Abstract
Nanoparticles are now widely used in various aspects of life, especially zinc oxide nanoparticles (ZnNPs) that used in mouth washing, cosmetics, sunscreens, toothpaste and root canal flings. This research aims to determine the impact of ZnNPs on healthy mice's brain tissue. ZnNPs have caused major changes in the brain monoamines (dopamine, norepinephrine and serotonin) and ions such as Ca2+, Na+, K+ and Zn2+. Concerning the histological picture, administration of ZnNPs caused some histopathological impairment in brain tissue. In addition, ZnNPs reduced the level of glutathione and catalase in brain tissue, although an increase in the level of nitrite / nitrate and ROS was observed, while the level of malondialdhyde was not significantly altered. Moreover, ZnNPs induced DNA fragmentation in brain of mice. Collectively, the obtained results revealed that ZnNPs affected the brain levels of investigated monamines, ions, enzymatic and non-enzymatic antioxidants thus they may have potential influence on central nervous system.
Keywords: Zinc oxide nanoparticles, Brain monoamines, Histopathology, Oxidative stress, DNA-fragmentation, Mice
1. Introduction
Over the last decade, nanotechnology is rapidly developing and has been one of the fastest-growing areas of science. Synthetic nanoparticles could be metal nanoparticles (e.g. gold and silver nanoparticles) or metal oxide nanoparticles (e.g. zinc oxide). Zinc oxide nanoparticles (ZnNPs) are widely used in food for medicinal purposes as Zn is a dietary supplement and food additive, which was applied to food packaging materials. Furthermore, ZnNPs are commonly used in medicine, because they used to be coated on dental implants because of their excellent antibacterial and antifungal abilities (Elshama et al., 2018).
The use of ZnNPs became controversial in recent years while ZnNPs can easily pass through the cell membrane and interact with cellular macromolecules leading to therapeutic effects like oxidative stress that has been found to have caused cytotoxic effects on certain organs (Elshama et al., 2018). Xiao et al., 2016, Elshama et al., 2018 reported that, due to their high solubility, ZnNPs have toxic effects on certain tissues, leading to cytotoxicity, oxidative stress and mitochondrial dysfunction. Meanwhile, ZnNPs have been approved by the Food and Drug Administration as a new and potent anticancer therapy. However, Reddy et al. (2007) reported that ZnNPs have minimal adverse effect on human cells. Due to the increasing use of metallic nanoparticles in medicine, more and more attention is paid to the safety of using them for the central nervous system (CNS) (Sawicki et al., 2019).
Therefore, both toxicological risks and therapeutic advantages for the use of ZnNPs in medicine are urgently required to be evaluated. In addition, it is necessary to determine whether their toxicity is reversible, and whether this toxicity depends on the concentration and period of particulate exposure (Elshama et al., 2018). Consequently, the purpose of this study is to investigate the neuro-biochemical effects of ZnNPs on healthy mice's brain.
2. Materials and methods
2.1. Characterization of ZnNPs
From the International Company for Scientific and Medical Supplies, Cairo, Egypt; zinc oxide nanoparticles were purchased. The powder material's morphology was examined by JEOL 6380 scanning electron microscopy (SEM) (JEOL Ltd., Tokyo, Japan). To know the structural detail in terms of SEM analysis, the prepared powder was coated on carbon tape fixed on sample holder and the unwanted material was eliminated from the air blower. The carbon tape with nanopowder materials sample holder was transferred to a specialized glass chamber and sputtered with platinum (~2–3 s). Once the sputtering was completed, sample holder was fixed in SEM and analyzed the materials morphology.
2.2. Animals and ZnNPs administration
The animals of this experiment were twenty male Swiss albino mice weighing 20–25 g (9 to11 weeks). All mice were fed a standard diet ad libitum and water as well. State authorities approved the experiment and followed Egyptian animal-protection rules. We divided animals into two groups (Ten mice per group). The first group was served as a control, where; each mouse received intrapretoneal (i.p.) injection of (100 μl − 0.9%) saline for 5 days. Mice of the second group were intrapretoneally received 5.6 mg/kg of ZnNPs (Xie et al., 2012) once daily for 5 days. At the end of the experimental phase, all animals were then cervically decapitated.
2.3. Tissue sampling
Brains were quickly excised from the skulls after rapid cervical decapitation, blotted out with filter paper. Brains were split into two hemispheres for measuring (1) potassium (K+), calcium (Ca2+), sodium (Na+) and zinc (Zn2+) levels, (2) the monoamines, dopamine (DA), norepinephrine (NE) and serotonin (5-HT) content, (3) the markers of the oxidative stress, glutathione (GSH), catalase (CAT), nitrite/nitrate, malondialdehyde (MDA) and Reactive Oxygen Species (ROS), (4) DNA fragmentation and (5) brain histopathology. For the neuro-biochemical investigations, the brain hemispheres were weighed quickly then stored at -80°C until being used. For the histopathological study, brains were fixed in formalin.
The brain index was calculated as ratio of brain weight (mg) to mice body weight (g).
2.4. Ions concentration in the brain
As mentioned in Murphy (1987), the brain ion centration of K+, Ca2+, Na+, and Zn2+ by using Perkin-Elmer 2380 atomic absorption.
2.5. Brain monoamines contents
Estimation of the brain monoamines: dopamine (DA), norepinephrine (NE) and serotonin (5-HT) were carried out according to the method of Ciarlone (1978).
2.6. Brain oxidative status
To estimate the markers of brain oxidative stress; the brain hemispheres were prepared in 50 mM Tris–HCl and 300 mM sucrose (Tsakiris et al., 2004). The used dilution of the brain homogenate was 1:10.
The reduced level of glutathione (GSH) was determined by Ellman (1959) in brain homogenates. To determine nitrite/nitrate level in brain homogenate, we used Green et al. (1982) method. The activity of catalase was determined by Aebi (1984) in brain homogenate samples. To estimate the malondialdehyde level in brain homogenate, Ohkawa et al. (1979) method was used. Finally, according to Vrablic et al. (2001) the reactive oxygen species (ROS) generation was determined.
2.7. Brain histology
After fast cervical decapitation, brain tissues of five mice from each group were immediately fixed in 10% formalin for 24 h. Then samples were dehydrated and processed for paraffin sectioning (5 µm sections). Thereafter, Sections were de-paraffinized and stained with hematoxylin and eosin (Drury and Wallington 1980).
2.8. DNA fragmentation assay
By using agarose gel electrophoresis; the assay of DNA fragmentation was done. Firstly; DNA was extracted from the brain of mouse according to Aljanabi and Martinez (1997). A gel was prepared with 2% agarose containing 200 µg/ml (0.1%) ethidium bromide. The DNA samples were mixed with loading buffer (bromophenol blue 0.25%, xylene cyanole FF 0.25% and glycerol 30%) and loaded into the DNA/lane wells (20 µl) with a standard molecular-sized ladder marker (Pharm’acia Biotech., USA). At a current of 50 mA; the gel was electrophoresed using the submarine gel electrophoresis machine for 1.5 h. Finally, with illumination under UV light, the DNA was visualized and photographed.
2.9. Statistical analysis
The data was shown as means ± standard error. According to the student t-test at p ≤ 0.05, the significance between the treated and the control animals was determined.
3. Results
The ZnNPs SEM characterization showed that the nanoparticles had a scale of 15–30 nm with a spherical form and smooth surfaces (Fig. 1). The administration of ZnNPs at a dose level of (5.6 mg/kg b.wt.) to mice induced a significant increase in the contents of brain DA, NE and 5-HT with a percentage change (41.15%, 142.88% and 52.47%) respectively; as compared to control group (Table 1). Mice of the ZnNPs showed a significant decrease in brain levels of K+, Ca2+ and Na+ with a percentage change −48.99% , −53.18% and −55.62% respectively; while the level of Zn2+ was increased significantly (P ≤ 0.05) with a percentage change of 1879.8% (Table 2).
Fig. 1.
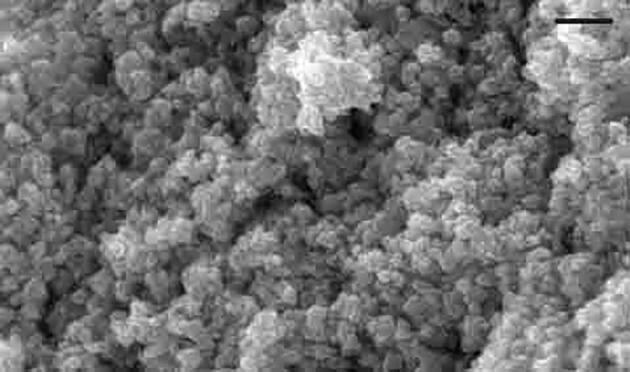
Characterization of ZnNPs by scanning electron microscopy. Scale = 100.
Table 1.
Effect of ZnNPs injection on brain dopamine (DA), Norepinephrine (NE) and Serotonin (5-HT) contents in male mice.
| Group | DA (µg/g) | NE (µg/g) | 5-HT (µg/g) |
|---|---|---|---|
| Control | 978.6 ± 43.0 | 312.0 ± 15.7 | 541.5 ± 22.2 |
| ZnNPs | 1755 ± 30.8(41.15%)* | 757.8 ± 27.2 (142.88%)* | 825.6 ± 32.8 (52.47%)* |
Values are expressed as mean ± SE. *: Significant against control group at P ≤ 0.05.
(): % difference with respect to control value.
Table 2.
Effect of ZnNPs on the level of mice brain Ca2+, Na+, K+ and Zn2+ for 5 consecutive days.
| Group | K+ (mg/g) | Ca2+ (mg/g) | Na+ (mg/g) | Zn2+ (mg/g) |
|---|---|---|---|---|
| Control | 105.2 ± 4.63 | 67.5 ± 3.65 | 95.15 ± 6.19 | 01.74 ± 0.02 |
| ZnNPs | 53.66 ± 2.35 (−48.99%)* | 31.6 ± 2.60 (−53.18%)* | 42.22 ± 2.23 (−55.62%)* | 34.45 ± 1.82 (1879.8%)* |
Values are expressed as mean ± SE. *: Significant against control group at P ≤ 0.05.
(): % difference with respect to control value.
Concerning the histological effects of ZnNPs on brain tissue; it was cleared that ZnNPs induced peri-neural vascularization, vascular congestion versus control group as shown in (Fig. 2).
Fig. 2.
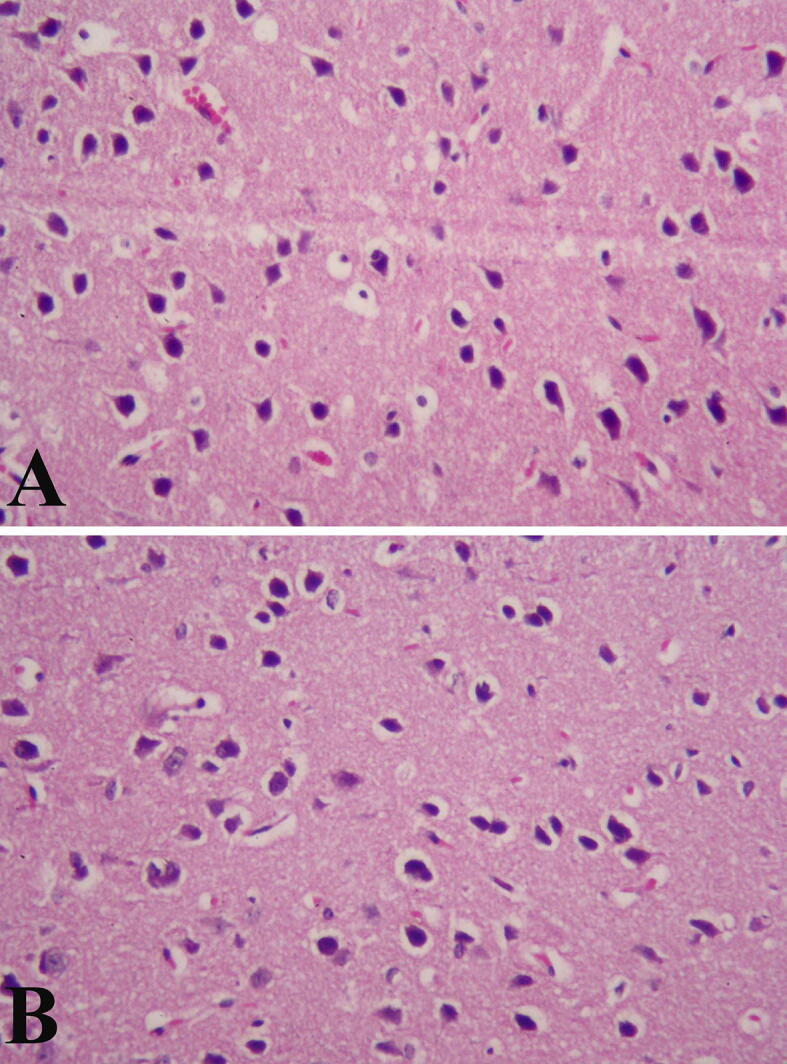
Histological picture of mice brain treated with ZnNPs (A) Control brain tissue, (B) Brain architecture after Nano zinc oxide injection. Sections were stained with H&E. Magnification × 100.
The data tabulated in Table 2 showed that ZnNPs induced a substantial increase in brain NO and ROS levels, with a percentage difference of 41.44% and 616.6%, respectively, while a non-significant change in brain MDA was reported at 17.68%. On the other hand, a significant decrease was observed at P ≤ 0.05 in brain GSH level and CAT activity with a percentage change of −27.44% and −60.13%, respectively (Table 3).
Table 3.
Effect of ZnNPs on brain GSH level, CAT activity, nitrite/nitrate, MDA and ROS levels in male Swiss albino mice.
| Group | GSH (mg/g) | CAT (U/g) | Nitrite/nitrate (μmol/g) | MDA (nmol/g) | ROS (µmol/g) |
|---|---|---|---|---|---|
| Control | 13.38 ± 0.57 | 1039 ± 28.8 | 69.20 ± 2.27 | 23.09 ± 0.94 | 0.061 ± 0.003 |
| Nano ZnO | 09.41 ± 0.31 (−27.44%)* | 406.3 ± 6.18 (−60.13%) * | 99.28 ± 2.23 (41.44%)* | 27.68 ± 1.48 (17.68%) | 0.43 ± 0.03 (616.6%)* |
Values are M ± SE. *, Significant against control group at P ≤ 0.05.
(): % difference with respect to control value.
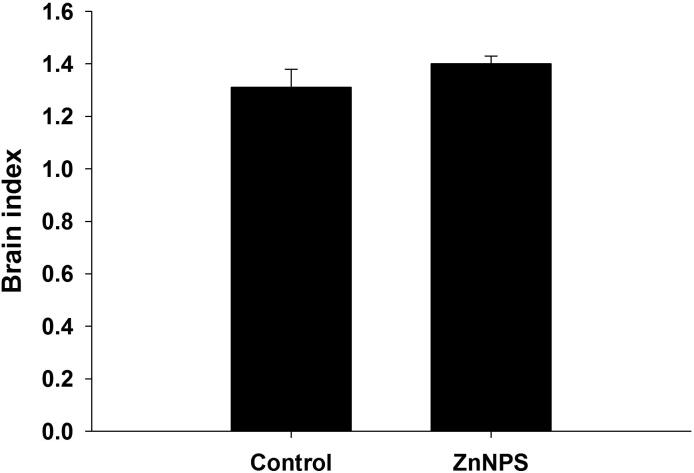
Healthy mice administered ZnNPs displayed apoptotic DNA fragmentation in brain tissue that was clearly demonstrated on the agarose gel and identified by ethidium bromide fluorescence (Fig. 3). However, the DNA of normal brain tissue did not reveal any ladder (Fig. 4, lane 1). A genomic DNA ladder was formed and observed when mice were inoculated with ZnNP (Fig. 4, lane 2). Finally, ZnNPs caused a non-significant change in the brain index (Fig. 3).
Fig. 3.
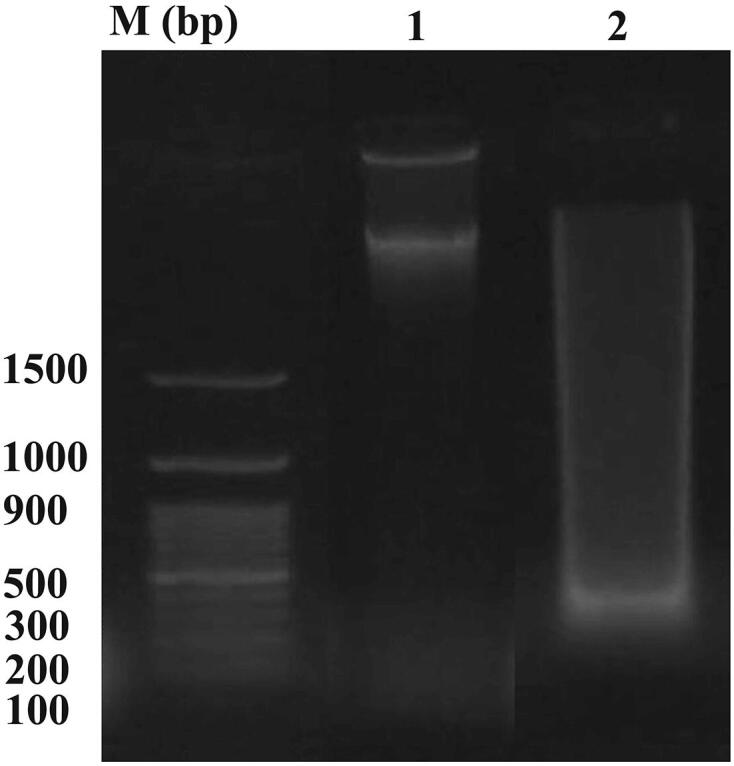
Effect of ZnNPs treatment on brain of healthy mice on agarose gel electrophoresis photograph of DNA extracted from brain tissue of normal and ZnNPs-inoculated mice. M−Marker, Lane1-Normal control group showed no DNA laddering, Lane 2- ZnNPs treated group showed DNA laddering band.
Fig. 4.
Effect of ZnNPs treatment on the brain index in healthy mice. Values are presented as mean ± SE.
4. Discussion
Researchers have tried in recent years to exploit the unique properties of nanoparticles, particularly ZnNPs, in the treatment of certain diseases (Elshama et al, 2018). Du et al. (2018) & Sruthi et al. (2018) reported for biomedical applications of ZnNPs; the size, shape, dose and exposure time should be determined and its toxicity based on its physical properties and dosage.
In the present study, the size of the ZnNPs under investigation was 15–30 nm with spherical shape and smooth surfaces. When the size of the particle decreases, more ZnNPs are taken up by the cells, leading to higher toxicity. Large surface area leads to exposure of more reactive sites at ZnNPs surface; thus better biological responses was obtained in the living cells (Siddiqi et al., 2018).
In the present study, ZnNPs induced a significant increase in brain monoamines “DA, NE & 5-HT” contents on the other hand, it decreased K+, Ca2+, and Na+ ions level significantly in brain homogenate as compared to control group. The present records go hand in hand with those of Kumar et al., 2010, Ryu et al., 2014, Sruthi et al., 2018.
Torabia et al. (2020) reported that ZnNPs affect neurotransimetters release. Moreover; Doboszewska et al. (2017) cleared that Zn2+ ions involved in the treatment of depression. Kumar et al. (2010) reported that Zn2+ ions are inhibitors of monoamine oxidase that leading to monoamines “DA, NE and 5-HT” accumulation at the nerve terminals. So the increment in monoamine contents in the present study may be due to Zn2+ ions act as monoamine oxidase inhibitor.
Ryu et al. (2014) demonstrated that ZnNPs alter various voltage-gated ion channels activities in hippocampal neurons and the neurons excitability of the brain. In addition, Mathie et al. (2006) revealed that ZnNPs regulate K+, Na+ and Ca2+ voltage-gated ionic conductance. Similarly, Bondarenko et al. (2013) suggested that in vitro nano ZnO modulate synaptic transmission and ionic homeostasis and regulate the neuronal physiological functions. In addition, Xie et al. (2012) reported that nano ZnO exposure activated K+ currents where its efflux was increased; these may explain the obtained reduction in brain K+ ions level after ZnNPs injection in the present study.
Since Magistretti et al. (2003) reported that Zn modulates the function of the Ca2+ voltage-gated channels, it was assumed that ZnNPs mediated the release of neurotransmitters, the physiological functions of the neurons and had potential influence on the CNS (Xie et al., 2012). In the same manner; Amara et al. (2015) showed that sub-acute ZnNPs dose resulted in a significant decrease of Ca2+ ions level in brain homogenate. Also, Thilsing and Jorgensen (2001) indicated the antagonism between Ca2+ and Zn2+ in dairy cows after zinc oxide gavage. Sruthi et al. (2018) revealed that ZnNPs treatment induced a significant Ca2+ ions level reduction in brain tissue.
Yongling et al. (2012) reported that ZnNPs ameliorate mice behavioral and cognitive impairments with depressive-like behaviors. In addition, Torabi et al. (2013) showed that Zn2+ deficiency might induce anxiety-like behavior in animals so; ZnNPs treatment to male rats at low and high doses induced analgesic, anxiolytic effects and reduced the locomotor activity in presence of acute stress. Furthermore; Kesmati et al. (2017) demonstrated that in animal models acute and chronic ZnNPs treatments (as novel sources of Zn2+) could be effective in modulation of pain perception and anxiety-like behaviors due to its effect on the neurochemical systems. In the presynaptic spaces; Zn2+ is co-released with the excitatory amino acid; glutamate that bounded to its receptor, charged ions (Na+ and Ca2+) pass through a channel (Koh and Choi, 1994, Paoletti et al., 1997).
Since; voltage-gated Na+ channels are essential for action potentials propagation along axons and control membrane excitability (Wood & Baker, 2001). So, blockers of voltage-gated Na+ channel have analgesic effects and are used to treat chronic pain in experimental models (Levinson et al., 2012). This may be explaining the reduction of Na+ ions level in this study. Similarly; Torabi et al. (2013) documented that Zn2+ promotes GABA release from inter neurons in the hippocampus, thus enhancing its inhibitory effects consequently decrease pre-synaptic glutamate release. This could explain the reduction in the levels of Ca2+ and Na+ ions in the current study. Nanoparticles of zinc oxide injection induced a significant increase in Zn2+ ions level in brain homogenate versus control group in the present investigations. These results are in agreement with (Cho et al., 2013). Both Cho et al. (2013) and Shim et al. (2014) stated that the NPs entered the brain tissue after administration of ZnNPs either by destroying the blood brain barrier or by neural transportation where toxic brain and blood effects were caused. NPs in the brain are able to enter neurons and move along axons or dendrites to other connected neurons. ZnNPs i.p. injection injured cerebral cortex and hippocampus and induced behavioral changes which attenuated the learning ability and memory. The highest accumulation in the brain was observed for NPs with a diameter of 18 nm after treatment by the oral administration (Han et al., 2011, Sawicki et al., 2019).
In the present study, some histological changes in mice 's brain were induced by ZnNPs and these results are in agreement with Lin et al. (2009), Najafzadeh et al. (2013) and Ben-Slama et al. (2015). Liang et al. (2018) noted, however, that in the brain, ZnNPs did not affect cell integrity or tissue morphology but caused minor damage. Ben-Slama et al. (2015) reported that the brain histological picture of rats treated with ZnNPs (10 mg/kg) for 5 consecutive days did not cause significant histological impairments; but caused edema and vascular congestion. Elshama et al. (2018) found that ZnNPs i.p. injection for prolonged time induced histopathological and ultrastructural changes in rat’s brains, depending on the dose and ROS generation.
Concerning the oxidative stress in the present study, ZnNPs induced a significant imbalance in antioxidant and oxidant system (a significant increase in nitrite/nitrate and ROS levels) in brain of mice as compared to control group, with exception; there was a non-significant change in MDA level after ZnNPs injection. Such findings are in line with Dkhil et al. (2015), Attia et al. (2018) and Torabia et al. (2018). In spite of Chitra & Annadurai (2013) reported that nano ZnO is non-toxic to human, another authors decided that in pathogens ROS were generated and oxidative stress was developed as a result of ZnNPs treatment; hence it is widely used as antibacterial, antifungal, antiviral and antiparasitic agents (Jiang et al., 2009, Dkhil et al., 2015).
Exposure to ZnNPs (2000 μg/L; high concentration) destructed the brain antioxidant system, while the low concentration (500 μg/l) supported the antioxidant activity (Sawicki et al., 2019). Moreover; Attia et al. (2018) decided that ZnNPs gavage (40 & 100 mg/kg, 7 days) caused a significant depletion of GSH level, SOD and CAT activities indicating that ZnNPs deteriorate brain antioxidant system with the subsequent oxidative and nitrosative stress. Torabi et al. (2018) indicated that in hippocampus; the highest dose of nano-ZnO (10 mg/kg) could decrease CAT activity and had no observable effect on MDA level. Also; Dawei et al. (2009) observed that nanoparticles of ZnO decreased MDA level. So, ZnONPs are dose and time dependent cytotoxicity (Lin et al., 2009, Najafzadeh et al., 2013).
Finally, our results proved that ZnNPs induced brain DNA fragmentation in mice. These findings are in agreement with Tian et al., 2015, Attia et al., 2018.
Tian et al. (2015) concluded that in vitro ZnNPs treatment caused DNA fragmentation in mice brain tumor cell lines. Meanwhile, according to in vivo study by Attia et al. (2018) showed that ZnNPs (40 or 100 mg/kg; <100 nm) induced brain DNA fragmentation. The authors attributed the DNA damage to ROS generation and oxidative stress after ZnNPs administration.
In conclusion, ZnNPs (with size particles: 15–30 nm) injection to mice induced alteration in brain monoamines, ions and some histological impairments as compared to control animals. Moreover; the investigated nanoparticles impaired the balance between antioxidant and free radicals levels while the level of MDA showed a non-significant change. Moreover, ZnNPs caused brain DNA fragmentation. However; further future investigations will be needed to precisely identify of the ZnNPs mechanism of action in brain tissue.
Acknowledgements
This study was supported by Research Supporting Project (RSP-2020/23), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Aljanabi S.M., Martinez I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucl. Acids Res. 1997;25(22):4692–4693. doi: 10.1093/nar/25.22.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S., Slama I.B., Omri K., Elghoul J., Lemir L., Ben Rhouma K., Abdelmelek H., Sakly M. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol. Ind. Health. 2015;31(12):1202–1209. doi: 10.1177/0748233713491802. [DOI] [PubMed] [Google Scholar]
- Attia H., Nounou H., Shalaby M. Zinc Oxide Nanoparticles Induced Oxidative DNA Damage, Inflammation and Apoptosis in Rat’s Brain after Oral Exposure. Toxics. 2018;6(2):29–49. doi: 10.3390/toxics6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Slama, I., Mrad, I., Rihane, N., EL Mir, L., Sakly, M., Amara, S., 2015. Sub-Acute Oral Toxicity of Zinc Oxide Nanoparticles in Male Rats. J. Nanomed. Nanotechnol. 6(3), 284-290.
- Bondarenko O., Juganson K., Ivask A., Kasemets K., Mortimer M., Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra K., Annadurai G. Antimicrobial activity of wet chemically engineered spherical shaped ZnO nanoparticles on food borne pathogen. Int. Food Res. J. 2013;20(1):59–64. [Google Scholar]
- Cho W.S., Kang B.C., Lee J.K., Jeong J., Che J.H., Seok S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlone A.E. Further modification of a fluorometric method for analyzing brain amines. Microchem. J. 1978;23:9–12. [Google Scholar]
- Dawei A.I., Zhisheng W., Angu Z. Protective effects of Nano-ZnO on the primary culture mice intestinal epithelial cells in in vitro against oxidative injury. Int. J. Nanotechnol. 2009;3:1–6. [Google Scholar]
- Dkhil M.A., Al-Quraishy S., Wahab R. Anticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunum. Int. J. Nanomed. 2015;10:1961–1968. doi: 10.2147/IJN.S79944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doboszewska, U., Wlaf, P., Nowak, G., RadziwoN-Zaleska, M., Cui, R., MByniec, K., 2017. Zinc in the Monoaminergic Theory of Depression: Its Relationship to Neural Plasticity. Neural Plast. 2017, 3682752. doi: 10.1155/2017/3682752. [DOI] [PMC free article] [PubMed]
- Du L.J., Xiang K., Liu J.H., Song Z.M., Liua Y., Cao A., Wang H. Intestinal injury alters tissue distribution and toxicity of ZnO nanoparticles in mice. Toxicol. Lett. 2018;295:74–85. doi: 10.1016/j.toxlet.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Drury R.A., Wallington E.A. 5th ed. Oxford University Press; Oxford, New York, Toronto: 1980. Carleton’s histological technique; pp. 188–291. [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Elshama S.S., Abdallah M.E., Abdel-Karim R.I. Zinc Oxide Nanoparticles: Therapeutic Benefits and Toxicological Hazards. Nanomed. J. 2018;5:16–22. [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Han D., Tian Y., Zhang T., Ren G., Yang Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J. Nanomed. 2011;6:1453–1461. doi: 10.2147/IJN.S18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Oberdörster G., Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009;11:77–89. [Google Scholar]
- Kesmati M., Zadehdarvish F., Jelodar Z., Torabi M. Vitamin C potentiate sedative effect of magnesium oxide nanoparticles on anxiety and nociception in the postpartum depression model. Nanomed. J. 2017;4(1):17–24. [Google Scholar]
- Koh J.Y., Choi D.W. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neurosci. 1994;60(4):1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Kumar M.V., Kumari B.N., Yellamma K. Zinc Toxicity to Aminergic Neurotransmitters in Rat Brain. Toxicol Int. 2010;17(2):52–58. doi: 10.4103/0971-6580.72670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S.R., Luo S., Henry M.A. The role of sodium channels in chronic pain. Muscle Nerve. 2012;46(2):155–165. doi: 10.1002/mus.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Chen A., Lai X., Liu J., Wu J., Kang Y., Wang X., Shao L. Neuroinflammation is induced by tongue-instilled ZnO nanoparticles via the Ca2+-dependent NF-κB and MAPK pathways. Part. Fibre Toxicol. 2018;15(1):39. doi: 10.1186/s12989-018-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Xu Y., Huang C.C., Ma Y., Shannon K.B., Chen D.R., Huang Y.W. Toxicity of nano and micro sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009;11:25–39. [Google Scholar]
- Magistretti J., Castelli L., Taglietti V., Tanzi F. Dual effect of Zn2+ on multiple types of voltage-dependent Ca2+ currents in rat palaeocortical neurons. Neurosci. 2003;117:249–264. doi: 10.1016/s0306-4522(02)00865-5. [DOI] [PubMed] [Google Scholar]
- Mathie A., Sutton G.L., Clarke C.E., Veale E.L. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Therapeut. 2006;11:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Murphy V.A. Method for determination of sodium, potassium, calcium, magnesium, chloride and phosphate in the rat choroid plexus by flame atomic absorption and visible spectroscopy. Anal. Biochem. 1987;161:144–151. doi: 10.1016/0003-2697(87)90664-6. [DOI] [PubMed] [Google Scholar]
- Najafzadeh H., Ghoreishi S.M., Mohammadian B., Rahimi E., Afzalzadeh M.R., Kazemivarnamkhasti M., Ganjealidarani H. Serum biochemical and histopathological changes in liver and kidney in lambs after Zinc Oxide nanoparticles administration. Vet. World. 2013;6:534–537. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P., Ascher P., Neyton J. High-Affinity Zinc Inhibition of NMDA NR1–NR2A Receptors. J. Neurosci. 1997;17(15):5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K.M., Feris K., Bell J., Wingett D.G., Hanley C., Punnoose A. Selective toxicity of Zinc Oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007;90(213902):2139021–2139023. doi: 10.1063/1.2742324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, H,J,, Seo, M.Y., Jung, S.K., Maeng, E.H., Lee, S.Y., Jang, D.H., Lee, T.J., Jo, K.Y., Kim, Y.R., Cho, K.B., Kim, M.K., Lee, B.J., Son, S.W., 2014. Zinc oxide nanoparticles: a 90-day repeated-dose dermal toxicity study in rats. Int. J. Nanomed. 9 Suppl. 2, 137-44. [DOI] [PMC free article] [PubMed]
- Sawicki K., Czajka M., Matysiak-Kucharek M., Fal B., Drop B., Męczyńska-Wielgosz S., Sikorska K., Kruszewski M., Kapka-Skrzypczak L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019;8:175–200. [Google Scholar]
- Siddiqi, K.S., ur Rahman, A., Tajuddin, S.M., Husen A., 2018. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 13, 141. [DOI] [PMC free article] [PubMed]
- Sruthi S., Ashtami J., Mohanan P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018;10:175–186. [Google Scholar]
- Thilsing T., Jorgensen R.J.R. Serum Calcium Response Following Oral Zinc Oxide Administrations in Dairy Cows. Acta Vet. Scand. 2001;42(2):271–278. doi: 10.1186/1751-0147-42-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Lin B., Lei W.L., Li K., Liu H., Xi Z. Neurotoxicity induced by zinc oxide nanoparticles: age-related differences and interaction. Sci. Rep. 2015;5:16117. doi: 10.1038/srep16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi M., Kesmati M., Harooni H.E., Varzi H.N. Different Efficacy of Nanoparticle and Conventional ZnO in an Animal Model of Anxiety. Neurophysiol. 2013;45(4):299–305. [Google Scholar]
- Torabia M., Kesmatia M., Pourrezab N., Najafzadeh V.H., Galehdari H. Neurobehavioral and biochemical modulation following administration of MgO and ZnO nanoparticles in the presence and absence of acute stress. Life Sci. 2018;203:72–82. doi: 10.1016/j.lfs.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Torabia M., Kesmatia M., Galehdaria H., Varzic H.N., Pourreza N. MgO and ZnO nanoparticles anti-nociceptive effect modulated by glutamate level and NMDA receptor expression in the hippocampus of stressed and non-stressed rats. Physiol. Behavior. 2020;214 doi: 10.1016/j.physbeh.2019.112727. [DOI] [PubMed] [Google Scholar]
- Tsakiris S., Schulpis K.H., Marinou K., Behrakis P. Protective effect of l-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol. Res. 2004;49:475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Vrablic A.S., Albright C.D., Craciunescu C.N., Salganik R.I., Zeisel S.H. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15(10):1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- Wood J.N., Baker M. Voltage-gated sodium channels. Curr. Opin. Pharmacol. 2001;1(1):17–21. doi: 10.1016/s1471-4892(01)00007-8. [DOI] [PubMed] [Google Scholar]
- Xiao L., Liu C., Chen X., Yang Z. Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem. Toxicol. 2016;90:76–83. doi: 10.1016/j.fct.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Xie Y., Wang Y., Zhang T., Ren G., Yang Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012;19:14. doi: 10.1186/1423-0127-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongling X., Wang Y., Zhang T., Ren G., Yang Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012;19:14. doi: 10.1186/1423-0127-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]