Abstract
Human Parainfluenza virus (HPIV) causes lower respiratory tract infections (LRTI) mostly in young children. Respiratory viral infections may decline T cells in circulation and display enhanced pathogenicity. This study is aimed to analyze T cells alterations due to HPIV in children with LRTIs. Children (N = 152) with bronchitis or pneumonia, admitted in tertiary care hospitals were included in the study. Respiratory samples (throat or nasopharyngeal swabs) were taken and HPIV genotypes (1–4) were analyzed through RT-PCR. Peripheral blood T cells, CD3+, CD4+, CD8+, and CD19+, were analyzed in confirmed HPIV positive and healthy control group children through flow cytometry. The positivity rate of HPIV was 24.34% and the most prevalent genotype was HPIV-3 (20.40%). HPIV-1 and HPIV-2 were detected in 0.66% and 02% children respectively. The T lymphocyte counts were observed significantly reduced in children infected with HPIV-3. CD4+ cell (1580 ± 97.87) counts did not change significantly but the lowest CD8+ T cell counts (518.5 ± 74.00) were recorded. Similarly, CD3+ and CD19 cell ratios were also reduced. The CD4/CD8 ratio was significantly higher (3.12 ± 0.59) in the study population as compared to the control group (2.18 ± 0.654). Changes in the count of CD8+ T cells were more pronounced in patients with bronchiolitis and pneumonia. It is concluded that CD8+ T cells show a reduced response to HPIV-3 in children with severe LRTIs suggesting a strong association of these cells with disease severity.
Keywords: Parainfluenza virus, T cells, Pneumonia, Bronchiolitis, Children
1. Introduction
The lower respiratory tract infections (LRTIs) remain a persistent health issue and a leading cause of hospitalization of about 11.9 million young children and mortality worldwide (Korsun et al., 2019, McAllister et al., 2019). LRTIs are responsible for 6.8% of deaths in neonates, 20% of deaths in children aged 1–12 months, and 12% of deaths in children aged 1–4 years (Sonawane et al., 2019). Common risk factors for LRTIs include indoor air pollution exposure, poor nutrition, overcrowding, low socioeconomic status, inadequate immunization, or premature birth (Dagvadorj et al., 2016).
Human parainfluenza virus (HPIV) is one of the most common pathogens associated with the serious and broad pathogenic spectrum of LRTI (Pawelczyk and Kowalski, 2017, Linster et al., 2018) such as pneumonia, bronchitis, and bronchiolitis especially in the pediatric, elder and immunocompromised host (Bose et al., 2019). HPIV infection does not confer complete protective immunity due to a lack of specific effective vaccines and recurrent infection reported throughout life (Bose et al., 2019). HPIV is the most important ubiquitous viral agent of the family Paramyxoviridae. There are four distinct types (HPIV 1–4) of HPIV are circulated (Linster et al., 2018, Bose et al., 2019).
Immunologically immature and immunocompromised individuals are more vulnerable to HPIVs and resulted in severe complications and disease. Usually, it has been taken that overwhelming diseases in children are linked to impaired responses of T cells, which are prerequisites to arbitrate viral clearance and ascertain long-lasting protective immunological memory (Meissner, 2016). The host T cells are being thoroughly studied in different respiratory viruses to find out the specific interaction of the virus with host immune responses. Some respiratory viruses like respiratory syncytial virus (RSV) has been reported with a decrease in circulating lymphocytes (Larranaga et al., 2009), while an increase in CD8+ T lymphocytes was observed in the child with severe adenovirus pneumonia (Tomoyo et al., 2000). HPIVs are respiratory pathogens responsible for serious infections of lower respiratory tract and as tere are no antivirals or vaccines available, it may become a serious health threat especially to children. Moreover, very little is known about T cells' responses to HPIV in association with LRTIs. So this study is designed to analyze the peripheral blood T cells count in HPIVs associated LRTIs in children.
2. Materials and methods
2.1. Study population
This was a cross-sectional study, designed for the diagnosis of HPIVs as well as for quantitative analysis of immune cells of the virus-positive patients and associated respiratory complications. The study includes children up to 10 years of age (N = 152) admitted to the pediatric wards or visited OPDs (outpatient department) of tertiary care hospitals in Peshawar Khyber Pakhtunkhwa province Pakistan. An inclusion criteria were setoff for the study population, such as; (i) children less than or up to 10 years of age (ii) children with the sign or symptoms/infections of LRTI. A child was thought to have LRTIs if they were showed the following signs or symptoms such as fever, cough (cough with phlegm in severe cases), sore throat, chest pain, dyspnea, panting, wheezing, or pneumonia and the abnormal number of blood cells according to clinical records. A control group (N = 20) with inclusion criteria, (i) with the same age and gender, (ii) with no present history of any clinical or virological or bacterial respiratory infections, were also included.
An informed written and oral consent was obtained from the parents/guardians’ of the study population at the time of sample collection. Demographic (age, gender) and clinical data (sign/symptoms of LRTIs, duration of infection, etc.) were collected through prescribed questionnaires. The study protocol was approved by the Ethical Committee, Centre of Biotechnology and Microbiology University of Peshawar Khyber Pakhtunkhwa Pakistan.
2.2. Sample collection
2.2.1. Respiratory specimens
Throat swab or nasopharyngeal swabs were collected with the help of trained medical persons. A spatula was used to press the tongue downward to the floor of the mouth and sterile cotton swab stick was used to swab both the tonsillar arches and the posterior nasopharynx. The samples were then transported in an appropriate viral transport medium to the Molecular Virology Lab, Zoology Department University of Peshawar Pakistan.
2.2.2. Blood
One mL venous blood was collected in sterile EDTA tubes after confirmation of HPIV by PCR. The samples were kept in a cold chain and transported to the laboratory within 1 h for T cells analysis.
2.3. HPIV detection and genotyping
HPIV RNA was extracted from diluted respiratory samples through TRIzolTM reagent (ThermoFisher USA) according to the suppliers’ instructions. One-step RT-PCR kit (ThermoFisher USA) was carried out in a thermal cycler (MyCycler™ Thermal Cycler System # 1709703) for single-step multiplex RT-PCR following the protocol of Bellau-Pujol et al. (2005). Briefly, HPIV 1–3 were amplified with primers specific for Hemagglutinin and Neuraminidase genes and HPIV4 with primers for Phosphoprotein gene, while primers to human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene were used as an internal control. The genotypes were confirmed with Hemi-nested multiplex PCR. All the samples were processed in duplicate. PCR amplified products were electrophoresed in 2% agarose gel and specific cDNA bands were visualized under UV transilluminator (Bio Rad 2000 #1708110EDU) and compared with 100 bp DNA ladder were used.
2.4. Immunological studies
Blood samples were analyzed for T cells subsets, CD3+, CD4+, CD8+, and CD19, through a flow cytometer (BD FACSCaliber USA). Standard protocols and fluorescently labeled antibodies were used for analysis. The cells counts were recorded as the number of cells per microliter (cells/µL). Normal cells ratio from a healthy control group for respective cells were used as standards for comparison of results. High blood count was recorded as cell count above the high level of the normal range and the low count was recorded as cells below the lower count of the normal range.
2.5. Data analysis
Demographic, clinical symptoms and age factor associated with the prevalence of virus were evaluated using chi square test or Fisher Exact test. Mean and the standard deviation was done for continuous variables. To assess the difference between HPIV patients and control group the unpaired t-test was used, and in case of significant differences between standard deviations, a non-parametric Mann Whitney U test was used for comparison. A two-sided value of p < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics of the study population
The demographic (age, gender, number) and clinical characteristics (hospitalization, sign/symptoms, clinical complications) of patients are shown in Table 1. A total of 152 study population and 20 children as a control group were included in the study. The mean age of study participants was 24.01 months. All the study population was hospitalized either in pediatric wards or in post-intensive care units (PICU). The mean duration of hospitalization was 3.5 days, and the PICU admission rate was 16.44%. Fever, cough, and dyspnea and panting were the main respiratory signs observed in the study population. The main clinical complications of the study population were pneumonia and bronchiolitis.
Table 1.
Baseline characteristics of the study population (N = 152).
| Variables | Frequency N (%) |
P-value | |
|---|---|---|---|
| Age range (months) | 24.01 | – | |
| Gender | Male | 93 (61.20) | 0.007 |
| Female | 59 (38.81) | ||
| Hospitalization | Ward | 127 (83.55) | 0.003 |
| Post ICU | 25 (16.44) | ||
| Hospital stay (mean days) | 3.5 | ||
| Signs | Fever | 98 (64.50) | 0.006 |
| Cough | 63 (41.44) | ||
| Cough with phlegm | 89 (58.55) | ||
| Difficult breathing | 109 (71.70) | ||
| Clinical Complications | Pneumonia | 83 (54.60) | 0.004 |
| Bronchiolitis | 69 (45.40) | ||
3.2. Viral etiologies and clinical blood cells
Out of all 152 patients with LRTIs tested for HPIV, 37 (24.34%) were found positive. HPIV-3 genotype showed the highest detection rate of 31 (20.40%), whereas the least one was HPIV-1 genotype-1 (0.66%) and HPIV-2 genotype (3, 2%). While the HPIV-4 genotype was not detected in any sample. Two samples (2%) were not amplified and considered untypable.
A high prevalence rate of HPIVs was observed in young children, that is, HPIV-3 was (19%) in infants (<12 months) and this proportion increased (31.4%) in young children (12–24 months). Although this proportion decreases as age increases, that is, 12% (25–60) and 5.5% (61–120) in the older age group (Table 2), which shows an association of infection by HPIV-3 with age factor. Cough (41.44%) and fever (64.50%) were the common symptoms observed in all three genotypes. But the ratio of cough with phlegm (58.55%) was high which is an indication of pneumonia. Croup infection was recorded in children with HPIV-1, while mild cold-like symptoms were observed in the patient with HPIV-2.
Table 2.
Frequency of HPIVs in different age groups.
| Age (months) | N | Positive cases |
||
|---|---|---|---|---|
| HPIV-1 N (%) |
HPIV-2 N (%) |
HPIV-3 N (%) |
||
| All ages | 152 | 1(0.65) | 3(2.0) | 31(20.4) |
| <12 | 58 | 01(1.70) | – | 11(19.0) |
| 12–24 | 51 | – | 3 (6.0) | 16 (31.4) |
| 25–60 | 25 | – | – | 3 (12.0) |
| 61–120 | 18 | – | – | 1 (5.5) |
Of the total HPIV positive patients, 17 refused for blood to be analyzed for T cells and the rest 20 were all HPIV-3 infected children. The clinical blood cell record of patients with HPIV-3 infection is shown in Table 3. An elevated number of WBCs, lymphocytes, and neutrophils were observed in HPIV-3 infected children. As these cells fight against pathogens so their high values give a sign of infectious agents in the blood. While a slightly low number of HGB levels and RBCs were observed in all the patients.
Table 3.
Analysis of blood cells in the study population.
| Cells | Patients (N = 20) | Control (N = 20) | P-value |
|---|---|---|---|
| Complete blood count | |||
| WBC | 10.02 ± 0.1414 | 7.85 ± 2.700 | 0.003 |
| RBC | 3.693 ± 0.75 | 5.45 ± 1.39 | 0.000 |
| HGB | 9.41 ± 1.293 | 14.3 ± 2.17 | 0.000 |
| Platelets | 200.75 ± 93.89 | 300 ± 79.47 | 0.001 |
| Neutrophil | 76.15 ± 6.222 | 60.25 ± 12.08 | 0.000 |
| Lymphocytes | 50.28 ± 6.0811 | 40.75 ± 6.74 | 0.001 |
| T cells count | |||
| CD3+ | 2098.5 ± 127.5 | 2585 ± 386.99 | 0.003 |
| CD4+ | 1580 ± 97.87 | 1690 ± 55.25 | 0.000 |
| CD8+ | 518.5 ± 74.00 | 840 ± 246.3 | 0.001 |
| CD19+ | 1785 ± 462.5 | 2525 ± 405.0 | 0.000 |
| CD4/CD8 ratio | 3.12 ± 0.59 | 2.18 ± 0.654 | 0.000 |
3.3. T cells count in the study population
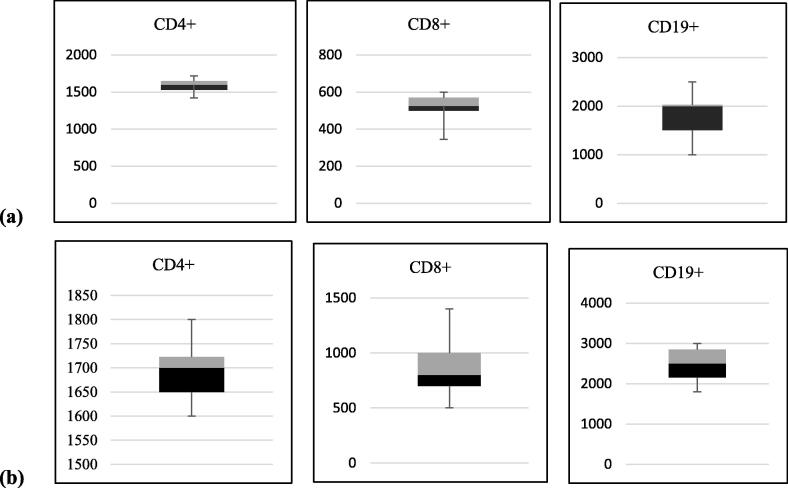
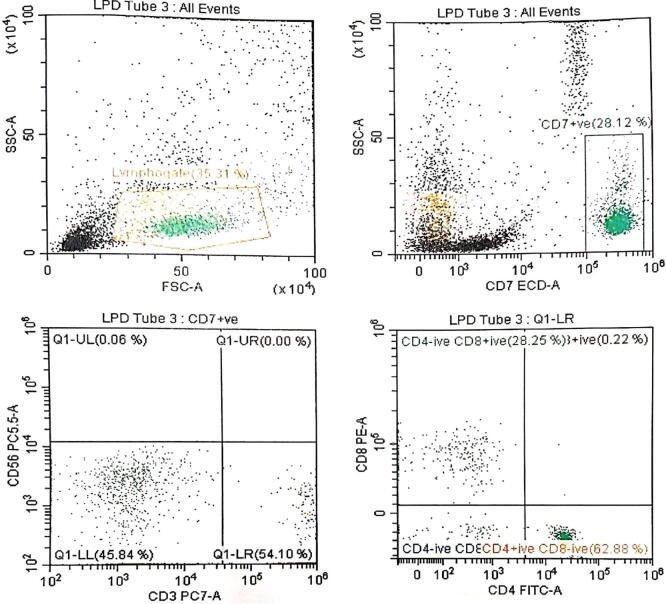
All the patients (N = 20) showed a marked change in their T cell subsets as compared to healthy control subjects. Lower count of lymphocytes was recorded in infected patients as compared to the control subject. CD4+ T cells show slight changes in infected children (1580 ± 97.87) as compared to the control group (1690 ± 53.85). A remarkable decrease was observed in CD8+ T cells count (518.5 ± 74.00) in children infected with HPIV-3 (Table 3). Fig. 1 shows T cell differences in HPIV-3 infected and control group children. There were no significant differences noted by gender or the lymphocyte subset counts in the study population. Table 4 shows a comparison of T cells count in mild, moderate, and severely ill patients. The results clearly showed the variations, displaying the low count of CD8+ T cells in severe patients. The immune cells from peripheral blood circulation of HPIV-3 infected children through flow cytometry plots are shown in Fig. 2.
Fig. 1.
showing the values of CD4+, CD8+ and CD19+ (a) in patients with PIV-3 infection and (b) control group. Data are plotted as medians with the 25th and 75th percentile score.
Table 4.
Comparison of T cells count with disease severity (N = 20).
| Disease | N (%) | Lymphocytes | CD4+ | CD8+ |
|---|---|---|---|---|
| Mild | 3 (15) | 3666.7 ± 577.3 | 1650 ± 50 | 1200 ± 100 |
| Moderate | 10 (50) | 3200 ± 421.6 | 1530 ± 103.27 | 540 ± 337.30 |
| Severe | 7 (35) | 3071.4 ± 188.9 | 1621.4 ± 69.86 | 200 ± 129.09 |
Fig. 2.
Immune cells from peripheral blood circulation. Representative flow cytometry plots from a patient with PIV-3 infection. Values are the percentages of T cells population gated on CD3+ T cells.
4. Discussion
The occurrence and increase in recurrent infection frequency are revealing the puzzling immune response to parainfluenza viruses and thus the main obstacle in the development of an effective vaccine (Hall, 2001). The pathogenesis of HPIV is still being explored (Henrickson, 2003), however reports showed that individuals with defective cell-mediated immunity tend to have a more severe infection, suggested the main role of T cells in disease (Parija and Marrie, 2019). It is supposed that CD4+ and CD8+ T-cells count in respiratory viral infections may be a valuable set of markers for recognition of respiratory viruses, their complications, and disease severity in young children (Schmidt and Verga, 2018). Cellular immunity are integrally linked during the initial induction of immunity against pathogens, these can become disconnected with developing pathology. This loss of correlation between T cells and neutralizing antibody responses varies according to different viruses, suggesting that independent time course measures of these separate immune responses are required over time for adequate recording of biomarkers of natural infection and vaccine efficacy or suggesting that T cell status may be most crucial measure of conferred long-term immunity(Monette and Mouland, 2019). The understanding of such immunological parameters in respiratory viral infections, clinical diagnostic models, and disease understanding may be refined.
In this study, HPIVs were detected in 24.34% and the prevalent genotype was HPIV-3 in the respiratory specimen of hospitalized children mostly aged 1–2 years. Previously, low HPIV prevalence (11.8%) was reported from Spain, but HPIV-3 was prevalent in hospitalized children, similar to the current study (Calvo et al., 2011). Similar findings of HPIV-3 high prevalence were reported in toddlers from the USA (Farichock et al., 2010), and Brazil (Mariana et al., 2008) while in contrast HPIV-4 was found prevalent in Korea (Gu et al., 2020) and in USA it is exhibited in fall-winter annually (Degroote et al., 2020). But in a study in Italy highest detection rate of HPIV-3 was reported in children aged >6 months to ≤3 years (Conto et al., 2019). In the current study, two samples did not amplify and were considered untypable, which might be due to the emergence of any possible mutation in the Hemi-nested primers target region or maybe also be attributed due handling.
WBCs and lymphocytes are responsible for immunity during any infection and their elevation during HPIV-3 infections shows that HPIV also strikes these cells. High WBCs and lymphocytes levels were observed in HPIV-3 infected children. The high counts of such immune cells were reported by some studies in children with Adenovirus infection (Ruuskanen et al., 1985, Pulliam et al., 2001). In another study, an elevated number of platelets were found in children infected with various respiratory viruses (Kim et al., 2016), which is in contrast to the current study findings. Neutrophils, important immune cells, were found elevated in HPIV-3 infected children of the current study. It is earlier suggested that neutrophils become prominent in the airway and the peripheral blood during acute viral infection (Trigg et al., 1996, Grunberg et al., 1997). Some studies showed that neutrophils are increased in the lungs and blood after infection with pathogenic influenza A virus in mice, humans, and ferrets (Watanabe et al., 2013, Long et al., 2013, Zhu et al., 2013). The increased level of neutrophils in the current study may be a sign of disease severity, as suggested by some previous studies that during severe viral complications like pneumonia an increase in the number of neutrophils is correlated with disease severity (Calore et al., 2011, Fukuyama and Kawaoka, 2011). These immune cells are important in viral infections as some studies suggested that the total neutrophil, lymphocyte counts can be used as markers of inflammation and infection (Jager et al., 2012, Gurol et al., 2015).
The present study demonstrated the variation in the count of T cells population in children with HPIV-3. The count of CD4+ T cells does not change significantly as compared to the control group. But there were significantly lower peripheral blood CD8+ T cells and CD19+ cells count recorded in these hospitalized patients. Many different respiratory viruses have also shown lower count of CD8+ cells during the acute phase of infection. This study revealed changes in T cells counts in children with HPIV-3 infection as previously the acquired immune cells were correlated with respiratory viruses as respiratory syncytial viruses (RSV). The current study results of lower counts of circulating CD8+ T cells and CD19+ cells in children with severe infection are coherent to that of RSV (Larranaga et al., 2009). While few studies documented a reduced number of T cells in influenza patients with severe pneumonia, whereas normal values of T cells were observed in moderate and control group (Kim et al., 2011). Similarly, changes in T cell subsets in bronchoalveolar lavage fluid during RSV infection was associated with the severity of pathologic responses (Openshaw, 1995). The exact reason for the lower count of C8+ T cells in peripheral blood to HPIV-3 infection is not clear yet. Although it is considered that due to the severity of infection CD8+ cells get exhausted leads to a low number of these cells in the blood or the cells may be migrated to lungs from circulation during severe infection. Although, some authors demonstrated in their studies that respiratory virus infection in mouse models results in an increase in the frequency and number of total and antigen-specific CD8 T cells in the lungs and airways. RSV-specific CD8 T cell responses typically reach peak numbers in the lung at approximately day 8 following acute infection (Chang et al., 2001, Knudson et al., 2014). While some authors suggested that acute respiratory infection by human metapneumovirus, influenza, and other viruses leads to CD8+ T cell functional impairment (Erickson et al., 2012, Chang and Braciale, 2002, Vallbracht et al., 2006, DiNapoli et al., 2008). A case report study from Yamaguchi University hospital Japan showed increases in CD8+ T cells and HLA-DR+CD8+ T cells in patients with adenovirus infection during the acute stage (Tomoyo et al., 2000). These contradictory reports of CD8+ T cells count in different respiratory viral infections may be used as an important immunological marker for assessment of disease severity in individual viral infections.
5. Conclusion
The study concluded variation in the number of T cells population during HPIV associate LRTIs in children. This might be an important immunological and clinical diagnostic tool for the determination of viral infections in children.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecherbonnier J., Pozzetto B., Ginevra C., Freymuth F. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J. Virol. Methods. 2005;126(1–2):53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M.E., Shrivastava S., He J., Nelson M.I., Bera J., Fedorova N. Sequencing and analysis of globally obtained human parainfluenza viruses 1 and 3 genomes. PLoS One. 2019;14(7):e0220057. doi: 10.1371/journal.pone.0220057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calore E.E., Uip D.E., Perez N.M. Pathology of the swine-origin influenza A (H1N1) flu. Pathol. Res. Pract. 2011;207:86–90. doi: 10.1016/j.prp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Calvo C., Garcia-Garcia M.L., Ambrona P., Rico M., Pozo F., Molinero M.D.M., Perez-Brena P., Casas I. The burden of infections by parainfluenza virus in hospitalized children in Spain. J. Ped. Infect. Dis. 2011;30(9):792–794. doi: 10.1097/INF.0b013e318212ea50. [DOI] [PubMed] [Google Scholar]
- Chang J., Braciale T.J. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- Chang J., Srikiatkhachorn A., Braciale T.J. Visualization and characterization of respiratory syncytial virus F-specific CD 8(+) T cells during experimental virus infection. J. Immunol. 2001;167(8):4254–4260. doi: 10.4049/jimmunol.167.8.4254. [DOI] [PubMed] [Google Scholar]
- Conto F.D., Conversano F., Medici M.C., Ferraglia F., Pinardi F., Arcangeletti M.C., Chezzi C., Calderaro A. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diag Microbiol. Inf. Dis. 2019;94(3):260–267. doi: 10.1016/j.diagmicrobio.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj A., Ota E., Shahrook S., Baljinnyam O.P., Takehara K., Hikita N. Hospitalization risk factors for children’s lower respiratory tract infection: A population-based, cross-sectional study in Mongolia. Sci. Rep. 2016;6:24615. doi: 10.1038/srep24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroote N.P., Haynes A.K., Taylor C., Killerby M.E., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human parainfluenza virus circulation, United States, 2011–2019. J. Clin. Virol. 2020;124:104261. doi: 10.1016/j.jcv.2020.104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli J.M., Murphy B.R., Collins P.L., Bukreyev A. Impairment of the CD8+ T cell response in lungs following infection with human respiratory syncytial virus is specific to the anatomical site rather than the virus, antigen, or route of infection. Virol. J. 2008;5:105. doi: 10.1186/1743-422X-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.J.P., Gilchuk A.K., Hastings S.J., Tollefson M., Johnson M.B., Downing K.L., Boyd J.E., Johnson A.S., Kim S., Joyce, Williams J.V. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farichock M.P., Martin E.T., Chambers S. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J. Clin. Virol. 2010;49:16–20. doi: 10.1016/j.jcv.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama S., Kawaoka Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr. Opin. Immunol. 2011;23:481–486. doi: 10.1016/j.coi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg K., Smits H.H., Timmers M.C., de Klerk E.P.A., Dolhain R.J.E.M., Dick E.C., Hiemstra P.S., Sterk P.J. Experimental rhino-virus 16 infection: effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care. Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- Gu Y.E., Park J.Y., Lee M.K., Lim I.S. Characteristics of human parainfluenza virus type 4 infection in hospitalized children in Korea. Pediatr Int. 2020;62(1):52–58. doi: 10.1111/ped.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurol G., Ciftci I.H., Terizi H.A., Atasoy A.R., Ozbek A., Koroglu M. Are there standardized cutoff values for neutrophil-lymphocyte ratios in bacteremia or sepsis? J. Microbiol. Biotechnol. 2015;25:521–525. doi: 10.4014/jmb.1408.08060. [DOI] [PubMed] [Google Scholar]
- Hall C.B. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Henrickson K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager D.C.P., Wever P.C., Gemen E.F., Kusters R., van Gageldonk-Lafeber A.B., van der Poll T. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7:46561. doi: 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Bauer S., Suk La K., Lee K.H., Choung J.T., Roh K.H., Lee C.K., Yoo Y. CD4+/CD8+ T lymphocytes imbalance in children with severe 2009 pandemic influenza A (H1N1) pneumoniaKorean. J. Pediatr. 2011;54(5):207–211. doi: 10.3345/kjp.2011.54.5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.K., Jeon J.K., Kim J.W., Kim G.Y. Correlation between abnormal platelet count and respiratory viral infection in patients from Cheonan, Korea. J. Clin. Lab. Ana. 2016;30:185–189. doi: 10.1002/jcla.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C.J., Weiss K.A., Hartwig S.M., Varga S.M. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J. Virol. 2014;88(16):9010–9016. doi: 10.1128/JVI.00329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsun N., Angelova S., Trifonova I., Georgieva I., Voleva S., Tzotcheva I., Mileva S., Ivanov I., Tcherveniakova T., Perenovska P. Viral pathogens associated with acute lower respiratory tract infections in children younger than 5 years of age in Bulgaria. Braz. J. Microbiol. 2019;50:117–125. doi: 10.1007/s42770-018-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larranaga C.L., Ampuero S.L., Luchsinger V.F., Carrion F.A., Aguilar N.V., Morales P.R., Palomino M.A.M., Tapia L.F., Avendano L.F. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr. Infect. Dis. J. 2009;28:867–873. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- Linster M., Do L.A.H., Minh N.N.Q. Clinical and molecular epidemiology of human parainfluenza viruses 1–4 in children from Viet Nam. Sci. Rep. 2018;8:6833. doi: 10.1038/s41598-018-24767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.P., Kotur M.S., Stark G.V., Warren R.L., Kasoji M., Craft J.L., Albrecht R.A., García-Sastre A., Katze M.G., Waters K.M., Vasconcelos D., Sabourin P.J., Bresler H.S., Sabourin C.L. Accumulation of CD11b(+)Gr-1(+) cells in the lung, blood and bone marrow of mice infected with highly pathogenic H5N1 and H1N1 influenza viruses. Arch. Virol. 2013;158:1305–1322. doi: 10.1007/s00705-012-1593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana M.M.F., Monteiro A.J., Fernanda E.A.M. Parainfluenza virus infections in a tropical city: clinical and epidemiological aspects. Braz. J. Infect. Dis. 2008;12(3):192–197. doi: 10.1590/s1413-86702008000300006. [DOI] [PubMed] [Google Scholar]
- McAllister D.A., Liu L., Shi T. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet. Global. Health. 2019;7(1):47–57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H.C. Viral bronchiolitis in children. N. Engl. J. Med. 2016;374(1):62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- Monette A., Mouland A.J. T lymphocytes as measurable targets of protection and vaccination against viral disorders. Int. Rev. Cell Mol. Biol. 2019;342:175–263. doi: 10.1016/bs.ircmb.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P.J.M. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am. J. Respir. Crit. Care Med. 1995;152:S59–S62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- Parija, S.C., Marrie, T.J., 2019. Human Parainfluenza Viruses (HPIV) and other parainfluenza viruses. Medscape. https://emedicine.medscape.com/article/224708-overview (accessed on 21.04.2020).
- Pawelczyk M., Kowalski M.L. The role of human parainfluenza virus infections in the immunopathology of the respiratory tract. Curr. Allergy. Asthma. Rep. 2017;17:16. doi: 10.1007/s11882-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam P.N., Attia M.W., Cronan K.M. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infections. Pediatrics. 2001;108:1275–1279. doi: 10.1542/peds.108.6.1275. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O., Meurman O., Sarkkinen H. Adenoviral diseases in children: A study of 105 cases. Pediatrics. 1985;76:79–83. [PubMed] [Google Scholar]
- Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane A.A., Shastri J., Bavdekar S.B. Respiratory pathogens in infants diagnosed with acute lower respiratory tract infection in a tertiary care hospital of Western India using multiplex real time PCR. Indian. J. Pediatr. 2019;86(5):433–438. doi: 10.1007/s12098-018-2840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyo M., Tamotsu I., Norimichi T., Kumiko K., Takashi M., Susumu F. Activation of peripheral blood CD8+ T cells in adenovirus infection. J. Ped. Infec. Dis. 2000;19(8):766–768. doi: 10.1097/00006454-200008000-00023. [DOI] [PubMed] [Google Scholar]
- Trigg C.J., Nicholson K.G., Wang J.H., Ireland D.C., Jordan S., Duddle J.M. Bronchial inflammation and the common cold: a comparison of atopic and non-atopic subjects. Clin. Exp. Allergy. 1996;26:665–676. doi: 10.1111/j.1365-2222.1996.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht S., Unsold H., Ehl S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur. J. Immunol. 2006;36:1434–1442. doi: 10.1002/eji.200535642. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tisoncik-Go J., Tchitchek N., Watanabe S., Benecke A.G., Katze M.G., Kawaoka Y. 1918 Influenza virus hemagglutinin (HA) and the viral RNA polymerase complex enhance viral pathogenicity, but only HA induces aberrant host responses in mice. J. Virol. 2013;87:5239–5254. doi: 10.1128/JVI.02753-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang D., Kelvin D.J., Li L., Zheng Z., Yoon S.W., Wong S.S., Farooqui A., Wang J., Banner D., Chen R., Zheng R., Zhou J., Zhang Y., Hong W., Dong W., Cai Q., Roehrl M.H., Huang S.S., Kelvin A.A., Yao T., Zhou B., Chen X., Leung G.M., Poon L.L., Webster R.G., Webby R.J., Peiris J.S., Guan Y., Shu Y. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science. 2013;341:183–186. doi: 10.1126/science.1239844. [DOI] [PubMed] [Google Scholar]