Abstract
An important goal of biomedical research is to translate basic research findings into practical clinical implementation. Despite the advances in the technology used in drug discovery, the development of drugs for central nervous system diseases remains challenging. The failure rate for new drugs targeting important central nervous system diseases is high compared to most other areas of drug discovery. The main reason for the failure is the poor penetration efficacy across the blood-brain barrier. The blood-brain barrier represents the bottleneck in central nervous system drug development and is the most important factor limiting the future growth of neurotherapeutics. Meanwhile, drug repositioning has been becoming increasingly popular and it seems a promising field in central nervous system drug development. In vitro blood-brain barrier models with high predictability are expected for drug development and drug repositioning. In this review, the recent progress of in vitro BBB models and the drug repositioning for central nervous system diseases will be discussed.
Keywords: Blood-brain barrier, central nervous system disease, drug development, drug repositioning, neurotherapeutics, penetration efficacy
1. Introduction
An important goal of biomedical research is to translate basic research findings into useful medical advances. Despite the advances in the technology used in drug discovery, the development of drugs for the central nervous system (CNS) diseases remains challenging [1-3]. The failure rate for new drugs targeting important CNS diseases is high compared to most other areas of drug discovery. Currently, the treatments available for CNS diseases are disappointing and primarily focus on relieving the symptoms rather than curing the disease. The main factors responsible for the failures in CNS drug development are a lack of understanding of the basic principles of CNS disease, the possibility of CNS side effects, and the inability of drugs to penetrate the blood-brain barrier (BBB).
The mechanisms underlying many CNS diseases have been understood for decades [3]. For example, Alzheimer’s disease (AD) is an age-related neurodegenerative disease and the most prevalent form of senile dementia in the world [4, 5]. Aβ peptides had been considered promising therapeutic targets for AD [6-8]. However, nowadays, the idea that AD is a multifactorial disease is growing in popularity, and many factors, including oxidative stress, neuroinflammation, energetic deficit, vascular damage, synaptic failure, axonal injury, tau pathology, and mitochondrial dysfunction, may contribute to AD onset and progression [9, 10]. Stroke is one of the most important causes of death and long-term disability in survivors [11]. Because many neuroprotective drugs validated in basic research have failed to show any effect against ischemic stroke in clinical settings, the concept of the neurovascular unit (NVU) has been proposed [12]. The NVU consists of endothelial cells, associated BBB tight junctions; basal lamina; pericytes; and parenchymal cells, including astrocytes, neurons, and interneurons [13]. The concept of NVU has not only changed and strengthened basic research into stroke but research into all CNS diseases [14, 15]. Drug development fails regardless of the progress of research into the mechanisms of CNS diseases; unfortunately, few drugs have been found to reproducibly improve outcomes in AD and stroke. AD drug development has proven to be unusually difficult, with a 99.6% failure from 2002 to 2012, and, currently, the success rate continues to be at the same low level [16]. Neuroprotective drugs for stroke have all failed in pivotal phase III efficacy trials [12, 17]. Brain metastasis is another challenging CNS disease. The metastasis of cancer to the CNS remains a devastating clinical reality, conveying an estimated survival time of less than one year, despite intensive treatment [18, 19]. Some primary tumors are more sensitive to therapies targeting specific molecular pathways, and a recent study found that these targeted therapies sometimes show activity in the brain and are delivered to the brain metastatic tumors [20, 21]. The medical needs of people suffering from brain metastases are still unmet, and much research and public attention are directed toward the treatment and prevention of primary cancer [22]. To date, there are no approved drugs for targeted brain metastasis.
One reason for the failure of clinical trials of CNS disease treatments is the CNS side effect, which is not experienced in animal studies; however, the main reason is the poor penetration efficacy across the BBB [3]. The BBB represents a bottleneck in CNS drug development and is the most important factor limiting the future growth of neurotherapeutics [23]. Essentially 100% of large-molecule pharmaceutics (>500 Da), including peptides, recombinant proteins, monoclonal antibodies, RNA interference (RNAi)-based drugs, and gene therapies and >98% of small-molecule drugs do not cross the BBB [24]. Despite emerging demands for CNS drug development, in the 2010s, several high-profile global pharmaceutical companies decided to shut down major research projects into CNS drug development [25]. The major reason being that the development of new CNS drugs has become an increasingly high-risk activity with a poor success rate [25]. There is a clear need for the development of adequate models to further investigate the mechanisms of transport across the BBB in order to design better brain delivery strategies [26]. Drug development studies (including for the CNS) have historically used both in vivo and in vitro techniques. In vivo models directly utilize entire living animals, while in vitro models are constructions of artificial environments using cultured cells to mimic in vivo structures [27]. While in vivo models provide environments close to the human phenotype, they require extraordinary amounts of funding, time, and manpower per test, and their use is facing increasing pressure regarding the ethical issues involved [27, 28]. In vitro models can ameliorate these issues by offering the same environments in numerous arrays, as well as having less budget, time, and ethical constraints [27]. As described above, in vitro BBB models with high predictability are required for each stage of CNS drug development, such as candidate exploration, toxicity evaluation, and safety evaluation. Furthermore, most of the drugs already on the market have not been tested for their permeability into the CNS. An ideal in vitro model of the BBB would facilitate mechanistic studies of BBB tight junctions, transporters, enzymes, and macromolecular and immune cell trafficking and signaling [29]. It would also be need to be suitable for the rapid screening for the BBB permeability of both new CNS drug candidates and previously untested marketed drugs.
The attempts to develop new treatments for CNS diseases have costly and disappointing results and require a long period of time and high expenditure, whereas the repurposing of safe existing drugs provides a cost-effective and time-saving alternative [30, 31]. Drug repositioning, also known as drug repurposing, is based on the promiscuous properties of approved drugs, which encourages the re-examination of marketed drugs for new indications, and is an alternative approach to speeding up drug discovery that has become increasingly popular in recent years [31]. To date, the conventional de novo drug discovery process requires an average of around 13-15 years and 2-3 billion US dollars to approve and launch a drug [32, 33]. Drug repurposing usually focuses on drugs that have already cleared phase I safety trials but have yet to show efficacy for the intended indication. The preclinical study can often be by-passed, thereby reducing the overall cost of drug development. It is speculated that drug repurposing requires an average of 6.5 years and 300 million US dollars to approve and launch a drug [32, 33]. The process has been applied to find potential new uses for approved drugs or compounds for various CNS diseases, including dementia, neurodegenerative diseases, acute stroke, cancer, rare or orphan diseases, and metabolic disorders, and as antiviral, anti-bacterial, and analgesic drugs [31, 33-38]. In this review, the recent progress of in vitro BBB models have been introduced and drug repositioning for CNS diseases has been discussed [39].
2. Blood-brain barrier
2.1. Structure and Function
The small capillaries of the brain are unique morphological and functional units that serve a variety of roles; they supply nervous tissue with oxygen and nutrients, such as glucose, amino acids, and neurotransmitter precursors, and are continuous nonfenestrated vessels with additional properties allowing them to tightly regulate the movement of molecules, ions, and cells between the blood and the CNS [29, 39]. This intensely restricting barrier capacity allows the BBB to firmly regulate CNS homeostasis, which is critical for proper neuronal function, as well as protect the CNS from toxins, pathogens, inflammation, injury, and disease [40-42]. Due to the absence of vascular fenestrae, nutrients, electrolytes, and metabolic waste are translocated across the endothelial cells via various transporters [43]. Glucose, amino acids, peptides, and proteins are actively transported down their concentration gradients from the vascular lumen to the brain parenchyma via carriers or receptors expressed at both the luminal and abluminal membranes of endothelial cells [41, 44-47]. Sodium pumps (Na+, K+-ATPase) in the abluminal membrane of the BBB regulate sodium influx into the brain in exchange for potassium [43, 47]. Potentially toxic substances, such as amyloid beta, a peptide implicated in the pathology of AD, are cleared from the brain parenchyma by receptors expressed at the abluminal membrane [41, 47]. Small lipophilic molecules, with molecular weights <400 Da, can cross the BBB by passive diffusion through the lipid bilayer membranes of ECs, which is counteracted by enzymatic metabolism or active efflux from the vascular endothelium into the blood [41, 44-47]. Active efflux is normally accomplished by ATP-binding cassette transporters (ABC), such as P-glycoprotein (P-gp), breast cancer resistance proteins (BCRPs), and multidrug resistance-associated proteins (MRPs) [41, 46, 47]. ABC transporters are transmembrane proteins that use the hydrolysis of adenosine triphosphate (ATP) to drive the translocation of the substrate against its concentration gradient [48]. The capillaries of the brain form complex structures, consisting of several cell types; endothelial cells constitute the capillary wall and form the actual barrier and are surrounded by pericytes [29, 49]. The precise amount of the vascular surface that is covered by pericytes is still unclear but varies 22%-70% according to some reports (and is believed to be approximately 30%) [29, 50]. The endothelial cells and pericytes are surrounded by the basement membrane, and more than 99% of the capillaries are covered by astrocyte endfeet [50]. Both pericytes and astrocytes contribute to BBB properties and development and to the unique endothelial phenotype [51]. These roles are mediated by the expression and release of soluble factors, and they are possible through their anatomic proximity to ECs [52]. The pericytes and the astrocytes that interact with ECs enhance tight junctions (TJ) and reduce the gap junctional area, thus demonstrating that these cells have an important role in the restricted permeability and integrity of the BBB [53]. Brain capillary endothelial cells and the surrounding accompanying cells, including not only astrocytes and pericytes but microglia, neurons, mast cells, as well as circulating immune cells, constitute the NVU, a term encompassing the specialized and unique cellular structure and function of the brain microvasculature [42, 54].
With the accelerated pace of brain research in recent years and a growing understanding of the complexity of the brain and various brain-associated neurological diseases, the need for effective tools to enhance drug screening, diagnosis, and basic research is increasing [55]. Highly representative models of the CNS can play a critical role in meeting these needs [55]. Unfortunately, in vivo animal models lack controllability, are difficult to monitor and do not model human-specific brain-behavior accurately. While in silico computational models struggle to comprehensively capture the intertwined biological, chemical, electrical, and mechanical complexity of the brain. Thus, the appearance of high-quality in vitro BBB models is highly anticipated. In this review, in vitro BBB models for the development of CNS drugs have been focussed.
2.2. In Vitro BBB Models
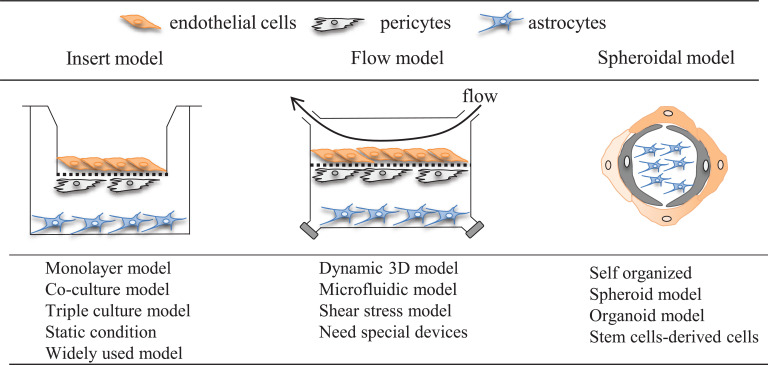
In general, it is difficult to prepare an in vitro BBB model that perfectly mimics in vivo functions and properties. Several reports have indicated that tight junction function, which is one of the characteristics of the BBB, is reduced in vitro compared with in vivo. Additionally, it has been pointed out that the expression levels and polarity of transporters change [56-59]. Despite the disadvantages of the in vitro BBB model, cell culture-based BBB models are widely accepted as powerful tools for evaluating BBB physiology, pathophysiology, and pharmacology and drug development. The in vitro system can provide detailed mechanism elucidation using a simplified experimental system and facilitate multi-sample processing. Indeed, a number of in vitro BBB models have been developed that use various culture devices and cells (Fig. 1).
Fig. (1).
Schematic overview of in vitro BBB models composed of brain endothelial cells, pericytes, and astrocytes. Consider the important role of astrocytes and pericytes on the induction of BBB properties, several groups reported the triple culture model. Endothelial cells are grown in the presence of both astrocytes and pericytes. In the case of the construction of BBB model using the insert membrane, brain endothelial cells are seeded onto the upper surface of the insert membrane while pericytes grow on the opposite surface of the membrane, and astrocytes are placed at the bottom of the well. In contrast to the static environment in standard culture conditions, blood vessels are exposed to shear stress in vivo. Based on this aspect, several flow-based BBB models, including the triple culture model, have been reported. To make a spheroid or organoid BBB model, mixed cells consist of endothelial cells, astrocytes, and pericytes are cultured in the same well and led to make 3D organized BBB models. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.3. Validation for In Vitro BBB Models
To develop cell culture-based in vitro BBB models, we need to confirm that (1) BBB function can be quantitatively evaluated, (2) mature BBB characteristics are developed, and (3) the model mimics the in vivo situation.
To date, a variety of cell sources (rodent, porcine, bovine, and human) are available for the culture of in vitro BBB models. In recent years, the use of a flow model has been taken into consideration. Endothelial cells are the most important components of the BBB, and, to select appropriate endothelial cells, it is necessary to confirm the formation of a thin monolayer structure and to use markers of endothelial cells, such as von Willebrand’s factor, factor VIII, and PECAM-1 [60-64]. In addition to confirming the expression of typical tight junction proteins by mRNA and western blot, it is also important to confirm their localization in cell-cell borders by immunostaining [65-67]. Typical examples of objective evaluations of tight junction function include transendothelial electrical resistance (TEER) and permeability tests [56, 68, 69]. TEER is a widely accepted quantitative technique for measuring tight junction function in cell culture models of endothelial monolayers [29, 70]. TEER values are used to accurately assess the integrity of the cellular barriers before they are used to evaluate drug or chemical transport [29, 70]. TEER measurements can be performed in real-time without any cell damage and are generally based on the measurement of ohmic resistance or impedance across a wide spectrum of frequencies. The non-invasiveness of TEER measurements allow us to use the endothelial cells for other experiments, such as permeability tests, western blotting, and immunocytochemistry. Other important BBB functions that should be confirmed are a solute carrier (SLC) and efflux transporters, and, in some cases, the transferrin receptor system [29, 71, 72]. Furthermore, it is also recommended to confirm that the function of endothelial cells is enhanced by the co-culture of surrounding cells, such as pericytes and astrocytes [73, 74]. Regardless of what kinds of endothelial cells are used, it is necessary to design experiments to confirm their BBB function.
2.4. TEER and Permeability Tests
TEER is a widely accepted quantitative technique for measuring tight junction function in cell culture models of endothelial monolayers. TEER values are used to accurately assess the integrity of the cellular barriers before they are used to evaluate drug or chemical transport. The measurements can be performed in real-time without any cell damage and are generally based on the measurement of ohmic resistance or impedance across a wide spectrum of frequencies. The non-invasiveness of TEER measurements allow us to use the endothelial cells for other experiments, such as permeability tests, western blotting, and immunocytochemistry. The classical setup for measurement of TEER consists of a cellular monolayer cultured on a semipermeable filter insert, which defines the partition for apical (or upper) and basolateral (or lower) compartments (a Transwell system is normally used) [70]. For electrical measurements, two electrodes are separated by the cellular monolayer, i.e., one electrode is placed in the upper compartment and the other in the lower compartment. In theory, the ohmic resistance can be determined by applying a direct current (DC) voltage to the electrodes and measuring the resulting current [70]. The ohmic resistance is calculated, based on Ohm’s law, as the ratio of the voltage to the current [29]. However, DC currents can damage both the cells and the electrodes; therefore, to overcome this issue, an alternating current (AC) voltage signal with a square waveform is applied. In a widely used and commercially available TEER measurement system known as an Epithelial Voltohmmeter (EVOM), an AC square wave at a frequency of 12.5 Hz is used to avoid any charging effects on the electrodes and the cell layer [29, 70]. Caution is needed when comparing the TEER values among published reports because the values vary to some extent, not only because of differences in the actual junctional tightness but also differences in the measuring equipment (chopstick electrodes, cup electrodes, impedance measurements etc.), temperature, and handling of the cells during measurements [29, 70]. Furthermore, TEER is sometimes difficult to translate to real functional tightness, since the tightness of the endothelial monolayer depends on both the composition of tight junction complexes and the size of the compound of interest. To validate functional tightness, permeability tests with tracer molecules, such as Lucifer yellow (444 Da), sodium fluorescein (376 Da), or sucrose (342 Da), are needed. TEER correlates with permeability for a given small hydrophilic molecule, but the correlation depends greatly on the size of the molecules and the experimental design [75-77].
3. Variety of in vitro BBB models
3.1. Parallel Artificial Membrane Permeability Assays (PAMPA)
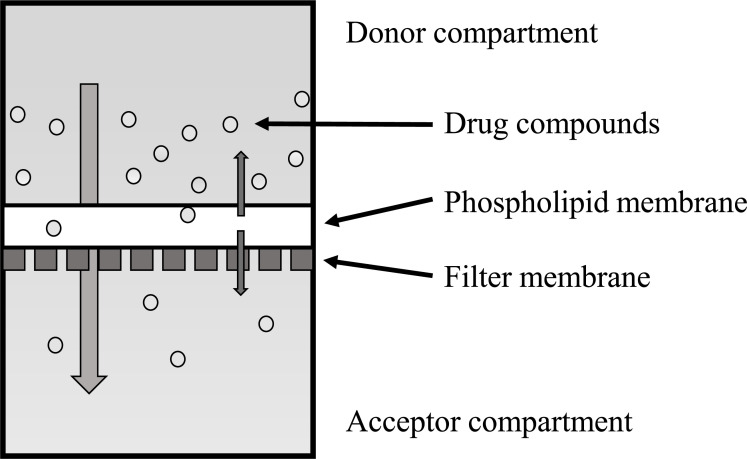
The parallel artificial membrane permeability assay (PAMPA) is a procedure developed for a rapid determination of passive transport permeability that is widely accepted, especially in pharmaceutical research. In PAMPA, a filter plate coated with a liquid artificial membrane is used to separate two compartments: one containing a buffer solution of compounds to be tested (defined as donor compartment) and the other containing an initial fresh buffer solution (defined as acceptor compartment) (Fig. 2). Permeation determined with PAMPA using filters impregnated with a solution of phospholipids or hexadecane provided significant correlations with gastrointestinal absorption in humans [78, 79]. The development of in vitro assays for modeling BBB functionality and the high-throughput testing of drug candidates is a critical area of research. BBB-specific PAMPAs are widely used, non-cell-based tools for predicting the passive diffusion of compounds through the BBB in a relatively high-throughput manner [80, 81]. These models could predict the passive diffusion of the compounds. With the same experimental conditions, the porcine brain lipid was the most discriminating as the BBB model [79]. These assays are useful for eliminating the costs, animal-to-human translation inaccuracies, and ethical quandaries associated with in vivo animal testing [80]. Könczöl et al. have shown that the simple modification of PAMPAs is a potentially valuable strategy in the CNS drug discovery environment [82]. PAMPA has been extensively used for drug development, but it is beneficial to understand its limitations and to use it only to predict passive transport [83]. The false-positive values obtained in the compounds could be attributed to the compounds being substrates for active efflux [79]. Caution should be used when interpreting the results of the system because it lacks the complexity to accurately model active transport processes [80, 81, 83].
Fig. (2).
Schematic diagram of PAMPA model. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Cell Culture Devices and Extracellular Matrix to Construct In Vitro BBB Model
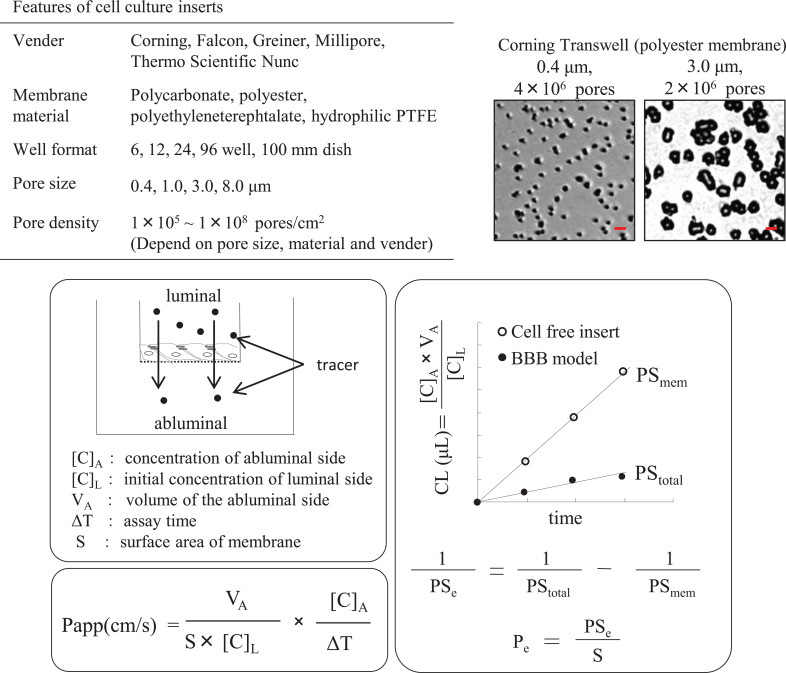
The discovery of cell culture inserts with porous filter membranes has allowed brain capillary endothelial cells to be used for permeability studies and the construction of co-culture in vitro models [84, 85]. So far, commercially available cell culture inserts have been applied most widely in the construction of in vitro BBB models. A researcher can choose from a variety of cell culture inserts with different pore diameters, pore densities, and membrane material, which affect the growth and function of endothelial cells, according to the research purpose. One benefit of using a culture insert is that it is possible to divide the space into two domains: the luminal (blood) and the abluminal (brain) side. Importantly, several reports have indicated that when culturing endothelial cells on large-pore-size membranes (more than a 3-μm pore size), the cells pass through the membrane and form a second layer on the undersurface of the insert membrane [86-88], and these in vitro models, which have a bi-endothelial layer, no longer represent the physiological BBB function. To avoid endothelial cells crossing the insert membrane, Vandenhaute et al. developed a new method involving the culture of endothelial cells on a dry-bottom insert (i.e., with no medium in the lower compartment) until a sufficient monolayer has developed. Additionally, properties of the insert membrane seem to affect the barrier function of the BBB model [88, 89]; therefore, selection of an appropriate insert membrane and optimization of culture conditions are critical in the model’s construction (Fig. 3).
Fig. (3).
Features of cell culture inserts and calculation of permeability. Commercially available cell-culture inserts have been used most widely to construct in vitro BBB models. A researcher has to choose from cell culture inserts from different pore diameters, pore densities, and membrane material according to the research purpose. Bar in the representative image of Transwell indicates 5 μm. In general, the Permeability of the substrate is calculated by apparent permeability (Papp) or transendothelial permeability coefficient (Pe). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The pore size and density of the membrane also affect the permeability of the substrate. Apparent permeability (Papp) is calculated using the following equation: Papp = (dQ/dt)/(S × C0), where dQ/dT is the cumulative amount of substrate in the receiver compartment versus time, S is the surface of the filter, and C0 is the initial concentration of the tracer in the luminal compartments. As the calculation of Papp includes the restriction effect of the insert membrane, Papp values tend to be low when using a small pore size and low pore density membrane. The influence of the membrane cannot be ignored, especially when examining the permeability of large molecules. In that case, the transendothelial permeability coefficient (Pe) can be used [90, 91]. Cleared volume is calculated from the concentration (C) of the tracer in the abluminal and luminal compartments and the volume (V) of the abluminal compartment by the following equation: Cleared volume = Cabluminal × Vabluminal /Cluminal. The average cleared volume is plotted vs. time, and the permeability × surface area product value for the endothelial monolayer (PSe) is calculated using the following formula: 1/PSendothelial = 1/PStotal - 1/PSinsert. PSe divided by the surface area generates the endothelial permeability coefficient. Although it is necessary to calculate the permeability of only the membrane using the transendothelial permeability coefficient, accurate permeability can be evaluated.
To promote cell attachment and growth on a culture device, several types of extracellular matrix (ECM) have been evaluated; collagen type I, type IV, and fibronectin are widely used in the construction of BBB models for their economy and other characteristics. BBB-related cells secrete several extracellular matrices: fibronectin, collagen IV, laminin, agrin, perlecan, SPARC, and nidogen-1 [92-97]. These ECM play an important role in the maintenance and development of the BBB as a component of a NVU [98-100]. There are some reports of the effects of ECM on the function of the in vitro BBB model. Endogenous ECM mixtures derived from cultured endothelial cells, pericytes, and astrocytes strengthen the barrier properties, compared with endogenous ECM derived from non-brain endothelial cells (aorta) [101, 102]. In addition, collagen IV, fibronectin, and laminin have the ability to up-regulate the barrier function of porcine brain endothelial cells [103]. Katt et al. reported that iPS-cell-derived endothelial cells have a higher TEER value when the cells are cultured in cross-linked collagen I gel coated with fibronectin and collagen IV [104].
While the cell culture inserts are useful for culturing endothelial cells, it is difficult to expose the cells to fluid-induced shear stresses similar to those occurring in vivo blood vessels. Therefore, dynamic cell culture devices with micro-holes, such as three-dimensional tube structure and micro-fluidic devices, have been developed for the construction of BBB models [105-107].
3.3. Cells for Constructing In Vitro BBB Models
3.3.1. Immortalized Endothelial Cells
A large number of in vitro BBB models have been developed using immortalized and primary cultured endothelial cells. Immortalized cell lines that are easy to handle and reproducible have been prepared from several species, such as mouse (bEND3, bEND5, MBEC4, TM-BBB, cEND), rat (RBE4, GP8.3, GPNT, TR-BBB), bovine (TBMEC P11, hTERT-BME), and human (hCMEC/D3, TY10, BB19, HBMEC/ciβ) sources [84, 89, 108-110]. Although leaky barriers are common disadvantages of these immortalized cells, some important BBB properties are retained; therefore, the cells have been used in various studies. The commercially available murine origin bEnd3 and bEND5 are widely used in BBB research, and the commercially available hCMEC/D3 cells, developed by Weksler et al., are well characterized by human brain endothelial cells. Reports indicate that hCMEC/D3 cells retain BBB properties, including several transporters and junctional proteins [111]. Among immortalized cell lines, cEND and cerebEND, which are developed from mouse cerebral and cerebellar capillaries, respectively, possess well-retained barrier properties similar to primary cultured brain endothelial cells [112].
To predict a drug’s ability to penetrate the BBB, non-cerebral cell lines, such as human bladder carcinoma (ECV304), Madin-Darby Canine Kidney (MDCK), and human colon carcinoma (Caco-2) cell lines, are used to construct surrogate BBB models. ECV304 cells present some features of endothelial cells, and a co-cultured model with C6 glioma cells has been used to develop an in vitro BBB model [113, 114]. The most prominent feature of MDCK and Caco-2 cells is their tight barrier function and restricted paracellular transport. Additionally, MDCK and Caco-2 that overexpress P-glycoprotein have been developed and applied to the prediction of BBB penetration by compounds [115-117]. While MDCK, Caco-2, and ECV304 cells have several advantages, depending on experimental design, these cell lines have different cell morphologies, TJ proteins, transporters, and enzymes compared with brain endothelial cells. Therefore, caution should be exercised when using them.
3.3.2. Primary Cultured Endothelial Cells
While primary cultures are expensive, time-consuming, and require expertise, primary cultured cells have the advantage of retaining many in vivo properties. To construct an in vitro BBB model using primary cultured cells, there are several methodical considerations: the purification of endothelial cells, culture conditions, source of brain cells, and whether to co-culture with other BBB-related cells.
3.3.3. Purification of Brain Endothelial Cells
One method for obtaining brain capillary endothelial cells is based on the successful isolation of brain capillaries [118]. Fractions of isolated brain capillaries contain several types of cells, such as endothelial cells, pericytes, and other brain-derived cells. Of the several attempts to eliminate contaminating cells, the development of a puromycin method has been successfully utilized to obtain pure endothelial cells [119]. This method is based on the fact that brain capillary endothelial cells express much higher amounts of P-glycoprotein than any other cells in the brain microvessel fractions, thus, they can tolerate toxic concentrations of P-glycoprotein ligand drugs while non-endothelial cells are eliminated. Puromycin is the best P-glycoprotein ligand to selectively kill contaminating cells. This selection appears to be more effective for capillary endothelial cells compared to large microvessels and could lead to tighter monolayers and better BBB models [120].
3.3.4. Culture Conditions
The culture conditions for in vitro BBB models are fundamental, as they can affect the properties of the BBB model. To appropriately maintain endothelial growth and function, several supplements are added to the culture medium. Glucocorticoid receptor agonists are widely used to improve the tightness of brain endothelial cells, whereas dexamethasone, corticosterone, and hydrocortisone can be used to strengthen barrier properties, increase TEER, and decrease Pe for paracellular markers [84]. Cyclic adenosine monophosphate (cAMP) is a prominent factor for inducing strong barrier properties in endothelial cells; cAMP increases barrier function through the upregulation of claudin-5 expression and phosphorylation of claudin-5 and myosin light chains [121, 122]. The elevation of cAMP using the cAMP analog, 8-(4-chlorophenylthio) cAMP (CPT-cAMP), a cell-permeable adenylate cyclase activator forskolin, and the phosphodiesterase (PDE) inhibitor are often applied to in vitro BBB models. However, for the examination of drugs, this model may influence intracellular cyclic nucleotides. Additionally, recent studies indicate that retinoic acid induces BBB properties in cultured brain endothelial cells and iPS-derived endothelial cells [123, 124]. Of note, retinoic acid-treated human pluripotent stem cell-derived BMECs showed significantly induced tightness and a high TEER value (~ 5,000 Ω x cm2) [124].
3.4. Co-culturing with Other BBB-related Cells
Brain capillary endothelial cells are a major component of the NVU and dynamically interact with neighboring cells, including astrocytes, pericytes, perivascular microglia, and neurons. Cross talk between the cells of the NVU is crucial for the formation and maintenance of a functional BBB and homeostasis of the CNS [125]. Reconstructing the cross-talk between BBB-related cells using a co-culture system of porous filter membranes enhances the construction of an in vitro BBB model.
Astrocytes are well-characterized regulators of brain capillary endothelial-specific properties, including the induction of barrier function and the upregulation of enzymes and transporters [126, 127]. These findings support the use of a brain capillary endothelial cell/astrocyte co-culture as an in vitro reconstruction model, which has become the most widespread type of in vitro BBB model. The most commonly used source species for astrocytes are rodents, and they can be co-cultured with several species of endothelial cells from primary cultured and immortal cell lines. In contrast to astrocytes, pericytes are rarely used in BBB co-culture systems. Pericytes embedded in the brain capillary basement membrane are the nearest neighbors of endothelial cells, and they have a fundamental role in the stabilization of the brain capillary structure in vivo [101, 128]. Consistent with in vivo observations, recent reports indicate that pericytes, like astrocytes, play an important role in inducing BBB properties [74]. The barrier tightening effects of rodent and human pericytes have been demonstrated for rodent and human endothelial cells [73, 129]. However, there are conflicting data about the effects of pericytes on porcine endothelial cells. Several studies have reported that the pericyte-endothelial interaction increased matrix metalloproteinase activities and did not improve the barrier tightness of porcine brain endothelial cells (PBEC) [130, 131]. On the other hand, Thomsen et al. indicated that the co-culture of PBEC with rat pericytes resulted in significantly increased tightness [132]. Overall, the co-culture model using endothelial cells and pericytes is one of the most important tools in BBB research.
Considering the importance of astrocytes and pericytes in the induction of BBB properties, several groups have constructed triple-culture models [74, 132, 133]. Endothelial cells are grown in the presence of both astrocytes and pericytes, and a synergetic effect on the induction of BBB properties can be observed. To construct a BBB model using an insert membrane, brain endothelial cells are seeded onto the upper surface of the membrane, while pericytes grow on the opposite surface, and astrocytes are placed at the bottom of the well. In contrast to the static environment in standard culture conditions, blood vessels in vivo are exposed to shear stress. Because of this, several flow-based BBB models, including the triple-culture model, have been used [105-107]. Research groups have developed a spheroid/organoid type of BBB model by culturing a mix of endothelial cells, astrocytes, and pericytes in the same well, resulting in a 3D structure [133-135]. The spheroid BBB model is primarily suitable for drug screening tests.
Among the other types of brain cells, neural precursor cells (NPCs) and oligodendrocytes precursor cells (OPCs) also have the ability to induce BBB properties. During in vivo BBB development, brain capillaries invade the immature neural environment and interact with each other [136]. Consistent with this, differentiating embryonic NPCs, which contain neuron and glial cells, have been shown to be able to induce BBB properties in co-culture models [137, 138]. Recently, some reports indicated that OPCs play an important role in the development and maintenance of BBB properties [139-141]. In vivo studies have shown that platelet-derived growth factor receptor-α (PDGFR-α)-positive OPCs exist in the perivascular and parenchymal space, and OPC-specific TGF-β-knock-out mice show leaky barrier properties. Furthermore, the OPCs interacted with endothelial cells and pericytes in vitro. Conditioned medium from cultured OPCs decreased the endothelial permeability of FITC-dextran through the TGF-β signaling pathway and decreased pericyte proliferation. These data indicate that OPCs can induce BBB properties. As no studies have focused on the construction of an OPC-endothelial cell co-culture model, further research on the development and characterization of this type of BBB model are needed.
3.5. Source of Endothelial Cells
A variety of endothelial cell sources exist to construct the in vitro BBB model (Table 1). We here discuss some of them, especially focusing on primary endothelial cell culture.
Table 1.
Different cells to construct in vitro blood-brain barrier model.
| - | Cells | Features | References | |
|---|---|---|---|---|
| Non-brain derived cells | ECV304 | Human bladder cancer derived epithelial cell line. Exhibit both endothelial and epithelial characteristics. |
[113, 142] | |
| Caco-2 | Human colon carcinoma cell line. High barrier properties. |
[115, 143] | ||
| MDCK | Madin-Darby Canine Kidneys High barrier properties. |
[115, 117] | ||
| Peripheral endothelium | HUVECs are widely used for endothelial biology. Co-cultured with astrocytes. |
[144, 145] | ||
| Brain-derived cells | Immortalized cell line | RBE4, GP8.3 TR-BBB |
Rat brain-derived cell lines. Low barrier properties. |
[109, 146] |
| bEnd.3, MBEC4 TM-BBB, cEND |
Mouse brain-derived cell lines. Low barrier properties. cEND possess well-retained barrier properties. |
[109, 147, 148] | ||
| hCMEC/D3 | Mouse brain-derived cell lines. Low barrier properties. One of the well-characterized cell line. |
[111, 149] | ||
| Primary cultured cells | Mouse, Rat | Low yield of endothelial cells from one brain. Possible to comparison with in vivo data. |
[73, 150] | |
| Bovine, porcine | High yield of endothelial cells from one brain. High barrier properties. |
[151, 152] | ||
| Monkey | High yield of endothelial cells from one brain. Primates model. |
[153, 154] | ||
| Human | Human data. Difficult to obtain normal tissue stably. |
[155] | ||
| Others | Artificial membrane (IAM, PAMPA) |
High throughput assay. Predict for passive transcellular transport. |
[79] | |
| iPS-derived cells | Several groups developed in vitro BBB model using iPS-derived cells. | [136] | ||
3.5.1. Rodent Endothelial Cells
In vitro BBB models using brain endothelial cells derived from mice and rats are one of the most widely used. Mice and rats have a long history as experimental animals in various research fields; therefore, there is a wealth of accumulated knowledge and experimental tools that can be applied to BBB research. Several groups have designed rodent in vitro models, including monolayer, co-culture, and triple-culture designs. Compared to immortalized cells, primary cultured cells show a tight barrier function. Rodent endothelial cells generally display low to medium barrier function (TEER: 100-300 Ω x cm2); however, the barrier function is upregulated when endothelial cells are co-cultured with astrocytes and/or pericytes (TEER: over 500 Ω x cm2) [156]. Accumulated evidence indicates that rodent co-culture models enhance the expression of several transporters, receptors, and enzymes compared to monolayer models. Here we introduce two major methods for isolating rodent endothelial cells.
3.5.2. Isolation of Rat Endothelial Cells
Primary cultures of rat brain capillary endothelial cells (RBEC) are prepared from 3-week-old rats, as previously described [73, 74]. Meninges are carefully removed from the forebrains and gray matter minced into small pieces of approximately 1 mm3 in ice-cold Dulbecco’s modified Eagle’s medium (DMEM), then dissociated by 25 up- and down-strokes with a 5-ml pipette in DMEM containing collagenase type 2 (1 mg/ml, Worthington Biochemical Corp., NJ, USA), 300 μl DNase (15 μg/ml), and gentamycin (50 μg/ml) and digested in a shaker for 1.5 h at 37 °C. The cell pellet is separated by centrifugation in 20% bovine serum albumin (BSA)-DMEM (1000 × g, 20 min). The microvessels obtained in the pellet are further digested with collagenase-dispase (1 mg/ml, Roche Applied Sciences, Basel, Switzerland) and DNase (6.7 μg/ml) in
DMEM for 1 h at 37°C. Microvessel endothelial cell clusters are separated on a 33% continuous Percoll (Pharmacia, Uppsala, Sweden) gradient, collected, and washed twice in DMEM before plating on 35 mm plastic dishes coated with collagen type IV and fibronectin (both 0.1 mg/ml). RBEC cultures are maintained in DMEM/F12 supplemented with 10% plasma-derived serum (PDS, Animal Technologies Inc., MD, USA), basic fibroblast growth factor (bFGF, Roche, Applied Sciences, Basel, Switzerland, 1.5 ng/mL), heparin (100 μg/ml), insulin (5 μg/ml), transferrin (5 μg/ml), sodium selenite (5 ng/ml) (insulin-transferrin-sodium selenite media supplement), gentamycin (50 μg/ml) and puromycin (4 μg/ml) (RBEC medium I) at 37°C with a humidified atmosphere of 5% CO2/95% air, for 2 days [119]. On the third day, the cells receive a new medium containing all the components of RBEC medium I except puromycin (RBEC medium II). When the cultures reached 80% confluency (4th day in vitro), the purified endothelial cells are passaged by a brief treatment with trypsin (0.05%, w/v)-EDTA (0.02%, w/v) solution, and used to construct various types of in vitro BBB models.
A major advantage of using the rat BBB model is that syngeneic co-cultures can be established, and the results obtained from rat BCECs can be correlated with in vivo data from the same species, and even strain, of the rat. In addition, the genome and transcriptome of rats are well understood, and a large set of antibodies are available for rat antigens.
3.5.3. Isolation of Mouse Endothelial Cells
The following describes the preparation of primary mouse BECs, according to Coisne et al., with modifications [157]. Briefly, meninges are carefully removed from forebrains and gray matter minced into small pieces. Preparations are pooled and ground with a Dounce homogenizer in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F12; Sigma-Aldrich Co., St. Louis, MO, USA) supplemented with gentamicin (50 µg/ml, Sigma-Aldrich Co., St. Louis, MO, USA). The resulting homogenate is mixed with 30% dextran (v/v, molecular weight 100,000–200,000, Sigma-Aldrich Co., St. Louis, MO, USA) in DMEM/F12 supplemented with 0.1% BSA; Sigma-Aldrich Co., St. Louis, MO, USA). The suspension is centrifuged at 3000 × g for 25 min at 4 °C. The pellet is suspended in DMEM/F12 and the supernatant centrifuged again under the same conditions. After the second centrifugation, the supernatant is discarded and the pellet re-suspended in DMEM/F12. Then the pellets are filtered through a 70 µm nylon mesh and digested in collagenase/dispase (2 mg/ml, Roche Applied Science, USA) and DNase I (10 µg/ml, Sigma-Aldrich Co., St. Louis, MO, USA) for 30 min. The digested solution is filtered through a 20 µm nylon mesh and seeded on collagen type IV/fibronectin-coated dishes (both form Sigma-Aldrich Co., St. Louis, MO, USA). Cultures are maintained in medium composed of DMEM/F12 supplemented with 10% PDS (Animal Technologies, Inc., USA), 1% GlutaMAX (Gibco), basic fibroblast growth factor (bFGF; Roche Applied Sciences), heparin, insulin, transferrin and sodium selenite supplement, and puromycin (4 µg/ml, Sigma-Aldrich Co., St. Louis, MO, USA) [119]. Twenty-four hours after plating, red blood cells, cell debris, and nonadherent cells are removed by washing with a medium. On the third day, the puromycin is removed from the medium. When the cultures reached 80% confluency (5th day in vitro), the purified endothelial cells are passaged by brief treatment with 0.25% Trypsin-EDTA (Invitrogen, Life Technologies) and used to construct in vitro BBB models.
The mouse is widely used as an animal model in biomedical research. This is not surprising as 99% of mouse genes have human gene counterparts, and its physiology and genetics have been studied extensively and can be easily compared with humans. Technologies, such as transgenics, have been developed over decades for studying mouse genetics and the function of specific genes. Many human disease model mice have been developed to advance the study of disease pathogenesis and to evaluate the efficacy and toxicity of various candidate drugs.
3.5.4. Porcine and Bovine BBB Models
Porcine and bovine brains are commonly used for the isolation of brain capillaries, owing to their brain size, economic advantages, and availability. Obtaining cells from laboratory animals is very expensive and requires large amounts of animal sacrifice. Primary cultures of porcine and bovine endothelial cells have been widely reported to maintain many important barrier characteristics and transport pathways [84, 151, 158, 159]. Therefore, it has been suggested that porcine and bovine in vitro BBB models are effective tools for researching BBB and in pharmaceutical permeability screening. A unique property of PBEC is that a physiological concentration of hydrocortisone considerably improves barrier properties in serum-free culture conditions [151]. Additionally, Thomsen et al. reported that a triple-cultured model using porcine endothelial cells, pericytes, and rodent astrocytes enhanced barrier function and the expression of claudin-5, occludin, and BCRP [132]. The astrocytes are often derived from rat pups, as they grow faster than astrocytes obtained from older animals. Thomsen et al. successfully created a triple-cultured model using cells isolated from 6-month-old domestic pig brains, and several groups have developed an in vitro BBB model using primary cultured bovine brain endothelial cells [132]. A major advantage of bovine brain endothelial cells is the high yield of endothelial cells per brain, thanks to the cells’ tolerance of several rounds of subculture [158]. Generally, BBB properties, especially barrier function, are rapidly lost during the subculture of rodent brain endothelial cells. Bovine endothelial cells have a strong barrier function and retain these properties even after subculture. The co-culture model of bovine endothelial cells and rat astrocytes is widely used in signaling and transport studies, as well as in the characterization of fundamental BBB properties [160].
3.5.5. Human and Monkey In Vitro BBB Model
Although the brain tissues of various species are used to obtain capillary endothelial cells, the human brain is also an important source. However, it is difficult to use normal human brain tissue because of ethics and constraints in obtaining the samples. In addition, when the brain sample contains pathological tissue, the endothelial cells isolated from the tissue may have a different property to normal human endothelial cells. Hence, to construct a human in vitro BBB model, commercially available brain endothelial or immortalized cells are mainly used. Furthermore, several groups have developed a human BBB model using iPS cell-derived BBB-related cells [136].
Some studies have used a commercially available monkey BBB model, constructed from monkey brain endothelial cells, rat pericytes, and astrocytes [154, 160, 161]. The monkey model displays a high TEER value (more than 800 Ω × cm2), expresses tight junction proteins (ZO-1, occludin, claudin-5) at the cell-cell junction, and can be applied to transport assays for chemical compounds and physiological peptides.
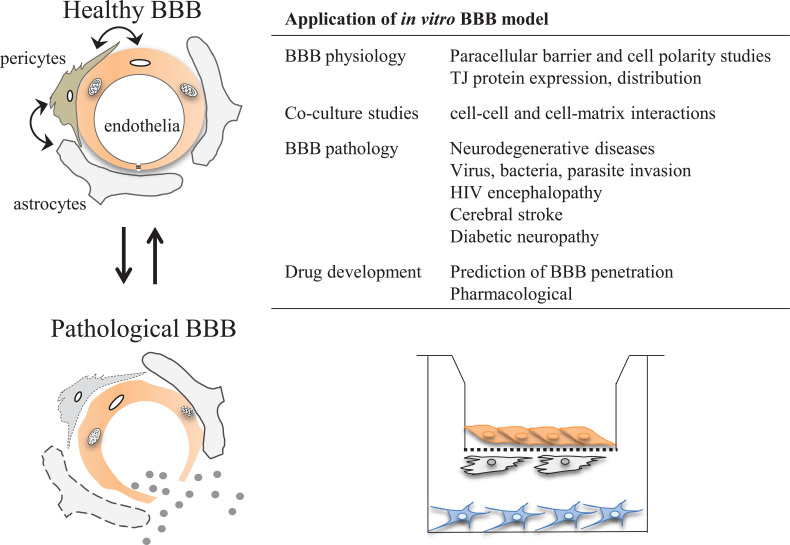
3.6. Application of In Vitro BBB Models (Fig. 4)
Fig. (4).
Schematic overview of application of in vitro BBB model. Cell-culture based BBB model is widely accepted as a powerful tool to evaluate the BBB physiology, pathophysiology, pharmacology, and drug development. The BBB acts as a biological device that maintains brain homeostasis due to selective uptake and restriction of substances that enter the central nervous system from the blood. Their special features are achieved by the interaction among components of the neurovascular unit. In vitro BBB model has been provided numerous insights about these complex mechanisms of development and maintenance of the BBB. Since BBB plays a critical role in the protection of the brain against harmful substances from peripheral fluids, disruption of the BBB evokes brain edema formation and allows the penetration of toxic substances and inflammatory cells, and leads to neuronal damage. In vitro BBB model is a powerful tool to evaluate the mechanism of BBB disruption under these pathophysiological conditions and search a BBB protective drug to prevent the development of CNS disease. The BBB is a key player to protect the brain, on the other hand, it acts as major barrier that is difficult to overcome for the development of CNS drugs. Several potential routes for permeation across the BBB are known, such as passive diffusion, ABC transporter efflux, carrier-mediated influx, receptor-mediated transcytosis, and adsorptive-mediated transcytosis. The prediction of effective drug penetration across the BBB using an in vitro BBB model is important for the development of centrally acting drugs. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.6.1. Using In Vitro BBB Models to Evaluate BBB Physiology
Endothelial cells of the blood vessels of different organs possess different characteristics and are regulated by specific local signals [162, 163]. Brain capillaries have a special function as the anatomical basis of the BBB, which acts to maintain brain homeostasis and has evolved to protect the health and activity of fragile neurons. The brain environment is maintained through the selective uptake and restriction of substances entering the CNS from the blood, which is achieved through interactions among components of the NVU: brain capillary endothelial cells, astrocytes, pericytes, perivascular microglia, and neurons. In addition to crosstalk along the NVU, substances in the blood fluid also affect the functions of the BBB. In vitro BBB models have provided numerous insights into these complex mechanisms behind the development and maintenance of the BBB.
3.6.2. In Vitro BBB Models and Evaluating BBB Pathophysiology
In vitro BBB models can be used to elucidate the role of BBB in disease states. Because the BBB plays a critical role in the protection of the brain against harmful substances in the peripheral fluids, disruption of the BBB evokes brain edema and allows the penetration of toxic substances and inflammatory cells, leading to neuronal damage. Accumulating evidence suggests that disruptions to the adhesion between brain capillary endothelial cells play a role in the onset and progression of several CNS disorders [42, 98]. In vitro BBB models are powerful tools to evaluate the mechanisms of BBB disruption under these pathophysiological conditions and in the search for BBB protective drugs to prevent the development of CNS diseases.
3.6.3. Using In Vitro BBB Models in Drug Development
The BBB protects the brain from harmful blood-borne substances. On the other hand, it is a major barrier to be overcome when developing CNS drugs, as it is the main regulator of drug transport into the CNS. Several potential routes for permeation across the BBB are known, such as passive diffusion, ABC transporter efflux, carrier-mediated influx, receptor-mediated transcytosis, and adsorptive-mediated transcytosis [71]. The presence of these complicated routes makes predicting a compound’s BBB permeability, based on its molecular weight and chemical properties, difficult. A variety of methods is developed for predicting drug permeability across the BBB. Considering the development of centrally acting drugs, it is important to use a model mimicking the in vivo human BBB as closely as possible. Among several methods to predict a drug permeability, in vivo model is one of the important techniques to predict the BBB permeability of new agents of interest, and a range of animal models and techniques to assess the permeability of compounds has been developed [164]. Though the rodent in vivo model is a relevant model for predicting permeability, the low throughput of this method restricts its application in an early stage of drug development possesses a large number of compounds. On the other hand, as the in vivo model provides reliable data of BBB permeability, these data available as a reliable reference for other methods.
Non-cell-based methods (PAMPA, IAM) and non-brain derived model (MDCK and Caco-2 model) are suitable for ranking a large number of compounds compared with in vivo methods. Although these methods have the advantage of multi-sample processing and easy handling, the reliability of data is not well achieved because of no or less expression of efflux and influx transporters. Therefore, after the ranking of compounds, it needs to validate the compound using other methods.
Another method to predict BBB permeability is using in vitro BBB models. Several reports indicated that immortalized cells less able to predict BBB permeability than primary cultured cells [165]. Although in vitro models using primary cultured cells are expensive and time-consuming compared with that of cell lines, primary cultured cells have the advantage of retaining many in vivo properties. In fact, some in vitro co-cultured BBB models have a good correlation to in vivo BBB permeability [74, 152]. Though in vitro BBB model using primary cultured cells is low to medium throughput, the prediction of effective drug penetration across the BBB using an in vitro BBB model is important for the development of centrally acting drugs [26]. Species differences, especially between humans and other species, have important implications for the drug discovery and development process. Using a quantitative targeted absolute proteomics method, Ohtsuki et al. showed that P-glycoprotein is expressed less abundantly in monkey and human brain capillaries than in mouse brain capillaries [166]. In addition, a PET study demonstrated that the brain penetration of an MDR1 substrate, such as [11C] GR205171, was greater in humans and monkeys than in rodents [167]. Considering these data, the triple co-culture model using monkey endothelial cells is a practical primate in vitro BBB model for basic research and drug permeability assays.
4. Drug repositioning
Decades of effort have gone into making in vitro BBB models, and the qualities of the models are improving, as mentioned previously, but the development of CNS drugs is still a major challenge [29]. The attempts to develop new treatments for CNS diseases too frequently meet costly and disappointing results and they require both long timespans and high expenditure [31]. However, the repurposing of safe existing drugs to new indications provides a cost-effective and time-saving alternative [30, 31]. Drug repositioning or repurposing uses the versatile properties of approved drugs to reassign then to a new purpose. This alternative approach using drug discovery fast-tracking is becoming increasingly popular [30]. Drug repositioning is particularly well-suited to the public sector, where off-patent agents can undergo high-throughput in vitro screening for their ability to interact with identified molecular targets [35]. The term drug repositioning is frequently used in the literature has several synonyms such as drug repurposing, drug reprofiling, which have been interchangeable. To date, no common consensus exists the definition of drug repositioning or similar terms. Drug repositioning is referred to the drugs approved for one disease which are used as a structural template for synthesis of derivatives active against another disease, while drug repurposing is referred to the old drugs that can be used without modification for new uses [168].
Historically, drug repositioning has been a largely unintentional, serendipitous process that takes place when a drug is found to have an off-target effect or a previously unrecognized on-target effect that could be applied to another purpose. Perhaps the most famous example of successful repositioning effort is sildenafil. Sildenafil, originally developed as angina pectoris, has been repurposed for the treatment of erectile dysfunction and, subsequently, pulmonary arterial hypertension. Another famous example is thalidomide, originally developed as a sleep-inducing drug but discontinued due to fetal teratogenicity, which is now used against multiple myeloma because of its anti-angiogenic effects [169]. Drug repositioning has the potential to facilitate drug discovery by physicians and pharmacists during daily clinical practice. In addition, drug repositioning also includes pharmaceuticals that are confirmed to be safe with clinical trials, but the effectiveness cannot be proven and their development is discontinued. The method of searching for new applications for marketed drugs that have been discontinued is called drug rescue; although, it may be classified as drug repurposing when their clinical application to other diseases is predicted using the known drug effects and side effects. In recent years, drug repositioning by drug reprofiling is becoming mainstream, and it is used to comprehensively analyze the actions of existing drugs at the molecular level using the latest analysis methods, and to examine the possibility of its use as a therapeutic drug for other diseases [170]. In vitro systems, such as high-throughput screening, are often used for effective discovery, and in silico systems, based on drug and disease databases, can be used for evaluation. Battah et al. recently reported an integrated screening protocol combining in silico and in vitro approaches to uncover the antimycobacterial potential of existing drugs. Their in silico system provided a series of marketed drugs possessing significant antitubercular activity levels, as assessed by different in vitro system [168].
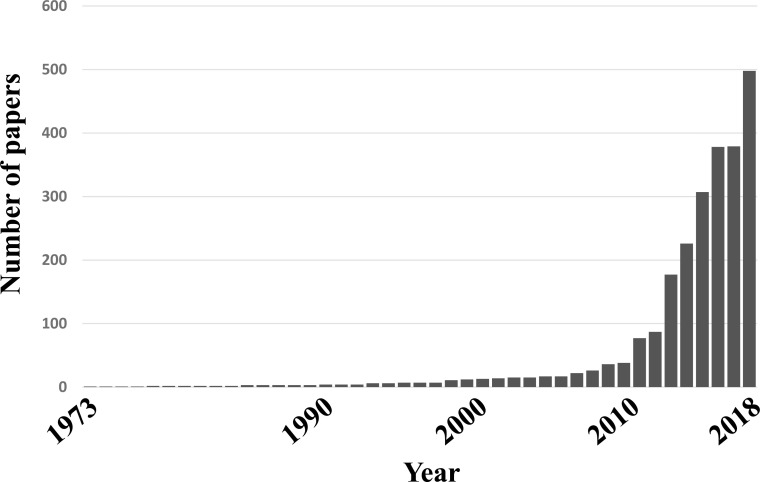
Drug repositioning is, simultaneously, a very old and very new method. In fact, the number of papers related to drug repositioning has been increasing in recent years. In PubMed, since 2013, more than 2300 publications have been indexed based on the keywords drug repositioning, and that number is increasing year on year (Fig. 5).
Fig. (5).
Publications indexed in PubMed based on keyword ‘drug repositioning’. More than 2300 publications have been indexed based on the keywords drug repositioning since 2013 and that number is increasing year on year.
The CNS is an important area for drug repositioning, and a well-known example of this is amantadine [171]. Amantadine was first recognized as an antiviral compound in the 1960s and was approved by the FDA for influenza prophylaxes in 1966. It was then repositioned for Parkinson’s disease, based on a case study in 1968 [172, 173]. The mechanisms are not fully elucidated, and, subsequently, a huge variety of indications of amantadine have been explored, from fatigue in multiple sclerosis, enuresis nocturna, ADHD, to pathological gambling and recovery after a head injury [172, 173]. Amantadine is currently used as an extended-release formulation for levodopa-induced dyskinesia in Parkinson’s disease.
The underlying pathophysiologies of CNS diseases are continuously being unraveled; therefore, it is beneficial to revisit the receptor profiling and mode of action of marketed drugs. However, most of the diseases within the CNS, especially psychiatric or neurological diseases, are still poorly understood in terms of their underlying pathophysiology and biological mechanisms [174]. To date, the exact mode of action by which many of the currently approved CNS drugs exert their effect is still not fully understood. Thus, the current disease characterizations within CNS are mainly based on clinical aspects, rather than the underlying pathophysiology [174]. It is not surprising that, despite the high prevalence of CNS disorders in the overall population and the high demand in this area, the discovery and development of drugs for CNS diseases has one of the lowest success rates. Therefore, drug repositioning for CNS diseases is becoming increasingly popular [175]. Successful drug repositioning examples in the CNS area are listed in Table 2.
Table 2.
Successful drug repositioning examples in CNS area.
| Drug Name | New Indication | Original Indication |
|---|---|---|
| Thalidomide | Multiple myeloma | Sleep inducer |
| Amantadine | Parkinson’s disease | Influenza |
| Valproic acid | Migraine | Epilepsy |
| Zonisamide | Parkinson’s disease | Epilepsy |
| Daburafenib | Parkinson’s disease | Melanoma |
| Edaravone | Amyotrophic lateral sclerosis | Stroke |
| Topiramate | Obesity | Epilepsy |
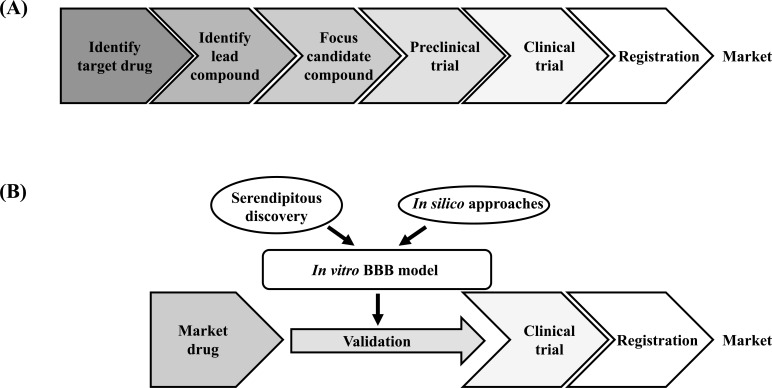
Since CNS drugs already on the market have been confirmed as possessing some effects on the CNS, it is entirely conceivable that they could act on other CNS diseases. In addition, the fact that the problem of side effects has already been overcome is also an advantage in terms of drug development. Caban et al. recently reported that the majority of CNS drugs successfully approved as a result of drug repositioning were originally for other CNS disorders [174]. On the other hand, the CNS drugs still in development presented a lower proportion of CNS drugs. Further research will be needed to validate their results, but one reason for this may be the problem of BBB penetration. Most drugs and drug candidates cannot reach the brain; however, already approved CNS drugs have a greater potential to pass through the BBB, which is the highest obstacle to the development of CNS drugs. Indeed, approximately 30% of CNS drugs have been repurposed two or more times as other CNS therapeutics [174]. In vitro BBB models can be powerful tools to validate the candidates of drug repositioning (Fig. 6A & B). In this perspective, we provide some representative CNS diseases that the focus of drug repositioning projects.
4.1. Alzheimer’s Disease
The underlying pathology of AD is complex and includes, amyloid beta (Aβ) plaque depositions, neurofibrillary tangles, brain atrophy and neurodegeneration, neuroinflammation, impaired neurogenesis, and vascular and BBB disruptions [176-183]. Despite recent advances in AD research, pathophysiological characterization remains incomplete, which hampers the development of effective treatments [184]. In fact, currently, there are no effective pharmacological treatments for AD [185]. In this situation, adopting a different strategy based on the repurposing of drugs that are approved for other disorders is attractive [186]. Kumar et al. studied the molecular interactions of already known antipsychotic drugs with the various protein targets implicated in AD using in silico studies [185]. They screened approximately 150 antipsychotic drugs and performed molecular docking on five major protein targets: acetylcholinesterase (AchE), butyrylcholinesterase (BuChE), beta secretase cleavage enzyme 1 (BACE 1), monoamine oxidase (MAO), and N-methyl-d-aspartate (NMDA). Their study has highlighted the potential of using leading antipsychotic drugs, such as pimozide, bromperidol, melperone, anisoperidone, benperidol, and anisopirol, against multiple targets associated with AD. In their study, benperidol was found to be the best candidate for the cholinergic (AchE and BuChE), monoaminergic (MAO A), and glutamatergic (NMDA) systems and beta secretase cleavage enzyme (BACE 1) [185]. Approximately 40% of clinical trials for AD currently in progress worldwide are using drug repositioning [6]. In these clinical trials, other original CNS drugs, such as rasagiline, levetiracetam, and riluzole, are being applied to AD, and there have been reports of other candidates, such as insulin, pioglitazone, leukotriene, and candesartan [6, 187-189]. This is likely to be a response to the recent announcement that AD is a complex multifactorial disorder, not dominated by one dominant biological factor, such as beta-amyloid, and including many relevant pathologic factors. Drug repositioning approaches may lead to a paradigm shift in the treatment of AD patients.
4.2. Metastatic Brain Tumor/Glioma
As there is very little research on metastatic brain tumors, there are few reports on drug repositioning studies, and they're only a few reports on breast cancer drug repositioning. The financial and time constraints of anticancer drug development are becoming increasingly greater barriers. It is becoming increasingly challenging to discover new anticancer drugs; therefore, the success rate is declining [190]. In the field of oncology, there are many examples of repurposing successes, proving the potential of this strategy. Aspirin, which is originally an antipyretic drug, was repurposed to treat colorectal cancer, and metformin, which is an anti-diabetes drug, was repurposed to treat breast cancer, prostate cancer, etc. [191, 192]. Previous epidemiologic studies reported that there are few patients with psychosis who develop cancers. In a study comparing schizophrenic patients with no history of cancer with a control group during a nine-year follow-up, the cancer incidence was 1.93% in the schizophrenic patient group, compared with 2.97% in the control group [193]. This result suggests that the likelihood of developing cancer among patients with schizophrenia was less than among the non-schizophrenic group. Other studies have shown an overall decreased cancer incidence in schizophrenic patients using neuroleptic drugs, implying that these drugs may have anticancer potential [194, 195]. In addition, some anti-schizophrenia drugs, such as trifluoperazine and chlorpromazine, have shown anticancer efficacies in preclinical studies [196, 197]. Fluphenazine hydrochloride is another commonly prescribed antipsychotic drug, and a few studies have reported its efficacy in the treatment of breast cancer [198]. As an anti-schizophrenia drug, Fluphenazine hydrochloride can penetrate the BBB to reach a relatively high concentration in the brain. Xu et al. evaluated the activity of fluphenazine hydrochloride in the treatment of triple negative breast cancer (TNBC) and brain metastases, and fluphenazine hydrochloride exhibited good anti-metastatic potential in a mouse brain metastasis model with an inhibition rate of 85% [199]. The drug also showed a strong inhibitory effect on spontaneous lung metastasis. Moreover, fluphenazine hydrochloride did not cause serious side effects in the mice. Their results prompted further preclinical and clinical investigations into repurposing fluphenazine hydrochloride for treating metastatic TNBC patients, who urgently need new treatment options. The exact mechanisms of fluphenazine hydrochloride’s anticancer effects are still unknown. Previous studies have shown that the various mechanisms of antipsychotic agents may be used to fight cancer, including the induction of autophagy, dysregulation of cholesterol homeostasis, and disruption of lysosomal homeostasis [196, 197, 200]. This suggests that some antipsychotic drugs can prevent the development of cancer, and further investigation is necessary.
4.3. Stroke
A common misperception of neuroprotection research is that, although many treatments work in animals, nothing works in people. This perception has been repeatedly reinforced by reports of unsuccessful or mixed outcomes in trials of candidate neuroprotectants in acute stroke patients [201, 202]. If animal experiments are indeed unable to inform clinical decision-making, then serious doubts are raised about the utility of animal models of stroke and about the ethics of continuing animal experiment practices. The differences among species may play a role in the fact that of 1,026 treatments tested on animal models for stroke therapy, nothing has been effective in clinical trials [202]. In this situation, drug repositioning is a realistic option. During a stroke, the occlusion of a cerebral vessel leads to a rapidly progressing cascade of events and to the destruction of critical brain tissue. This includes not only neurons but also other cells, including glial and vascular cell types and the intervening matrix [203]. Promising targets have been identified for vascular protection after strokes, such as the inhibition of endogenous mediators of vascular damage (superoxide, endothelin, matrix metalloproteases, cytokines, and caspases) and the stimulation of endogenous protectors (nitric oxide, angiopoietin-1, vascular endothelial growth factor, and superoxide dismutases) [203]. Several of these targets can be approached with repurposed drugs, including statins, angiotensin II receptor blockers, minocycline, and growth factors such as erythropoietin [204]. Other vascular protection strategies that have been successful in experimental models of cerebral ischemia are lowering of blood pressure [205, 206], melatonin [207], minocycline [208, 209], statins [210, 211], and fasudil, a Rho-kinase inhibitor [212].
Vascular protection seems to be a promising strategy for improving stroke outcome, as vascular function is critical to both cardiovascular diseases and ischemic cerebrovascular diseases [31]. Vascular-function-related biological processes and pathways may be the key links between cardiovascular diseases and ischemic cerebrovascular diseases. Zhao et al. reported that a multi-database, in silico target identification, gene function enrichment, and network pharmacology analysis integration method was applied to investigate the 119 FDA-approved cardiovascular disease drugs for ischemic cerebrovascular diseases repurposing. As a multi-target pleiotropic drug, carvedilol was investigated for its potential as an ischemic cerebrovascular disease therapy. Carvedilol is a nonselective β-adrenergic blocking agent with α1-blocking activity, and it shows pleiotropicity by effecting 17 targets. Their results indicated that the mode of action of carvedilol for ischemic cerebrovascular disease treatment may be closely linked to vascular function regulation, and the mechanism is multi-target and multi-signaling pathway-related [31]. In the future, this method of computational drug discovery and drug repositioning based on the biomolecular profile is expected to increase.
Attempts to develop new drugs for acute ischemic stroke are still struggling; however, acute stroke therapy has now entered a new era of highly effective reperfusion through the development of the stent retriever and vacuum aspiration devices that can directly remove the thrombus from inside the occluded cerebral artery directly [12]. The recent publication of five positive thrombectomy trials in 2015 and subsequent publications, including the American Heart Association/American Stroke Association guidelines, support the evidence for the effects of mechanical thrombectomy [213-219]. Even in the era of rapid and effective recanalization using endovascular approaches, the percentage of patients with good outcomes varies between 33 and 71%. Furthermore, these trials used various forms of pre-thrombectomy imaging criteria and/or time limitations to exclude patients with significant infarcted cores. Thus, while clinical outcomes continue to lag behind the high rates of technical success, there may be an opportunity to use adjuvant neuroprotectants to extend the window for intervention or to reverse damage that is currently viewed as unsalvageable based on radiographic imaging alone, thereby increasing the number of patients eligible for thrombectomy [220]. Considering these facts, combining neuroprotection with intravenous or intra-arterial reperfusion therapy is now an important next step in the development of acute stroke therapies. Mechanical thrombectomy is now at the forefront of the treatment of large-vessel acute ischemic stroke [221]. Selective intra-arterial access has opened a new avenue for neuroprotection in acute ischemic stroke that has the potential to maximize the local benefits while minimizing systemic effects [220, 221].
In animal models of acute ischemic stroke, verapamil, nitroglycerin, erythropoietin, and platelet-rich plasma have been evaluated via the intra-arterial route [220, 222, 223]. Among them, one possible candidate neuroprotectant for stroke therapy, verapamil, a calcium channel blocker, was an effective intra-arterial adjunct in a preclinical mouse focal ischemia model [220, 221]. Fraser et al. recently reported on an in vivo intra-arterial dose-response evaluation of their mouse stroke model. Furthermore, they have already conducted a human Phase I trial, Superselective Administration of Verapamil During Recanalization in Acute Ischemic Stroke (SAVER-I), to evaluate the feasibility and safety of the drug’s administration in the human disease. The SAVER-I clinical trial showed no evidence that IA verapamil increased the risk of intracranial hemorrhage or other adverse effects/procedural complications in human subjects [220]. Since mechanical thrombectomy is now the gold standard for acute ischemic stroke treatment, neuroprotective strategies via the intra-arterial route are highly anticipated.
4.4. Parkinson’s Disease
The exact pathophysiological mechanisms underlying neurodegeneration in Parkinson’s disease (PD) are not well understood. De novo drug discovery and development in PD is time-consuming, expensive, and risky process same as other CNS diseases. Therefore, it is reasonable that pharmaceutical companies are facing a paradigm shift in how drugs for PD are discovered and developed. To overcome the high attrition in drug development, pharmaceutical companies are increasingly exploring drug repositioning. In fact, amantadine, originally developed in the 1960s as a prophylactic against several forms of influenza, has approved for the treatment for PD. This repositioning from an anti-flu compound to an anti-Parkinson drug was initiated by a case observation in 1968 of a 58-year-old woman with moderately severe Parkinson’s disease. This patient told the treating neurologist that while talking amantadine hydrochloride 100 mg tablets to prevent the flu, she experienced a remarkable remission in her symptoms of rigidity, tremor, and akinesia [172]. Although the compound is hydrophilic it easily penetrates the blood-brain barrier, due to active transport probably via a proton-coupled organic cation antiporter [224]. Interestingly, there have been facts emerging, amantadine has a great variety of action in other indications such as multiple sclerosis, traumatic brain injury, cancer pain. The mechanisms of action of amantadine related to the various indications will be totally different among the indications. Zonisamide is another representative drug repositioning example. It has been developed as an anti-epileptic drug in the 1980s. Murata et al. used zonisamide to treat epilepsy in a patient with PD and serendipitously found that not only epilepsy but symptoms related to PD were improved [225]. The anti-epilepsy effect of zonisamide has been related to the inhibition of voltage-dependent sodium channels and T-type calcium channels. While the anti-PD effect of zonisamide may be related to the inhibition of dopamine metabolism due to inhibition of monoamine oxidase-B, the stimulation of dopamine release from striatum, and the blockade of T-type calcium channels. Based on these data and subsequent clinical trials, zonisamide was approved in Japan as an anti-PD drug in 2009.
Currently available treatment for PD primarily focus on stimulation of dopaminergic signaling and can provide symptomatic relief for a limited time but little effect on nonmotor symptoms. And none of the drugs have shown to affect the progressive pathological and clinical decline. As with other multifactorial genetic disorders, genome-wide association studies (GWASs) found multiple risk loci for PD, although their clinical significance remains uncertain. Uenaka et al. report the identification of candidate drugs for PD by a method using GWAS data and in silico databases [226]. They identified 57 Food and Drug Administration-approved drug families as candidate neuroprotective drugs for PD. Among them, dabrafenib, which is known as a B-Raf kinase inhibitor and is approved for the treatment of malignant melanoma, showed remarkable cytoprotective effects. Their data indicated that dabrafenib exerts protective effects against neurotoxicity associated with PD. They also confirmed the effectiveness of this in silico screening method by using animal model. Drug repurposing is a promising strategy for drug development because the safety data of these drugs in human patients are already available [227]. However, it is inefficient to examine all FDA-approved drugs, because of their vast number. In silico drug screening, which links the data of GWAS to drug/protein-protein interaction databases, may narrow down candidate drugs for many polygenic diseases and save cost and time.
Conclusion
In vitro BBB models can be essential tools for basic research and pharmaceutical screening and drug repositioning by mimicking clinical settings.
Drug repositioning can minimize the cost and time span of CNS drug development. To date, two-thirds of repositioned drugs are the result of serendipitous discovery through careful observations by the treating physicians. In addition, pharmacists, and other medical staff, as well as basic researchers, should pay attention to the daily observations of patients, the appropriate conduct of clinical trials, and the drug’s un-anticipated effects on patients. Furthermore, in silico drug repositioning, which involves network analysis, data mining, and machine learning, is also expected to play an important role in future treatment developments.
Fig. (6).
(A, B) A comparison of traditional drug discovery and development for central nervous system diseases versus drug repositioning using in vitro Blood-Brain Barrier (BBB) model.. The attempts to develop new treatments for CNS diseases have costly and disappointing results, and require a long period of time and high expenditure, whereas the repurposing of safe existing market drugs provides a cost-effective and time-saving alternative.
Acknowledgements
We would like to show our gratitude to the Masami Niwa (Emeritus Professor of Nagasaki University, Pharma-CoCell Company Ltd.) and Maria A. Deli (Hungarian Academy of Sciences) for sharing their pearls of wisdom with us for preparing the manuscript. We wish to thank Takashi Fujimoto, Kei Sato, Yuki Matsunaga, Yusuke Iki, Kenta Ujifuku, Nobutaka Horie, Tsuyoshi Izumo, Takeo Anda, and Takayuki Matsuo for providing insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations/conclusions of this paper.
We also thank Enago (www.enago.jp) for the English language review.
Consent for Publication
Not applicable.
Funding
This study was partially funded by JSPS and HAS under the Japan-Hungary Research Cooperative Program (to Y.M. and S.N.), Grants-in-Aid for Scientific Research (Fostering Joint International Research) 15KK0349 (to Y.M.), Grants-in-Aid for Scientific Research (C) 17K10840 (to Y.M.), and (C) 17K10838 (to S.N.).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Abbott A. Novartis to shut brain research facility. Nature. 2011;480(7376):161–162. doi: 10.1038/480161a. [DOI] [PubMed] [Google Scholar]
- 2.Miller G. Is pharma running out of brainy ideas? Science. 2010;329(5991):502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 3.Gribkoff V.K., Kaczmarek L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. 2017;120:11–19. doi: 10.1016/j.neuropharm.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellani R.J., Rolston R.K., Smith M.A. Alzheimer disease. Dis. Mon. 2010;56(9):484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ihara M., Saito S. Brain Nerve. 2019;71(9):961–970. doi: 10.11477/mf.1416201388. [Drug Repositioning for Alzheimer’s Disease]. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 8.Kang J., Lemaire H.G., Unterbeck A., et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 9.Cenini G., Voos W. Mitochondria as potential targets in alzheimer disease therapy: an update. Front. Pharmacol. 2019;10:902. doi: 10.3389/fphar.2019.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G., Sommerlad A., Orgeta V., et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw J.M., Murray V., Berge E., del Zoppo G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014;(7):CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savitz S.I., Baron J.C., Yenari M.A., Sanossian N., Fisher M. Reconsidering Neuroprotection in the Reperfusion Era. Stroke. 2017;48(12):3413–3419. doi: 10.1161/STROKEAHA.117.017283. [DOI] [PubMed] [Google Scholar]
- 13.del Zoppo G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010;267(2):156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacchigna S., Lambrechts D., Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 16.Cummings J. Lessons learned from alzheimer disease: clinical trials with negative outcomes. Clin. Transl. Sci. 2018;11(2):147–152. doi: 10.1111/cts.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savitz S.I., Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann. Neurol. 2007;61(5):396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 18.Markesbery W.R., Brooks W.H., Gupta G.D., Young A.B. Treatment for patients with cerebral metastases. Arch. Neurol. 1978;35(11):754–756. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 19.Freilich R.J., Seidman A.D., DeAngelis L.M. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995;76(2):232–236. doi: 10.1002/1097-0142(19950715)76:2<232:AID-CNCR2820760212>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Davies M.A., Saiag P., Robert C., et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spagnolo F., Picasso V., Lambertini M., Ottaviano V., Dozin B., Queirolo P. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: a systematic review. Cancer Treat. Rev. 2016;45:38–45. doi: 10.1016/j.ctrv.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Lowery F.J., Yu D. Brain metastasis: unique challenges and open opportunities. Biochim. Biophys. Acta Rev. Cancer. 2017;1867(1):49–57. doi: 10.1016/j.bbcan.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardridge W.M. Blood-brain barrier delivery. Drug Discov. Today. 2007;12(1-2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Wegener G., Rujescu D. The current development of CNS drug research. Int. J. Neuropsychopharmacol. 2013;16(7):1687–1693. doi: 10.1017/S1461145713000345. [DOI] [PubMed] [Google Scholar]
- 26.Cecchelli R., Berezowski V., Lundquist S., et al. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007;6(8):650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 27.Booth R., Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip. 2012;12(10):1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 28.Antoni D., Burckel H., Josset E., Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int. J. Mol. Sci. 2015;16(3):5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms H.C., Abbott N.J., Burek M., et al. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016;36(5):862–890. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekins S., Williams A.J., Krasowski M.D., Freundlich J.S. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov. Today. 2011;16(7-8):298–310. doi: 10.1016/j.drudis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q.Q., Li X., Luo L.P., Qian Y., Liu Y.L., Wu H.T. Repurposing of approved cardiovascular drugs against ischemic cerebrovascular disease by disease-disease associated network-assisted prediction. Chem. Pharm. Bull. (Tokyo) 2019;67(1):32–40. doi: 10.1248/cpb.c18-00634. [DOI] [PubMed] [Google Scholar]
- 32.Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534(7607):314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard J.E., O’Mara T.A., Glubb D.M. Enhancing the promise of drug repositioning through genetics. Front. Pharmacol. 2017;8:896. doi: 10.3389/fphar.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei H., Xia T., Feng G., Zhu J., Lin S.M., Qiu Y. Opportunities in systems biology to discover mechanisms and repurpose drugs for CNS diseases. Drug Discov. Today. 2012;17(21-22):1208–1216. doi: 10.1016/j.drudis.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Fagan S.C. Drug repurposing for drug development in stroke. Pharmacotherapy. 2010;30(7 Pt. 2):S51–S54. doi: 10.1592/phco.30.pt2.51S. [DOI] [PubMed] [Google Scholar]
- 36.Xu M., Lee E.M., Wen Z., et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016;22(10):1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015;14(12):821–832. doi: 10.1038/nrd4675. [DOI] [PubMed] [Google Scholar]
- 38.Finsterer J., Frank M. Repurposed drugs in metabolic disorders. Curr. Top. Med. Chem. 2013;13(18):2386–2394. doi: 10.2174/15680266113136660166. [DOI] [PubMed] [Google Scholar]
- 39.Banks W.A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016;15(4):275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 40.Daneman R. The blood-brain barrier in health and disease. Ann. Neurol. 2012;72(5):648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 41.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Abdul Razzak R., Florence G.J., Gunn-Moore F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019;20(12):3108. doi: 10.3390/ijms20123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 45.Al-Ahmady Z.S. Selective drug delivery approaches to lesioned brain through blood brain barrier disruption. Expert Opin. Drug Deliv. 2018;15(4):335–349. doi: 10.1080/17425247.2018.1444601. [DOI] [PubMed] [Google Scholar]
- 46.Mikitsh J.L., Chacko A.M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Medicin. Chem. 2014;6:11–24. doi: 10.4137/PMC.S13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robey R.W., Pluchino K.M., Hall M.D., Fojo A.T., Bates S.E., Gottesman M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer. 2018;18(7):452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banks W.A., Kovac A., Morofuji Y. Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow Metab. 2018;38(6):1104–1118. doi: 10.1177/0271678X17740793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 51.Gastfriend B.D., Palecek S.P., Shusta E.V. Modeling the blood-brain barrier: beyond the endothelial cells. Curr Opin Biomed Eng. 2018;5:6–12. doi: 10.1016/j.cobme.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sá-Pereira I., Brites D., Brito M.A. Neurovascular unit: a focus on pericytes. Mol. Neurobiol. 2012;45(2):327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 53.Obermeier B., Verma A., Ransohoff R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016;133:39–59. doi: 10.1016/B978-0-444-63432-0.00003-7. [DOI] [PubMed] [Google Scholar]
- 54.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33(12):579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Heidari H., Taylor H. Review article: capturing the physiological complexity of the brain’s neuro-vascular unit in vitro. Biomicrofluidics. 2018;12(5):051502. doi: 10.1063/1.5045126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butt A.M., Jones H.C., Abbott N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kniesel U., Wolburg H. Tight junctions of the blood-brain barrier. Cell. Mol. Neurobiol. 2000;20(1):57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyck R., Ruderisch N., Moll A.G., et al. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J. Cereb. Blood Flow Metab. 2009;29(9):1491–1502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- 59.Roux F., Couraud P.O. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell. Mol. Neurobiol. 2005;25(1):41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowman P.D., Ennis S.R., Rarey K.E., Betz A.L., Goldstein G.W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann. Neurol. 1983;14(4):396–402. doi: 10.1002/ana.410140403. [DOI] [PubMed] [Google Scholar]
- 61.Rubin L.L., Hall D.E., Porter S., et al. A cell culture model of the blood-brain barrier. J. Cell Biol. 1991;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaffe E.A., Hoyer L.W., Nachman R.L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J. Clin. Invest. 1973;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorovini-Zis K., Huynh H.K. Ultrastructural localization of factor VIII-related antigen in cultured human brain microvessel endothelial cells. J. Histochem. Cytochem. 1992;40(5):689–696. doi: 10.1177/40.5.1573250. [DOI] [PubMed] [Google Scholar]
- 64.Müller A.M., Hermanns M.I., Skrzynski C., Nesslinger M., Müller K.M., Kirkpatrick C.J. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp. Mol. Pathol. 2002;72(3):221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 65.Nitta T., Hata M., Gotoh S., et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furuse M., Hirase T., Itoh M., et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123(6 Pt. 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morita K., Sasaki H., Furuse M., Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crone C., Olesen S.P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 69.Ohno K., Pettigrew K.D., Rapoport S.I. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am. J. Physiol. 1978;235(3):H299–H307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- 70.Srinivasan B., Kolli A.R., Esch M.B., Abaci H.E., Shuler M.L., Hickman J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015;20(2):107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbott N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 72.Khan A.I., Liu J., Dutta P. Iron transport kinetics through blood-brain barrier endothelial cells. Biochim. Biophys. Acta, Gen. Subj. 2018;1862(5):1168–1179. doi: 10.1016/j.bbagen.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakagawa S., Deli M.A., Nakao S., et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell. Mol. Neurobiol. 2007;27(6):687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakagawa S., Deli M.A., Kawaguchi H., et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009;54(3-4):253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Helms H.C., Hersom M., Kuhlmann L.B., Badolo L., Nielsen C.U., Brodin B. An electrically tight in vitro blood-brain barrier model displays net brain-to-blood efflux of substrates for the ABC transporters, P-gp, Bcrp and Mrp-1. AAPS J. 2014;16(5):1046–1055. doi: 10.1208/s12248-014-9628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patabendige A., Skinner R.A., Morgan L., Abbott N.J. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res. 2013;1521:16–30. doi: 10.1016/j.brainres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watson P.M., Paterson J.C., Thom G., Ginman U., Lundquist S., Webster C.I. Modelling the endothelial blood-CNS barriers: a method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci. 2013;14:59. doi: 10.1186/1471-2202-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ottaviani G., Martel S., Carrupt P.A. Parallel artificial membrane permeability assay: a new membrane for the fast prediction of passive human skin permeability. J. Med. Chem. 2006;49(13):3948–3954. doi: 10.1021/jm060230+. [DOI] [PubMed] [Google Scholar]
- 79.Mensch J., Melis A., Mackie C., Verreck G., Brewster M.E., Augustijns P. Evaluation of various PAMPA models to identify the most discriminating method for the prediction of BBB permeability. Eur. J. Pharm. Biopharm. 2010;74(3):495–502. doi: 10.1016/j.ejpb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 80.TA R M K. Challenges and opportunities in central nervous system drug discovery. Trends in Chemistry. 2019;1:612–624. doi: 10.1016/j.trechm.2019.04.009. [DOI] [Google Scholar]
- 81.Di L., Kerns E.H., Fan K., McConnell O.J., Carter G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003;38(3):223–232. doi: 10.1016/S0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 82.Könczöl A., Müller J., Földes E., et al. Applicability of a blood-brain barrier specific artificial membrane permeability assay at the early stage of natural product-based CNS drug discovery. J. Nat. Prod. 2013;76(4):655–663. doi: 10.1021/np300882f. [DOI] [PubMed] [Google Scholar]
- 83.Passeleu-Le Bourdonnec C., Carrupt P.A., Scherrmann J.M., Martel S. Methodologies to assess drug permeation through the blood-brain barrier for pharmaceutical research. Pharm. Res. 2013;30(11):2729–2756. doi: 10.1007/s11095-013-1119-z. [DOI] [PubMed] [Google Scholar]
- 84.Deli M.A., Abrahám C.S., Kataoka Y., Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joó F. The cerebral microvessels in culture, an update. J. Neurochem. 1992;58(1):1–17. doi: 10.1111/j.1471-4159.1992.tb09272.x. [DOI] [PubMed] [Google Scholar]
- 86.Biegel D., Pachter J.S. Growth of brain microvessel endothelial cells on collagen gels: applications to the study of blood-brain barrier physiology and CNS inflammation. In Vitro Cell. Dev. Biol. Anim. 1994;30A(9):581–588. doi: 10.1007/BF02631256. [DOI] [PubMed] [Google Scholar]
- 87.Vandenhaute E., Drolez A., Sevin E., Gosselet F., Mysiorek C., Dehouck M.P. Adapting coculture in vitro models of the blood-brain barrier for use in cancer research: maintaining an appropriate endothelial monolayer for the assessment of transendothelial migration. Lab. Invest. 2016;96(5):588–598. doi: 10.1038/labinvest.2016.35. [DOI] [PubMed] [Google Scholar]
- 88.Wuest D.M., Wing A.M., Lee K.H. Membrane configuration optimization for a murine in vitro blood-brain barrier model. J. Neurosci. Methods. 2013;212(2):211–221. doi: 10.1016/j.jneumeth.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 89.Eigenmann D.E., Xue G., Kim K.S., Moses A.V., Hamburger M., Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS. 2013;10(1):33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dehouck M.P., Jolliet-Riant P., Brée F., Fruchart J.C., Cecchelli R., Tillement J.P. Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J. Neurochem. 1992;58(5):1790–1797. doi: 10.1111/j.1471-4159.1992.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 91.Gaillard P.J., de Boer A.G. Relationship between permeability status of the blood-brain barrier and in vitro permeability coefficient of a drug. Eur. J. Pharm. Sci. 2000;12(2):95–102. doi: 10.1016/S0928-0987(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 92.Grimpe B., Probst J.C., Hager G. Suppression of nidogen-1 translation by antisense targeting affects the adhesive properties of cultured astrocytes. Glia. 1999;28(2):138–149. doi: 10.1002/(SICI)1098-1136(199911)28:2<138:AID-GLIA5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 93.Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001;153(5):933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vincent A.J., Lau P.W., Roskams A.J. SPARC is expressed by macroglia and microglia in the developing and mature nervous system. Dev. Dyn. 2008;237(5):1449–1462. doi: 10.1002/dvdy.21495. [DOI] [PubMed] [Google Scholar]
- 96.Webersinke G., Bauer H., Amberger A., Zach O., Bauer H.C. Comparison of gene expression of extracellular matrix molecules in brain microvascular endothelial cells and astrocytes. Biochem. Biophys. Res. Commun. 1992;189(2):877–884. doi: 10.1016/0006-291X(92)92285-6. [DOI] [PubMed] [Google Scholar]
- 97.Wolburg H., Noell S., Wolburg-Buchholz K., Mack A., Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15(2):180–193. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 98.Hermann D.M., ElAli A. The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci. Signal. 2012;5(236):re4. doi: 10.1126/scisignal.2002886. [DOI] [PubMed] [Google Scholar]
- 99.Kangwantas K., Pinteaux E., Penny J. The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J. Neuroinflammation. 2016;13:25. doi: 10.1186/s12974-016-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milner R., Hung S., Wang X., Berg G.I., Spatz M., del Zoppo G.J. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39(1):191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartmann C., Zozulya A., Wegener J., Galla H.J. The impact of glia-derived extracellular matrices on the barrier function of cerebral endothelial cells: an in vitro study. Exp. Cell Res. 2007;313(7):1318–1325. doi: 10.1016/j.yexcr.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 102.Zobel K., Hansen U., Galla H.J. Blood-brain barrier properties in vitro depend on composition and assembly of endogenous extracellular matrices. Cell Tissue Res. 2016;365(2):233–245. doi: 10.1007/s00441-016-2397-7. [DOI] [PubMed] [Google Scholar]
- 103.Tilling T., Korte D., Hoheisel D., Galla H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 104.Katt M.E., Linville R.M., Mayo L.N., Xu Z.S., Searson P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids Barriers CNS. 2018;15(1):7. doi: 10.1186/s12987-018-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown T.D., Nowak M., Bayles A.V., et al. A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. Transl. Med. 2019;4(2):e10126. doi: 10.1002/btm2.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cucullo L., Hossain M., Tierney W., Janigro D. A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prabhakarpandian B., Shen M.C., Nichols J.B., et al. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip. 2013;13(6):1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamiichi A., Furihata T., Kishida S., et al. Establishment of a new conditionally immortalized cell line from human brain microvascular endothelial cells: a promising tool for human blood-brain barrier studies. Brain Res. 2012;1488:113–122. doi: 10.1016/j.brainres.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 109.Terasaki T., Hosoya K. Conditionally immortalized cell lines as a new in vitro model for the study of barrier functions. Biol. Pharm. Bull. 2001;24(2):111–118. doi: 10.1248/bpb.24.111. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe T., Dohgu S., Takata F., et al. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol. Pharm. Bull. 2013;36(3):492–495. doi: 10.1248/bpb.b12-00915. [DOI] [PubMed] [Google Scholar]
- 111.Weksler B.B., Subileau E.A., Perrière N., et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19(13):1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 112.Silwedel C., Förster C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J. Neuroimmunol. 2006;179(1-2):37–45. doi: 10.1016/j.jneuroim.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 113.Easton A.S., Abbott N.J. Bradykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood-brain barrier. Brain Res. 2002;953(1-2):157–169. doi: 10.1016/S0006-8993(02)03281-X. [DOI] [PubMed] [Google Scholar]
- 114.Neuhaus W., Plattner V.E., Wirth M., et al. Validation of in vitro cell culture models of the blood-brain barrier: tightness characterization of two promising cell lines. J. Pharm. Sci. 2008;97(12):5158–5175. doi: 10.1002/jps.21371. [DOI] [PubMed] [Google Scholar]
- 115.Hellinger E., Veszelka S., Tóth A.E., et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012;82(2):340–351. doi: 10.1016/j.ejpb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 116.Lohmann C., Hüwel S., Galla H.J. Predicting blood-brain barrier permeability of drugs: evaluation of different in vitro assays. J. Drug Target. 2002;10(4):263–276. doi: 10.1080/10611860290031903. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q., Rager J.D., Weinstein K., et al. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier. Int. J. Pharm. 2005;288(2):349–359. doi: 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 118.Joó F., Karnushina I. A procedure for the isolation of capillaries from rat brain. Cytobios. 1973;8(29):41–48. [PubMed] [Google Scholar]
- 119.Perrière N., Demeuse P., Garcia E., et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J. Neurochem. 2005;93(2):279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- 120.Ge S., Song L., Pachter J.S. Where is the blood-brain barrier ... really? J. Neurosci. Res. 2005;79(4):421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- 121.Ishizaki T., Chiba H., Kojima T., et al. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp. Cell Res. 2003;290(2):275–288. doi: 10.1016/S0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 122.Soma T., Chiba H., Kato-Mori Y., et al. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp. Cell Res. 2004;300(1):202–212. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Bonney S., Siegenthaler J.A. Differential effects of retinoic acid concentrations in regulating blood-brain barrier properties. eNeuro. 2017;4(3):4. doi: 10.1523/ENEURO.0378-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lippmann E.S., Al-Ahmad A., Azarin S.M., Palecek S.P., Shusta E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McConnell H.L., Kersch C.N., Woltjer R.L., Neuwelt E.A. The translational significance of the neurovascular unit. J. Biol. Chem. 2017;292(3):762–770. doi: 10.1074/jbc.R116.760215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haseloff R.F., Blasig I.E., Bauer H.C., Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell. Mol. Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sobue K., Yamamoto N., Yoneda K., et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 1999;35(2):155–164. doi: 10.1016/S0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 128.Allt G., Lawrenson J.G. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169(1):1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 129.Nakagawa S., Castro V., Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J. Cell. Mol. Med. 2012;16(12):2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thanabalasundaram G., Pieper C., Lischper M., Galla H.J. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res. 2010;1347:1–10. doi: 10.1016/j.brainres.2010.05.096. [DOI] [PubMed] [Google Scholar]
- 131.Zozulya A., Weidenfeller C., Galla H.J. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res. 2008;1189:1–11. doi: 10.1016/j.brainres.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 132.Thomsen L.B., Burkhart A., Moos T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS One. 2015;10(8):e0134765. doi: 10.1371/journal.pone.0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nzou G., Wicks R.T., Wicks E.E., et al. Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci. Rep. 2018;8(1):7413. doi: 10.1038/s41598-018-25603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho C.F., Wolfe J.M., Fadzen C.M., et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017;8:15623. doi: 10.1038/ncomms15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Logan S., Arzua T., Canfield S.G., et al. Studying human neurological disorders using induced pluripotent stem cells: from 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 2019;9(2):565–611. doi: 10.1002/cphy.c180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lippmann E.S., Al-Ahmad A., Palecek S.P., Shusta E.V. Modeling the blood-brain barrier using stem cell sources. Fluids Barriers CNS. 2013;10(1):2. doi: 10.1186/2045-8118-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim J.C., Wolpaw A.J., Caldwell M.A., Hladky S.B., Barrand M.A. Neural precursor cell influences on blood-brain barrier characteristics in rat brain endothelial cells. Brain Res. 2007;1159:67–76. doi: 10.1016/j.brainres.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 138.Weidenfeller C., Svendsen C.N., Shusta E.V. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J. Neurochem. 2007;101(2):555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Itoh K., Maki T., Lok J., Arai K. Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015;1623:135–149. doi: 10.1016/j.brainres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maki T., Maeda M., Uemura M., et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci. Lett. 2015;597:164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seo J.H., Maki T., Maeda M., et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS One. 2014;9(7):e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuchler-Bopp S., Delaunoy J.P., Artault J.C., Zaepfel M., Dietrich J.B. Astrocytes induce several blood-brain barrier properties in non-neural endothelial cells. Neuroreport. 1999;10(6):1347–1353. doi: 10.1097/00001756-199904260-00035. [DOI] [PubMed] [Google Scholar]
- 143.Faassen F., Vogel G., Spanings H., Vromans H. Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int. J. Pharm. 2003;263(1-2):113–122. doi: 10.1016/S0378-5173(03)00372-7. [DOI] [PubMed] [Google Scholar]
- 144.Hayashi Y., Nomura M., Yamagishi S., Harada S., Yamashita J., Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19(1):13–26. doi: 10.1002/(SICI)1098-1136(199701)19:1<13:AID-GLIA2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 145.Isobe I., Watanabe T., Yotsuyanagi T., et al. Astrocytic contributions to blood-brain barrier (BBB) formation by endothelial cells: a possible use of aortic endothelial cell for in vitro BBB model. Neurochem. Int. 1996;28(5-6):523–533. doi: 10.1016/0197-0186(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 146.Greenwood J., Pryce G., Devine L., et al. SV40 large T immortalised cell lines of the rat blood-brain and blood-retinal barriers retain their phenotypic and immunological characteristics. J. Neuroimmunol. 1996;71(1-2):51–63. doi: 10.1016/S0165-5728(96)00130-0. [DOI] [PubMed] [Google Scholar]
- 147.Dohgu S., Takata F., Yamauchi A., et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038(2):208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 148.Förster C., Silwedel C., Golenhofen N., et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005;565(Pt. 2):475–486. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Czupalla C.J., Liebner S., Devraj K. In vitro models of the blood-brain barrier. Methods Mol. Biol. 2014;1135:415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- 150.Nakagawa S., Aruga J. Sphingosine 1-phosphate signaling is involved in impaired blood-brain barrier function in ischemia-reperfusion injury. Mol. Neurobiol. 2020;57(3):1594–1606. doi: 10.1007/s12035-019-01844-x. [DOI] [PubMed] [Google Scholar]
- 151.Hoheisel D., Nitz T., Franke H., et al. Hydrocortisone reinforces the blood-brain barrier properties in a serum free cell culture system. Biochem. Biophys. Res. Commun. 1998;244(1):312–316. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- 152.Lundquist S., Renftel M., Brillault J., Fenart L., Cecchelli R., Dehouck M.P. Prediction of drug transport through the blood-brain barrier in vivo: a comparison between two in vitro cell models. Pharm. Res. 2002;19(7):976–981. doi: 10.1023/A:1016462205267. [DOI] [PubMed] [Google Scholar]
- 153.MacLean A.G., Orandle M.S., MacKey J., Williams K.C., Alvarez X., Lackner A.A. Characterization of an in vitro rhesus macaque blood-brain barrier. J. Neuroimmunol. 2002;131(1-2):98–103. doi: 10.1016/S0165-5728(02)00256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tominaga N., Kosaka N., Ono M., et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bernas M.J., Cardoso F.L., Daley S.K., et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat. Protoc. 2010;5(7):1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eigenmann D.E., Dürig C., Jähne E.A., et al. In vitro blood-brain barrier permeability predictions for GABAA receptor modulating piperine analogs. Eur. J. Pharm. Biopharm. 2016;103:118–126. doi: 10.1016/j.ejpb.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 157.Coisne C., Dehouck L., Faveeuw C., et al. Mouse syngenic in vitro blood-brain barrier model: a new tool to examine inflammatory events in cerebral endothelium. Lab. Invest. 2005;85(6):734–746. doi: 10.1038/labinvest.3700281. [DOI] [PubMed] [Google Scholar]
- 158.Cecchelli R., Dehouck B., Descamps L., et al. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999;36(2-3):165–178. doi: 10.1016/S0169-409X(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 159.Smith M., Omidi Y., Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J. Drug Target. 2007;15(4):253–268. doi: 10.1080/10611860701288539. [DOI] [PubMed] [Google Scholar]
- 160.Yamamoto Y., Liang M., Munesue S., et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019;2:76. doi: 10.1038/s42003-019-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Amano M., Salcedo-Gómez P.M., Zhao R., et al. A modified p1 moiety enhances in vitro antiviral activity against various multidrug-resistant HIV-1 variants and in vitro central nervous system penetration properties of a novel nonpeptidic protease inhibitor, GRL-10413. Antimicrob. Agents Chemother. 2016;60(12):7046–7059. doi: 10.1128/AAC.01428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aird W.C. Vascular bed-specific thrombosis. J. Thromb. Haemost. 2007;5(Suppl. 1):283–291. doi: 10.1111/j.1538-7836.2007.02515.x. [DOI] [PubMed] [Google Scholar]
- 163.Regan E.R., Aird W.C. Dynamical systems approach to endothelial heterogeneity. Circ. Res. 2012;111(1):110–130. doi: 10.1161/CIRCRESAHA.111.261701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith Q.R. A review of blood-brain barrier transport techniques. Methods Mol. Med. 2003;89:193–208. doi: 10.1385/1-59259-419-0:193. [DOI] [PubMed] [Google Scholar]
- 165.Garberg P., Ball M., Borg N., et al. In vitro models for the blood-brain barrier. Toxicol. In Vitro. 2005;19(3):299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 166.Ohtsuki S., Uchida Y., Kubo Y., Terasaki T. Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: methodology, advantages, strategy, and prospects. J. Pharm. Sci. 2011;100(9):3547–3559. doi: 10.1002/jps.22612. [DOI] [PubMed] [Google Scholar]
- 167.Syvänen S., Lindhe O., Palner M., et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab. Dispos. 2009;37(3):635–643. doi: 10.1124/dmd.108.024745. [DOI] [PubMed] [Google Scholar]
- 168.Battah B., Chemi G., Butini S., et al. A repurposing approach for uncovering the anti-tubercular activity of FDA-approved drugs with potential multi-targeting profiles. Molecules. 2019;24(23):24. doi: 10.3390/molecules24234373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim J.H., Scialli A.R. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011;122(1):1–6. doi: 10.1093/toxsci/kfr088. [DOI] [PubMed] [Google Scholar]
- 170.Tanaka H. Brain Nerve. 2019;71(9):981–989. doi: 10.11477/mf.1416201390. [Artificial Intelligence-based Drug Discovery and Drug Repositioning]. [DOI] [PubMed] [Google Scholar]
- 171.Murteira S., Ghezaiel Z., Karray S., Lamure M. Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: reassessment of nomenclature. J. Mark. Access Health Policy. 2013;1:1. doi: 10.3402/jmahp.v1i0.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schwab R.S., England A.C., Jr, Poskanzer D.C., Young R.R. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208(7):1168–1170. doi: 10.1001/jama.1969.03160070046011. [DOI] [PubMed] [Google Scholar]
- 173.Schwab R.S., Poskanzer D.C., England A.C., Jr, Young R.R. Amantadine in Parkinson’s disease. Review of more than two years’ experience. JAMA. 1972;222(7):792–795. doi: 10.1001/jama.1972.03210070026008. [DOI] [PubMed] [Google Scholar]
- 174.Caban A., Pisarczyk K., Kopacz K., et al. Filling the gap in CNS drug development: evaluation of the role of drug repurposing. J. Mark. Access Health Policy. 2017;5(1):1299833. doi: 10.1080/20016689.2017.1299833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pangalos M.N., Schechter L.E., Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007;6(7):521–532. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 176.Michael J., Marschallinger J., Aigner L. The leukotriene signaling pathway: a druggable target in Alzheimer’s disease. Drug Discov. Today. 2019;24(2):505–516. doi: 10.1016/j.drudis.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 177.De Strooper B., Karran E. The cellular phase of alzheimer’s disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 178.Cotman C.W., Su J.H. Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol. 1996;6(4):493–506. doi: 10.1111/j.1750-3639.1996.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 179.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 180.Unger M.S., Marschallinger J., Kaindl J., et al. Early changes in hippocampal neurogenesis in transgenic mouse models for alzheimer’s disease. Mol. Neurobiol. 2016;53(8):5796–5806. doi: 10.1007/s12035-016-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van de Haar H.J., Burgmans S., Jansen J.F., et al. Blood-brain barrier leakage in patients with early alzheimer disease. Radiology. 2016;281(2):527–535. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 182.Montagne A., Zhao Z., Zlokovic B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017;214(11):3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao L.B., Jia Z.Y., Lu G.D., Zhu Y.S., Jing L., Shi H.B. Establishment of a canine model of acute pulmonary embolism with definite right ventricular dysfunction through introduced autologous blood clots. Thromb. Res. 2015;135(4):727–732. doi: 10.1016/j.thromres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 184.Vargas D.M., De Bastiani M.A., Zimmer E.R., Klamt F. Alzheimer’s disease master regulators analysis: search for potential molecular targets and drug repositioning candidates. Alzheimers Res. Ther. 2018;10(1):59. doi: 10.1186/s13195-018-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar A., Singh A. Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol. Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 186.de Castro A.A., da Cunha E.F.F., Pereira A.F., et al. Insights into the drug repositioning applied to the alzheimer’s disease treatment and future perspectives. Curr. Alzheimer Res. 2018;15(12):1161–1178. doi: 10.2174/1567205015666180813150703. [DOI] [PubMed] [Google Scholar]
- 187.Saito S., Ihara M. New therapeutic approaches for Alzheimer’s disease and cerebral amyloid angiopathy. Front. Aging Neurosci. 2014;6:290. doi: 10.3389/fnagi.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Saito S., Kojima S., Oishi N., et al. A multicenter, randomized, placebo-controlled trial for cilostazol in patients with mild cognitive impairment: the COMCID study protocol. Alzheimers Dement. 2016;2(4):250–257. doi: 10.1016/j.trci.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saito S., Yamamoto Y., Ihara M. Development of a multicomponent intervention to prevent alzheimer’s disease. Front. Neurol. 2019;10:490. doi: 10.3389/fneur.2019.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Basso J., Miranda A., Sousa J., Pais A., Vitorino C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018;428:173–183. doi: 10.1016/j.canlet.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 191.Dilly S.J., Clark A.J., Marsh A., et al. A chemical genomics approach to drug reprofiling in oncology: antipsychotic drug risperidone as a potential adenocarcinoma treatment. Cancer Lett. 2017;393:16–21. doi: 10.1016/j.canlet.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 192.Gupta S.C., Sung B., Prasad S., Webb L.J., Aggarwal B.B. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013;34(9):508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 193.Chou F.H., Tsai K.Y., Su C.Y., Lee C.C. The incidence and relative risk factors for developing cancer among patients with schizophrenia: a nine-year follow-up study. Schizophr. Res. 2011;129(2-3):97–103. doi: 10.1016/j.schres.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 194.Dalton S.O., Johansen C., Poulsen A.H., et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br. J. Cancer. 2006;95(7):934–939. doi: 10.1038/sj.bjc.6603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mortensen P.B. Neuroleptic treatment and other factors modifying cancer risk in schizophrenic patients. Acta Psychiatr. Scand. 1987;75(6):585–590. doi: 10.1111/j.1600-0447.1987.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 196.Zong D., Zielinska-Chomej K., Juntti T., et al. Harnessing the lysosome-dependent antitumor activity of phenothiazines in human small cell lung cancer. Cell Death Dis. 2014;5:e1111. doi: 10.1038/cddis.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shin S.Y., Lee K.S., Choi Y.K., et al. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis. 2013;34(9):2080–2089. doi: 10.1093/carcin/bgt169. [DOI] [PubMed] [Google Scholar]
- 198.Klutzny S., Lesche R., Keck M., et al. Functional inhibition of acid sphingomyelinase by fluphenazine triggers hypoxia-specific tumor cell death. Cell Death Dis. 2017;8(3):e2709. doi: 10.1038/cddis.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu F., Xia Y., Feng Z., et al. Repositioning antipsychotic fluphenazine hydrochloride for treating triple negative breast cancer with brain metastases and lung metastases. Am. J. Cancer Res. 2019;9(3):459–478. [PMC free article] [PubMed] [Google Scholar]
- 200.Wu L., Liu Y.Y., Li Z.X., et al. Anti-tumor effects of penfluridol through dysregulation of cholesterol homeostasis. Asian Pac. J. Cancer Prev. 2014;15(1):489–494. doi: 10.7314/APJCP.2014.15.1.489. [DOI] [PubMed] [Google Scholar]
- 201.Pound P., Ebrahim S., Sandercock P., Bracken M.B., Roberts I. Reviewing animal trials systematically g. Where is the evidence that animal research benefits humans? BMJ. 2004;328(7438):514–517. doi: 10.1136/bmj.328.7438.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.O’Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., van der Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59(3):467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 203.Fagan S.C., Hess D.C., Hohnadel E.J., Pollock D.M., Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35(9):2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 204.Fagan S.C., Hess D.C., Machado L.S., Hohnadel E.J., Pollock D.M., Ergul A. Tactics for vascular protection after acute ischemic stroke. Pharmacotherapy. 2005;25(3):387–395. doi: 10.1592/phco.25.3.387.61592. [DOI] [PubMed] [Google Scholar]
- 205.Fagan S.C., Kozak A., Hill W.D., et al. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J. Hypertens. 2006;24(3):535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 206.Elewa H.F., Kozak A., Johnson M.H., Ergul A., Fagan S.C. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J. Hypertens. 2007;25(4):855–859. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 207.Chen H.Y., Chen T.Y., Lee M.Y., et al. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J. Pineal Res. 2006;41(2):175–182. doi: 10.1111/j.1600-079X.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 208.Murata Y., Rosell A., Scannevin R.H., Rhodes K.J., Wang X., Lo E.H. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39(12):3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Machado L.S., Sazonova I.Y., Kozak A., et al. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke. 2009;40(9):3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Elewa H.F., Kozak A., El-Remessy A.B., et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J. Pharmacol. Exp. Ther. 2009;330(2):532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Morofuji Y., Nakagawa S., So G., et al. Pitavastatin strengthens the barrier integrity in primary cultures of rat brain endothelial cells. Cell. Mol. Neurobiol. 2010;30(5):727–735. doi: 10.1007/s10571-010-9497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gibson C.L., Srivastava K., Sprigg N., Bath P.M., Bayraktutan U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J. Neurochem. 2014;129(5):816–826. doi: 10.1111/jnc.12681. [DOI] [PubMed] [Google Scholar]
- 213.Goyal M., Menon B.K., van Zwam W.H., et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 214.Goyal M., Demchuk A.M., Menon B.K., et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 215.Powers W.J., Derdeyn C.P., Biller J., et al. 2015 American heart association/american stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46(10):3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 216.Saver J.L., Goyal M., Bonafe A., et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 217.Campbell B.C., Mitchell P.J., Kleinig T.J., et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 218.Jovin T.G., Chamorro A., Cobo E., et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 219.Berkhemer O.A., Fransen P.S., Beumer D., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 220.Fraser J.F., Maniskas M., Trout A., et al. Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke. J. Cereb. Blood Flow Metab. 2017;37(11):3531–3543. doi: 10.1177/0271678X17705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Griauzde J., Ravindra V.M., Chaudhary N., Gemmete J.J., Pandey A.S. Neuroprotection for ischemic stroke in the endovascular era: a brief report on the future of intra-arterial therapy. J. Clin. Neurosci. 2019;69:289–291. doi: 10.1016/j.jocn.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 222.Maniskas M.E., Roberts J.M., Trueman R., et al. Intra-arterial nitroglycerin as directed acute treatment in experimental ischemic stroke. J. Neurointerv. Surg. 2018;10(1):29–33. doi: 10.1136/neurintsurg-2016-012793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang R., Wu X., Liang J., et al. Intra-artery infusion of recombinant human erythropoietin reduces blood-brain barrier disruption in rats following cerebral ischemia and reperfusion. Int. J. Neurosci. 2015;125(9):693–702. doi: 10.3109/00207454.2014.966354. [DOI] [PubMed] [Google Scholar]
- 224.Suzuki T., Aoyama T., Suzuki N., et al. Involvement of a proton-coupled organic cation antiporter in the blood-brain barrier transport of amantadine. Biopharm. Drug Dispos. 2016;37(6):323–335. doi: 10.1002/bdd.2014. [DOI] [PubMed] [Google Scholar]
- 225.Murata M., Horiuchi E., Kanazawa I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci. Res. 2001;41(4):397–399. doi: 10.1016/S0168-0102(01)00298-X. [DOI] [PubMed] [Google Scholar]
- 226.Uenaka T., Satake W., Cha P.C., et al. In silico drug screening by using genome-wide association study data repurposed dabrafenib, an anti-melanoma drug, for Parkinson’s disease. Hum. Mol. Genet. 2018;27(22):3974–3985. doi: 10.1093/hmg/ddy279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hurle M.R., Yang L., Xie Q., Rajpal D.K., Sanseau P., Agarwal P. Computational drug repositioning: from data to therapeutics. Clin. Pharmacol. Ther. 2013;93(4):335–341. doi: 10.1038/clpt.2013.1. [DOI] [PubMed] [Google Scholar]