Abstract
Advances in molecular research techniques have enabled a new frontier in discerning the mechanisms responsible for monogenic diseases. In this review, we discuss the current research on the molecular pathways governing blood pressure disorders with a Mendelian inheritance pattern, each presenting with a unique pathophysiology. Glucocorticoid Remediable Aldosteronism (GRA) and Apparent Mineralocorticoid Excess (AME) are caused by mutations in regulatory enzymes that induce increased production of mineralocorticoids or inhibit degradation of glucocorticoids, respectively. Geller syndrome is due to a point mutation in the hormone responsive element of the promotor for the mineralocorticoid receptor, rendering the receptor susceptible to activation by progesterone, leading to hypertension during pregnancy. Pseudohypoaldosteronism type II (PHA-II), also known as Gordon’s syndrome or familial hyperkalemic hypertension, is a more variable disorder typically characterized by hypertension, high plasma potassium and metabolic acidosis. Mutations in a variety of intracellular enzymes that lead to enhanced sodium reabsorption have been identified. In contrast, hypertension in Liddle’s syndrome, which results from mutations in the Epithelial sodium Channel (ENaC), is associated with low plasma potassium and metabolic alkalosis. In Liddle’s syndrome, truncation of one the ENaC protein subunits removes a binding site necessary protein for ubiquitination and degradation, thereby promoting accumulation along the apical membrane and enhanced sodium reabsorption. The myriad effects due to mutation in phosphodiesterase 3A (PDE3A) lead to severe hypertension underlying sodium-independent autosomal dominant hypertension with brachydactyly. How mutations in PDE3A result in the phenotypic features of this disorder are discussed. Understanding the pathologies of these monogenic hypertensive disorders may provide insight into the causes of the more prevalent essential hypertension and new avenues to unravel the complexities of blood pressure regulation.
Keywords: Hypertension, monogenic, aldosteronism, mineralocorticoid, liddle’s syndrome, ENaC, WNK, PDE3A
1. INTRODUCTION
The purpose of this paper is to describe human hypertension disorders that are due to alterations in single genes. These include Glucocorticoid Remediable Aldosteronism (GRA), Liddle’s syndrome, Apparent Mineralocorticoid Excess (AME), Pseudohypoaldosteronism type II (PHA-II), Geller syndrome, and Autosomal Dominant Hypertension with Brachydactyly (ADHB). The research done on these rare genetic disorders has shed much light on the pathways and mechanisms involved in blood pressure regulation. These disorders are often categorized as low renin forms of hypertension. Patients with these disorders present with unique pathological states offer new insight into the causes of primary hypertension. Genes responsible for hereditary forms of pheochromocytoma and familial paraganglioma have also been identified but are beyond the scope of this review.
1.1. Background
Hypertension is a chronic medical condition characterized by elevated arterial blood pressure. Hypertension has been considered as a major risk factor of coronary heart disease [1], stroke, aneurysms, kidney failure and arterial disease, and is classified as either primary or secondary. Primary hypertension, also called essential or idiopathic hypertension, is a form of elevated blood pressure with no identifiable cause. Studies have shown that out of all cases of diagnosed hypertension, 95% of cases are diagnosed as this type [2]. The underlying mechanisms are currently believed to be a combination of genetic and environmental factors. Secondary hypertension results from an identifiable underlying cause, often involving kidney or endocrine diseases and tumors. It can also occur as a side effect of certain medications. Only 5% of hypertensive cases are diagnosed as secondary. Of these, a subgroup can be further identified to be due to mutations in a single gene product or protein. According to the Center of Disease Control and Prevention, about 70 million American adults are hypertensive. Annual costs of health care and medication to treat people with hypertension are over $46 billion. In 2013, more than 360,000 deaths in the United States reported hypertension as a primary or secondary cause [3, 4] .
2. GLUCOCORTICOID REMEDIABLE ALDO- STERONISM
GRA is an autosomal dominant disorder that results in hypertension, high serum aldosterone, low renin activity and abnormal adrenal steroid production. It is also referred to as aldosterone synthase hyperactivity, glucocorticoid suppressible hyperaldosteronism or familial hyperaldo-steronism type I.
2.1. Background
GRA was first discovered and studied in the 1960’s, and since then has been studied by multiple investigators across the scientific community. Sutherland et al. (1966) and Salti et al. (1969) first described a father and son who showed symptoms of hypertension with increased aldosterone secretion but low renin activity [5, 6]. Ganguly et al. (1981) diagnosed a 7-year-old boy with GRA, which led to the discovery that his mother and grandmother also had an identical phenotype. A urinary analysis failed to identify an absolute “aldosterone stimulating factor.” This suggested that GRA was a distinct disorder from idiopathic aldosteronism [7]. Another study reported a family diagnosed with GRA when a saline infusion failed to lower aldosterone levels and administration of a loop diuretic and low Na+ diet did not increase renin activity. This study also showed that the decline in plasma aldosterone secretion when a patient is upright, seen in aldosterone producing adenomas, is also observed in GRA. Gordon et al. (1995) reported the phenotypic heterogeneity of GRA in over 21 members from a kindred with more than 1,000 decedents of an English convict, who was sent to Australia in 1837 for highway robbery. Gordon was the first to refer to GRA as familial hyperaldosteronism type I [8]. Shortly thereafter, Gates et al. (1996) reported two large pedigrees with GRA that were confirmed my genetic analysis. At the time, GRA patients were clinically indistinguishable from patients with essential hypertension. Gates suggested that GRA is an underdiagnosed condition [9]. Stowasser et al. (1999) studied ten normotensive subjects with GRA that took no medication. Results showed normal plasma and normal upright aldosterone levels. However, in subjects with GRA and hypertension, the results demonstrated a failure of aldosterone to increase at least 50% (normal) in subjects sitting in an upright position, as well as a failure to raise aldosterone after an angiotensin II infusion. Studies also showed that aldosterone levels correlated significantly with cortisol levels, but not with plasma renin activity. Aldosterone levels were suppressed after treatment with dexamethasone, suggesting that Adrenocorticotropic Hormone (ACTH) was regulating aldosterone production [10].
In 2000, Stowasser et al. studied GRA patients with varying degrees of hypertension. They showed that males were affected more severely than females and that the degree of hybrid gene-induced aldosterone overproduction contributed to the severity of hypertension [11]. Multatero et al. (2002) discovered the presence of a chimeric gene in the affected GRA members of through four of five-generations in a pedigree from Sardinia [12].
2.2. Physiology
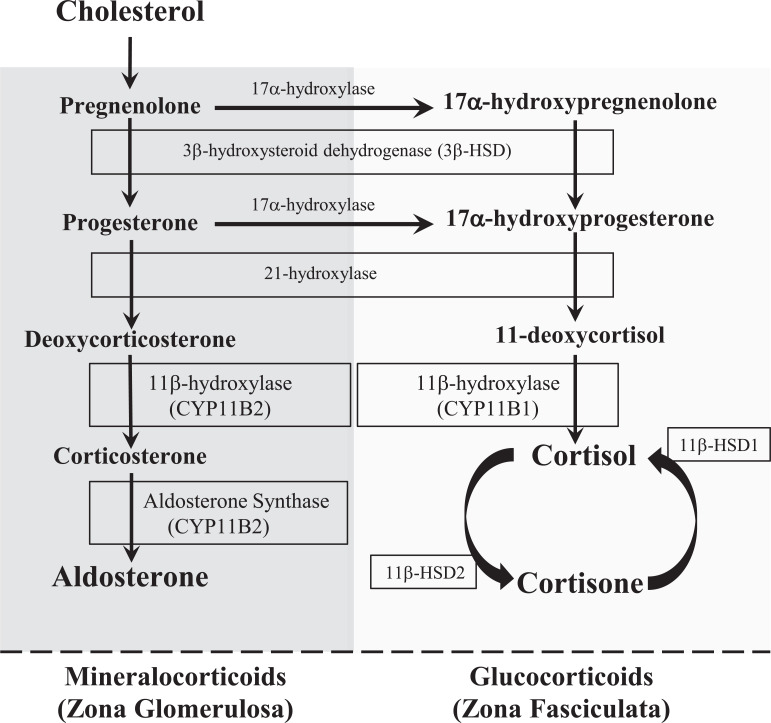
The adrenal glands are endocrine glands that produce a variety of hormones including: glucocorticoids (cortisol), mineralocorticoids (aldosterone), androgens (dehydroepi-androsterone [DHEA]), epinephrine and norepinephrine. Glucocorticoids, mineralocorticoids, and androgens are steroid hormones and share the same biosynthetic pathways. Each is unique in their evolution, regulation, and effects. They are all derived from cholesterol and synthesized in differentiated layers within the cortex of the adrenal gland (Fig. 1). The zona glomerulosa is the outermost layer of the cortex. This is the main site of mineralocorticoid (aldosterone) synthesis [13].
Fig. (1).
Pathways for mineralocorticoid and glucocorticoid hormone synthesis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Aldosterone is involved in the regulation of electrolytes and blood pressure. The final step is the formation of aldosterone from corticosterone by the enzyme aldosterone synthase. Aldosterone synthase is a steroid hydroxylase cytochrome P450 enzyme and is exclusively expressed in mitochondria of the zona glomerulosa [14]. Aldosterone synthase is encoded on chromosome 8 by the CYP11B2 gene. It converts corticosterone the final product aldosterone [13].
Studies show that calcium (Ca2+) acts as a transcription factor for aldosterone synthase though interaction at the 5’-flanking region of the CYP11B2 gene [15]. Aldosterone is stimulated by multiple factors: a decrease in blood pressure via the stretch receptors in the heart, acidosis, an increase in circulating angiotensin II (ANG II), or elevated plasma K+ concentration. Increases in plasma K+ concentrations are the most potent stimulator, and act by depolarizing the cell and opening voltage-gated Ca2+ channels, which stimulate aldosterone synthase expression and activity. Studies have shown that sustained aldosterone production requires a higher intracellular Ca2+ concentration [16, 17].
In normal conditions, Adrenocorticotropic Hormone (ACTH) has only a minor role in regulating aldosterone and acts indirectly by stimulating the expression of its precursor molecules.
Aldosterone exerts its effects mainly in the distal tubule and collecting ducts of the nephron. By binding to the nuclear mineralocorticoid receptor of the principal cells, it upregulates and/or activates the basolateral Na+/K+ pumps (Na/K-ATPase), leading to greater K+ secretion. Aldosterone also upregulates the expression of epithelial sodium channels (ENaC) on the apical membrane of the principal cells of the renal collecting ducts. This leads to an increase in sodium (Na+) reabsorption and allows chloride (Cl-) to be reabsorbed to maintain electrochemical balance. It also stimulates hydrogen (H+) secretion in exchange for K+ to regulate pH. Lastly, the urinary loss of K+ results in lower plasma K+ concentration, thereby indirectly stimulating reabsorption of NaCl by the thiazide-sensitive sodium-chloride cotransporter (NCC) [13].
The zona fasciculate is the middle layer of the cortex. This is the main site of glucocorticoid (cortisol) synthesis. It is the largest layer, accounting for up to 80% of the volume of the entire adrenal cortex. Cortisol is also a steroid hormone and is involved in regulating aspects of multiple systems that pertain to metabolism, electrolyte balance, stress, and immune responses. It is regulated by the hypothalamus and pituitary glands. Within the paraventricular nucleus of the hypothalamus, Corticotrophin-Releasing Hormone (CRH) is produced. When released into the median eminence, CRH travels through the hypothalamo-hypophyseal portal system to the anterior pituitary. CRH stimulates corticotropes to secrete ACTH, a peptide hormone that stimulates the secretion of glucocorticoids. The ACTH receptor is expressed primarily on adrenocortical cells in the zona glomerulosa. It has rapid effects that stimulate delivery of cholesterol to the mitochondria, for the first step of steroidogenesis via the P450 side chain cleavage enzyme (P450scc). It also stimulates lipoprotein influx into the cortical cells. Chronically, it stimulates the transcription of the genes coding for steroidogenic enzymes (P450scc) and steroid 11β-hydroxylase. It also stimulates the transcription of genes encoding subunits of the mitochondrial oxidative phosphorylation system, which is thought to be necessary for the enhanced energy needs due to ACTH stimulation [18].
Cortisol is synthesized from cholesterol and is the main hormone secreted from the adrenal cortex in humans and many other species. Cortisol is metabolized from its precursor 11-deoxycortisol via the steroid enzyme 11β-hydroxylase in a final step of the pathway. The 11β-hydroxylase, also referred to as cytochrome P450 11B1 and is encoded by the CYP11B1 gene. White et al. (1987) stated that the CYP11B1 gene is present on chromosome 8 and later localized to chromosome 8q21 [19, 20]. 11β-Hydroxylase expression is found on the inner mitochondrial membrane of the zona glomerulosa and the zona fasciculata. It is not only responsible for the metabolism of cortisol, but also for the conversion of deoxycorticosterone to corticosterone, the precursor to aldosterone. Cortisol is also metabolized by the 11β-hydroxysteroid dehydrogenase system (11βHSD) which requires two enzymes: 11β-HSD1, which converts inactive cortisone to active cortisol and requires NADPH as a cofactor; 11β-HSD2, which converts cortisol to cortisone and requires an NAD+ cofactor [21]. Cortisol’s main effects on the kidneys include increasing glomerular filtration rate, renal plasma flow, Na+ reabsorption, K+ secretion and water diuresis.
The zona reticularis is the inner most layer of the cortex and is superficial to the medulla. This layer is responsible for the production of precursor weak androgens such as Dehydroepiandreosterone (DHEA) and androstenedione, from cholesterol.
2.3. Pathophysiology
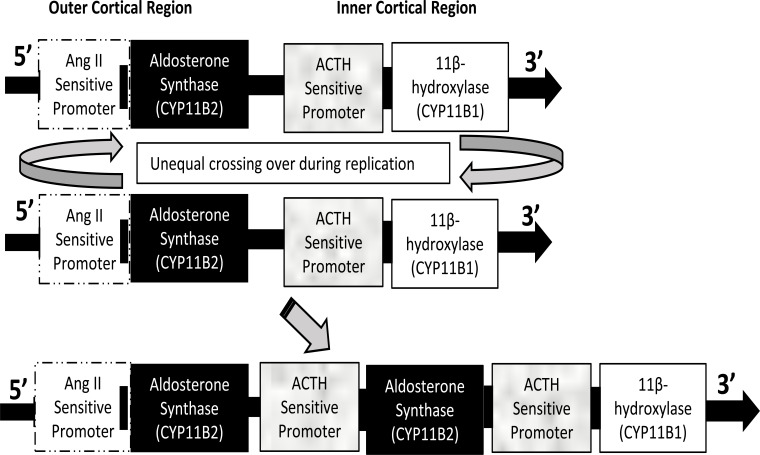
GRA is a cause of primary hyperaldosteronism. The genes encoding 11β-hydroxlase and aldosterone synthase, CYP11B1 and CYP11B2, respectively, are both on chromosome 8 within close proximity to each other. The two genes encoding each enzyme share 95% sequence homology and have the same intron-exon structures (Fig. 2). During DNA replication in patients with GRA, an unequal crossing over of the genes occurs, resulting in the 5’ regulatory region of the 11β-hydroxylase gene inserted in the coding region of aldosterone synthase gene [22]. The new chimeric gene encodes for an aldosterone synthase that is activated not only by its normal stimulators: ANG II, high serum K+, low blood volume, but can also be directly activated by ACTH. Aldosterone synthase, as a result becomes expressed in the zona fasciculata and therefore aldosterone is produced along with cortisol upon stimulation with ACTH [23]. The overall effect of the ACTH-sensitive aldosterone synthase is a significant increase in aldosterone concentration (Fig. 3). This leads to an increase in ENaC and Na/K-ATPase expression and activity within the principal cells of the kidney collecting ducts, resulting in increased NaCl and water reabsorption and increased K+ secretion.
Fig. (2).
Chimeric gene duplication in GRA. The genes CYP11B2 and CYP11B1 that code for aldosterone synthase and 11β-hydroxylase, respectively, possess 95% homology. They are located on chromosome 8 and lie adjacent to each other. Unequal crossing over during replication results in a chimeric gene where CYP11B2 comes under the regulation of the promotor regulated by ACTH. Thus, transcription of aldosterone synthase is enhanced by ACTH and leads to increased formation of aldosterone. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
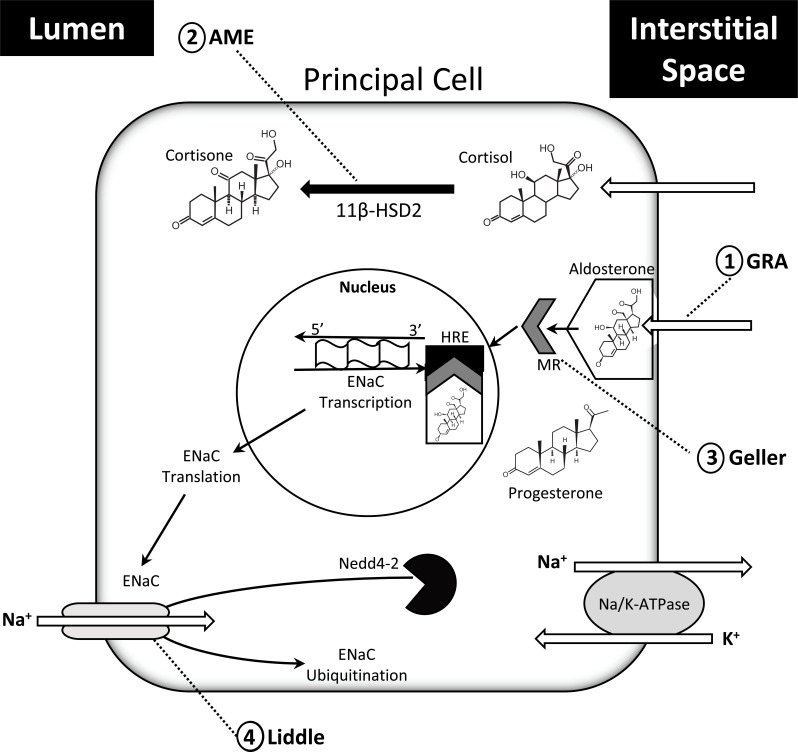
Fig. (3).
Schematic representation of the physiology of steroid hormone action and sites of pathologic relevance within the principal cells of the collecting tubules and collecting ducts of the kidney. 1) Glucocorticoid remediable aldosteronism (GRA) is caused by a chimeric gene formation that produces aldosterone under the stimulation of ACTH. 2) Apparent mineralocorticoid Excess (AME) occurs due to a mutation in the gene encoding 11βHSD2 that results in reduced oxidation of cortisol, thereby permitting cortisol activation of the MR. 3) Geller syndrome (Geller) is caused by a missense, gain-of-function mutation on the MR that permits binding of progesterone. During pregnancy, progesterone levels are elevated, leading to excess activation of the MR pathway. 4) Liddle’s syndrome (Liddle) is the result of mutation of either beta or gamma subunits of ENaC that prevents Nedd4-2 binding. As a result, ENaC avoids ubiquitination and degradation. (See text for additional information). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.4. Clinical Findings, Diagnosis, and Treatment
The most common side effects of GRA include severe hypertension with low plasma renin activity (PRA) and mild hypernatremia. The onset of the disease is often seen early in life. Most GRA patients have normal or only mildly decreased plasma levels of K+, despite the apparent hyperaldosteronism effects. The reason why is not well understood. In a retrospective study on 27 GRA pedigrees, early hemorrhagic stroke (EHS) was an associated feature in nearly half of the pedigrees. There were no symptoms of EHS in the GRA negative family members in each pedigree.
GRA is often misdiagnosed as essential hypertension. Early signs of GRA include early onset of hypertension, family history, and refractory hypertension when given standard treatment. Testing for the plasma aldosterone to PRA ratio may help screening test to identify high aldosterone with suppressed PRA [24]. This ratio and a dexamethasone suppression test, used to measure urinary hybrid steroids (18-oxocortisol and 18-hydroxycortisol), can both be used as diagnostic aids to differentiate elevated aldosterone that is abnormally under the influence of ACTH. Direct genetic analysis is the best way to diagnose GRA and treatment can be achieved with multiple approaches. Low dose glucocorticoids are effective as a treatment due to negative feedback on ACTH, thereby removing its stimulation of aldosterone. Mineralocorticoid Receptor (MR) antagonists such as spironolactone or eplerenone are effective alternatives due to the competitive inhibition of the MR therefore suppressing the effect of aldosterone. ENaC antagonists like amiloride have also been used effectively to inhibit ENaC, thereby diminishing Na+ reabsorption and K+ secretion. Antihypertensive drugs affecting the renin-angiotensin system, such as ACE-inhibitors or β-blockers, are ineffective in treating GRA because PRA is already suppressed.
3. LIDDLE’S SYNDROME
Liddle’s syndrome is an autosomal dominant genetic disorder characterized by severe high blood pressure associated with low plasma renin and aldosterone. Very low plasma K+ concentrations and metabolic alkalosis are also present.
3.1. Background
Liddle’s syndrome was first described by Liddle et al. (1963) as hypertension associated with hypokalemia that was not caused by hyperaldosteronism but by problems in the distal tubule. Family studies of patients with this disease displayed an autosomal dominant inheritance. Several investigators confirmed the original findings by Liddle and colleagues [25-28]. They discovered that either amiloride or triamterene - both K+ sparing diuretics that inhibit ENaC - along with a low Na+ diet provide an effective treatment of both the hypertension and hypokalemia. Importantly, they showed that spironolactone, a competitive inhibitor of the Mineralocorticoid Receptor (MR), was not an effective treatment for Liddle’s syndrome.
Botero-Velez et al. (1994) studied a brother and sister who presented with hypertension and hypokalemia with low aldosterone levels. High ratios of Na+: K+ in sweat and saliva, and a lack of effect by spironolactone to treat the hypertension excluded mineralocorticoid excess as the cause. They postulated that constitutive activation of any component of ENaC or the MR within the collecting tubule could explain the syndrome [29]. Thirty-five years later, the first in vivo results showing an increase in ENaC activity in a patient with Liddle’s Syndrome by measuring transnasal potential difference were reported. Nasal potential difference measurements are now used as a simple clinical test [30]. Shimkets et al. (1994) discovered a mutation in the beta subunit of ENaC involving a premature stop codon that causes a truncated C terminus in the cytoplasm. This same premature stop or frameshift mutation in the same C terminus domain was identified in four additional family members [31]. Hansson et al. (1995) also showed that Liddle’s Syndrome can also result in truncation of the C terminus of the gamma subunit of ENaC [32]. Truncation of the C termini increases expression of ENaC on the apical membrane, thereby enhancing Na+ reabsorption by the collecting duct. They discovered a conserved motif in the C terminus of all three subunits of ENaC that, when mutated, produce the effects observed with Liddle’s Syndrome. Results showed that the truncation increased the surface expression of ENaC on the apical membrane [33].
3.2. Physiology
Under normal conditions, hypertension and low potassium would both suppress aldosterone production and secretion (see above).
Within the kidney aldosterone acts on the nuclear MR localized in the collecting duct, specifically in principal cells. Aldosterone upregulates and activates the Na+/K+-ATPase on the basolateral membrane. This creates a gradient for the reabsorption of Na+ from the tubular fluid and secretion of K+ into the lumen of the collecting duct. Upregulation of ENaC expression on the apical surface of the distal nephron is induced by aldosterone [13, 34]. ENaC plays a vital role in Na+ and K+ homeostasis in coordination with the basolateral Na+/K+-ATPase. The function of ENaC is critical and contributes crucially to the maintenance of total body Na+ content and blood pressure. ENaC structure consists of three different subunits (alpha, beta and gamma) and is a heterotrimeric protein [34]. Each subunit contains two transmembrane helices and an extracellular loop. The extracellular loops contain multiple, highly conserved cysteine residues, proposed to function as receptors. Both the amino- and carboxy-termini are intracellular. The C-terminus contains proline-rich motifs (PPxY). Studies have suggested that ENaC contains a central ion channel along its central symmetry axis between the three subunits. Opening of the ion pathway is thought to involve a coordinated rotation of the second transmembrane spanning domain of each subunit [35]. The complex regulation of ENaC synthesis and trafficking are reviewed thoroughly by Palmer et al. (2012) [36].
Retrieval of ENaC from the apical membrane is also carefully regulated. ENaC is marked for retrieval and degradation by ubiquitination via the E3 ubiquitin ligase, Nedd4-2. The main function of ubiquitin protease system is to degrade marked proteins in the cell by proteolysis. The gene for NEDD4 is highly conserved in all eukaryotes. It is widely expressed in the body and is involved in the regulation of multiple metabolic processes [37]. It binds to ENaC at the C-terminus of the beta and/or gamma subunits to mark it for degradation [38, 39].
3.3. Pathophysiology
Liddle’s syndrome is caused by heterozygous mutations in the SCNN1B and/or the SCNN1G genes located on chromosome 16, which result in a truncated C-terminus on either the beta or gamma subunits of ENaC. The mutant ENaC protein no longer has a binding site for Nedd 4-2 and therefore, cannot mark ENaC for proteolytic degradation.
The result of the inhibited breakdown of ENaC leads to increased ENaC expression on the apical membrane which causes increased Na+ reabsorption. This correlates with the severe hypertension seen in patients with Liddle’s syndrome [31]. The increase in Na+ entry into the principal cell activates the Na+/K+-ATPase, resulting in increased flux of K+ into the cell at the basolateral side thereby facilitating K+ secretion into the lumen (Fig. 3). The result is low urinary Na+ and high urinary K+ excretion culminating in hypertension and hypokalemia. The influx of Na+ also leads to a more negative lumen potential that is permissive for H+ secretion by nearby type A intercalated cells and results in metabolic alkalosis.
3.4. Clinical Findings, Diagnosis, and Treatment
Patients with Liddle’s Syndrome have salt-sensitive hypertension that develops early in childhood, hypokalemia, metabolic alkalosis, low PRA and low aldosterone. Patients are recommended to eat a low Na+ diet and are treated with a K+ sparing diuretic such as amiloride or triamterene. Both amiloride and triamterene are direct inhibitors of ENaC and therefore block Na+ reabsorption via the channel. By inhibiting Na+ influx, the hypokalemia is also alleviated though patients may require potassium supplementation as well. The increased arterial pressure suppresses aldosterone via negative feedback, and due to this, competitive MR inhibitors of aldosterone such as spironolactone are not an effective treatment.
4. APPARENT MINERALOCORTICOID EXCESS
AME is an autosomal recessive disease that results in hypertension and hypokalemia with low levels of renin and aldosterone.
4.1. Background
Initial publications of documented clinical cases that presented with symptoms of AME began in the 1970’s, with patients displaying effects of mineralocorticoid excess but with low to undetectable levels of aldosterone [40, 41]. The hypertensive symptoms in these cases responded well to diuretics such as triamterene and spironolactone. Studies showed an increase in the half-life of cortisol, implying a deficiency in the enzyme 11β-Hydroxysteroid Dehydrogenase type 2 (11βHSD2) that converts cortisol to its less active form, cortisone [42, 43]. It was believed that AME results in a lack of specificity by the Mineralocorticoid Receptor (MR) [44]. Several studies have shown that the MR has equal affinity to both cortisol and aldosterone in vitro [44, 45]. In studies done in vivo, the MR has substantially higher affinity for aldosterone. Finally, in 1983, it was proposed that cortisol was the mineralocorticoid that induced hypertension. Patients later diagnosed with AME were treated with the glucocorticoid hormone, hydrocortisone, but unexpectedly experienced aggravated effects of hypertension and hypokalemia [43].
Studies were conducted to identify 11βHSD as the cause of AME. The search first led to the discovery of 11βHSD1 in rat liver homogenates. 11βSD1 is also an oxoreductase but favors the conversion of cortisone to cortisol. No mutations were found on the gene encoding 11βHSD1 in patients with AME. Additional studies done on the distal nephron in rabbits showed a separate NAD-dependent isoform of 11βHSD. In 1994, cDNA-encoding 11βHSD2 was isolated and cloned in kidney cells of sheep, humans and rabbits. One year later, the first identification of a mutation on the 11βHSD2 gene was discovered within an Iranian mutations have since been discovered in the 11βHSD2 gene that abolishes enzyme activity, resulting in AME [46, 47].
AME is very rare. In the past 25 years less than 100 patients worldwide have been diagnosed with the disease. Such rare autosomal recessive mutations are usually explained by endogamy, consanguinity, or the founder effect. A study done in 1995 showed that seven of the eight families with AME appeared to conform to one of the three explanations [48].
4.2. Physiology
In the kidneys, active circulating cortisol is degraded to its inactive form cortisone via 11βHSD2 [49]. Cortisol is a steroid hormone produced in the zona fasciculata of the adrenal gland. Its release is regulated by stress and low blood glucose levels. Its functions include stimulating gluconeogenesis and anti-inflammatory pathways and regulating electrolyte balance. Within the kidneys, cortisol activates its glucocorticoid receptor which stimulates water diuresis, glomerular filtration rate, and renal plasma flow [50]. Cortisol is oxidized by 11βHSD2, which is from a family of oxidoreductases that act on donor hydroxyl groups and converts them to keto-groups, forming cortisone. This reaction is dependent on the ratio of NADP+/NADPH. 11βHSD2 is transcribed from the HSD11B2 gene, located on chromosome 16q22. The gene is approximately 6kb in length containing 5 exons. The two isoforms of 11βHSD only contain 20% sequence homology, suggesting they belong to different gene families. 11βHSD2 is colocalized with the MR in the distal tubules and collecting ducts of the nephron and is specifically localized in the endoplasmic reticulum of principal cells as well as in epithelial tissues and in the brainstem. In contrast to cortisol, cortisone binds very poorly to MR. Thus, by facilitating the conversion of cortisol to cortisone, 11βHSD2 activity in the principal cell prevents activation of MR by cortisol such that the MR in the distal nephron responds almost exclusively to aldosterone.
4.3. Pathophysiology
AME is due to a deficiency in the enzyme 11βHSD2, resulting in an increase in serum cortisol (Fig. 3). Circulating cortisol levels are normally 100-to 1000-fold higher than those of aldosterone, particularly in the morning. Failure of cortisol conversion to cortisone results in inappropriate activation of the MR by cortisol [51]. When the MR is activated by mineralocorticoids and even glucocorticoids, clinical signs of hyperaldosteronism ensue. The MR is expressed in many tissues: kidney, CNS, heart and sweat glands. In the principal cells of the kidney, MR activation specifically increases Na+ reabsorption through ENaC, leading to an increase in extracellular volume. K+ secretion is concurrently increased within these cells via the Renal Outer Medullary K+ channel (ROMK) for the combined effect hypertension and hypokalemia. The resultant change in intratubular electrical potential also facilitates H+ secretion by adjacent α-intercalated cells, leading to enhanced bicarbonate reabsorption and metabolic alkalosis [52-54]. AME-like clinical presentation can also be induced by ingesting large amounts of licorice, which contains glycyrrhizic acid that is hydrolyzed to glycyrrhetinic acid. In turn, glycyrrhetinic acid is metabolized into 3-beta-D-(monoglucuronyl)-18-beta-glycyrrhetinic acid which is capable of inhibiting 11βHSD2. The increased levels of cortisol in the collecting duct leading to MR activation and an “acquired” form of AME. Notably, glycyrrhizic acid is absent from licorice produced in the United States, but may still be found in licorice flavored chewing tobacco or imported licorice [55].
4.4. Clinical Findings, Diagnosis, and Treatment
AME presents early on in life with clinical features of low birth weight and severe hypertension. It is associated with metabolic alkalosis and severe hypokalemia. Diagnosis can be made by measuring the ratio of urinary cortisol metabolites, tetrahydrocortisol and allotetrahydrocortisol to the urinary concentration of tetrahydrocortisone. A normal ratio is 1:1. In a patient with AME, ratios ranged from 6.7 to 33 [56]. The optimal diagnostic test is the measurement of urine samples following 11-tritiated cortisol injection. In patients with AME, the infused cortisol is converted to detectable cortisone only 0-6% of what is observed in healthy individuals. However, this technique is not widely used due to the rarity of tritiated cortisol. Patients with AME are favorably treated with spironolactone to lower Na+ reabsorption and K+ secretion.
5. GELLER SYNDROME
Geller Syndrome is a form of hypertension caused by a heterozygous mutation of the MR that leads to altered nuclear receptor ligand selectivity and activation. In Geller syndrome, steroid hormones such as progesterone have increased affinity for the MR, leading to enhanced activation of mineralocorticoid signaling cascades that increase Na+ reabsorption and K+ secretion. Patients with this mutation often present at a young age with hypertension, diminished PRA, and low serum aldosterone. During pregnancy when progesterone levels are dramatically elevated, this mutation induces severe hypertension.
5.1. Background
Pregnancy-related hypertension results in complications in 6-10% of pregnancies and leads to increases in maternal and perinatal mortality [57]. Hypertension occurring during gestation is characterized by one of four conditions: pre-existing hypertension, gestational hypertension and pre-eclampsia, pre-existing hypertension plus superimposed gestational hypertension with proteinuria and unclassified hypertension. In many of these cases, delivery of the infant resolves symptoms, suggesting pregnancy-specific factors as a pathologic source. Geller et al. (2000) tested the hypothesis that gain-of-function mutations in the MR could induce hypertension. Seventy-five patients with early onset of hypertension were screened, of which a 15-year-old boy with severe idiopathic hypertension was found to be heterozygous for a missense mutation in the MR hormone binding domain. Eleven of 23 relatives of this child were evaluated and were found to have been diagnosed with severe hypertension before the age of 20. Three individuals died due to heart failure before the age of 50. The early onset of hypertension in this pedigree co-segregated with the MR mutation, strongly suggesting a Mendelian inheritance pattern.
5.2. Physiology
As described in prior sections, the binding of aldosterone to the MR in the principal cell of the kidney tubules leads to translocation to the nucleus where it binds to the hormone response element of the promotor region of genes regulated by aldosterone. The result is increased expression of ENaC and Na/K-ATPase. The Glucocorticoid Receptor (GR) and MR possess close structural similarity such that cortisol has high affinity for both GR and MR, indicating common ancestry [58]. However, aldosterone demonstrates clear MR specificity. To study the specificity of MR to aldosterone, Rogerson et al. created a series of chimeras from both receptors. Using this strategy, they identified amino acids 804-874 of the MR ligand-binding domain that were essential for aldosterone binding. Further analysis of these chimeras from this group and others found that the amino acid region 820-844 on the surface of the molecule determines ligand-binding specificity for each receptor subtype. Site-directed mutagenesis found 12 of the 16 amino acids that differ between GR and MR in the 820-844 region were responsible for directing specificity to aldosterone [59].
5.3. Pathophysiology
Wild type MR (MRWT) requires a steroid 21-hydroxyl group to permit binding and activation by steroid hormones such as aldosterone - a group that is not found on the progesterone molecule. Ligand binding induces various conformational changes involving the bending of several receptor helices to form a surface for coactivator binding [60]. A point mutation causing substitution of a leucine for a serine at codon 810 (S810L; MRL810), located on chromosome 4q31 alters the conformation of the hormone-binding domain. Subsequent studies indicate that the substituted lysine in MRL810 permits bending of the side-chain that forms van der Waals interactions when bound by smaller steroids such as progesterone. These interactions significantly increase binding affinity when compared to that of aldosterone. Progesterone levels typically increase extensively during pregnancy, suggesting that the MRL810 mutation could result in increases in ENaC and Na/K-ATPase expression and activity, thereby augmenting Na+ reabsorption and provoking gestational hypertension (Fig. 3). Indeed, in one such pregnant patient blood pressure rose to levels of 210/120 mmHg with low serum potassium levels low and nearly undetectable aldosterone levels.
In men and non-pregnant women, the severe hypertension observed with the MRL810 mutation is not likely explained by elevated progesterone levels. Cortisol levels are significantly more abundant than aldosterone in men and bind the MR with similar affinity [61, 62]. However, in healthy individuals, MR activation is prevented by metabolism of cortisol to corticosterone. Interestingly, in vitro analysis of MRL810 activity in the presence of cortisol by-products clearly demonstrated the ability of corticosterone and 11-dehydrocorticosterone to bind and activate the MR with high affinity. These results suggest cortisol by-products as the pathologic source in men and non-pregnant women with the MRL810 mutation [63].
5.4. Clinical Findings, Diagnosis and Treatment
Patients with Geller syndrome need to consume a low salt diet. Pregnancy should be closely monitored. Due to the altered binding parameters of the ligand binding domain, MRL810 has increased affinity for the traditional MR antagonist, spironolactone. This may result in paradoxical activation of the MR, inducing an agonist reaction and exacerbating the hypertension and electrolyte disturbances.
6. PSEUDOHYPOALDOSTERONISM TYPE II
PHA is a heterogeneous group of genetic disorders involved in electrolyte balance. Although, there are multiple types and subsets of PHA, this review focuses on PHA-II, also known as Gordon’s syndrome, since it is associated with hypertension. PHA-II is an autosomal dominant disorder characterized by hypertension, hyperkalemia and metabolic acidosis. It is associated with both low renin and low aldosterone levels [64-67]. In contrast, PHA-I results in salt wasting and hypotension, which is beyond the focus of this review. For more information on PHA-I, the reader is referred to excellent recent reviews [68, 69].
6.1. Background
In 1970, Gordon et al. reported a case of hyperkalemia and hypertension with low PRA and aldosterone that responded to dietary salt restriction [70]. Brautbar et al. (1978) treated a similar patient who had four family members with the same symptoms. The inability to increase K+ excretion by exogenous mineralocorticoid treatment pointed to a defect in K+ regulation in the distal nephron. Treatment with an ion exchange resin reduced the hyperkalemia and ameliorated the hyperchloremic acidosis [71]. Several other reports followed [72-75]. Schambelan et al. (1981) first referred to Gordon’s syndrome as PHA-II; a syndrome of chronic mineralocorticoid-resistant hyperkalemia and hypertension [76]. Pasman et al. (1989) studied a 14-year-old boy with Gordon’s syndrome that presented with secondary hyperkalemic periodic paralysis. Treatment with hydrochlorothiazide normalized plasma K+ levels, aldosterone and PRA and cured the periodic paralysis [77]. Take et al. (1991) studied a Japanese family with three affected members and were the first to suggest increased NaCl reabsorption in the thiazide-sensitive segment of the nephron and autosomal dominant transmission [78], later shown to be a linkage disorder with chromosome 1 and 17 [65].
Wilson et al. (2001) identified two genes that associated with PHA-II. These genes encoded isoforms of the with-no-lysine (WNK) family of serine-threonine kinases, WNK1 and WNK4. Identification of these proteins propelled research and precipitated advances in the understanding of their actions within the kidney. Further research lead to the discovery of two additional proteins, CUL3 and KLHL3, that are involved in the proteasomal degradation of WNK proteins. Mutations in these have also been associated with PHA-II [66, 67].
6.2. Physiology
The WNK1 gene is located on chromosome 12 and produces four alternatively spliced isoforms: L-WNK1 and KS-WNK1 are most relevant to Gordon’s syndrome. L-WNK1 is the full-length isoform and is expressed throughout the body. Kidney-specific WNK1 (KS-WNK1) is expressed exclusively in the kidneys [79, 80]. Both proteins help maintain electrolyte homeostasis by regulating the thiazide transporter, NaCl-cotransporter (NCC) and/or the renal outer medullary potassium channel (ROMK). NCC is expressed apically in the distal tubules and is responsible for the reabsorption of ~5-10% of filtered NaCl. It is inhibited by thiazides. ROMK is an ATP-dependent K+ channel expressed in the thick ascending limb and in the principal cells of the collecting ducts. It is involved in K+ recycling and K+ secretion, respectively [81, 82].
The regulation of Na+ reabsorption and K+ secretion by the WNK kinases is complex and the mechanisms continue to be updated. L-WNK1 activates SPS-1-related proline/alanine-rich kinase (SPAK) via phosphorylation. In turn, SPAK phosphorylates NCC thereby increasing its trafficking and membrane expression [83]. By stimulating NCC activity, WNK1 promotes increased NaCl reabsorption. Moreover, L-WNK1 directly inhibits ROMK activity in the collecting duct. Inhibition of ROMK promotes decreased K+ secretion [84, 85]. KS-WNK1, in turn, can inhibit L-WNK1 and L-WNK1 can interact with WNK4 (via a conserved HQ domain), to promote further “fine tuning” of the intracellular regulatory mechanisms controlling Na+ and K+ handling by this nephron segment [86].
The gene encoding WNK4 is located on chromosome 17. The function of WNK4 has been more difficult to elucidate due to apparent paradoxical effects. WNK4 stimulates NCC and inhibits surface expression of ROMK via a dynamin-dependent mechanism that does not require ubiquitination [85, 87]. WNK4 has also been implicated in increasing paracellular Cl- reabsorption by phosphorylation of claudins [88-91]. WNK4 also regulates ENaC, but its major effects appear to be on NCC as a knockout of WNK4 results in a Gitelman-like phenotype [92]. Notably, intracellular Cl- concentration modulates WNK4 function via a chloride-sensing motif [93]. WNK4 maintains basal phosphorylation of NCC via SPAK. Under conditions of low intracellular Cl-, NCC is stimulated whereas high intracellular Cl- inhibits. Moreover, membrane potential at the apical surface of the distal convoluted tubule cells (related to extracellular K+) also plays a role. Mutations in WNK1 or WNK4 have been identified in a relative small number of individuals with PHA-II. This observation suggested the presence of mutations in other intracellular proteins. The ubiquitin proteasome system is a protein degradation pathway that is critical to processes such as cell cycle progression, signal transduction, protein expression and quality control [87]. Proteolytic specificity occurs via a protein complex comprised of an E3-ubiquitin ligase, a Cullin protein and an adaptor protein. The Cullins provide a scaffold for E3 ligase subunits, which are enzymes that confer substrate specificity and are required for ubiquitination of targeted proteins. In turn, the Cullin proteins interact with a family of adaptor proteins. More specifically, Cullin3 (CUL3) binds to a domain on Kelch-like protein 3 (KLHL3), a subclass of complex/tram-track/bric-a-brac proteins. Within the nephron, CUL3 binds with KLHL3. Kelch domains within KLHL3 bind to WNK proteins and target them for degradation [94-98].
6.3. Pathophysiology
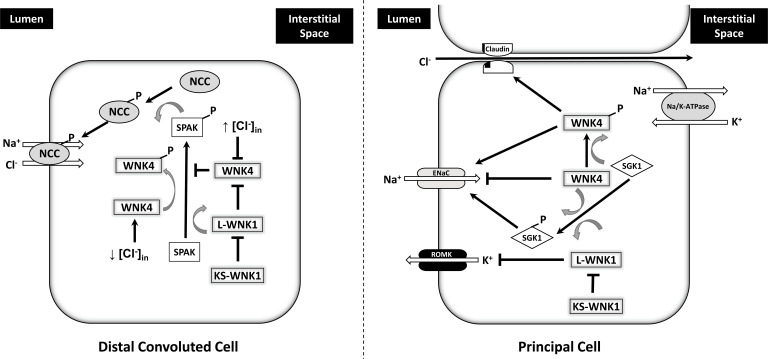
Direct mutations in the WNK1 and WNK4 genes are associated with PHA-II, but account for only a small number of reported cases (Fig. 4). Mutant WNK1 genes lead to overexpression of L-WNK1. The resultant activation of SPAK leads to enhanced phosphorylation of NCC, increased NaCl reabsorption and hypertension. Overexpression of WNK1 can inhibit WNK4 activity, further promoting additional NCC phosphorylation and NaCl reabsorption. Concomitant inhibition of ROMK results in decreased K+ secretion and hyperkalemia.
Fig. (4).
Simplified scheme of WNK pathways in the cell of the distal convoluted tubule and principal cell of the collecting tubules and ducts. In the distal tubule, when normal or high intracellular Cl- concentrations exist, L-WNK1 inhibits WNK4. Under conditions of low intracellular Cl- concentration (usually associated with low extracellular K+), WNK4 and LWNK1 are disinhibited and can phosphorylate and activate SPAK which, in turn, phosphorylates NCC. This leads to increase NCC trafficking to the apical membrane and enhanced Na+ and Cl- reabsorption. KS-WNK1 also inhibits L-WNK1. In the principal cells, WNK pathways have been primarily studied in vitro. WNK4 can inhibit ENaC independent of its kinase activity; however, WNK4 and L-WNK1 have been shown to phosphorylate serum glucocorticoid kinase 1 (SGK1) thereby regulating in increased ENaC expression and Na+ reabsorption. WNK4 has also been shown to enhance paracellular Cl- reabsorption via activation of phosphorylation of claudins. L-WNK1 inhibits ROMK and KS-WNK1 can block this function of L-WNK1. In vitro studies have shown conflicting results and definitive in vivo confirmation is required. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Mutations in WNK4 genes tend to cluster within a non-catalytic domain that is otherwise highly conserved and is critical for binding to KLHL3. Disruption of the binding to KLHL3, ubiquitination and subsequent proteolysis are impaired leading to increased levels of WNK4 and downstream phosphorylation of NCC via SPAK. Together with simultaneous inhibition of ROMK, these mutations in WNK4 also lead to the PHA-II phenotype [99-101].
Mutations in CUL3 or KHLH3 also result in PHA-II. The pathologic changes in the CUL3 gene to date have resulted in variants that impair splicing of exon 9. There are 16 currently known mutations to CUL3 which ultimately result in the aberrant splicing of exon 9 and the subsequent in-frame fusion of exons 8 and 10. The product is a protein lacking 57 amino acids that is unable to develop ubiquitin-ligase complexes or bind substrates. The resultant in-frame deletion of this motif in CUL3 disrupts proteolytic degradation of the WNKs. Likewise, pathologic dominantly inherited changes in the KLHL3 gene tend to be missense mutations that produce an abnormal C-terminal Kelch propeller domain that alters substrate binding. In contrast, recessively inherited KLHL3 mutations are more widely distributed along the gene and include frameshift or truncation of the protein. All in all, these variants disrupt binding to CUL3, WNK4 or WNK1 such that WNK4 levels are increased due to attenuated proteolysis. The mechanisms have been discussed in greater detail in the recent review by Murthy et al. [98].
6.4. Clinical Findings, Diagnosis and Treatment
Importantly, the clinical phenotype of patients with PHA-II, or Gordon’s syndrome, is the same for mutations in any of the four proteins: WNK1, WNK4, CUL3 or KLHL3. Mutations in CUL3, however, are associated with a more severe presentation with higher blood pressures, more profound hyperkalemia and acidosis and an earlier onset [66]. Dramatic improvement in PHA-II is observed with inhibition of NCC by thiazide diuretics. A Na+ and K+ restricted diet is also advisable [75, 76, 98].
7. AUTOSOMAL DOMINANT HYPERTENSION WITH BRACHYDACTYLY
Autosomal dominant hypertension with brachydactyly (ADHB) is a rare autosomal dominant disorder characterized by brachydactyly type E, short stature and severe age-dependent hypertension. When left untreated, death due to stroke often occurs before the age of 50.
7.1. Background
Bilginturan et al. (1973) first described a Turkish family with brachydactyly involving the metacarpal and phalangeal bones that co-segregated 100% with hypertension. An extensive pedigree of the family revealed an autosomal dominant inheritance pattern [102]. More than twenty years later, investigation of six members from the same family, five affected and one unaffected individuals, revealed that the affected patients were not salt sensitive and had normal renin, aldosterone and catecholaminergic responses to volume depletion and volume expansion. Investigators were able to pinpoint the disorder to chromosome 12p12.2-p11.2 by linkage analysis. Further studies of the original pedigree as well as several other reports from families unrelated to the original pedigree showed mutations in the same gene [102-104]. Toka et al. (1998) also reported that the brachydactyly was type E; due to the association with short stature and severe hypertension [105].
Magnetic resonance imaging (MRI) with angiography identified unilateral or bilateral posterior inferior cerebellar artery or vertebral artery loops contacting the ventrolateral medulla in the posterior fossa in all affected patients. Unaffected individuals did not display these loops. They proposed that the loops could be responsible for hypertension [106-108].
Linkage analysis of the hypertension with brachydactyly and the neurovascular anomaly with chromosome 12 markers revealed a LOD score of 9.2; meaning the odds that the two traits being linked are better than 1,000,000,000:1. Gong et al. (2003) performed a genome-wide parametric linkage analysis and discovered a locus for essential hypertension on chromosome 12 (HYT4), that overlaps with the locus associated with ADHB. They proposed two candidate genes as the underlying cause of the hypertension: phosphodiesterase 3A (PDE3A) and the sulfonylurea receptor 2 (SUR2) which is a subunit of an ATP-sensitive K+ channel [109].
7.2. Physiology
When the PDE3A gene is normally expressed, it encodes phosphodiesterase 3A, a member of the cGMP-inhibited cyclic nucleotide phosphodiesterase (cGI-PDE) family. The cGI-PDEs are capable of hydrolyzing both cGMP and cAMP and are expressed prominently in platelets, oocytes, heart and vascular smooth muscle cells (VSMC). These enzymes play a vital role in many cellular processes by regulating the duration and amplitude of intracellular cyclic nucleotide signals [110]. Low levels of cAMP can promote cell proliferation. PDEs also can mediate platelet aggregation and regulate vascular smooth muscle contraction, arteriogenesis and arterial remodeling [111].
PDEs are also involved during early stages of osteogenesis [112]. Studies show PDE3A expression in developing limbs and chondrogenesis in mice [113]. Parathyroid related protein (PTHrP) is a member of the parathyroid hormone family that acts as an autocrine, endocrine, intracrine and paracrine hormone. PTHrP can inhibit VSMC proliferation and regulate endochondral bone development and growth by maintaining the endochondral growth plate at a constant width. Endochondral ossification is one of the two essential processes in fetal development of the mammalian skeletal system, vital in the rudimentary formation, growth and healing of long bones. PDEs share an identical N-terminus as PTH and can stimulate most of the effects of PTH, including enhanced bone resorption and distal tubule Ca2+ reabsorption, while inhibiting proximal tubular phosphate transport [114, 115].
7.3. Pathophysiology
Bahring et al. (2008) studied all affected members of the four Turkish, Canadian and American families and showed that all had mutations on chromosome 12. They predicted the single exon is a stem-loop structure essential in noncoding RNA processing [116].
Maass et al. (2015) performed whole-exome sequencing on the Turkish family and identified a heterozygous missense mutation in the PDE3A gene. Studies on six different families revealed six independently clustered heterozygous missense mutations in exon 4. The altered amino acids at residues: 445, 447 and 449, belong to a highly conserved domain. Adjacent to the mutations are two residues, Ser428 and Ser438, which can be activated by either protein kinase A or protein kinase C. This gain of function results in increased cAMP hydrolysis and lowered cAMP levels. Functional analysis revealed the mutations increase protein kinase A-mediated PDE3A phosphorylation. This results in a gain of function in PDE3A activity. The increase in cAMP hydrolysis causes reduced levels of phosphorylated vasodilator-stimulated phosphoprotein, [110, 117, 118]. In vitro studies show an increased expression of smooth muscle actin alpha, calponin and transgelin in mesenchymal stem-cell-derived VSMCs from affected individuals. Thus, enhanced activity of PDE3A promotes lower cAMP levels in VSMC which, in turn, increases neointimal proliferation and remodeling of the arteries and neurovascular structures. Hypertension ensues from the attendant increases in vasoconstriction and peripheral vascular resistance.
Although platelet function was not altered, dysregulation of parathyroid-related protein (PTHrP) which regulates epithelial-mesenchymal interactions and endochondral bone development was observed. They hypothesized that these mutations cause the hypertension by contributing to an increase in peripheral vascular resistance along with the characteristic skeletal changes [112, 119].
7.4. Clinical Signs, Diagnosis and Treatment
Patient with ADHB typically present with short stature, severe salt-independent hypertension and altered baroreflex regulation. They will also suffer from type E brachydactyly, which is characterized by thickening and shortening of metacarpals and phalanges. The disease can be hard to diagnose in prepubescent children. Neurovascular contact at the rostroventrolateral medulla is also associated with ADHB and likely contributes to the barodysregulation [108, 120]. Hypertension occurs in childhood and progresses in a time-dependent manner. Stroke is typically associated with untreated ADHB, with early mortality. Diagnosis is typically made during childhood and early treatment can reduce the chances of stroke [121]. Studies have shown significant reduction of hypertension in combination or single drug therapy with beta-blockers, alpha-blockers, Ca2+ channel blockers, or an ACE inhibitor. Measures to increase cyclic GMP to offset the cAMP deficiency are being studied but not yet approved [122].
CONCLUSION
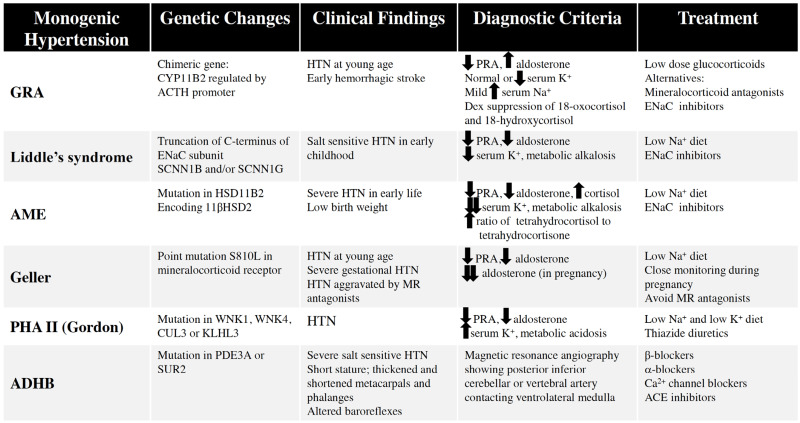
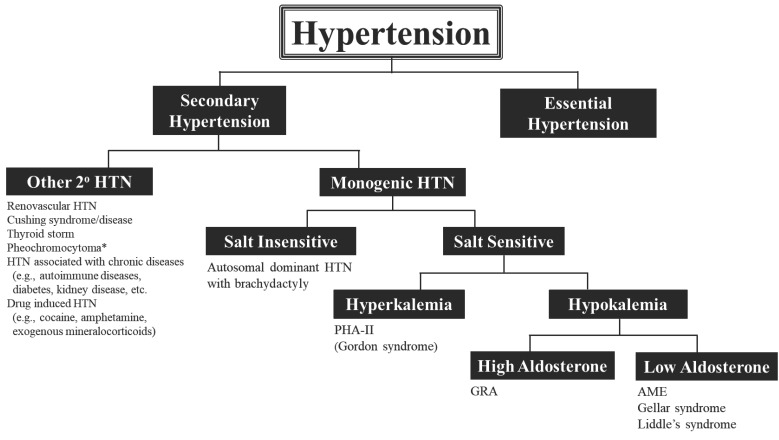
Hypertension remains a major health issue as the leading cause of cardiovascular morbidity and mortality in the U.S. and the world. In the overwhelming number of cases, the mechanism(s) underlying pathological sustained elevations in arterial pressure remain unknown. Table 1 summarizes the six categories of monogenic hypertension discussed in this review and their clinical and diagnostic parameters. In addition, Fig. 5 shows a simplified diagnostic approach for identifying individuals who may potentially possess one of these genetic forms of hypertension. Ultimately, genetic confrimation is the only definitive test.
Table 1.
Summary of key aspects of monogenic hypertension types.
Fig. (5).
Diagnostic approach to secondary hypertension with emphasis on monogenic hypertensive disorders. Diagnostic tests are summarized in Table 1. *Note that familial forms of pheochromocytoma have also been associated with genetic changes. All are autosomal dominant and are listed here with their respective genes: Von-Hippel-Lindau (VHL), multiple endocrine neoplasia type 2 (RET-proto-oncogene), neurofibromatosis type 1 (NF1) and familial paraganglioma (SDH and its subunit genes). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1. Summary of key aspects of monogenic hypertension types.
Although these disorders may encompass only a small proportion of individuals suffering from hypertension, they provide invaluable insights into the genetics and complex regulatory systems involved in blood pressure regulation. They have also shed light on some of the cellular functions that underscore hypertension that may be multifactorial. These unique pathologic states have provided a framework for the discovery of additional mechanisms of hypertension and innovative targets for therapeutic development.
ACKNOWLEDGEMENTS
Alexander Diaczok and Peter Levanovich contributed equally to the review of the literature, analysis of existing reports and initial drafts of the review article. Noreen Rossi oversaw the literature review and analysis, composed the final draft of the manuscript and illustrated the figures. The authors appreciate the contribution of Dr. James Sondheimer who read and critiqued the manuscript prior to submission.
LIST OF ABBREVIATIONS
- ACE
Angiotensin Converting Enzyme
- ACTH
Adrenocorticotropic Hormone
- ADHB
Autosomal Dominant Hypertension with Brachydactyly
- AME
Apparent Mineralocorticoid Excess
- ANG II
Angiotensin II
- CUL3
Cullin 3
- cGI-PDE
cGMP-Inhibited Cyclic Nucleotide Phosphodiesterase
- ENaC
Epithelial Sodium Channel
- GRA
Glucocorticoid Remediable Aldosteronism
- GS
Gordon Syndrome
- HRE
Hormone Response Element
- KLHL3
Kelch-Like Protein 3
- LS
Liddle’s Syndrome
- MR
Mineralocorticoid Receptor
- NCC
Thiazide-Sensitive Sodium Chloride Cotransporter
- PHA II
Pseudohypoaldosteronism Type II
- PRA
Plasma Renin Activity
- RET
Rearranged During Transfection
- ROMK
Renal Outer Medullary Potassium Channel
- SDH
Succinyl Dehydrogenase
- SPAK
SPS-1-Related Proline Alanine Rich Kinase
- VHS
Von Hippel Lindau
- VSMC
Vascular Smooth Muscle Cell
- WNK
With-No-Lysine Kinase
Consent for Publication
Not applicable.
FUNDING
This work is funded by a grant from the Department of Veterans Affairs to NFR.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R., Prospective Studies C., Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Carretero O.A., Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101(3):329–335. doi: 10.1161/01.CIR.101.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Nwankwo T., Yoon S.S., Burt V., Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;133(133):1–8. [PubMed] [Google Scholar]
- 4.Mozaffarian D., Benjamin E.J., Go A.S., et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland D.J., Ruse J.L., Laidlaw J.C. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can. Med. Assoc. J. 1966;95(22):1109–1119. [PMC free article] [PubMed] [Google Scholar]
- 6.Salti I.S., Stiefel M., Ruse J.L., Laidlaw J.C. Non-tumorous “primary” aldosteronism. I. Type relieved by glucocorticoid (glucocorticoid-remediable aldosteronism). Can. Med. Assoc. J. 1969;101(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguly A., Grim C.E., Weinberger M.H. Anomalous postural aldosterone response in glucocorticoid-suppressible hyperaldosteronism. N. Engl. J. Med. 1981;305(17):991–993. doi: 10.1056/NEJM198110223051706. [DOI] [PubMed] [Google Scholar]
- 8.Gordon R.D. Heterogeneous hypertension. Nat. Genet. 1995;11(1):6–9. doi: 10.1038/ng0995-6. [DOI] [PubMed] [Google Scholar]
- 9.Gates L.J., MacConnachie A.A., Lifton R.P., Haites N.E., Benjamin N. Variation of phenotype in patients with glucocorticoid remediable aldosteronism. J. Med. Genet. 1996;33(1):25–28. doi: 10.1136/jmg.33.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowasser M., Huggard P.R., Rossetti T.R., Bachmann A.W., Gordon R.D. Biochemical evidence of aldosterone overproduction and abnormal regulation in normotensive individuals with familial hyperaldosteronism type I. J. Clin. Endocrinol. Metab. 1999;84(11):4031–4036. doi: 10.1210/jcem.84.11.6159. [DOI] [PubMed] [Google Scholar]
- 11.Stowasser M., Bachmann A.W., Huggard P.R., Rossetti T.R., Gordon R.D. Severity of hypertension in familial hyperaldosteronism type I: Relationship to gender and degree of biochemical disturbance. J. Clin. Endocrinol. Metab. 2000;85(6):2160–2166. doi: 10.1210/jc.85.6.2160. [DOI] [PubMed] [Google Scholar]
- 12.Mulatero P., di Cella S.M., Williams T.A., et al. Glucocorticoid remediable aldosteronism: Low morbidity and mortality in a four-generation italian pedigree. J. Clin. Endocrinol. Metab. 2002;87(7):3187–3191. doi: 10.1210/jcem.87.7.8647. [DOI] [PubMed] [Google Scholar]
- 13.Nussey S, Whitehead S. 2001. [PubMed]
- 14.Curnow K.M., Tusie-Luna M.T., Pascoe L., et al. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol. Endocrinol. 1991;5(10):1513–1522. doi: 10.1210/mend-5-10-1513. [DOI] [PubMed] [Google Scholar]
- 15.Bassett M.H., White P.C., Rainey W.E. The regulation of aldosterone synthase expression. Mol. Cell. Endocrinol. 2004;217(1-2):67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Williams G.H., Dluhy R.G. Aldosterone biosynthesis. Interrelationship of regulatory factors. Am. J. Med. 1972;53(5):595–605. doi: 10.1016/0002-9343(72)90156-8. [DOI] [PubMed] [Google Scholar]
- 17.Brown R.D., Strott C.A., Liddle G.W. Site of stimulation of aldosterone biosynthesis by angiotensin and potassium. J. Clin. Invest. 1972;51(6):1413–1418. doi: 10.1172/JCI106937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanukoglu I., Feuchtwanger R., Hanukoglu A. Mechanism of corticotropin and cAMP induction of mitochondrial cytochrome P450 system enzymes in adrenal cortex cells. J. Biol. Chem. 1990;265(33):20602–20608. doi: 10.1016/S0021-9258(17)30545-8. [DOI] [PubMed] [Google Scholar]
- 19.Chua S.C., Szabo P., Vitek A., Grzeschik K.H., John M., White P.C. Cloning of cDNA encoding steroid 11 beta-hydroxylase (P450c11). Proc. Natl. Acad. Sci. USA. 1987;84(20):7193–7197. doi: 10.1073/pnas.84.20.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White P.C. Genetics of steroid 21-hydroxylase deficiency. Recent Prog. Horm. Res. 1987;43:305–336. doi: 10.1016/b978-0-12-571143-2.50014-9. [DOI] [PubMed] [Google Scholar]
- 21.Lifton R.P., Dluhy R.G. The molecular basis of a hereditary form of hypertension, glucocorticoid-remediable aldosteronism. Trends Endocrinol. Metab. 1993;4(2):57–61. doi: 10.1016/S1043-2760(05)80016-5. [DOI] [PubMed] [Google Scholar]
- 22.Lifton R.P., Dluhy R.G., Powers M., et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 23.Fisher A., Friel E.C., Bernhardt R., et al. Effects of 18-hydroxylated steroids on corticosteroid production by human aldosterone synthase and 11beta-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86(9):4326–4329. doi: 10.1210/jcem.86.9.7797. [DOI] [PubMed] [Google Scholar]
- 24.Montori VM, Young WF., Jr 2002.
- 25.Liddle G.W., Island D.P., Ney R.L., Nicholson W.E., Shimizu N. Nonpituitary neoplasms and Cushing’s syndrome. Ectopic “adrenocorticotropin” produced by nonpituitary neoplasms as a cause of Cushing’s syndrome. Arch. Intern. Med. 1963;111:471–475. doi: 10.1001/archinte.1963.03620280071011. [DOI] [PubMed] [Google Scholar]
- 26.Wang C., Chan T.K., Yeung R.T., Coghlan J.P., Scoggins B.A., Stockigt J.R. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J. Clin. Endocrinol. Metab. 1981;52(5):1027–1032. doi: 10.1210/jcem-52-5-1027. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J.A., Biglieri E.G., Schambelan M. Pseudohyperaldosteronism with renal tubular resistance to mineralocorticoid hormones. Trans. Assoc. Am. Physicians. 1981;94:172–182. [PubMed] [Google Scholar]
- 28.Nakada T., Koike H., Akiya T., et al. Liddle’s syndrome, an uncommon form of hyporeninemic hypoaldosteronism: Functional and histopathological studies. J. Urol. 1987;137(4):636–640. doi: 10.1016/S0022-5347(17)44161-9. [DOI] [PubMed] [Google Scholar]
- 29.Botero-Velez M., Curtis J.J., Warnock D.G. Brief report: Liddle’s syndrome revisited--a disorder of sodium reabsorption in the distal tubule. N. Engl. J. Med. 1994;330(3):178–181. doi: 10.1056/NEJM199401203300305. [DOI] [PubMed] [Google Scholar]
- 30.Baker E., Jeunemaitre X., Portal A.J., et al. Abnormalities of nasal potential difference measurement in Liddle’s syndrome. J. Clin. Invest. 1998;102(1):10–14. doi: 10.1172/JCI1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimkets R.A., Warnock D.G., Bositis C.M., et al. Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79(3):407–414. doi: 10.1016/0092-8674(94)90250-X. [DOI] [PubMed] [Google Scholar]
- 32.Hansson J.H., Nelson-Williams C., Suzuki H., et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: Genetic heterogeneity of Liddle syndrome. Nat. Genet. 1995;11(1):76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- 33.Snyder P.M., Price M.P., McDonald F.J., et al. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995;83(6):969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 34.Kellenberger S., Schild L. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 2002;82(3):735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 35.Carattino M.D. Structural mechanisms underlying the function of epithelial sodium channel/acid-sensing ion channel. Curr. Opin. Nephrol. Hypertens. 2011;20(5):555–560. doi: 10.1097/MNH.0b013e328348bcac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frindt G., Palmer L.G. Regulation of epithelial Na+ channels by adrenal steroids: Mineralocorticoid and glucocorticoid effects. Am. J. Physiol. Renal Physiol. 2012;302(1):F20–F26. doi: 10.1152/ajprenal.00480.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anan T., Nagata Y., Koga H., et al. Human ubiquitin-protein ligase Nedd4: expression, subcellular localization and selective interaction with ubiquitin-conjugating enzymes. Genes Cells. 1998;3(11):751–763. doi: 10.1046/j.1365-2443.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- 38.Bhalla V., Soundararajan R., Pao A.C., Li H., Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am. J. Physiol. Renal Physiol. 2006;291(4):F714–F721. doi: 10.1152/ajprenal.00061.2006. [DOI] [PubMed] [Google Scholar]
- 39.Wiemuth D., Ke Y., Rohlfs M., McDonald F.J. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem. J. 2007;405(1):147–155. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.New M.I., Levine L.S., Biglieri E.G., Pareira J., Ulick S. Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J. Clin. Endocrinol. Metab. 1977;44(5):924–933. doi: 10.1210/jcem-44-5-924. [DOI] [PubMed] [Google Scholar]
- 41.Ulick S., Levine L.S., Gunczler P., et al. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J. Clin. Endocrinol. Metab. 1979;49(5):757–764. doi: 10.1210/jcem-49-5-757. [DOI] [PubMed] [Google Scholar]
- 42.Shackleton C.H., Honour J.W., Dillon M.J., Chantler C., Jones R.W. Hypertension in a four-year-old child: Gas chromatographic and mass spectrometric evidence for deficient hepatic metabolism of steroids. J. Clin. Endocrinol. Metab. 1980;50(4):786–02. doi: 10.1210/jcem-50-4-786. [DOI] [PubMed] [Google Scholar]
- 43.Oberfield S.E., Levine L.S., Carey R.M., Greig F., Ulick S., New M.I. Metabolic and blood pressure responses to hydrocortisone in the syndrome of apparent mineralocorticoid excess. J. Clin. Endocrinol. Metab. 1983;56(2):332–339. doi: 10.1210/jcem-56-2-332. [DOI] [PubMed] [Google Scholar]
- 44.Krozowski Z.S., Funder J.W. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc. Natl. Acad. Sci. USA. 1983;80(19):6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arriza J.L., Weinberger C., Cerelli G., et al. Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 46.Atanasov A.G., Ignatova I.D., Nashev L.G., et al. Impaired protein stability of 11beta-hydroxysteroid dehydrogenase type 2: A novel mechanism of apparent mineralocorticoid excess. J. Am. Soc. Nephrol. 2007;18(4):1262–1270. doi: 10.1681/ASN.2006111235. [DOI] [PubMed] [Google Scholar]
- 47.Nunez B.S., Rogerson F.M., Mune T., et al. Mutants of 11beta-hydroxysteroid dehydrogenase (11-HSD2) with partial activity: Improved correlations between genotype and biochemical phenotype in apparent mineralocorticoid excess. Hypertension. 1999;34(4 Pt 1):638–642. doi: 10.1161/01.HYP.34.4.638. [DOI] [PubMed] [Google Scholar]
- 48.Mune T., Rogerson F.M., Nikkilä H., Agarwal A.K., White P.C. Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. Nat. Genet. 1995;10(4):394–399. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- 49.Seckl J.R., Walker B.R. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 50.Raisz L.G., McNeely W.F., Saxon L., Rosenbaum J.D. The effects of cortisone and hydrocortisone on water diuresis and renal function in man. J. Clin. Invest. 1957;36(6 Part 1):767–779. doi: 10.1172/JCI103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anagnostis P., Athyros V.G., Tziomalos K., Karagiannis A., Mikhailidis D.P. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 52.Bailey M.A., Unwin R.J., Shirley D.G. In vivo inhibition of renal 11beta-hydroxysteroid dehydrogenase in the rat stimulates collecting duct sodium reabsorption. Clin. Sci. (Lond.) 2001;101(2):195–198. doi: 10.1042/cs1010195. [DOI] [PubMed] [Google Scholar]
- 53.Gaeggeler H.P., Gonzalez-Rodriguez E., Jaeger N.F., et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J. Am. Soc. Nephrol. 2005;16(4):878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- 54.Choi K.B. Hypertensive hypokalemic disorders. Electrolyte Blood Press. 2007;5(1):34–41. doi: 10.5049/EBP.2007.5.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farese R.V., Jr, Biglieri E.G., Shackleton C.H., Irony I., Gomez-Fontes R. Licorice-induced hypermineralocorticoidism. N. Engl. J. Med. 1991;325(17):1223–1227. doi: 10.1056/NEJM199110243251706. [DOI] [PubMed] [Google Scholar]
- 56.Palermo M., Quinkler M., Stewart P.M. Apparent mineralocorticoid excess syndrome: An overview. Arq. Bras. Endocrinol. Metabol. 2004;48(5):687–696. doi: 10.1590/S0004-27302004000500015. [DOI] [PubMed] [Google Scholar]
- 57.WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia 2011. [PubMed]
- 58.Baker M.E. Adrenal and sex steroid receptor evolution: environmental implications. J. Mol. Endocrinol. 2001;26(2):119–125. doi: 10.1677/jme.0.0260119. [DOI] [PubMed] [Google Scholar]
- 59.Rogerson F.M., Yao Y.Z., Elsass R.E., Dimopoulos N., Smith B.J., Fuller P.J. A critical region in the mineralocorticoid receptor for aldosterone binding and activation by cortisol: Evidence for a common mechanism governing ligand binding specificity in steroid hormone receptors. Mol. Endocrinol. 2007;21(4):817–828. doi: 10.1210/me.2006-0246. [DOI] [PubMed] [Google Scholar]
- 60.Moras D., Gronemeyer H. The nuclear receptor ligand-binding domain: Structure and function. Curr. Opin. Cell Biol. 1998;10(3):384–391. doi: 10.1016/S0955-0674(98)80015-X. [DOI] [PubMed] [Google Scholar]
- 61.Arriza J.L., Simerly R.B., Swanson L.W., Evans R.M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 62.Hellal-Levy C., Couette B., Fagart J., Souque A., Gomez-Sanchez C., Rafestin-Oblin M. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 1999;464(1-2):9–13. doi: 10.1016/S0014-5793(99)01667-1. [DOI] [PubMed] [Google Scholar]
- 63.Rafestin-Oblin M.E., Souque A., Bocchi B., Pinon G., Fagart J., Vandewalle A. The severe form of hypertension caused by the activating S810L mutation in the mineralocorticoid receptor is cortisone related. Endocrinology. 2003;144(2):528–533. doi: 10.1210/en.2002-220708. [DOI] [PubMed] [Google Scholar]
- 64.Disse-Nicodème S., Achard J.M., Desitter I., et al. A new locus on chromosome 12p13.3 for pseudohypoaldosteronism type II, an autosomal dominant form of hypertension. Am. J. Hum. Genet. 2000;67(2):302–310. doi: 10.1086/303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansfield T.A., Simon D.B., Farfel Z., et al. Multilocus linkage of familial hyperkalaemia and hypertension, pseudohypoaldosteronism type II, to chromosomes 1q31-42 and 17p11-q21. Nat. Genet. 1997;16(2):202–205. doi: 10.1038/ng0697-202. [DOI] [PubMed] [Google Scholar]
- 66.Boyden L.M., Choi M., Choate K.A., et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482(7383):98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis-Dit-Picard H., Barc J., Trujillano D., et al. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat. Genet. 2012;44(4):456–460. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 68.Hanukoglu I., Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016;579(2):95–132. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zennaro M.C., Fernandes-Rosa F. 30 Years of the mineralocorticoid receptor: Mineralocorticoid receptor mutations. J. Endocrinol. 2017;234(1):T93–T106. doi: 10.1530/JOE-17-0089. [DOI] [PubMed] [Google Scholar]
- 70.Gordon R.D., Geddes R.A., Pawsey C.G., O’Halloran M.W. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas. Ann. Med. 1970;19(4):287–294. doi: 10.1111/imj.1970.19.4.287. [DOI] [PubMed] [Google Scholar]
- 71.Brautbar N., Levi J., Rosler A., et al. Familial hyperkalemia, hypertension, and hyporeninemia with normal aldosterone levels. A tubular defect in potassium handling. Arch. Intern. Med. 1978;138(4):607–610. doi: 10.1001/archinte.1978.03630280069022. [DOI] [PubMed] [Google Scholar]
- 72.Roy C. Familial pseudohypoaldosteronism (apropos of 5 cases). Arch. Fr. Pediatr. 1977;34(1):37–54. [PubMed] [Google Scholar]
- 73.Limal JM, Rappaport R, Dechaux M, Morin C. Familial dominant pseudohypoaldosteronism. 1978. [DOI] [PubMed]
- 74.Lee M.R., Morgan D.B. Familial hyperkalaemia responsive to benzothiadiazine diuretic. Lancet. 1980;1(8173):879. doi: 10.1016/S0140-6736(80)91378-1. [DOI] [PubMed] [Google Scholar]
- 75.Licht J.H., Amundson D., Hsueh W.A., Lombardo J.V. Familiar hyperkalaemic acidosis. Q. J. Med. 1985;54(214):161–176. [PubMed] [Google Scholar]
- 76.Schambelan M., Sebastian A., Rector F.C., Jr Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 1981;19(5):716–727. doi: 10.1038/ki.1981.72. [DOI] [PubMed] [Google Scholar]
- 77.Pasman J.W., Gabreëls F.J., Semmekrot B., Renier W.O., Monnens L.A. Hyperkalemic periodic paralysis in Gordon’s syndrome: A possible defect in atrial natriuretic peptide function. Ann. Neurol. 1989;26(3):392–395. doi: 10.1002/ana.410260314. [DOI] [PubMed] [Google Scholar]
- 78.Take C., Ikeda K., Kurasawa T., Kurokawa K. Increased chloride reabsorption as an inherited renal tubular defect in familial type II pseudohypoaldosteronism. N. Engl. J. Med. 1991;324(7):472–476. doi: 10.1056/NEJM199102143240707. [DOI] [PubMed] [Google Scholar]
- 79.Xu B., English J.M., Wilsbacher J.L., Stippec S., Goldsmith E.J., Cobb M.H. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275(22):16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z., Yang C.L., Ellison D.H. Comparison of WNK4 and WNK1 kinase and inhibiting activities. Biochem. Biophys. Res. Commun. 2004;317(3):939–944. doi: 10.1016/j.bbrc.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 81.Faure S., Delaloy C., Leprivey V., et al. WNK kinases, distal tubular ion handling and hypertension. Nephrol. Dial. Transplant. 2003;18(12):2463–2467. doi: 10.1093/ndt/gfg426. [DOI] [PubMed] [Google Scholar]
- 82.Hebert S.C., Gamba G. Molecular cloning and characterization of the renal diuretic-sensitive electroneutral sodium-(potassium)-chloride cotransporters. Clin. Investig. 1994;72(9):692–694. doi: 10.1007/BF00212991. [DOI] [PubMed] [Google Scholar]
- 83.Moriguchi T., Urushiyama S., Hisamoto N., et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 2005;280(52):42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 84.Yang C.L., Zhu X., Wang Z., Subramanya A.R., Ellison D.H. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J. Clin. Invest. 2005;115(5):1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazrak A., Liu Z., Huang C.L. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc. Natl. Acad. Sci. USA. 2006;103(5):1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadchouel J., Ellison D.H., Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu. Rev. Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 87.Segref A., Hoppe T. Think locally: control of ubiquitin-dependent protein degradation in neurons. EMBO Rep. 2009;10(1):44–50. doi: 10.1038/embor.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahle K.T., Wilson F.H., Lalioti M., Toka H., Qin H., Lifton R.P. WNK kinases: Molecular regulators of integrated epithelial ion transport. Curr. Opin. Nephrol. Hypertens. 2004;13(5):557–562. doi: 10.1097/00041552-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Tatum R., Zhang Y., Lu Q., Kim K., Jeansonne B.G., Chen Y.H. WNK4 phosphorylates ser(206) of claudin-7 and promotes paracellular Cl(-) permeability. FEBS Lett. 2007;581(20):3887–3891. doi: 10.1016/j.febslet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Yu A.S. Claudins and the kidney. J. Am. Soc. Nephrol. 2015;26(1):11–19. doi: 10.1681/ASN.2014030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamauchi K., Rai T., Kobayashi K., et al. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc. Natl. Acad. Sci. USA. 2004;101(13):4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takahashi D., Mori T., Nomura N., et al. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci. Rep. 2014;34(3):e00107. doi: 10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terker A.S., Zhang C., Erspamer K.J., Gamba G., Yang C.L., Ellison D.H. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89(1):127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furukawa M., He Y.J., Borchers C., Xiong Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 2003;5(11):1001–1007. doi: 10.1038/ncb1056. [DOI] [PubMed] [Google Scholar]
- 95.Geyer R., Wee S., Anderson S., Yates J., Wolf D.A. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell. 2003;12(3):783–790. doi: 10.1016/S1097-2765(03)00341-1. [DOI] [PubMed] [Google Scholar]
- 96.Xu L., Wei Y., Reboul J., et al. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425(6955):316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- 97.Zimmerman E.S., Schulman B.A., Zheng N. Structural assembly of cullin-RING ubiquitin ligase complexes. Curr. Opin. Struct. Biol. 2010;20(6):714–721. doi: 10.1016/j.sbi.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murthy M., Kurz T., O’Shaughnessy K.M. WNK signalling pathways in blood pressure regulation. Cell. Mol. Life Sci. 2017;74(7):1261–1280. doi: 10.1007/s00018-016-2402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson F.H., Kahle K.T., Sabath E., et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl. Acad. Sci. USA. 2003;100(2):680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Golbang A.P., Murthy M., Hamad A., et al. A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension. 2005;46(2):295–300. doi: 10.1161/01.HYP.0000174326.96918.d6. [DOI] [PubMed] [Google Scholar]
- 101.Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am. J. Physiol. Renal Physiol. 2005;288(2):F245–F252. doi: 10.1152/ajprenal.00311.2004. [DOI] [PubMed] [Google Scholar]
- 102.Bilginturan N., Zileli S., Karacadag S., Pirnar T. Hereditary brachydactyly associated with hypertension. J. Med. Genet. 1973;10(3):253–259. doi: 10.1136/jmg.10.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hattenbach L.O., Toka H.R., Toka O., Schuster H., Luft F.C. Absence of hypertensive retinopathy in a Turkish kindred with autosomal dominant hypertension and brachydactyly. Br. J. Ophthalmol. 1998;82(12):1363–1365. doi: 10.1136/bjo.82.12.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chitayat D, Grix A, Balfe JW, et al. 1997.
- 105.Toka H.R., Bähring S., Chitayat D., et al. Families with autosomal dominant brachydactyly type E, short stature, and severe hypertension. Ann. Intern. Med. 1998;129(3):204–208. doi: 10.7326/0003-4819-129-3-199808010-00008. [DOI] [PubMed] [Google Scholar]
- 106.Schuster H., Wienker T.E., Bähring S., et al. Severe autosomal dominant hypertension and brachydactyly in a unique Turkish kindred maps to human chromosome 12. Nat. Genet. 1996;13(1):98–100. doi: 10.1038/ng0596-98. [DOI] [PubMed] [Google Scholar]
- 107.Luft F.C., Toka O., Toka H.R., Jordan J., Bahring S. Mendelian hypertension with brachydactyly as a molecular genetic lesson in regulatory physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285(4):R709–R714. doi: 10.1152/ajpregu.00174.2003. [DOI] [PubMed] [Google Scholar]
- 108.Naraghi R., Schuster H., Toka H.R., et al. Neurovascular compression at the ventrolateral medulla in autosomal dominant hypertension and brachydactyly. Stroke. 1997;28(9):1749–1754. doi: 10.1161/01.STR.28.9.1749. [DOI] [PubMed] [Google Scholar]
- 109.Gong M., Zhang H., Schulz H., et al. Genome-wide linkage reveals a locus for human essential (primary) hypertension on chromosome 12p. Hum. Mol. Genet. 2003;12(11):1273–1277. doi: 10.1093/hmg/ddg135. [DOI] [PubMed] [Google Scholar]
- 110.Maurice D.H., Palmer D., Tilley D.G., et al. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol. Pharmacol. 2003;64(3):533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 111.Begum N., Hockman S., Manganiello V.C. Phosphodiesterase 3A (PDE3A) deletion suppresses proliferation of cultured murine vascular smooth muscle cells (VSMCs) via inhibition of mitogen-activated protein kinase (MAPK) signaling and alterations in critical cell cycle regulatory proteins. J. Biol. Chem. 2011;286(29):26238–26249. doi: 10.1074/jbc.M110.214155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wakabayashi S., Tsutsumimoto T., Kawasaki S., Kinoshita T., Horiuchi H., Takaoka K. Involvement of phosphodiesterase isozymes in osteoblastic differentiation. J. Bone Miner. Res. 2002;17(2):249–256. doi: 10.1359/jbmr.2002.17.2.249. [DOI] [PubMed] [Google Scholar]
- 113.Maass P.G., Rump A., Schulz H., et al. A misplaced lncRNA causes brachydactyly in humans. J. Clin. Invest. 2012;122(11):3990–4002. doi: 10.1172/JCI65508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song G.J., Fiaschi-Taesch N., Bisello A. Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol. Endocrinol. 2009;23(10):1681–1690. doi: 10.1210/me.2009-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Potts J.T., Gardella T.J. Progress, paradox, and potential: Parathyroid hormone research over five decades. Ann. N. Y. Acad. Sci. 2007;1117:196–208. doi: 10.1196/annals.1402.088. [DOI] [PubMed] [Google Scholar]
- 116.Bähring S., Kann M., Neuenfeld Y., et al. Inversion region for hypertension and brachydactyly on chromosome 12p features multiple splicing and noncoding RNA. Hypertension. 2008;51(2):426–431. doi: 10.1161/HYPERTENSIONAHA.107.101774. [DOI] [PubMed] [Google Scholar]
- 117.Wechsler J., Choi Y.H., Krall J., Ahmad F., Manganiello V.C., Movsesian M.A. Isoforms of cyclic nucleotide phosphodiesterase PDE3A in cardiac myocytes. J. Biol. Chem. 2002;277(41):38072–38078. doi: 10.1074/jbc.M203647200. [DOI] [PubMed] [Google Scholar]
- 118.Zhao H., Guan Q., Smith C.J., Quilley J. Increased phosphodiesterase 3A/4B expression after angioplasty and the effect on VASP phosphorylation. Eur. J. Pharmacol. 2008;590(1-3):29–35. doi: 10.1016/j.ejphar.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 119.Maass P.G., Aydin A., Luft F.C., et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat. Genet. 2015;47(6):647–653. doi: 10.1038/ng.3302. [DOI] [PubMed] [Google Scholar]
- 120.Schuster H., Toka O., Toka H.R., et al. A cross-over medication trial for patients with autosomal-dominant hypertension with brachydactyly. Kidney Int. 1998;53(1):167–172. doi: 10.1046/j.1523-1755.1998.00732.x. [DOI] [PubMed] [Google Scholar]
- 121.Toka O., Maass P.G., Aydin A., et al. Childhood hypertension in autosomal-dominant hypertension with brachydactyly. Hypertension. 2010;56(5):988–994. doi: 10.1161/HYPERTENSIONAHA.110.156620. [DOI] [PubMed] [Google Scholar]
- 122.Toka O., Tank J., Schächterle C., et al. Clinical effects of phosphodiesterase 3A mutations in inherited hypertension with brachydactyly. Hypertension. 2015;66(4):800–808. doi: 10.1161/HYPERTENSIONAHA.115.06000. [DOI] [PubMed] [Google Scholar]