Abstract
Background: Prevention and treatment of chronic inflammatory diseases require effective and low-toxic medicines. Molecular hybridization is an effective strategy to enhance the biological activity of new compounds. Triterpenoid scaffolds are in the focus of attention owing to their anti-inflammatory, antiviral, antiproliferative, and immunomodulatory activities. Heteroprostanoids have different pleiotropic effects in acute and chronic inflammatory processes.
Objective: The study aimed to develop structurally new and low toxic anti-inflammatory agents via hybridization of betulinic acid with azaprostanoic acids.
Methods: A series of betulinic acid-azaprostanoid hybrids was synthesized. The synthetic pathway included the transformation of betulin via Jones' oxidation into betulonic acid, reductive amination of the latter and coupling obtained by 3β-amino-3-deoxybetulinic acid with the 7- or 13-azaprostanoic acids and their homo analogues. The hybrids 1-9 were investigated in vivo on histamine-, formalin- and concanavalin A-induced mouse paw edema models and two models of pain - the acetic acid-induced abdominal writhing and the hot-plate test. The hybrids were in vitro evaluated for cytotoxic activity on cancer (MCF7, U-87 MG) and non-cancer humane cell lines.
Results: In the immunogenic inflammation model, the substances showed a pronounced anti-inflammatory effect, which was comparable to that of indomethacin. In the models of the exudative inflammation, none of the compounds displayed a statistically significant effect. The hybrids produced weak or moderate analgesic effects. All the agents revealed low cytotoxicity on human immortalized fibroblasts and cancer cell lines compared with 3β-amino-3-deoxybetulinic acid and doxorubicin.
Conclusion: The results indicate that the principal anti-inflammatory effect of hybrids is substantially provided with the triterpenoid scaffold and in some cases with the azaprostanoid scaffold, but the latter makes a significant contribution to reducing the toxicity of hybrids. Hybrid 1 is of interest as a potent low toxic agent against immune-mediated inflammation.
Keywords: Analgesic activity, anti-inflammatory activity, azaprostanoids, betulinic acid, cytotoxicity, hybrids
1. INTRODUCTION
Inflammation is a pivotal protective mechanism of host defense against an injury, wounds or infection. However, the persistent inflammation generates an excessive production of pro-inflammatory molecules and reactive species which could be the pathogenic factors initiating numerous chronic diseases, such as arthritis, atherosclerosis, diabetes and even cancer [1]. Many trials for finding new effective anti-inflammatory agents are being conducted [2]. Plant-derived substances are a valuable source of potent anti-inflammatory pharmacons [3], which have been used for structural modification by medicinal chemistry [4]. Triterpene scaffolds are in the focus of attention owing to their anti-inflammatory, antimicrobial, antiviral, antiproliferative, hepatoprotective and immunomodulatory activities [5-7]. The anti-inflammatory effect of pentacyclic triterpenoids is based on the inhibition of 5-LOX, iNOS, COX-2 and nuclear factor-kB (NF-kB) activities [8]. Betulinic acid (BA) is a naturally occurring pentacyclic triterpenoid, safe and non-toxic at doses up to 500 mg/kg body weight in mice [9]. BA is reported to have anti-inflammatory and immunomodulatory activities in numerous experimental models, including sepsis [10-13]. BA decreases pro-inflammatory mediators NO and PGE2 in LPS-stimulated RAW 264.7 macrophages via the inhibition of inducible iNOS and COX-2. It also suppresses NF-κB pathway by blocking the phosphorylation of IκB-α [14]. The BA anti-inflammatory effect is mediated by the macrophages’ induction and modulation of the immune response [8, 14].
The prostaglandins (PGs) are increased in inflamed tissue and usually thought to have pro-inflammatory effects such as: dolor (pain), calor (heat), rubor (redness) and tumor (swelling) [15]. Although the pro-inflammatory effects are well documented, the evidence that PGs could resolve inflammation was found. Cyclopentenone prostaglandins have been shown to possess anti-inflammatory activity [16, 17], which is revealed through the activation of peroxisome proliferator-activated receptor-g [18, 19] or through the direct inhibition and modification of the IKKβ subunit of IKK in NF-κB pathway [20]. Heteroprostanoids not only have anti-inflammatory, but also different pleiotropic effects [21]. Thus, 7- and 13-azapros- tanoids and their homo analogues [22], synthesized on the base of 2-acylcycloalkane-1,3-diones, exhibit anti-ulcerative, anti-arrhythmic and anti-ischemic activities [23, 24]. Molecular hybridization is an effective strategy to enhance the biological activity of new molecules [25, 26]. Keeping in view the fact that triterpenoids and azaprostanoids have different targets of anti-inflammatory effect, we supposed their hybridization could improve the pharmacological activities of the compounds.
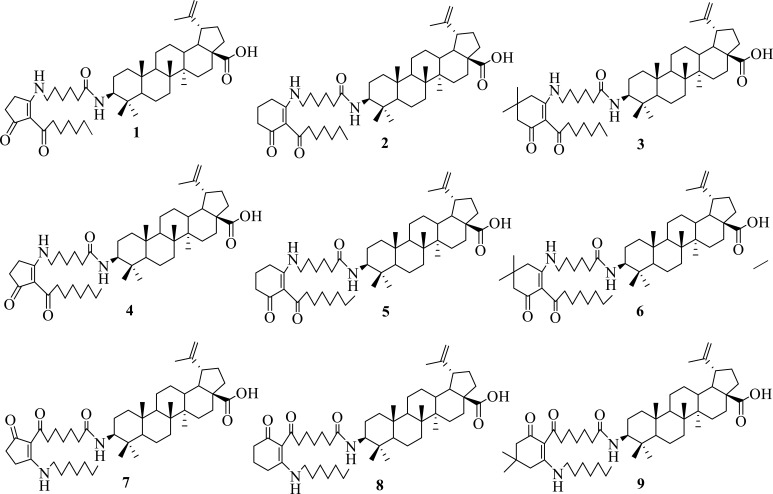
In our continuing search for bioactive hybrid compounds [27-29], we present in the article the synthesis and in vivo investigation of anti-inflammatory and analgesic effects of nine betulinic acid-azaprostanoid hybrids including hybrids with 7-azaprostanoids 1-6 and hybrids with 13-azaprostanoids 7-9 (Fig. 1). Furthermore, we evaluated the influence of azaprostanoid fragment on the cytotoxicity of a triterpenoid hybrid. To our knowledge, this is the first time report where 7- and 13-azaprostanoids were conjugated with the lupane skeleton.
Fig. (1).
New synthesized and bioevaluated hybirds.
2. MATERIALS AND METHODS
2.1. Chemistry
1H and 13C NMR spectra were recorded on a Bruker AVANCE 500 spectrometer (500 MHz for 1H, 125 MHz for 13C). Chemical shift values are given in δ (ppm) relative to the residual solvent signals for δH 7.26 ppm and δC 77.16 ppm (CDCl3), and for δH 2.04 ppm and δC 20.00 ppm (CD3COOD). Melting points were measured on a Boetius apparatus and are uncorrected. High-resolution mass spectra (HRMS) were recorded on a 6550 iFunnel Q-TOF LC/MS (Agilent Technologies) micro mass spectrometer by electrospray ionization (ESI). The elemental analyses were performed on a Eurovector EA3000 CHNS-O analyzer. Follow-up of the reactions and evaluation of the purity of the compounds were performed by thin layer chromatography (TLC) on Silica gel (60 Å medium pore diameter) on TLC Al foils with a fluorescent indicator 254 nm (Sigma-Aldrich). Chemicals were purchased from Sigma-Aldrich or Acros Organics and used as received. Solvents were dried and freshly distilled according to the common practice.
2.1.1. General Procedure for the Synthesis of Target Compounds 1-9
To a solution of azaprostanoic acids 11-19 (1 mmol) in methylene chloride (30 ml), N,N′-dicyclohexylcarbodiimide (160 mg, 1 mmol) was added. After stirring for 2 hr at room temperature, 3β-amino-3-deoxybetulinic acid (10) (460 mg, 1 mmol) and 4-dimethylaminopyridine (10 mg) were added to the mixture. After stirring for 16 hr, the precipitate was filtered and the filtrate was evaporated under reduced pressure. The residue was purified by silica gel column chromatography with petroleum-ethyl acetate as an eluent to give the corresponding hybrids 1-9.
2.1.1.1. 3-Deoxy-3β-((6-(2-heptanoyl-3-oxocyclo- pent-1-en-1-yl)amino)hexanamido))betulinic Acid (1)
Yield 55%; White solid; mp 123-127°C; 1H NMR (500 MHz, CDCl3) δ: 0.71-2.27 (m, 43H, CH, CH2, CH3), 0.71 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.83 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.37-2.47 (m, 2H, CH2), 2.63-2.65 (m, 2H, CH2), 2.88 (t, J = 7.5 Hz, 2H, CH2), 3.00 (td, J = 10.7, 4.8 Hz, 1H, H-19), 3.35 (q, J = 6.7 Hz, 2H, CH2), 3.64 (ddd, J = 14.2, 10.0, 4.5 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.71 (brs, 1H, H-29), 5.43 (d, J = 9.9 Hz, 1H, NH), 10.38 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 14.8, 16.2, 16.2, 16.6, 18.6, 19.4, 20.9, 22.7, 24.2, 24.3, 25.4, 25.6, 25.8, 26.5, 28.5, 29.3, 29.5, 29.8, 30.4, 30.7, 31.9, 32.3, 33.6, 34.3, 36.9, 37.2, 37.9, 38.5, 39.2, 40.6, 40.7, 42.5, 43.9, 47.1, 49.3, 50.5, 56.0, 56.4, 56.5, 109.8, 110.3, 150.6, 172.3, 181.3, 181.8, 200.0, 200.4. HR-MS (ESI): m/z calcd for C48H76N2NaO5 [M+Na]+ 783.5652; found 783.5623.
2.1.1.2. 3-Deoxy-3β-((6-(2-heptanoyl-3-oxocyclo- hex-1-en-1-yl)amino)hexanamido))betulinic Acid (2)
Yield 60%; White solid; mp 138-142°C. 1H NMR (500 MHz, CDCl3) δ: 0.72-2.27 (m, 45H, CH, CH2, CH3), 0.72 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.39 (t, J = 6.3 Hz, 2H, CH2), 2.57 (t, J = 6.1 Hz, 2H, CH2), 2.93 (t, J = 7.6 Hz, 2H, CH2), 2.97-3.03 (m, 1H, H-19), 3.31 (q, J = 6.4 Hz, 2H, CH2), 3.64 (td, J = 11.1 Hz, 10.0 Hz, 4.2 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.39 (d, J = 9.3 Hz, 1H), 12.57 (t, J = 5.2 Hz, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 14.8, 16.2, 16.6, 18.7, 19.4, 19.9, 20.9, 22.8, 25.4, 25.6, 25.8, 26.6, 27.2, 28.6, 29.1, 29.4, 29.8, 30.7, 32.0, 32.3, 34.3, 36.9, 37.2, 37.9, 38.5, 38.6, 39.2, 40.7, 42.5, 43.5, 43.9, 47.1, 49.4, 50.5, 56.1, 56.5, 56.6, 108.8, 109.8, 150.6, 172.3, 173.0, 181.4, 195.0, 204.4. HR-MS (ESI): m/z calcd for C49H78N2NaO5 [M+Na]+ 797.5808; found 797.5839.
2.1.1.3. 3-Deoxy-3β-((6-((2-heptanoyl-5,5-dime- thyl-3-oxocyclohex-1-en-1-yl)amino)hexanamido)) betulinic Acid (3)
Yield 65%; White solid; mp 158-162°C. 1H NMR (500 MHz, CDCl3) δ: 0.72-2.25 (m, 43H, CH, CH2, CH3), 0.73 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.05 (s, 6H, 2CH3), 1.68 (s, 3H, CH3), 2.29 (s, 2H, CH2), 2.41 (s, 2H, CH2), 2.93-3.03 (m, 3H, H-19, CH2), 3.32 (q, J = 6.3 Hz, 2H, CH2), 3.65 (ddd, J = 14.1 Hz, 9.6 Hz, 4.2 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.37 (d, 1H, J = 10.1 Hz, NH), 12.70 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.3, 14.8, 16.2, 16.6, 18.7, 19.5, 20.9, 22.8, 25.4, 25.6, 25.9, 26.6, 28.5, 28.6, 29.2, 29.5, 29.8, 30.7, 32.0, 32.3, 34.3, 36.9, 37.2, 37.9, 38.5, 39.3, 40.6, 40.8, 42.6, 43.6, 43.9, 47.1, 49.4, 50.5, 52.1, 56.1, 56.5, 56.6, 107.6, 109.8, 150.6, 172.1, 172.3, 181.2, 194.5, 204.5. HR-MS (ESI): m/z calcd for C51H82N2NaO5 [M+Na]+ 825.6121; found 825.6150.
2.1.1.4. 3-Deoxy-3β-((6-(2-octanoyl-3-oxocyclo- pent-1-en-1-yl)amino)hexanamido))betulinic Acid (4)
Yield 54%; White solid; mp 191-194°C. 1H NMR (500 MHz, CDCl3) δ: 0.72-2.28 (m, 45H, CH, CH2, CH3), 0.72 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.44-2.47 (m, 2H, CH2), 2.65-2.67 (m, 2H, CH2), 2.88 (td, J = 7.4, 2.1 Hz, m, 2H, CH2), 3.01 (td, J = 10.8, 4.8 Hz, 1H, H-19), 3.36 (q, J = 6.8 Hz, 2H, CH2), 3.65 (ddd, J = 14.3 Hz, 10.1 Hz, 4.4 Hz, 1H, H-3), 4.60 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.56 (d, J = 9.4 Hz, 1H, NH), 10.41 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.3, 14.8, 16.2, 16.3, 16.7, 18.7, 19.5, 20.9, 22.8, 24.3, 24.4, 25.5, 25.6, 25.8, 26.5, 28.6, 29.4, 29.5, 29.6, 29.8, 30.7, 31.9, 32.3, 33.6, 34.3, 36.8, 37.2, 37.3, 37.9, 38.6, 39.3, 40.7, 40.8, 42.6, 44.0, 47.1, 49.4, 50.5, 56.1, 56.5, 56.7, 109.8, 110.4, 150.6, 172.4, 181.2, 182.0, 200.0, 200.4. HR-MS (ESI): m/z calcd for C49H78N2NaO5 [M+Na]+ 797.5808; found 797.5830.
2.1.1.5. 3-Deoxy-3β-((6-(2-octanoyl-3-oxocyclo- hex-1-en-1-yl)amino)hexanamido))betulinic Acid (5)
Yield 59%; White solid; mp 131-135°C. 1H NMR (500 MHz, CDCl3) δ: 1H NMR (500 MHz, CDCl3) δ: 0.67-2.28 (m, 47H, CH, CH2, CH3), 0.73 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.39 (t, J = 6.0 Hz, 2H), 2.57 (t, J = 5.7 Hz, 2H), 2.93 (t, J = 7.7 Hz, 2H, CH2), 2.97-3.03 (m, 1H, H-19), 3.32 (q, J = 6.5 Hz, 2H, CH2), 3.64 (ddd, J =14.1, 10.1, 4.4 Hz, 1H, H-3), 4.60 (brs, 1H, H-29), 4.73 (brs, 1H, H-29), 5.35 (d, J = 9.9 Hz, 1H, NH), 12.58 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.3, 14.8, 16.2, 16.6, 18.7, 19.5, 19.9, 20.9, 22.8, 25.4, 25.5, 25.6, 25.9, 26.6, 27.2, 28.6, 29.1, 29.4, 29.7, 29.8, 30.7, 32.0, 32.3, 34.4, 36.9, 37.2, 37.9, 38.5, 39.3, 40.8, 42.6, 43.5, 43.9, 47.1, 49.4, 50.6, 56.1, 56.5, 56.6, 108.8, 109.8, 150.6, 172.3, 173.1, 181.1, 195.0, 204.5. HR-MS (ESI): m/z calcd for C50H80N2NaO5 [M+Na]+ 811.5965; found 811.5984.
2.1.1.6. 3-Deoxy-3β-((6-(5,5-dimethyl-2-octanoyl-3-oxocyclohex-1-en-1-yl)amino)hexanamido)) betulinic Acid (6)
Yield 62%; White solid; mp 146-149°C. 1H NMR (500 MHz, CDCl3) δ: 0.64-2.25 (m, 45H, CH, CH2, CH3), 0.73 (s, 3H, CH3), 0.79 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.05 (s, 6H, 2CH3), 1.68 (s, 3H, CH3), 2.26 (s, 2H, CH2), 2.41 (s, 2H, CH2), 2.93-3.03 (m, 3H, CH2, H-19), 3.31 (q, 2H, CH2), 3.65 (ddd, J = 12.1, 9.8, 4.3 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.34 (d, J = 9.8 Hz, 1H, NH), 12.68 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.3, 14.8, 16.2, 16.6, 18.7, 19.5, 20.9, 22.8, 25.4, 25.5, 25.6, 28.5, 28.6, 29.3, 29.4, 29.7, 29.8, 30.7, 30.8, 32.0, 32.3, 34.4, 37.0, 37.2, 37.9, 38.5, 39.3, 40.6, 40.8, 42.6, 43.5, 43.9, 47.1, 49.4, 50.6, 52.2, 56.1, 56.5, 56.6, 107.7, 109.8, 150.6, 172.0, 172.3, 181.2, 194.5, 204.0. HR-MS (ESI): m/z calcd for C52H84N2NaO5 [M+Na]+ 839.6278; found 839.6309.
2.1.1.7. 3-Deoxy-3β-((7-(2-heptylamino-5-oxocyc- lopent-1-enyl)-7-oxoheptanamido))betulinic Acid (7)
Yield 65%; White solid; mp 130-133°C. 1H NMR (500 MHz, CDCl3) δ: 0.73-2.28 (m, 45H, CH, CH2), 0.73 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.41-2.43 (m, 2H, CH2), 2.64-2.66 (m, 2H, CH2), 2.83-3.03 (m, 3H, H-19, CH2), 3.34 (q, J = 6.8 Hz, 2H, CH2), 3.64 (ddd, J=12.2, 9.9, 4.4 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.54 (d, J = 9.9 Hz, 1H, NH), 10.36 (t, J = 6.3 Hz, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 14.8, 16.2, 16.6, 18.7, 19.5, 20.9, 22.7, 23.9. 24.2, 25.6, 25.8, 25.9, 26.9, 28.5, 28.9, 29.0, 29.8, 30.7, 31.7, 32.3, 33.7, 34.4, 37.1, 37.2, 37.9, 38.5, 39.3, 40.3, 40.8, 42.6, 44.3, 47.1, 49.4, 50.6, 56.1, 56.4, 56.5, 109.8, 110.3, 150.6, 173.0, 181.1, 181.8, 199.9, 200.1. HRMS (ESI): m/z calcd for C49H78N2NaO5 [M+Na]+ 797.5808; found 797.5831.
2.1.1.8. 3-Deoxy-3β-((7-(2-heptylamino-6-oxocyc- lohex-1-enyl)-7-oxoheptanamido))betulinic Acid (8)
Yield 55%; White solid; mp 122-125°C. 1H NMR (500 MHz, CDCl3) δ: 0.73-2.27 (m, 47H, CH, CH2), 0.73 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.68 (s, 3H, CH3), 2.38 (t, J = 6.5 Hz, 2H, CH2), 2.58 (t, J = 6.3 Hz, 2H, CH2), 2.90-3.03 (m, 3H, H-19, CH2), 3.30 (td, J = 7.2 Hz, 5.3 Hz, 2H, СH2), 3.64 (ddd, J = 12.1 Hz, 9.8 Hz, 4.3 Hz, 1H, H-3), 4.59 (brs, 1H, H-29), 4.72 (brs, 1H, H-29), 5.57 (d, J = 9.9 Hz, 1H, NH), 12.57 (br s, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 14.8, 16.2, 16.6, 18.7, 19.5, 19.9, 20.9, 22.7, 24.9, 25.6, 25.8, 26.0, 27.0, 27.2, 28.5, 29.0, 29.1, 29.5, 29.8, 30.7, 31.7, 32.3, 34.4, 37.1, 37.2, 37.9, 38.5, 38.6, 39.3, 40.8, 42.6, 43.5, 43.9, 47.1, 49.4, 50.6, 56.1, 56.4, 56.5, 108.7, 109.8, 150.6, 173.0, 173.1, 181.2, 194.9, 204.0. HR-MS (ESI): m/z calcd for C50H80N2NaO5 [M+Na]+ 811.5965; found 811.5996.
2.1.1.9. 3-Deoxy-3β-((7-(4,4-dimethyl-2-heptyla- mino-6-oxocyclohex-1-enyl)-7-oxoheptanamido)) betulinic Acid (9)
Yield 50%; White solid; mp 127-130°C. 1H NMR (500 MHz, CDCl3) δ: 0.73-2.27 (s, 45H, CH, CH2), 0.73 (s, 3H, CH3), 0.80 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.05 (s, 6H, 2CH3), 1.68 (s, 3H, CH3), 2.26 (s, 2H, CH2), 2.88-3.02 (m, 3H, CH2, H-19), 3.30 (td, J =7.1, 5.2 Hz, 2H, CH2), 3.64 (ddd, J =12.3, 9.9, 4.3 Hz, 1H, H-3), 4.60 (brs, 1H, H-29), 4.73 (brs, 1H, H-29), 5.56 (d, 1H, J = 9.8 Hz, NH), 12.68 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 14.3, 14.8, 16.2, 16.6, 18.7, 19.5, 20.9, 22.7, 24.9, 25.6, 25.8, 26.0, 27.0, 28.5, 28.6, 29.0, 29.1, 29.6, 29.8, 30.7, 31.7, 32.3, 34.4, 37.1, 37.2, 37.9, 38.5, 39.3, 40.6, 40.8, 42.6, 43.5, 43.9, 47.1, 49.4, 50.6, 52.2, 56.1, 56.4, 56.5, 107.5, 109.8, 150.6, 172.0, 173.2, 181.2, 194.5, 203.6. HR-MS (ESI): m/z calcd for C52H84N2NaO5 [M+Na]+ 839.6278; found 839.6305.
2.1.2. Synthesis of 3β-amino-3-deoxybetulinic Acid (10)
To a solution of betulonic acid (1.36 g, 3 mmol) in dry methanol, ammonium acetate (2.22 g, 30 mmol) and cyanoborohydride (0.19 g, 3 mmol) were added. After stirring at room temperature for 12 hr, methanol was evaporated under reduced pressure. To the residue, water (100 ml) was added and the pH of aqueous layer was adjusted to 9 using 1M sodium hydroxide. The precipitate was filtered and purified by silica gel column chromatography using a mixture of chloroform:petroleum as an eluent at a ratio of 20:1 to 10:1 to afford 0.68 g (50% yield) of 3β-amino-3-deoxybetulinic acid (10) as white solid; mp 271-274°С. 1H NMR (500 MHz, CD3COOD) δ: 1.00-2.57 (m, 22H, CH, CH2), 1.13 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.23 (s, 3H, CH3), 1.28 (s, 3H, CH3), 1.31 (s, 3H, CH3), 1.96 (s, 3H, CH3), 3.24-3.30 (m, 2H, H-3, H-19), 4.87 (brs, 1H, H-29), 5.00 (brs, 1H, H-29). 13C NMR (125 MHz, CD3COOD) δ: 15.1, 16.1, 16.3, 16.5, 19.1, 21.7, 24.0, 26.5, 28.1, 30.6, 31.4, 32.9, 35.1, 37.4, 37.9, 38.0, 39.1, 39.4, 41.6, 43.4, 48.1, 50.1, 51.4, 56.6, 57.4, 61.6, 110.5, 151.4, 183.1. Anal. Calcd for C30H49NO2: C, 79.07; H, 10.84; N, 3.07. Found: C, 79.15; H, 10.91; N, 3.12.
2.1.3. Synthesis of 7-aza- and 13-azaprostanoic Acids 11-19
To a solution of compound 32-37, 44-46 (1 mmol) in the mixture of tetrahydrofuran (THF) (5 ml) and H2O (2 ml), sodium hydroxide (2 mmol) was added. After stirring at room temperature for 2 hr, THF was evaporated under reduced pressure. To the residue, 3% HCl (30 ml) was added and the resulting mixture was extracted with chloroform (3 x 10 ml). Combined organic layers were dried (anhydrous Na2SO4), and the solvent was evaporated under reduced pressure to give the desired acids 11-19.
2.1.3.1. 6-((2-Heptanoyl-3-oxocyclopent-1-en-1-yl)amino)hexanoic Acid (11)
Yield 95%; White solid; mp 61-63°С. 1H NMR (500 MHz, CDCl3) δ: 0.84 (t, 3H, CH3), 1.18-1.71 (m, 14H, 7CH2), 2.35 (t, J = 7.3 Hz, 2H, CH2), 2.44-2.47 (m, 2H, CH2), 2.64-2.66 (m, 2H, CH2), 2.87 (t, J = 7.5 Hz, 2H, CH2), 3.35 (q, J = 6.7 Hz, 2H, CH2), 10.40 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 22.6, 24.3, 26.2, 29.2, 29.4, 30.4, 31.8, 33.5, 33.8, 40.6, 44.0, 110.3, 177.8, 182.0, 200.4, 200.5. Anal. Calcd for C18H29NO4: C, 66.84; H, 9.04; N, 4.33. Found: C, 66.93; H, 9.16; N, 4.39.
2.1.3.2. 6-((2-Heptanoyl-3-oxocyclohex-1-en-1-yl)amino)hexanoic Acid (12)
Yield 98%; Colorless oil. 1H NMR (500 MHz, CDCl3) δ: 0.86 (t, J = 7.0 Hz, 3H, CH3), 1.25-1.35 (m, 6H, 3CH2), 1.43-1.49 (m, 2H, CH2), 1.52-1.58 (m, 2H, CH2), 1.65-1.73 (m, 4H, 2CH2), 1.88-1.93 (m, 2H, CH2), 2.37 (t, J = 7.3 Hz, 2H, CH2), 2.44 (t, J = 6.5 Hz, 2H, CH2), 2.58 (t, J = 6.3 Hz, 2H, CH2), 2.92-2.95 (m, 2H, CH2), 3.34 (td, J = 7.1 Hz, 5.3 Hz, 2H, CH2), 12.61 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 19.8, 22.7, 24.3, 25.3, 26.4, 27.2, 29.1, 29.4, 31.9, 33.8, 38.1, 43.6, 44.0, 108.7, 173.3, 178.1, 195.3, 204.6. Anal. Calcd for C19H31NO4: C, 67.63; H, 9.26; N, 4.15. Found: C, 67.71; H, 9.32; N, 4.19.
2.1.3.3. 6-((2-Heptanoyl-5,5-dimethyl-3-oxocyclo- hex-1-en-1-yl)amino)hexanoic Acid (13)
Yield 95%; White solid; mp 42-44°С. 1H NMR (500 MHz, CDCl3) δ: 0.85 (t, J = 6.9 Hz, 3H, CH3), 1.05 (s, 6H, 2CH3), 1.24-1.35 (m, 6H, 3CH3), 1.44-1.58 (m, 4H, 2CH2), 1.65-1.73 (m, 4H, 2CH2), 2.30 (s, 2H, CH2), 2.37 (t, J = 7.3 Hz, 2H, CH2), 2.42 (s, 2H, CH2), 2.93-2.96 (m, 2H, CH2), 3.33 (td, J = 7.0 Hz, 5.3 Hz, 2H, CH2), 12.71 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 22.8, 24.3, 25.4, 26.4, 28.4, 29.2, 29.4, 30.7, 31.9, 33.8, 40.6, 43.6, 43.9, 51.9, 107.6, 172.2, 178.2, 194.8, 204.1. Anal. Calcd for C21H35NO4: C, 69.01; H, 9.65; N, 3.83. Found: C, 68.93; H, 9.56; N, 3.80.
2.1.3.4. 6-((2-Octanoyl-3-oxocyclopent-1-en-1-yl) amino)hexanoic Acid (14)
Yield 98%; White solid; mp 47-50°С. 1H NMR (500 MHz, CDCl3) δ: 0.85 (t, J = 6.9 Hz, 3H, CH3), 1.19-1.34 (m, 8H, 4CH2), 1.41-1.47 (m, 2H, CH2), 1.52-1.58 (m, 2H, CH2), 1.64-1.71 (m, 4H, CH2), 2.36 (t, J = 7.3 Hz, 2H, CH2), 2.45-2.48 (m, 2H, CH2), 2.64-2.67 (m, 2H, CH2), 2.88 (t, J = 7.5 Hz, 2H, CH2), 3.36 (q, J = 6.8 Hz, 2H, CH2), 10.41 (t, J = 6.1 Hz, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 22.8, 24.3, 24.4, 26.2, 29.3, 29.4, 29.5, 31.9, 33.5, 33.8, 40.6, 44.0, 110.4, 178.0, 182.0, 200.4, 200.6. Anal. Calcd for C19H31NO4: C, 67.63; H, 9.26; N, 4.15. Found: C, 67.55; H, 9.19; N, 4.11.
2.1.3.5. 6-((2-Octanoyl-3-oxocyclohex-1-en-1-yl) amino)hexanoic Acid (15)
Yield 93%; Colorless oil. 1H NMR (500 MHz, CDCl3) δ: 0.85 (t, J = 6.9 Hz, 3H, CH3), 1.20-1.34 (m, 8H, 4CH2), 1.42-1.49 (m, 2H, CH2), 1.52-1.58 (m, 2H, CH2), 1.64-1.72 (m, 4H, 2CH2), 1.87-1.92 (m, 2H, CH2), 2.36 (t, J = 7.4 Hz, 2H, CH2), 2.41 (t, J = 6.5 Hz, 2H, CH2), 2.57 (t, 2H, J = 6.2 Hz, CH2), 2.91-2.94 (m, 2H, CH2), 3.31-3.35 (m, 2H, CH2), 12.59 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 19.8, 22.8, 24.3, 25.4, 26.4, 27.1, 29.1, 29.4, 29.7, 31.9, 33.8, 38.3, 43.5, 43.9, 108.8, 173.1, 178.0, 195.3, 204.5. Anal. Calcd for C20H33NO4: C, 68.34; H, 9.46; N, 3.99. Found: C, 68.46; H, 9.51; N, 4.02.
2.1.3.6. 6-((5,5-Dimethyl-2-octanoyl-3-oxocyclo- hex-1-en-1-yl)amino)hexanoic Acid (16)
Yield 98%; Colorless oil. 1H NMR (500 MHz, CDCl3) δ: 0.85 (t, J = 6.9 Hz, 3H, CH3), 1.05 (s, 6H, CH3), 1.22-1.35 (m, 8H, 4CH2), 1.43-1.49 (m, 2H, CH2), 1.52-1.58 (m, 2H, CH2), 1.65-1.73 (m, 4H, 2CH2), 2.30 (s, 2H, CH2), 2.37 (t, J = 7.3 Hz, 2H, CH2), 2.42 (s, 2H, CH2), 2.93-2.96 (m, 2H, CH2), 3.31-3.35 (m, 2H, CH2), 12.71 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.2, 22.8, 24.3, 25.4, 26.4, 28.4, 29.2, 29.4, 29.7, 30.7, 32.0, 33.8, 40.6, 43.6, 43.9, 51.9, 107.6, 172.2, 178.2, 194.7, 204.1. Anal. Calcd for C22H37NO4: C, 69.62; H, 9.83; N, 3.69. Found: C, 69.75; H, 9.87; N, 3.71.
2.1.3.7. 7-(2-(Heptylamino)-5-oxocyclopent-1-en-1-yl)-7-oxoheptanoic Acid (17)
Yield 95%; White solid; mp 76-78°С. 1H NMR (500 MHz, CDCl3) δ: 0.87 (t, J = 6.8 Hz, 3H, CH3), 1.23-1.42 (m, 10H, 5CH2), 1.57-1.68 (m, 6H, 3CH3), 2.33 (t, J = 7.6 Hz, 2H, CH2), 2.43-2.45 (m, 2H, CH2), 2.64-2.66 (m, 2H, CH2), 2.90 (t, J = 7.4 Hz, 2H, CH2), 3.33 (q, J = 6.8 Hz, 2H, CH2), 10.36 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.1, 22.7, 24.0, 24.2, 24.8, 26.8, 28.9, 29.8, 31.7, 33.6, 34.1, 40.3, 44.3, 110.3, 178.7, 181.8, 200.0, 200.2. Anal. Calcd for C19H31NO4: C, 67.63; H, 9.26; N, 4.15. Found: C, 67.54; H, 9.20; N, 4.11.
2.1.3.8. 7-(2-(Heptylamino)-6-oxocyclohex-1-en-1-yl)-7-oxoheptanoic Acid (18)
Yield 95%; White solid; mp 84-86°С. 1H NMR (500 MHz, CDCl3) δ: 0.87 (t, J = 6.6 Hz, 3H, CH3), 1.22-1.44 (m, 10H, 5CH2), 1.56-1.69 (m, 6H, 3CH2), 1.90 (q, J = 6.5 Hz, 2H, CH2), 2.33 (t, J = 7.6 Hz, 2H, CH2), 2.39 (t, 2H, J = 6.5 Hz, 2H, CH2), 2.92-2.95 (m, 2H, CH2), 3.30 (q, J = 6.8 Hz, 2H, CH2), 12.55 (brs, 1H, NH). 13C NMR (125 MHz, CDCl3) δ: 14.1, 19.8, 22.7, 24.8, 24.9, 27.0, 27.1, 28.9, 29.1, 29.4, 31.7, 34.2, 38.4, 43.5, 43.8, 108.7, 173.1, 179.0, 195.3, 204.0. Anal. Calcd for C20H33NO4: C, 68.34; H, 9.46; N, 3.99. Found: C, 68.45; H, 9.52; N, 4.03.
2.1.3.9. 7-(2-(Heptylamino)-4,4-dimethyl-6-oxo- cyclohex-1-en-1-yl)-7-oxoheptanoic Acid (19)
Yield 95%; White solid; mp 65-67°С. 1H NMR (500 MHz, CDCl3) δ: 0.87 (t, J = 6.7 Hz, 3H, CH3), 1.04 (s, 6H, 2CH3), 1.22-1.44 (m, 10H, 5CH2), 1.55-1.69 (m, 6H, 3CH2), 2.27 (s, 2H, CH2), 2.33 (t, J = 7.6 Hz, 2H, CH2), 2.41 (s, 2H, CH2), 3.04-2.88 (m, 2H, CH2), 3.30 (td, J = 7.1 Hz, 5.3 Hz, 2H, CH2), 12.66 (t, J = 5.4 Hz, 1H, NH). 13C NMR (126 MHz, CDCl3) δ: 14.1, 22.7, 24.8, 24.9, 26.9, 28.4, 28.9, 29.1, 29.5, 30.7, 31.7, 34.2, 40.5, 43.5, 43.9, 52.0, 107.5, 172.1, 178.9, 194.8, 203.5. Anal. Calcd for C22H37NO4: C, 69.62; H, 9.83; N, 3.69. Found: C, 69.53; H, 9.79; N, 3.65.
2.2. Pharmacological Activity
2.2.1. Animals
The pharmacological studies were carried out on outbred albino male mice or C57BL/6 female mice weighing 20-25 g with eight animals in each group. All the animals were taken from SPF-vivarium of the Institute of Cytology and Genetics of the Siberian Branch of Russian Academy of Sciences. Mice were housed in wire cages at 22-25°C on a 12 hr light-dark cycle. The animals had free access to standard pellet diet, tap water was available ad libitum. All the experimental procedures were approved by the Bio-Ethical Committee of the N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian Academy of Sciences in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, and the requirements and recommendations of the Guide for the Care and Use of Laboratory Animals.
2.2.2. In vivo Anti-inflammatory Activity
The anti-inflammatory activity of hybrids 1-9, aminobetulinic acid 10 and azaprostanoic acid 17 was evaluated in three acute inflammation models - the histamine-induced, the formalin-induced and concanavalin A-induced mouse paw edema. In accordance to the model, 0.05 mL of 0.1% histamine dihydrochloride solution or 3% formalin solution or 0.02 ml of 0.5% concanavalin A solution was injected into the aponeurosis of the mouse hind paw. The test compounds 1-9 were dissolved in saline containing 0.5% Tween 80 just before the use and were administered at the dose of 20 mg/kg 1 hr before histamine, formalin or concanavalin A injection. In concanavalin A-induced edema model, test compounds were administered twice 24 hr and 1 hr before phlogogen. The reference agent indomethacin (Fluka) was administered in the same manner and in the same dose as test substances. The control group of animals received an equivalent volume of water-tween mixture. Animals were sacrificed by cervical dislocation under the light ether anesthesia 5 hr after injection of the phlogogenic agent (histamine or formalin) or 1 hr after the last concanavalin A injection. The mouse paws were cut off at the ankle joint and weighed. The ratio of the difference in weight between the treated and untreated hind paws to the weight of the untreated hind paw was used as an inflammatory edema index (IEI).
2.2.3. Antinociceptive Evaluation
The antinociceptive activity of hybrids 1-9, aminobetulinic acid 10 and azaprostanoic acid 17 was evaluated in two standard experimental pain models - the acetic acid-induced abdominal writhing and the hot-plate test. The test compounds dissolved in saline containing 0.5% Tween 80 were administered i.p. at a dose of 20 mg/kg 1 hr before testing. Indomethacin at a dose of 20 mg/kg was used as a reference drug. In the acetic acid-induced writhing test, the pain reaction was determined by the number of abdominal convulsions recorded during 3 min after 5 min of 0.75% acetic acid injection (0.1 mL/10 g body weight i.p.) [30]. The percentage of pain reaction inhibition was calculated according to the following equation: % inhibition = 100 × (A - B)/A, where A was the mean number of writhes in the control group, and B was the mean number of writhes in the test group. In the hot-plate test, animals were placed individually on the hot metallic plate which was heated to a constant temperature 53 ± 0.5°C and surrounded by acrylic cage. The latency to respond was observed with hind paw licking, flick or jumping and the time of response was recorded using a stopwatch [31].
2.2.4. Cytotoxicity Assay
DMEM with 10% fetal bovine serum (Gibco, United States) was used for culturing. Optical density was measured on a Multiskan RC spectrophotometer (LabSystems). The MTT test was carried out according to the standard procedure [32] using the reagent 3-(4,5-dimethylthiazol-2-yl)-2,5-diphen-yltetrazolium bromide (MTT, Sigma, United States). Cell lines were obtained from the American Type Culture Collection: U-87 MG (ATCC number HBT-14), MCF7 (ATCC number HTB-22). Immortalized human fibroblasts used as a negative control were provided by A. Schilov (Institute of Cytology and Genetics of Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia). The viability for DMSO (negative control) was calculated as 100%. All culture experiments were carried out independently three times with two repeats in the experiment, and the final result is presented as concentration, which caused 50% inhibition of cell population growth (IC50 ± SEM).
2.2.5. Statistical Analysis
Data was represented as mean ± standard error (SEM) from the groups of animals. The statistical analysis was applied with parametric and non-parametric methods using “STATISTICA 6” software. The differences were significant at P < 0.05.
3. RESULTS AND DISCUSSION
3.1. Chemistry
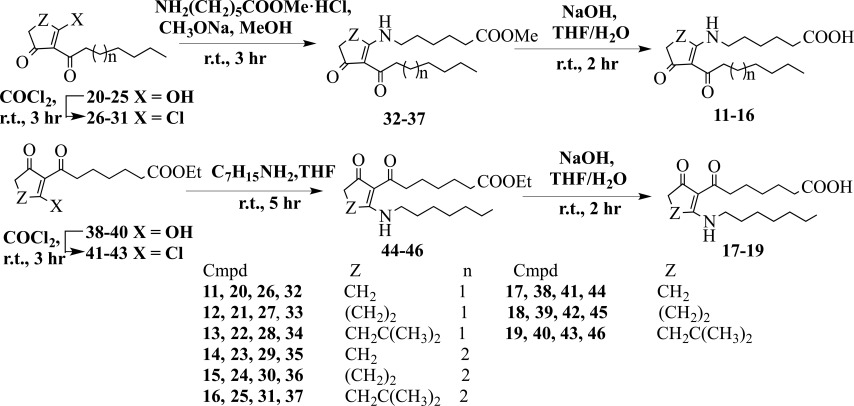
The synthesis of the desired betulinic acid-azaprostanoid hybrids 1-9 with amide linker was accomplished as outlined in Scheme 1.
Scheme 1.
A synthetic route for the preparation of hybirds 1-9.
As a starting compound for the synthesis of hybrids 1-9, we took naturally accessible lupane triterpenoid betulin [33]. Betulin was converted into betulonic acid by using Jones’oxidation according to a published procedure [34]. Reductive amination of betulonic acid under an action of cyanoborohydride in the presence of ammonium acetate in dry methanol resulted in a mixture of β- and α-isomers [35]. Target 3β-amino-3-deoxybetulinic acid (10) was isolated by us using silica gel column chromatography of the mixture in a yield of 50%. Triterpenoid hybrids 1-9 were synthesized via the condensation of prepared aminobetulinic acid 10 with 7-azaprostanoic acids 11-16 or 13-azaprostanoic acids 17-19 under an action of N,N′-dicyclohexylcarbodiimide (DCC) in the presence of 4-dimethylaminopyridine (DMAP) in methylene chloride in yields of 54-65%.
Azaprostanoic acids and their homo analogues 11-19 were prepared on the basis of cyclic β-trik- etones with 2-alkanoyl or 2-(ω-alkoxycarbonylal- kanoyl) side chains under known methods (Scheme 2) [22]. A treatment of 2-heptanoyl- or 2-octanoylcycloalkane-1,3-diones 20-25 with oxalyl
Scheme 2.
Synthesis of azaprostanoic acids 11-19.
chloride gave the vinylogous chlorides 26-31. An interaction of the latter with methyl-ε-aminohexa-noate hydrochloride in methanol led to 7-aza-prostanoids 32-37. 13-Azaprostanoids 44-46 were synthesized via the transformation of 2-(ω-ethoxy-carbonylalkanoyl)cycloalkane-1,3-diones 38-40 under an action of oxalyl chloride into the vinylogous chlorides 41-43 followed by the treatment of the latter with a twofold excess of heptylamine in chloroform. The obtained azaprostanoids 32-37, 44-46 were hydrolyzed with sodium hydroxide in THF/H2O to give the acids 11-19 in 93-98% yields.
All the target compounds have not been previously reported in the literature, and the structures were identified by physicochemical and spectral means, and both the analytical and spectral data of the synthesized compounds were totally consistent with their molecular structures.
3.2. Pharmacology
The anti-inflammatory and analgesic activities of hybrids 1-9, 3β-amino-3-deoxybetulinic acid (10) and 13-aza-7,9-dioxo-8(12)prostenoic acid (17) were investigated in vivo in comparison with indomethacin. Three models of mouse paw inflammatory edema induced by histamine, formalin or concanavalin A injection were used (Table 1). In the first two models of the exudative inflammation, none of the compounds at dose 20 mg/kg i.p. displayed a statistically significant effect. In the immunogenic (concanavalin A-induced) inflammation model, the betulinic acid-7-azaprostanoid hybrids 1, 2, 4-6 displayed a statistically significant effect by lowering the edema index 1.3-1.7 times relative to the control group. Among the hybrids with 13-azaprostanoids 7-9, only hybrid 9 exhibited a statistically significant effect by lowering the edema index 1.4 times relative to the control group (Table 1).
Table 1.
The influence of compounds 1-10, 17 on mice paw edema indexes in three models of inflammation.
| Agent | Dose, mg/kg | Mean Indexes of Paw Edema, % | ||
|---|---|---|---|---|
| Histamine-Induced Edema | Formalin-Induced Edema | Concanavalin A-Induced Edema | ||
| Control | - | 25.27 ± 8.69 | 71.58 ± 7.78 | 24.93 ± 2.14 |
| 1 | 20 | 19.83 ± 5.55 | 70.17 ± 4.85 | 14.77 ± 2,99*** |
| 2 | 20 | 23.76 ± 7.48 | 69.78 ± 10.82 | 18.76 ± 2.88*** |
| 3 | 20 | 26.67 ± 10.89 | 72.33 ± 6.61 | 21.88 ± 4.25# |
| 4 | 20 | 27.15 ± 9.43 | 71.47 ± 18.77 | 18.44 ± 4.65** |
| 5 | 20 | 21.99 ± 4.64 | 75.02 ± 12.88 | 18.59 ± 5.79* |
| 6 | 20 | 24.98 ± 5.46 | 72.87 ± 8.28 | 18.12 ± 4.52** |
| 7 | 20 | 19.92 ± 4.25 | 84.19 ± 8.85 | 24.57 ± 6.81# |
| 8 | 20 | 20.24 ± 4.05 | 73.71 ± 18.41 | 21.96 ± 4.73# |
| 9 | 20 | 22.37 ± 8.70 | 74.14 ± 17.32 | 17.25 ± 3.79*** |
| 10 | 20 | 22.27 ± 5.22 | 73.90 ± 17.34 | 16.42 ± 3.23*** |
| 17 | 20 | 17.88 ± 4.91 | 71.02 ± 18.66 | 21.44 ± 3.9*# |
| Indomethacin | 20 | 16.86 ± 3.31* | 48.02 ± 5.00* | 17.07 ± 2.70*** |
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001- difference with control are significant.
#P ≤ 0.05- difference with indomethacin are significant.
It is noteworthy that the aforementioned hybrids have anti-inflammatory activity, which is comparable to that of indomethacin in the immunogenic inflammation model, whereas in the exudative inflammation models, the activity of those substances was lower to that of indomethacin. The inflammatory response induced by histamine or formalin injection is mediated mainly through the activation of endothelial secretion of the pro-inflammatory and nociceptive (pain-generative) mediators as histamine, serotonin, bradykinin, calcitonin. They increase the excitation of primary afferent neurons and vascular leakage resulted in local edema and neutrophil infiltration [36]. Lectin concanavalin A injection causes the macrophage and mast cell activation and degranulation resulted in allergic-like reaction and edema. Obviously, the anti-inflammatory effect of hybrid agents is provided by their interactions mainly with the cells involved in inflammatory/immunogenic processes. In this case, the triterpenoid structure contributes significantly to this effect as it has been shown that the aminobetulinic acid 10 decreased allergic-like edema more potently compared to 13-azapros- tanoic acid 17 (respectively 1.5 times versus 1.2 times relative to the control group) (Table 1).
Antinociceptive properties of compounds 1-10, 17 were investigated using models of visceral and tactile pain. In the visceral pain model induced by intraperitoneal injection of 0.75% acetic acid, hybrids 1-9 displayed an analgesic effect ranging from weak to moderate (Table 2). Hybrids with
Table 2.
Analgesic activity of compounds 1-10, 17.
| Agent | Dose, mg/kg | A Pain Reaction of Mice in Visceral and Tactile Pain Models | |
|---|---|---|---|
|
Model of Visceral Pain,
Number of Writhes During 3 Minutes |
Model of Tactile Pain,
Time Until Licking of the Hind Paw, Sec |
||
| Control | - | 16.63 ± 3,70 | 6.63 ± 1.84 |
| 1 | 20 | 14.88 ± 6,85 | 6.25 ± 1.28 |
| 2 | 20 | 14.63 ± 5,42 | 5.63 ± 0.74 |
| 3 | 20 | 13.75 ± 3,42 | 5.63 ± 1.30 |
| 4 | 20 | 13.38 ± 6,12* | 7.00 ± 1,77 |
| 5 | 20 | 13.63 ± 3,66* | 5.38 ± 1.77 |
| 6 | 20 | 12.38 ± 2.67* | 6.38 ± 1.06 |
| 7 | 20 | 10.50 ± 2.33* | 7.75 ± 2.43 |
| 8 | 20 | 13.63 ± 2.77* | 7.00 ± 1.60 |
| 9 | 20 | 7.63 ± 3.44** | 6.25 ± 1.16 |
| 10 | 20 | 9.75 ± 2.12** | 5.25 ± 0.89 |
| 17 | 20 | 15.00 ± 3.51 | 6.50 ± 1.51 |
| Indomethacin | 20 | 4.25 ± 1.83*** | 7.50 ± 1.85 |
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 - difference with control are significant.
13-azaprostanoids 7-9 displayed more significant analgesic action by lowering the number of writhes 1.2-2.2 times relative to the control group, whereas the similar effect of hybrids with 7-azaprostanoids 1-6 did not exceed 1.3 times relative to the control group. Hybrid 9 and amino acid 10 were the most active among the tested compounds and had an activity lower to that of indomethacin 1.8 and 2.3 times, respectively. The results indicated that the antinociceptive effect of hybrids was due to their triterpenoid structure while azaprostanoic acid hadn’t visible activity. In the tactile pain model induced by heating, no significant differences in pain threshold in the investigated groups versus control were found.
The cytotoxic activity of the synthesized hybrids 1-9 was evaluated in vitro by the MTT assay on immortalized human fibroblasts, human breast cancer (MCF7) and human glioblastoma cell lines (U-87 MG) using doxorubicin, 3β-amino-3-deoxy- betulinic acid (10) and 13-aza-7,9-dioxo-8(12) pro- stenoic acid (17) as reference compounds (Table 3).
Table 3.
Concentrations of half-maximal inhibition (IC50) of compounds 1-10, 17 on U-87 MG, MCF7 cell lines and immortalized human fibroblasts.
| Compound | IC50 ± SEM, µM | ||
|---|---|---|---|
| U-87 MG | MCF7 |
Immortalized Human
Fibroblasts |
|
| Doxorubicin | 4.02 ± 1.17 | 5.51 ± 1.74 | 5.74 ± 2.04 |
| 1 | > 100 | 98.71 ± 10.02 | > 100 |
| 2 | > 100 | 41.4 ± 7.88 | 84.26 ± 11.08 |
| 3 | > 100 | > 100 | 51.19 ± 7.44 |
| 4 | 70.36 ± 10.12 | > 100 | 82.16 ± 10.52 |
| 5 | 66.6 ± 9.84 | > 100 | > 100 |
| 6 | 31.75 ± 4.87 | 17.96 ± 4.54 | > 100 |
| 7 | 74.7 ± 9.08 | > 100 | 78.64 ± 10.11 |
| 8 | 38.53 ± 6.78 | > 100 | 32.78 ± 5.61 |
| 9 | 59.92 ± 8.09 | > 100 | 78.43 ± 9.88 |
| 10 | < 10 | < 10 | < 10 |
| 17 | 8.28 ± 2.11 | 70.36 ± 5.96 | 61.27 ± 3.76 |
All the hybrids exhibited much lower cytotoxicity than doxorubicin, aminobetulinic acid 10 and 13-azaprostanoic acid 17 (last in case of U-87 MG). Some agents were more toxic than azaprostanoid 17: hybrids 2, 6 in MCF7 cell line and hybrids 3, 8 in immortalized fibroblasts.
CONCLUSION
In summary, in the current work, we developed a simple synthesis of novel betulinic acid-azaprostanoid hybrids containing amide linkage. The synthetic pathway includes the transformation of betulin via Jones’ oxidation into betulonic acid, reductive amination of the latter and coupling obtained by 3β-amino-3-deoxybetulinic acid with the appropriate 7- or 13-azaprostanoic acids and their homo analogues. The synthesized hybrids 1-9 were evaluated for in vivo anti-inflammatory activity using mouse paw edema models induced by the histamine, formalin and concanavalin A. In the immunogenic inflammation model, most of the synthesized compounds showed pronounced anti-inflammatory effect at 20 mg/kg, which was comparable to that of indomethacin. Compound 1 showed the most potent inhibitory effect towards immunogenic inflammation among all hybrids and their precursors - 3β-amino-3-deoxybetulinic acid (10) and azaprostanoic acid 17. In the visceral pain model, the hybrids 1-9 displayed analgesic effects ranged from weak to moderate which were less than that of indomethacin. The in vitro cytotoxicity study of the synthesized hybrids on cancer (MCF7, U-87MG) and non-cancer humane cell lines indicated that the hybridization of betulinic acid with azaprostanoids enhanced viability of the tested cells. Thus, the principal pharmacological effect of hybrids is the anti-inflammatory activity, substantially provided with the triterpenoid scaffold and in some cases, with the azaprostanoid scaffold, but the latter makes a significant contribution to reducing the toxicity of hybrids. These findings also showed the potential of molecular hybridization methods in the development of structurally new and safe anti-inflammatory agents. The hybrid 1 is suggested for further trials as a low toxic anti-inflammatory agent against immune-mediated inflammatory diseases.
Acknowledgements
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the experimental procedures were approved by the Bio-Ethical Committee of the N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian Academy of Sciences Russian Academy of Sciences, Russia, (Resolution N7, 05.16.2017).
HUMAN AND ANIMAL RIGHTS
No humans were used in the study that is the basis of this research. All the experimental protocols on animals were in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, and the requirements (European Communities Council Directives of 24 November 1986, 86/609/EEC) and recommendations of the Guide for the Care and Use of Laboratory Animals.
Consent for Publication
Not applicable.
Availability of Data and Materials
Not applicable.
Funding
This work was financially supported by National Academy of Sciences of Belarus (grant X15SO-001) and Siberian Branch of the Russian Academy of Sciences (project 0302-2015-0003).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sun L.D., Wang F., Dai F., Wang Y.H., Lin D., Zhou B. Development and mechanism investigation of a new piperlongumine derivative as a potent anti-inflammatory agent. Biochem. Pharmacol. 2015;95(3):156–169. doi: 10.1016/j.bcp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello C.A. Anti-inflammatory agents: present and future. Cell. 2010;140(6):935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azab A., Nassar A., Azab A.N. Anti-inflammatory activity of natural products. Molecules. 2016;21(10):1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg C.M. Natural product scaffolds of value in medicinal chemistry. In: Bräse S., editor. Privileged scaffolds in medicinal chemistry. Design, synthesis, evaluation. Cambridge, UK: Royal Society of Chemistry; 2016. pp. 348–378. [Google Scholar]
- 5.Tolstikova T.G., Sorokina I.V., Tolstikov G.A., Tolstikov A.G., Flekhter O.B. [Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives]. Bioorg. Khim. 2006;32(1):42–55. doi: 10.1134/s1068162006010031. [DOI] [PubMed] [Google Scholar]
- 6.Xu G.B., Xiao Y.H., Zhang Q.Y., Zhou M., Liao S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018;145:691–716. doi: 10.1016/j.ejmech.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Jonnalagadda S.C., Suman P., Morgan D.C., Seay J.N. 2017. Recent developments on the synthesis and applications of betulin and betulinic acid derivatives as therapeutic agents. [DOI] [Google Scholar]
- 8.Ríos J.L. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010;128(1):1–14. doi: 10.1016/j.jep.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 9.Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24(1):90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 10.Lingaraju M.C., Pathak N.N., Begum J., Balaganur V., Ramachandra H.D., Bhat R.A., Ram M., Singh V., Kandasamy K., Kumar D., Kumar D., Tandan S.K. Betulinic acid attenuates renal oxidative stress and inflammation in experimental model of murine polymicrobial sepsis. Eur. J. Pharm. Sci. 2015;70:12–21. doi: 10.1016/j.ejps.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Kim K.S., Lee D.S., Kim D.C., Yoon C.S., Ko W., Oh H., Kim Y.C. Anti-inflammatory effects and mechanisms of action of coussaric and betulinic acids isolated from Diospyros kaki in lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules. 2016;21(9):1206. doi: 10.3390/molecules21091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa J.F.O., Barbosa-Filho J.M., Maia G.L., Guimarães E.T., Meira C.S., Ribeiro-dos-Santos R., de Carvalho L.C.P., Soares M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014;23(2):469–474. doi: 10.1016/j.intimp.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Yun Y., Han S., Park E., Yim D., Lee S., Lee C.K., Cho K., Kim K. Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Arch. Pharm. Res. 2003;26(12):1087–1095. doi: 10.1007/BF02994763. [DOI] [PubMed] [Google Scholar]
- 14.Patlolla J.M.R., Rao C.V. Triterpenoids for cancer prevention and treatment: current status and future prospects. Curr. Pharm. Biotechnol. 2012;13(1):147–155. doi: 10.2174/138920112798868719. [DOI] [PubMed] [Google Scholar]
- 15.Smyth E.M., Grosser T., Wang M., Yu Y., FitzGerald G.A. Prostanoids in health and disease. J. Lipid Res. 2009;50(Suppl.):S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sykes L., MacIntyre D.A., Teoh T.G., Bennett P.R. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction. 2014;148(2):R29–R40. doi: 10.1530/REP-13-0587. [DOI] [PubMed] [Google Scholar]
- 17.Gilroy D.W., Colville-Nash P.R., Willis D., Chivers J., Paul-Clark M.J., Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 18.Ricote M., Li A.C., Willson T.M., Kelly C.J., Glass C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 19.Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403(6765):103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 21.Lakhvich F.A., Pashkovskii F.S., Koroleva E.V. Heteroprostanoids: synthesis and biological activity. Russ. Chem. Rev. 1992;61:243–266. doi: 10.1070/RC1992v061n02ABEH000943. [DOI] [Google Scholar]
- 22.Lakhvich F.A., Khlebnikova T.S. Synthesis of 7-aza- and 13-azaprostanoids on the base of 2-acylcycloalkane-1,3-diones. Vestsi Akad. Nav. BSSR. Ser. Khim. Nav. 1989;4:39–45. [Google Scholar]
- 23.Kuz′mitskii, B.B.; Golubeva, M.B.; Mizulo, N.A.; Romanova, V.N.; Khlebnikova, T.S.; Lakhvich, F.A. Anti-ulcerative and anti-inflammatory activity of some 7-aza-13-keto- and 13-aza-7-ketoprostanoids. Vestsi Akad. Nav. BSSR. Ser. Khim. Nav. 1989;6:82–86. [Google Scholar]
- 24.Kuz′mitskii, B.B.; Ignat′eva, T.N.; Khlebnikova, T.S.; Lakhvich, F.A. Antiischemic and antiarrhythmic activity of some 7-aza- and 13-azaprostanoids. Vestsi Akad. Nav. BSSR. Ser. Khim. Nav. 1989;5:65–68. [Google Scholar]
- 25.Choudhary S., Singh P.K., Verma H., Singh H., Silakari O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018;151:62–97. doi: 10.1016/j.ejmech.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Shaveta M., Mishra S., Singh P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016;124:500–536. doi: 10.1016/j.ejmech.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Khlebnicova T.S., Piven Y.A., Baranovsky A.V., Lakhvich F.A., Shishkina S.V., Zicāne D., Tetere Z., Rāviņa I., Kumpiņš V., Rijkure I., Mieriņa I., Peipiņš U., Turks M. Synthesis of novel lupane triterpenoid-indazolone hybrids with oxime ester linkage. Steroids. 2017;117:77–89. doi: 10.1016/j.steroids.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Khlebnikova T.S. Piven′, Y.A.; Lakhvich, F.A. Synthesis of new betulonic acid conjugates with 2-perfluoroacylcycloalkane-1,3-diones using polymethylenediamine linkers. Chem. Nat. Compd. 2017;53:486–491. doi: 10.1007/s10600-017-2028-x. [DOI] [Google Scholar]
- 29.Vasilevsky S.F., Govdi A.I., Shults E.E., Shakirov M.M., Sorokina I.V., Tolstikova T.G., Baev D.S., Tolstikov G.A., Alabugin I.V. Efficient synthesis of the first betulonic acid-acetylene hybrids and their hepatoprotective and anti-inflammatory activity. Bioorg. Med. Chem. 2009;17(14):5164–5169. doi: 10.1016/j.bmc.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Koster R., Anderson M., Deber E.I. Acetic acid for analgetic screening. Fed. Proc. 1959;18:412–414. [Google Scholar]
- 31.Eddy N.B., Leimbach D. Studies of anesthetics. J. Pharmacol. Exp. Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Alakurtti S., Mäkelä T., Koskimies S., Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006;29(1):1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim D.S.H.L., Chen Z., van Tuyen N., Pezzuto J.M., Qiu S., Lu Z-Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997;27:1607–1612. doi: 10.1080/00397919708006099. [DOI] [Google Scholar]
- 35.Kim D.S.H.L., Pezzuto J.M., Pisha E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg. Med. Chem. Lett. 1998;8(13):1707–1712. doi: 10.1016/S0960-894X(98)00295-9. [DOI] [PubMed] [Google Scholar]
- 36.Aman R., Schulidoi R., Lanz I., Donnerer J. Hystamine-induced edema in the rat paw - effect of capcaicin denervation and a CGRP receptor antagonist. Eur. J. Pharm. 1995;279:227–231. doi: 10.1016/0014-2999(95)00169-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
Not applicable.