (0.88-1.18) | 0.85 (0.61-1.19) | 0.75 (0.52-1.09) | 0.88 (0.78-1.00) | .05 | |
Abbreviations: HR, hazard ratio; NA, not applicable.
Calculated by entering frequency of consumption as a continuous variable.
Adjusted for age (continuous) and total energy intake (continuous).
Adjusted for age (continuous), total energy intake (continuous), sex (female or male), Eastern Cooperative Oncology Group performance status (0 or 1-2), prior adjuvant chemotherapy (yes or no), chemotherapy backbone (leucovorin, fluorouracil, and oxaliplatin [mFOLFOX6] or leucovorin, fluorouracil, and irinotecan [FOLFIRI]), assigned treatment arm (bevacizumab, cetuximab, or bevacizumab plus cetuximab), body mass index (continuous), and physical activity (continuous).
Cutoffs for intake included never, less than 1, 1, or at least 2 cups/d.
Improved outcomes were also found when caffeinated or decaffeinated coffee was individually analyzed (Table). Compared with those who never consumed coffee, those who consumed at least 2 cups/d of decaffeinated coffee had a multivariable HR for OS of 0.64 (95% CI, 0.43-0.95; P = .003 for trend) and for PFS of 0.75 (95% CI, 0.52-1.09; P = .05 for trend). Consumption of at least 4 cups/d of caffeinated coffee was associated with improved OS with a multivariable HR of 0.66 (95% CI, 0.47-0.94; P = .04 for trend), whereas no significant association was noted between caffeinated coffee consumption and PFS (HR, 0.85; 95% CI, 0.62-1.17; P = .15 for trend).
To eliminate potential differences between participants who drank coffee and those who did not, we analyzed survival among those who drank any amount of coffee. Higher total coffee intake continued to be associated with improved OS (HR for 1-cup/d increment, 0.94; 95% CI, 0.89-0.99) and PFS (HR for 1-cup/d increment, 0.96; 95% CI, 0.92-1.01) in this subset of patients. To further address the possible influence of occult cancer or impending death on dietary habits, we performed a sensitivity analysis by extending the exclusion period to 180 days. Higher total coffee intake remained significantly associated with improvement in OS (HR for 1-cup/d increment, 0.94; 95% CI, 0.89-0.99); the association with PFS was generally unchanged, although slightly attenuated (HR for 1-cup/d increment, 0.96; 95% CI, 0.92-1.01).
Subgroup Analysis
In subgroup analyses (Figure 3), the association of increasing coffee consumption with improved OS was stronger in patients with BMI of less than 25.0 than those with BMI of at least 25.0 (HR, 0.87 [95% CI, 0.80-0.95] vs 0.96 [95% CI, 0.91-1.02]; P = .05 for interaction). The association with PFS was stronger in female patients (HR, 0.88 [95% CI, 0.81-0.96] vs 1.01 [95% CI, 0.95-1.07]; P = .01 for interaction). No other statistically significant interactions were detected.
Figure 3. Multivariable-Adjusted Hazard Ratios (HRs) and 95% CIs for Overall and Progression-Free Survival.
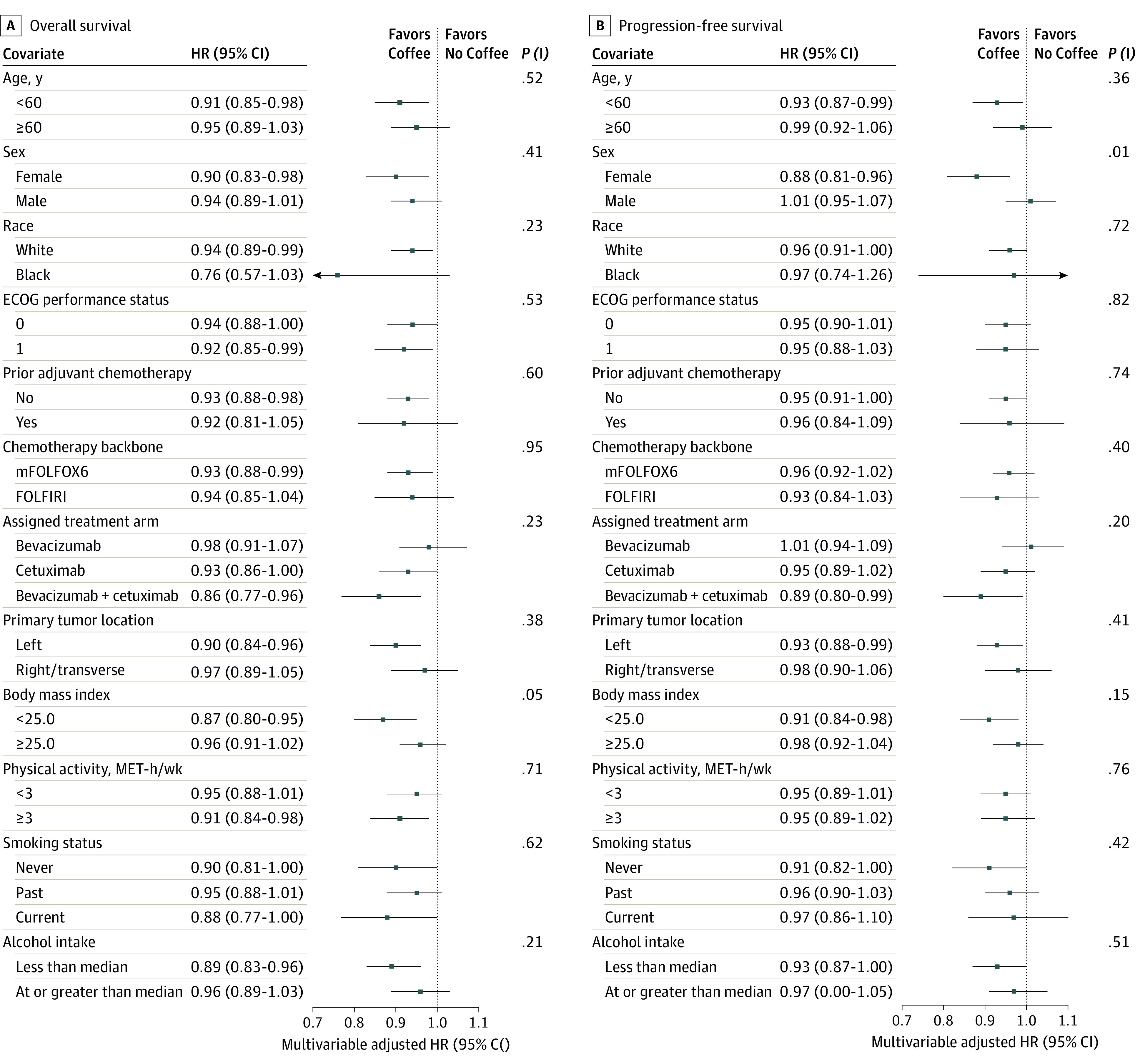
Data are calculated per 1-cup/d increment in total coffee consumption, across strata of covariates and adjusted for age (continuous), total energy intake (continuous), sex (female or male), Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1-2), prior adjuvant chemotherapy (yes or no), chemotherapy backbone (leucovorin, fluorouracil, and oxaliplatin [mFOLFOX6] or leucovorin, fluorouracil, and irinotecan [FOLFIRI]), assigned treatment arm (bevacizumab, cetuximab, or bevacizumab plus cetuximab), body mass index (continuous), and physical activity (continuous), excluding the stratification variable. MET indicates metabolic equivalent; P (I), P value for interaction.
Discussion
In 1171 patients with advanced or metastatic CRC, we found that increased total coffee intake was associated with a statistically significant improvement in both OS and PFS. When caffeinated coffee and decaffeinated coffee were considered separately, both were associated with improved OS, whereas the association with PFS was attenuated for caffeinated coffee. The significant association between higher coffee intake and improved patient outcomes remained even after multivariate adjustment for potential confounding and prognostic variables.
Previous studies have suggested an association between coffee consumption and CRC risk and outcome, with mixed findings. A recent meta-analysis examining coffee consumption and CRC risk26 detected a significant protective effect of coffee in 7 US studies but not among the total 26 studies. A subsequent study among participants in the UK Biobank27 found no association between coffee consumption and CRC risk. Owing to the conflicting data, coffee consumption is not currently recognized as a risk factor for CRC by the World Cancer Research Fund/American Institute for Cancer Research.28 Regarding coffee consumption and CRC survival, higher coffee intake was associated with reduced cancer recurrence and death in 953 patients with stage III colon cancer from the CALGB 89803 (Alliance) Diet and Lifestyle Companion study.13 In another study of 1599 patients with stages I to III CRC from 2 prospective cohorts,12 higher coffee intake was associated with a lower risk of overall and CRC-specific mortality, particularly among those with stage III disease.
One possible hypothesis to explain the underlying biological mechanism of the anticancer effects of coffee consumption involves coffee’s effects on the insulin pathway. Caffeine increases insulin sensitivity of tissues and therefore has antihyperinsulinemic effects. Studies have associated indicators of insulin resistance, such as increased levels of circulating C-peptide (a marker of insulin secretion)6 and higher dietary insulin load,5,7 with worse survival in patients with CRC. Although the insulin-modulating effects of caffeine have been most extensively studied,29 cross-sectional studies have also shown correlations between consumption of decaffeinated coffee and circulating levels of C-peptide and adiponectin (a marker of insulin sensitivity).10,30 The present study detected more marked associations with OS in participants with lower BMI and with PFS in women. Interestingly, both women and individuals with lower BMI have higher levels of insulin sensitivity.31 These findings are considered exploratory in nature and bear further examination in future research.
A recent analysis of the UK Biobank32 found an inverse association between coffee consumption and all-cause mortality for all types of coffee, including instant, ground, and decaffeinated, suggesting a role for noncaffeine constituents of coffee in this association. One potential etiologic molecule is chlorogenic acid, which has been shown to modify blood glucose levels and tolerance in humans and rats.33,34 The present study detected a statistically significant survival benefit associated with consumption of coffee but did not find a similar association with total caffeine intake. This may indicate a stronger role for these noncaffeine components of coffee in CRC outcomes.
Alternative hypotheses focus on coffee’s other biological effects. Coffee is the largest source of dietary antioxidants in the United States.35 Studies have implicated high oxidative stress in CRC development and metastases.36,37 As such, a greater consumption of antioxidants could potentially slow the development of such cancers. In addition, kahweol is another component in coffee with anti-inflammatory and proapoptotic effects that may decrease mutagenesis and cancer progression.8,9,38 Further research is ongoing to identify other bioactive molecules in coffee and their effect on cancer in humans. Both insulin sensitization and other anticancer effects may work in combination to slow tumor growth and development.
The data analyzed for this study were derived from a large, multicenter, randomized clinical trial. This source of data is advantageous owing to the trial’s detailed patient eligibility criteria, standardized treatment procedures, and careful follow-up for end points such as OS and PFS. Dietary information was prospectively collected using a validated food frequency questionnaire. Our results remained statistically significant after controlling for demographic, lifestyle, disease, and treatment-related variables thought to affect outcomes of patients with CRC, which were collected at the time of patient enrollment in the clinical trial.
Limitations
Despite these considerations, our results may have been confounded by factors that were not captured in the clinical trial or associated questionnaire, such as sleep habits, employment, physical activity not related to dedicated exercise, or changes in coffee consumption after cancer diagnosis. Furthermore, most patients who consume coffee while being treated for cancer likely consumed coffee before the cancer diagnosis, so these results do not allow us to discern whether the consumption of coffee acts directly on active tumors or whether coffee drinkers tend to develop less aggressive tumors. In a population of patients with advanced cancer, we considered the possibility that coffee drinkers may be more robust or have a lesser burden of cancer; however, we did not find any significant differences in performance status or disease characteristics across categories of coffee consumption. The population of patients with cancer enrolled in CALGB/SWOG 80405 may not be representative of the general population of patients with CRC. However, this clinical trial recruited patients from academic and community hospitals across North America, and similar randomized clinical trials are routinely used to determine the standards of care for treatment of CRC and other forms of cancer. In addition, these results do not represent a causal relationship between coffee and survival, which requires randomized intervention studies.
Conclusions
This large cohort study detected an association between increased consumption of coffee and improved CRC outcomes. These findings are consistent with those of previous epidemiological studies, although this is the first such study, to our knowledge, to show a protective effect of coffee consumption in patients with advanced or metastatic CRC. Further research is needed to elucidate the specific mechanism driving these associations.
eMethods. Determination of KRAS and NRAS Mutation Status
eTable. Baseline Characteristics by Frequency of Total Coffee Consumption
References
- 1.Kindler HL, Shulman KL. Metastatic colorectal cancer. Curr Treat Options Oncol. 2001;2(6):459-471. doi: 10.1007/s11864-001-0068-7 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.van Zutphen M, Kampman E, Giovannucci EL, van Duijnhoven FJB. Lifestyle after colorectal cancer diagnosis in relation to survival and recurrence: a review of the literature. Curr Colorectal Cancer Rep. 2017;13(5):370-401. doi: 10.1007/s11888-017-0386-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18(8):e457-e471. doi: 10.1016/S1470-2045(17)30411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan C, Bao Y, Sato K, et al. Influence of dietary insulin scores on survival in colorectal cancer patients. Br J Cancer. 2017;117(7):1079-1087. doi: 10.1038/bjc.2017.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27(2):176-185. doi: 10.1200/JCO.2008.17.9945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Oyarvide V, Yuan C, Babic A, et al. Dietary insulin load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803 (Alliance). J Natl Cancer Inst. 2019;111(2):170-179. doi: 10.1093/jnci/djy098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bøhn SK, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol Nutr Food Res. 2014;58(5):915-930. doi: 10.1002/mnfr.201300526 [DOI] [PubMed] [Google Scholar]
- 9.Cárdenas C, Quesada AR, Medina MA. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS One. 2011;6(8):e23407. doi: 10.1371/journal.pone.0023407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hang D, Kværner AS, Ma W, et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am J Clin Nutr. 2019;109(3):635-647. doi: 10.1093/ajcn/nqy295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita K, Yatsuya H, Muramatsu T, Toyoshima H, Murohara T, Tamakoshi K. Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: a cross-sectional study. Nutr Diabetes. 2012;2:e33. doi: 10.1038/nutd.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Ding M, Yuan C, et al. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology. 2018;154(4):916-926.e9. doi: 10.1053/j.gastro.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guercio BJ, Sato K, Niedzwiecki D, et al. Coffee intake, recurrence, and mortality in stage III colon cancer: results from CALGB 89803 (Alliance). J Clin Oncol. 2015;33(31):3598-3607. doi: 10.1200/JCO.2015.61.5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563-572. doi: 10.1056/NEJMoa0808268 [DOI] [PubMed] [Google Scholar]
- 15.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672-680. doi: 10.1200/JCO.2008.19.8135 [DOI] [PubMed] [Google Scholar]
- 16.Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379. doi: 10.1200/JCO.2007.12.5906 [DOI] [PubMed] [Google Scholar]
- 17.Guercio BJ, Zhang S, Ou FS, et al. Associations of physical activity with survival and progression in metastatic colorectal cancer: results from cancer and leukemia group B (Alliance)/SWOG 80405. J Clin Oncol. 2019;37(29):2620-2631. doi: 10.1200/JCO.19.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. doi: 10.1001/jama.2017.7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790-796. doi: 10.1016/0002-8223(93)91754-E [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 21.Brown JC, Zhang S, Ou FS, et al. Diabetes and clinical outcome in patients with metastatic colorectal cancer: CALGB 80405 (Alliance). J Natl Cancer Inst Cancer Spectr. 2019;4(1):pkz078. doi: 10.1093/jncics/pkz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4)(suppl):1220S-1228S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 25.Cox DR. Regression Models and Life-tables: Breakthroughs in Statistics. Springer; 1992:527-541. doi: 10.1007/978-1-4612-4380-9_37 [DOI] [Google Scholar]
- 26.Sartini M, Bragazzi NL, Spagnolo AM, et al. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2019;11(3):E694. doi: 10.3390/nu11030694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran KT, Coleman HG, McMenamin UC, Cardwell CR. Coffee consumption by type and risk of digestive cancer: a large prospective cohort study. Br J Cancer. 2019;120(11):1059-1066. doi: 10.1038/s41416-019-0465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira AR, Abar L, Chan DSM, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28(8):1788-1802. doi: 10.1093/annonc/mdx171 [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Xue W, Liang S, Zhao J, Zhang X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: a systematic review and meta-analysis. Nutr J. 2016;15(1):103. doi: 10.1186/s12937-016-0220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T, Willett WC, Hankinson SE, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in US women. Diabetes Care. 2005;28(6):1390-1396. doi: 10.2337/diacare.28.6.1390 [DOI] [PubMed] [Google Scholar]
- 31.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(suppl 1):60-75. doi: 10.1016/j.genm.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loftfield E, Cornelis MC, Caporaso N, Yu K, Sinha R, Freedman N. Association of coffee drinking with mortality by genetic variation in caffeine metabolism: findings from the UK Biobank. JAMA Intern Med. 2018;178(8):1086-1097. doi: 10.1001/jamainternmed.2018.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunnicliffe JM, Eller LK, Reimer RA, Hittel DS, Shearer J. Chlorogenic acid differentially affects postprandial glucose and glucose-dependent insulinotropic polypeptide response in rats. Appl Physiol Nutr Metab. 2011;36(5):650-659. doi: 10.1139/h11-072 [DOI] [PubMed] [Google Scholar]
- 34.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78(4):728-733. doi: 10.1093/ajcn/78.4.728 [DOI] [PubMed] [Google Scholar]
- 35.Svilaas A, Sakhi AK, Andersen LF, et al. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr. 2004;134(3):562-567. doi: 10.1093/jn/134.3.562 [DOI] [PubMed] [Google Scholar]
- 36.Babbs CF. Free radicals and the etiology of colon cancer. Free Radic Biol Med. 1990;8(2):191-200. doi: 10.1016/0891-5849(90)90091-V [DOI] [PubMed] [Google Scholar]
- 37.Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer. 2002;101(4):395-397. doi: 10.1002/ijc.10610 [DOI] [PubMed] [Google Scholar]
- 38.Choi DW, Lim MS, Lee JW, et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol Ther (Seoul). 2015;23(2):128-133. doi: 10.4062/biomolther.2014.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Determination of KRAS and NRAS Mutation Status
eTable. Baseline Characteristics by Frequency of Total Coffee Consumption