Abstract
Non-destructive methods have been widely recognized for evaluating fruit quality traits of many horticultural crops and food processing industry. Destructive (analytical) test, and non-destructive evaluation of the quality traits were investigated and compared for ‘Red Rose’ tomato (Solanum lycopersicum L.) fruit grown under protected environment. Fresh tomato fruit at five distinctive maturity stages namely; breaker (BK), turning (TG), pink (PK), light-red (LR), and red (RD) were labeled and scanned using the handheld near infra-red (NIR) enhanced spectrometer at a wavelength range of 285–1200 nm. The labeled tomato samples were then measured analytically for flesh firmness, lycopene, β-carotene, total phenolic content (TPC) and total flavonoids content (TFC). The results revealed that quality traits could be estimated using NIR spectroscopy with a relatively high coefficient of determination (R2): 0.834 for total phenolic content, 0.864 for lycopene, 0.790 for total flavonoid content, 0.708 for β-carotene; and 0.679 for flesh firmness. The accumulation of Lyco and β-Car rapidly increased in tomatoes harvested between the TG and the LR maturity stages. Harvesting tomatoes at BK maturity stage resulted in significantly higher flesh firmness than harvesting at the later maturity stages. Tomato fruits had the lowest TPC and TFC contents at the earliest maturity stage (BK), while they had intermediate TPC and TFC levels at LR and RD maturity stages. NIR spectroscopic measurements of fruit firmness and lipophilic antioxidants in tomato fruit at various maturity stages were partially in accordance with those estimated by destructive (analytical) methods. Based on these findings, we recommend using non-destructive NIR spectroscopy as an effective tool for predicting tomato fruit quality during harvest stage and postharvest processing.
Keywords: Solanum lycopersicum, NIR spectroscopy, Postharvest handling, Spectral analysis, Red Rose tomato, Quality attributes
1. Introduction
Tomato (Solanum lycopersicum L.) fruit is a rich source of health promoting antioxidants, dietary fibers, phenolic compounds, flavonoids, proteins, minerals and vitamins (Wilbur, 1992). Its consumption helps in reducing cancer cases and assists in minimizing the risks of cardiovascular and related illnesses (Cheng et al., 2017). Further of being a dominant carotene in tomatoes, lycopene acts as an intermediate of carotene biosynthesis. It is effective in the pigmentation phenomenon of both plants and algae. Among all the 600 known natural carotenes, lycopene has the highest antioxidant activity as well as the highest reactive oxygen species (ROS) quenching ability (Alda et al., 2009). Several factors influence lycopene accumulation in tomato fruit which include cultural practices, environmental conditions, genotypes and phonological stages (Serio et al., 2007). The beneficial effects of consuming tomatoes predominantly come from their dietary antioxidants (Çelik et al., 2017) which are known to counteract the adverse effects of free radical ions (FRI) and ROS.
Lipids in fresh fruits and vegetables serve number of physiological functions. Specifically, these molecules contribute to their organoleptic (aroma, flavor, color, texture) and nutritional (essential fatty acids, vitamins and metabolic energy) characteristics (Baeza-Jiménez et al., 2017). However, the auto-oxidation of lipids by FRI and ROS can adversely affect their physiological functions, which ultimately leads to deterioration of their quality, in addition to the shorter shelf-life.
During the maturity process, tomato fruit undergoes a series of nutritional, biochemical and physiological modifications which affect their functional and proximate quality characteristics (Abdullahi et al., 2016). The instrumental assessment of these changes are preferred over the sensory evaluation, to minimize the tangential variations. Additionally, the instrumental characterization of internal quality of fresh produce is more precise that may have commercial application in the processing industry (Barrett et al., 2010).
Destructive techniques of measuring quality indices in fresh produce to identify the optimum time of fruit harvesting have a variety of drawbacks that highlights the need for non-destructive and dependable tools (Pedro and Ferreira 2005). Additionally, most of their commercial applications are on-site measurements, which require portable tools.
Being accurate, cheap, fast, and user-friendly, NIR spectroscopy technique is gaining worldwide popularity in assessing the quality of fruits and vegetables especially in the food processing industry (Porep et al., 2015). NIR spectroscopy is a potential tool for non-destructive assessment of quality traits of fresh produce and agricultural products (Sirisomboon, 2018). In this respect, Walsh et al., (2020) reviewed the applications of NIR spectroscopy in the field of postharvest decision support systems. The NIR spectroscopy is being commercially used in the postharvest processing industry of some fruits and vegetables (Cattaneo and Stellari, 2019). Such non-invasive data is a powerful tool in the assessment of fruit maturation and ripening (Baranska et al., 2006). The short wave NIR (720–1100 nm) region is generally used for penetrating through biological tissues. Based on interactance spectra, Khuriyati et al. (2004) used the spectrometric technique in the development of a dry matter partial least square (PLS) model for tomatoes with a coefficient of determination (R2) of 0.88; however, the model was not tested on independent population of the fruit.
Previously, Alenazi et al. (2020) investigated the status of dietary antioxidants of tomato fruit during maturity stages. They reported that the highest contents of ascorbic acid, phenolic compounds, and flavonoids were between the (PK) and (LR) stages. In addition, the highest β-carotene and lycopene levels were found in fruits harvested at the ‘red’ stage. This study was conducted to compare NIR spectroscopic (non-destructive) evaluation of maturity-dependent dietary antioxidants accumulation and flesh firmness of ‘Red Rose’ tomato fruit grown under the protected environment, with their analytical (destructive/subjective) assessment.
2. Material and methods
2.1. Chemicals
Reference standards; gallic acid (GA), quercetin (QE), L-ascorbic acid) and Folin-Ciocalteu (FC) phenol reagent were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Other chemicals/reagents (2,4-dinitrophenylhydrazine, H2SO4, potassium acetate) and organic solvents (ethanol, acetone, hexane, acetic acid, petroleum ether) of analytical grade were locally sourced from Somatco Scientific Supplies (Riyadh, Saudi Arabia).
2.2. Study layout
Forty-day ‘Red Rose’ tomato (Reimer Seeds, Saint Leonard MD, USA) seedlings were transplanted in the greenhouse facility at King Saud University (24.7256° N, 46.6153° E), Riyadh, Saudi Arabia. The plastic pots (25-cm diameter, with growth media consisted of sand and peat in the 1:1 ratio) were arranged in completely randomized design with four replications. The pots were placed in the greenhouse at 50 cm apart with 1 m distance between the rows. All plants received similar cultural practices as recommended for greenhouse grown tomatoes (Maynard and Hochmuth, 2006).
2.3. Fruit sampling and NIR spectroscopy test
Based on visual assessment (California Tomato Commission, 2020), fifty fruits from each replication were randomly harvested at five maturity stages: breaker (BK), turning (TG), pink (PK), light-red (LR), and red (RD). Fresh tomato fruit were labeled and scanned using the handheld near infra-red enhanced spectrometer (F-750, Produce Quality Mater, Felix Instruments, Camas WA, USA), at wavelength range (285–1200 nm). The labeled tomato samples were then measured analytically for flesh firmness, lycopene (Lyco), β-carotene (β-Car), total phenolic content (TPC), and total flavonoid content (TFC).
2.4. Analytical (destructive) methods
2.4.1. Flesh firmness
The flesh firmness of tomato fruits was measured as described by Subedi and Walsh (2009) by pushing a plunger tip (6 mm) into their opposite pared surfaces along the equatorial region using a handheld penetrometer (FT 40, Wagner Instruments, Greenwich CT, USA) and the values were expressed as newton (N).
2.4.2. Dietary antioxidants
The seeds and the placental tissues of the fruit were discarded and the pericarp slices (r = 4) were divided into two groups. The levels of oxygen-free carotenoids (Lycopene and β-Car) were immediately analyzed in the first group to avoid isomerization and photo-degradation. The second group of pericarp slices was freeze-dried and stored at –20 °C until needed (maximum 3-week storage) for determination of TPC and TFC.
2.4.2.1. Oxygen-free carotenoids
The levels of oxygen-free carotenoids (Lyco, and β-Car) were estimated according to Nagata and Yamashita (1992). One gram of tomato pericarp slices was extracted in 5 mL of acetone-hexane (4:6) solution using a general laboratory homogenizer (Model: GLH 850 with 5 mm tip, Omni International, Inc. Kennesaw Georgia, USA), under the dim-light environment. Supernatant optical densities (ODs) were recorded at 453, 505, 645, and 663 nm (Ultrospec 2000 UV/VIS, Amersham Pharmacia Biotech, Little Chalfont, UK). The concentrations of β-Carotene and Lycopene (μg g−1, fm) were calculated according to Nagata and Yamashita, (1992) as follows:
| (1) |
| (2) |
2.4.2.2. Total phenolic content (TPC)
TPC of the tomato extract was estimated following the method of Surana et al. (2016), with minor modifications. One millilitre (1 mL) of the stock extract was mixed with 1 mL of Gallic Acid (GA) standard solution in a 25-mL volumetric flask. Then, 1.5 mL of FC reagent mixed in 10 mL of deionized water (DW) was added. The mixture was kept to react at 23 ± 1 °C for 10 min. The aqueous solution of sodium carbonate (4 mL) was added and the total volume of the mixture was adjusted to 25 mL with DW. After incubation at 23 ± 1 °C, the supernatant OD was recorded at 765 nm. To construct a standard curve, similar procedure was followed for GA solutions at 20, 40, 60, 80, and 100 mg L–1 concentrations. TPC of the fruit extract (μg GA equivalent g−1 fm) was calculated against the standard curve.
2.4.2.3. Total flavonoid content (TFC)
TFC in the tomato extract was estimated according to Nour et al. (2015) with minor modifications. In short, 0.5 mL of tomato extract was diluted with ethanol (1:10) in a glass test tube. Then, 0.1 mL of 10% aluminium chloride, 0.1 mL of molar of potassium acetate aqueous solution, and 4.3 mL of ethanol were added to the test tube. The mixture was kept to react at 23 ± 1 °C for 40 min. The supernatant OD was recorded at 415 nm and the (QE) was used as a standard reference to plot a standard curve against its various concentrations. The TFC of the extract (μg QE g−1 fm) was estimated by comparing their ODs against the standard curve.
2.5. Non–destructive spectral scanning
The quality indices (reference values) of tomato at different maturity stages were measured by spectral scanning. Procedure in the prediction model of tomato fruits quality was conducted by creating training set for represented samples of tomato fruit, measuring corresponding quality traits, extracting spectrum data from the instrument into Data Viewer Software, building tomato fruit model based on the spectrum and fruit quality indices using Model Builder, and finally validating the developed model based on the newly measured quality indices and the predicted model. The spectrum data were analyzed using (F-750 Data Viewer Software, Version: v1.1.0.51). To correlate reflectance (1st and 2nd derivatives) at 285–1200 nm, the Model Builder (Version: v1.1.0.105) was used as illustrated in Alhamdan and Atia, 2017, Alhamdan et al., 2019.
2.6. Statistical analysis
The data obtained by the destructive analysis of tomato fruit were subjected to analysis of variance (ANOVA) following the PROC GLM procedure using SAS 9.2 software (SAS Institute 2009). The effects of maturity stages on flesh firmness, and levels of carotenes and hydrophilic antioxidants (phenolic and flavonoid compounds) in ‘Red Rose’ tomato fruit were assessed using ANOVA. The least significant differences (LSDs) were calculated by the Fisher’s test (F-test) with a significance level at P ≤ 0.05.
For comparison with the non-destructive evaluation, Felix Model Builder was utilized to calculate coefficient of determination (R2), Root Mean Square Error (RMSE), and Principle Component Analysis (PCA). In general, the best results can be obtained when the predicted values have the lowest RMSE and the highest R2. As a standard regression analysis, PCA was performed both for calibration and cross-validation of samples for each of the quality traits studied.
3. Results and discussion
3.1. Proximate quality characteristics
The analytical (subjective) assessments of oxygen-free carotenes in the present investigation were focused on Lyco and β-Car. The accumulation of Lyco and β-Car rapidly increased in tomatoes harvested between the TG and the LR stages of fruit maturity (Table 1). The highest levels of carotenes (Lyco = 67.5 μg g–1, fm; β-Car = 67.3 μg g–1, fm) were found in fruits harvested at the RD stage of maturity. Lyco remained below the detection level until the fruit entered the TG stage of maturity. Whereas it stayed in the range of 11.8–67.5 μg g–1 during the course of next stages of fruit maturity, with the lowest average of 11.8 μg g–1 at TG and the highest average of 67.5 μg g–1 at RD maturity stage (Table 1). A rapid rise of β-Car content in tomatoes was observed between BK and LR maturity stages, with no significant differences (P ≤ 0.05) between the levels of β-Car in tomato fruit harvested at LR or RD maturity stages. The highest accumulation of carotenoids upon the developmental progress of fruit maturity (Table 1) might be attributed to chlorophyll degradation. Tigist et al. (2015) reported that increaed lycopene, β-carotene contents were associated with increased chlorophyll degradation. With advancing maturity, the chloroplasts at BK stage transform into the chromoplasts which are plastids containing high levels of carotenoids such as Lyco (Tigist et al. 2015).
Table 1.
Flesh firmness and levels of dietary antioxidants of the greenhouse-grown ‘Red Rose’ tomatoes at various stages of fruit maturity (subjective analysis).
| Stage of fruit maturity | Firmness (N) | Lycopene (μg g−1, fm) | β-carotene (μg g−1, fm) | TPC (μg g−1, fm) | TFC (μg g−1, fm) |
|---|---|---|---|---|---|
| Breaker (BK) | 21.7 ± 2.6a | ND | 25.8 ± 3.7c | 1363.1 ± 82.8d | 1258.7 ± 57.7d |
| Turning (TG) | 15.5 ± 1.6ab | 11.8 ± 1.7c | 40.9 ± 4.2b | 1572.1 ± 59.0c | 1675.0 ± 86.1c |
| Pink (PK) | 16.7 ± 2.5b | 28.4 ± 0.9b | 45.1 ± 3.5b | 1813.3 ± 69.3a | 1873.5 ± 100.8a |
| Light red (LR) | 9.50 ± 1.3c | 60.8 ± 3.8a | 66.1 ± 4.2a | 1692.5 ± 92.2b | 1727.1 ± 22.6ab |
| Red (RD) | 5.90 ± 1.9d | 67.5 ± 2.1a | 67.3 ± 2.3a | 1659.1 ± 52.9ab | 1682.6 ± 33.4bc |
N: Newton (force), TPC: Total phenolic content, TFC: Total flavonoid content, fm: Fresh mass. The data are given as mean values ± standard error of 4 replicates. The values within a column sharing the same alphabetical letters are not significant at P ≤ 0.05.
Harvesting tomatoes at BK maturity stage resulted in significantly higher flesh firmness than harvesting at the later maturity stages (Table 1). Whereas, tomatoes harvested at LR and RD maturity stages exhibited statistically similar firmness (9.5 N and 5.9 N, respectively). Verheul et al., (2015) harvested cherry tomato fruits at three maturity stages from commercial greenhouses and evaluated their quality traits at storage, transport, and supermarket conditions. They reported that flesh firmness of BK harvested fruits was 30% higher than for those harvested at RD stages.
Loss in firmness of fresh produce during maturity leads to deterioration of quality and causes higher incidence of mechanical damages during handling, storage and transportation (Parker and Maalekuu, 2013). Tomato fruit get softer as it matures. Softening of tomatoes at later stages of fruit maturity (Table 1) may be ascribed to the enzymatic breakdown of pectin and related substances in the epidermal layers of the fruit (Macnish et al., 1997).
At the earliest maturity stage (BK), tomato fruits had the lowest TPC and TFC contents (1363.1 and 1258.7 μg g−1, fm, respectively (Table 1). The highest TPC and TFC contents were found in tomato fruits harvested at (PK) maturity stage (1813.3 and 1873.5 μg g−1, fm, respectively. Fruits harvested at (LR) and (RD) maturity stages had intermediate TPC and TFC levels. These results are in accordance with Nikolaos et al., (2018) who indicated that the highest polyphenols content of tomato fruits was observed in the red colour fruits, while the mature green fruits had the lowest polyphenols content.
3.2. Spectral analysis
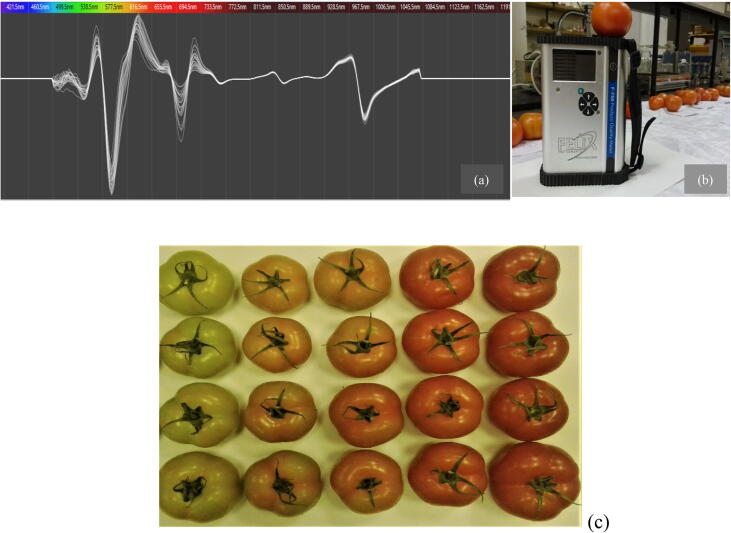
A spectral curve in the wavelength range of 350–1250 nm of tomato at the five maturity stages and the sample measurement are shown in Fig. 1. The positive and negative peaks in the spectra indicate the strong and the weak absorbance characteristics of the fruit within the range studied, respectively.
Fig 1.
Raw spectra curves of all tomato samples at various stages of fruit maturity (a), NIR measurements of tomatoes (b), and the five stages of fruit maturity in vertical rows (c): Vertical rows from left to right; (1) Breaker, (2) Turning, (3) Pink, (4) Light-red, (5) Red. Fresh tomato fruits were labeled and scanned using the handheld near infra-red enhanced spectrometer (F-750, Produce Quality Mater, Felix Instruments, Camas WA, USA), at wavelength range (285–1200 nm).
There were apparent differences between the maturity stages of tomato fruits in shape and magnitude that might indicate the variations of fruit properties. This might be attributed to moisture content and surface texture of fruit samples as well as other properties (Jaiswal et al., 2012). In addition, the light absorption of oxygen-free carotenoids in tomatoes at the BK stage of maturity has strong masking spectra which resulted in other compounds with minor absorption intensity being invisible. These masking effects are due to overlapping light absorption by different pigments. Similarly, Qin and Lu, (2008) observed noticeable changes in absorption spectra at wavelength range 500 and 730 nm for tomato fruit with different maturity stages (green, pinkish, and red). They reported relatively stable and modest absorption values at wavelength range 730–900 nm which were similar to what was found in this study.
The quality indices of ‘Red Rose’ tomatoes that were predicted with NIR are shown in Table 2. The results revealed a fair high coefficient of determination (R2) for various quality traits of tomato fruit. The R2 values were 0.966 for TPC, 0.959 for lyco, 0.984 for TFC, 0.938 for β-Car; and 0.919 (fair) for flesh firmness. To examine the influence of distribution on the prediction performance of calibration models, Partial Least Squares Regression (PLSR) was re-calculated with logarithmic transformed values. For the firmness validation model, the prediction with R2 = 0.679 were RMSECV = 0.640 and PRESS of 1.048 as shown in Table 2. These results are lower than those reported by Gómez et al., (2006) for mandarin with R2 = 0.83 and root mean square error of prediction (RMSEP) = 8.53 N, but were higher than those reported by McGlone and Kawano (1998) for kiwifruit with R2 = 0.76 and RMSEP = 0.70 that is due to composition variations of fruits. Fan et al., (2020) used a portable (Vis/NIR) device to evaluate internal qualities of apple fruit on-tree and during storage. They indicated that the device can be practically used for detecting soluble solids content (SSC).
Table 2.
Flesh firmness and dietary antioxidants in the greenhouse-grown ‘Red Rose’ tomatoes at various fruit maturity stages (NIR model performance).
| Quality indices | Principle components | R2 | CVR2 | RMSEC | RMSECV | PRESS | PRESSCV |
|---|---|---|---|---|---|---|---|
| Flesh Firmness | 08 | 0.919 | 0.6790 | 0.462 | 0.6400 | 1.0480 | 1.454 |
| Lycopene | 11 | 0.959 | 0.8647 | 1.770 | 1.0291 | 3.1329 | 1.059 |
| β-carotene | 12 | 0.938 | 0.7082 | 0.163 | 1.1404 | 0.0260 | 1.301 |
| TPC | 12 | 0.966 | 0.8343 | 2.506 | 1.7980 | 6.2811 | 3.234 |
| TFC | 12 | 0.984 | 0.7900 | 1.541 | 1.8230 | 2.3770 | 3.326 |
TPC: Total phenolic content, TFC: Total flavonoid content, R2: Coefficient of determination of calibration; CVR2: Coefficient of determination of cross validation; RMSEC: Root mean squared error calibration; RMSECV: Root mean squared error cross validation; PRESS: Predicted residual error sum of squares; PRESSCV: Predicted residual error sum of squares cross validation.
Saad et al., (2016) used visible/near-infrared (VIS/NIR) spectroscopy to investigate the tomato fruit quality traits during storage. They reported that the PLSR calibration model with SSC had the highest coefficient of determination (R2) = 0.91, RMSEP = 0.285, while R2 for lycopene was the lowest (0.73). They concluded that during storage, significant changes in quality traits occur in tomato fruit at turning stage. These changes have practical use in post-harvest industry. On the other hand, Ibáñez et al., (2019) used NIR diffuse reflectance to predict taste-related quality traits in tomato. They reported a good performance for the prediction of acids, sugars and soluble solids. The data created by the non-destructive methods can be used to indicate key variables that correlate with fruit maturity stages (Wu and Sun, 2013). These variables can be utilized in regression models to assess fruit quality and ripeness.
The influence of wavelength on the detection of fruit quality traits are shown in Fig. 2. The firmness detection in which the highest influences of spectra were in the range of 1050–1250 nm is shown in Fig. 2a while Fig. 2b exhibits the highest wavelength influence on Lyco at NIR region of 1185 nm. Fig. 2c and d illustrate the influence of wavelength on β-Car and TPC at 1150 nm and Fig. 2e shows the influence of the wavelength on TFC at the peak of 1100 nm. There are relative similarities of the occurrence of spectra influence at high wavelength for firmness, Lyco, β-Car, TPC, and TFC traits. However, prediction influence was higher (∼19.4) for β-Car and TPC traits compared to other traits indicating a more important pixels for prediction. Furthermore, wavelengths with high prediction level (β-Car and TPC traits) are weighted more heavily than firmness, Lyco, and TFC traits.
Fig 2.
Wave-length influence according to the estimation of fruit quality for: (a) firmness; (b) Lycopene; (c) β-Carotene; (d) TPC; and (e) TFC. Fresh tomato fruit were labeled and scanned using the handheld near infra-red enhanced spectrometer (F-750, Produce Quality Mater, Felix Instruments, Camas WA, USA), at wavelength range (285–1200 nm).
3.3. Reflectance analysis
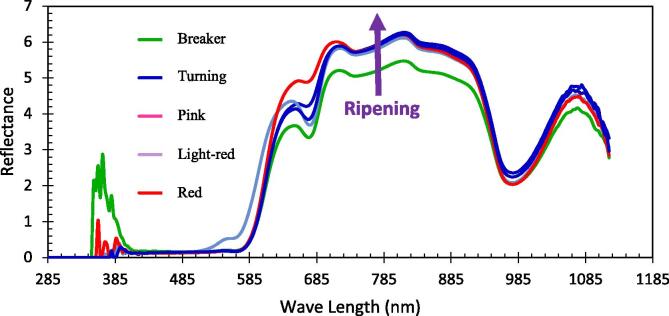
The reflection spectra (350–1200 nm) were initiated with the spectrophotometer (Model: Felix F-750) around the equator (approximately 120°), at three equidistant positions for each fruit. It can be observed from Fig. 3 that tomato at earlier stage of maturity (BK) had lower reflectance compared to the more mature ones (PK–RD) at the NIR region. These variations might be attributed to the differences in color and chemical composition of tomato fruit at different stages of maturity. VIS/NIR reflectance spectroscopy detect reflected light which depends on the light absorption by fruit surface. The fruit physical and chemical quality traits determines the amount of reflectance which indicates the stage of ripeness (Li et al., 2018).
Fig 3.
Spectra reflectance (in wave length range 350–1200 nm) of tomato fruit at different maturity stages. The arrow shows the direction of ripening in progress. The reflection spectra was initiated with the spectrophotometer (Model: Felix F-750) around the equator (approximately 120°), at three equidistant positions for each fruit.
Typically, the reflectance spectra occur in the visible range (Jiang et al., 2016). Fig. 3 illustrates the reflectance spectra for tomato fruit samples at different of maturity stages. The variations of reflectance curves of maturity stages were noticeable within the visible region of 585 to 685 nm. Liu, (2016) reported similar pattern of these spectral curves and indicated that there were clear distinctions among reflectance curves at wavelengths of 405–700 nm (visible region) of tomato fruit with various maturity stages whereas they were substantially lower at 780–970 nm (infrared region). The nature of those spectra curves are closely associated with the physiological maturity of tomato fruit. The upward shift of the spectra curves reflects the transition of ripening stages of fruit maturity. Similar findings were reported by Alhamdan and Atia (2016) and Alhamdan et al, (2019) on Barhi date fruit.
3.4. Absorbance analysis
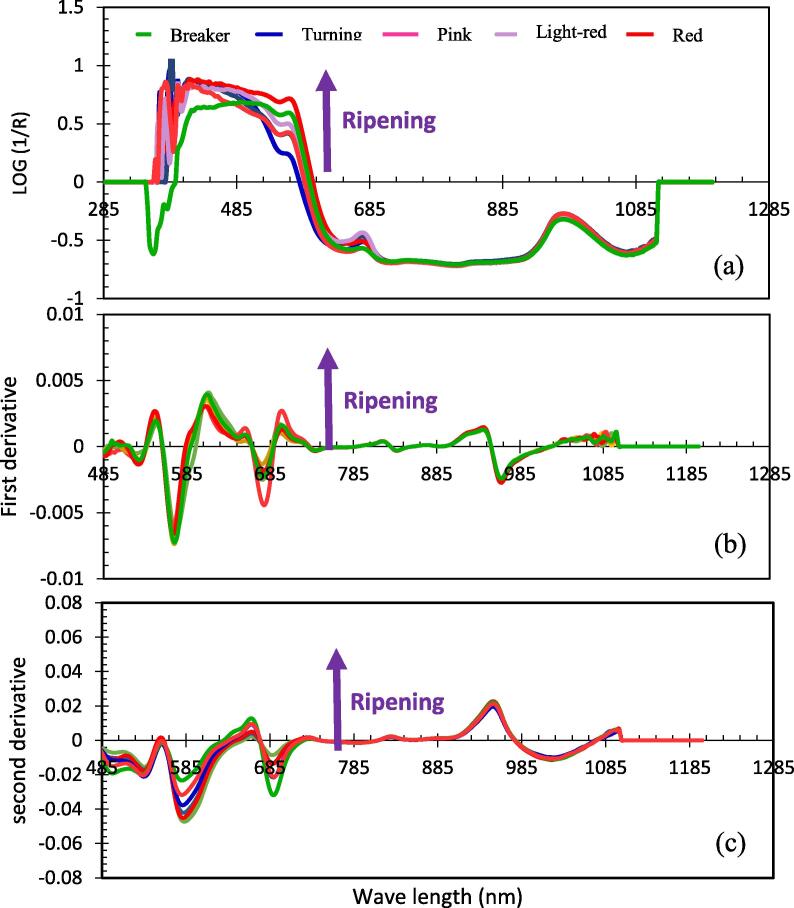
Fig. 4 shows the spectral absorbance of tomatoes from five maturity stages at the wavelength range of 400–1200 nm. Fig. 4(a) exhibits log (1/R) absorbance while 4(b) shows the 1st derivative spectra, whereas 4(c) displays the 2nd derivative spectra. The 2nd derivative absorbance curves were utilized to show two additional positive satellite bands on both sides of the main band. The wavelength range of 750–1200 (Fig. 4c) showed the variations of spectral patterns more clearly for different stages of fruit maturity which could be attributed to the levels of carotenoids and possibly photosynthetic pigments (e.g., chlorophylls) for each maturity stage. With the advancement of tomato fruit maturity stages from BK to RD, the levels of photosynthetic pigments decrease whereas the levels of carotenoids and phenolic pigments increase (Qin and Lu, 2008).
Fig 4.
Absorbance of tomato fruit at different stages of maturity: (a) Absorbance (log (1/R)); (b) Absorbance of the 1st derivative; and (c) Absorbance of the 2nd derivative. The arrow shows the direction of ripening in progress. Fresh tomato fruits were labeled and scanned using the handheld near infra-red enhanced spectrometer (F-750, Produce Quality Mater, Felix Instruments, Camas WA, USA), at wavelength range (285–1200 nm). The statistical details (e.g means and standard errors) of all quality parameters are shown in Table 1.
The wavelength region (900–1183 nm) is highly influenced by the broad water bonds (O-H bonds), specifically at 970 nm and 1180 nm. The Absorption by carbohydrates in the wavelength region (800–1050) nm is well known for food produces (Norris and Williams, 2001). Moreover, the wavelength region (850–920 nm) revealed more insight on SSC and firmness differences of tomatoes at different maturity stages in which firmness levels decrease as sugars content increase in the fruit. Feng et al., (2019) demonstrated that cherry tomatoes could be well distinguished through either of the quality traits (firmness, SSC, and pH) or the NIR spectroscopic characteristics. They reported that extreme learning machine (ELM) algorithm combined with raw spectra performed better in predicting the proximate quality characteristics of cherry tomatoes, in comparison with Partial least square (PLS), support vector machine (SVM). Their results suggested that NIR spectroscopy when combined with chemo-metric analysis might be accurately used to predict cherry tomatoes quality traits during cold storage.
4. Conclusion
The investigation of this study demonstrated that the stage of fruit maturity at harvest substantially influences the fruit flesh firmness and the levels of both lipophilic and hydrophilic antioxidants in ‘Red Rose’ tomato fruit grown under protected environment. Fruits harvested at the ‘Pink’ stage of maturity had higher levels of hydrophilic antioxidants whereas those harvested at ‘Light-red’ or ‘Red’ stage of maturity exhibited higher levels of lipophilic antioxidants. The NIR spectroscopic measurements of firmness and lipophilic antioxidants in tomato fruit at various maturity stages were partially in accordance with those estimated by destructive (analytical) methods. The prediction with NIR presented in this study can be utilized for online measurements of quality indices. These results have implications for the improvement of quality control in handling and processing industry of tomatoes and possibly other fruits and vegetables.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RGP-1438-011. The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullahi I.I., Abdullahi N., Abdu A.M., Ibrahim A.S. Proximate, Mineral and vitamin analysis of fresh and canned tomato. Biosci. Biotechnol. Res. Asia. 2016;13:1163–1169. doi: 10.13005/bbra/2147. [DOI] [Google Scholar]
- Alda M.L., Gogoaşă I., Bordean D.M., Gergen I., Alda S., Moldovan C., Niţă L. Lycopene content of tomatoes and tomato products. J. Agroaliment. Proc. Technol. 2009;15:540–542. [Google Scholar]
- Alenazi M.M., Shafiq M., Alsadon A.A., Alhelal I.M., Alhamdan A.M., Solieman T.H.I., Ibrahim A.A., Shady M.R., Al-Selwey W.A. Improved Functional and Nutritional Properties of Tomato Fruit during Cold Storage. Saudi J. Biol. Sci. 2020;27(6):1467–1474. doi: 10.1016/j.sjbs.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamdan A.M., Atia A. Non-destructive method to predict Barhi dates quality at different stages of maturity utilising near-infrared (NIR) spectroscopy. Int. J. Food Prop. 2017;20(sup3):S2950–S2959. doi: 10.1080/10942912.2017.1387794. [DOI] [Google Scholar]
- Alhamdan A.M., Fickak A., Atia A.R. Evaluation of sensory and texture profile analysis properties of stored Khalal Barhi dates nondestructively using Vis/NIR spectroscopy. J. Food Process Eng. 2019;42(6):1–8. doi: 10.1111/jfpe.13215. [DOI] [Google Scholar]
- Baeza-Jiménez, R., LópezMartínez, L.X., GarcíaVarela, R., García, H.S., 2017. Lipids in Fruits and Vegetables, Chemistry and Biological Activities. Pp: 423-450. In: Yahia, E.M. (Ed). Fruit and Vegetable Phytochemicals: Chemistry and Human Health. John Wiley & Sons, Inc., Bridgewater, NJ, USA. 10.1002/9781119158042.ch20. [DOI]
- Baranska M., Schütze W., Schulz H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006;78:8456–8461. doi: 10.1021/ac061220j. [DOI] [PubMed] [Google Scholar]
- Barrett D., Beaulieu J.C., Shewfelt R.L. Color, Flavor, Texture and Nutritional Quality of Fresh-cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010;50:369–389. doi: 10.1080/10408391003626322. [DOI] [PubMed] [Google Scholar]
- California Tomato Commission Guide to ripening stages. 2020. http://wwwlagoriocom/assets/pdf/lagorio-tomato-guidepdf Retrieved on May 1, 2020 from.
- Cattaneo, T.M.P., Stellari, A., 2019. NIR Spectroscopy as a Suitable Tool for the Investigation of the Horticultural Field. Agronomy, 9(9), 503; 10.3390/agronomy9090503. [DOI]
- Çelik Ö., Ayan A., Atak Ç. Enzymatic and non-enzymatic comparison of two different industrial tomato (Solanum lycopersicum) varieties against drought stress. Botan. Stud. 2017;58:32–44. doi: 10.1186/s40529-017-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.M., Koutsidis G., Lodge J.K., Ashor A., Siervo M., Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Fan, S., Wang, Q., Tian, X., Yang, G., Xia, Y., Li, J., Huang., W., 2020. Non-destructive evaluation of soluble solids content of apples using a developed portable Vis/NIR device. Biosyst. Eng.. 193,138-148. 10.1016/j.biosystemseng.2020.02.017. [DOI]
- Feng L., Zhang M., Adhikari B., Guo Z. Nondestructive Detection of Postharvest Quality of Cherry Tomatoes Using a Portable NIR Spectrometer and Chemometric Algorithms. Food Anal. Methods. 2019;12:914–925. doi: 10.1007/s12161-018-01429-9. [DOI] [Google Scholar]
- Gómez A.H., He Y., Pereira A.G. Non-destructive measurement of acidity, soluble solids and firmness of Satsuma mandarin using Vis/NIR-spectroscopy techniques. J. Food Eng. 2006;77:313–319. doi: 10.1016/j.jfoodeng.2005.06.036. [DOI] [Google Scholar]
- Ibáñez, G., ebolla-Cornejo, J., Marti, R., Rosello, S., Valcárcel., M., 2019. Non-destructive determination of taste-related compounds in tomato using NIR spectra. J. Food Eng., vol. 263, pp. 237–242. 10.1016/j.jfoodeng.2019.07.004. [DOI] [PubMed]
- Jaiswal P., Jha S.N., Bharadwaj R. Non-destructive prediction of quality of intact banana using spectroscopy. Sci. Hortic. 2012;135:14–22. doi: 10.1016/j.scienta.2011.11.021. [DOI] [Google Scholar]
- Jiang J., Qiao X., He R. Use of Near-Infrared Hyperspectral Images to Identify Moldy Peanuts. J. Food Eng. 2016;169:284–290. doi: 10.1016/j.jfoodeng.2015.09.013. [DOI] [Google Scholar]
- Khuriyati N., Matsuoka T., Kawano S. Precise near Infrared Spectral Acquisition of Intact Tomatoes in Interactance Mode. J. Near Infrared Spectrosc. 2004;12:391–395. doi: 10.1255/jnirs.448. [DOI] [Google Scholar]
- Li, B., Lecourt, J., Bishop, G., 2018. Advances in Non-Destructive Early Assessment of Fruit Ripeness towards Defining Optimal Time of Harvest and Yield Prediction—A Review. Plants 2018, vol. 7, 1, 3; 10.3390/plants7010003. [DOI] [PMC free article] [PubMed]
- Liu, Y., 2016. Non-destructive phenotyping of postharvest quality traits of tomatoes and strawberries. M Sc Minor Thesis. 49 pp. Wageningen University, Wageningen, The Netherlands.
- Macnish A.J., Joyce D.C., Shorter A.J. A simple non-destructive method for laboratory evaluation of fruit firmness. Aust. J. Exp. Agric. 1997;37:709–713. doi: 10.1071/EA97033. [DOI] [Google Scholar]
- Maynard, D.N., Hochmuth, G.J., 2007. Knott’s Handbook for Vegetable Growers. John Wiley & Sons Inc. Hoboken, New Jersey, USA. 630 P.
- McGlone V.A., Kawano S. Firmness, dry-matter and soluble-solids assessment of postharvest kiwifruit by NIR spectroscopy. Postharvest Biol. Technol. 1998;13:131–141. doi: 10.1016/S0925-5214(98)00007-6. [DOI] [Google Scholar]
- Nagata M., Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Japan. Soc. Food Sci. Technol. 1992;39:925–928. [Google Scholar]
- Nikolaos, G., Alexandros, P., Evangelia, L., Vassiliki, T., Maria-Nektaria, N. 2018. Effect of ripening stage on the total phenolics content, lycopene and antioxidant activity of tomato fruits grown to a geothermal greenhouse. Annals of the University of Craiova. Food produce processing technology. Environ. Eng., vol. 23 (59), pp.115 –120.
- Norris, K., Williams, P., 2001. Near-infrared technology in the agricultural and food industries. American Association of Cereal Chemists, Inc., St. Paul, Minnesota, USA. 296 P.
- Nour, V., Ionica, M.E., Trandafir, I., 2015. Bioactive compounds, antioxidant activity and color of hydroponic tomato fruits at different stages of ripening. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 43, 404-412. 10.15835/nbha43210081 [DOI]
- Parker R., Maalekuu B.K. The effect of harvesting stage on fruit quality and shelf-life of four tomato cultivars (Lycopersicon esculentum Mill) Agric. Biol. J. North Am. 2013;4:252–259. doi: 10.5251/abjna.2013.4.3.252.259. [DOI] [Google Scholar]
- Pedro A.M., Ferreira M.M. Nondestructive determination of solids and carotenoids in tomato products by near-infrared spectroscopy and multivariate calibration. Anal. Chem. 2005;77:2505–2511. doi: 10.1021/ac048651r. [DOI] [PubMed] [Google Scholar]
- Porep J., Kammerer D.R., Carle R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015;46:211–230. [Google Scholar]
- Qin J., Lu R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008;49:355–365. doi: 10.1016/j.postharvbio.2008.03.010. [DOI] [Google Scholar]
- Saad A., Jha S.N., Jaiswal P., Srivastava N., Helyes L. Non-destructive quality monitoring of stored tomatoes using VIS-NIR spectroscopy. Eng. Agric. Environ. Food. 2016;9:158–164. doi: 10.1016/j.eaef.2015.10.004. [DOI] [Google Scholar]
- SAS Institute 2009. SAS/STAT 9.2; Users' Guide, SAS Institute, Cary, NC, USA.
- Serio F., Leo L., Parente A., Santamaria P. Potassium nutrition increases the lycopene content of tomato fruit. J. Hortcul. Sci. Biotechnol. 2007;82:941–945. doi: 10.1080/14620316.2007.11512330. [DOI] [Google Scholar]
- Sirisomboon P. NIR Spectroscopy for Quality Evaluation of Fruits and Vegetables. Mater. Today Proc. 2018;5:22481–22486. doi: 10.1016/j.matpr.2018.06.619. [DOI] [Google Scholar]
- Subedi P.P., Walsh K.B. Non-invasive techniques for measurement of fresh fruit firmness. Postharvest Biol. Technol. 2009;51(3):297–304. doi: 10.1016/j.postharvbio.2008.03.004. [DOI] [Google Scholar]
- Surana A.R., Kumbhare M.R., Wagh R.D. Estimation of total phenolic and total flavonoid content and assessment of in vitro antioxidant activity of extracts of Hamelia patens jacq. stems. Res. J. Phytochem. 2016;10:67–74. https://scialert.net/abstract/?doi=rjphyto.2016.67.74 [Google Scholar]
- Tigist N.T., Ali M.I., Wosene G.A. Degradation and formation of fruit color in tomato (Solanum lycopersicum L.) in response to storage temperature. Am. J. Food Technol. 2015;10:147–157. https://scialert.net/abstract/?doi=ajft.2015.147.157 [Google Scholar]
- Verheul M.J., Slimestad R., Tjøstheim I.H. From Producer to Consumer: Greenhouse Tomato Quality As Affected by Variety, Maturity Stage at Harvest, Transport Conditions, and Supermarket Storage. J. Agric. Food. Chem. 2015;63:5026–5034. doi: 10.1021/jf505450j. [DOI] [PubMed] [Google Scholar]
- Walsh K.B., McGlone V.A., Han D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020;163:111139. doi: 10.1016/j.postharvbio.2020.111139. [DOI] [Google Scholar]
- Wilbur, A.G., 1992. Composition of Tomatoes. pp. 433–450. In: Gould, W.A. (Ed). Tomato Production, Processing and Technology. Woodhead Publ., Cambridge, UK. 10.1533/9781845696146.3.433. [DOI]
- Wu D., Sun D.W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innov. Food Sci. Emerg. Technol. 2013;19:1–14. doi: 10.1016/j.ifset.2013.04.014. [DOI] [Google Scholar]