Abstract
This study explored the effects of natural growth promoters (phytogenic feed additives and organic acids) on animal performance, carcass characteristics, blood parameters, gut microflora composition, and microbe–host interactions in broiler chickens over a 42-day feeding period. Two-hundred-fifty-day-old chicks were randomly assigned to one of five treatments: (i) control diets (CON); (ii) control diets + 40 g/tons antibiotic growth promoter (AB); (iii) control diets + 3 kg/tons organic acids (ORG); (iv) control diets + 3 kg/tons phytogenic feed additives (PHY); (v) control diets + 3 kg/tons organic acids + phytogenic feed additive combination (COM). A non-significant differences (p > 0.05) were observed in broiler performance among treatments at 21 days of age; however, a gradually increasing body weight gain and reduced feed conversion ratio were observed at 42 days in treatments versus control group. Biochemical indices were non-significant (p > 0.05) except for decreased cholesterol (p < 0.05) and increased A/G ratio (p < 0.05) recorded in the treatment groups. The addition of PHY and ORG improved total counts of Enterococcus spp. and Lactobacillus spp. (p < 0.05) as well as reduced caecal and ileal Campylobacter spp. and Escherichia coli (p < 0.05). Correlation analysis elucidated beneficial bacteria (Enterococcus spp. and Lactobacillus spp.) were positively and pathogenic bacteria (Campylobacter spp. and E. coli) were negatively correlated (p < 0.05) with host weight gain. The findings indicated that dietary supplementation of PHY and ORG sustained balanced gut microflora, which in turn improved body weight. This study broadens the significance of using PHY and ORG as safe alternatives to antibiotic growth promoters for achieving healthier and economical broiler production.
Keywords: Broiler, Dietary modulation, Gut microbiota, Organic acids, Phytogenic feed additives, Weight gain
1. Introduction
Gut microflora plays a character role in host energy, immunity, and metabolism, especially in commercial poultry birds. The gastrointestinal tract (GIT) is densely populated with a variety of organisms such as viruses, fungi, bacteria, and protozoans (Sommer and Backhed, 2013). Over the years, various studies have highlighted the influential impacts of gut bacteria in hosts which include (1) epithelial barrier maintenance; (2) inhibition of pathogenic bacterial adhesion to the intestinal surface; (3) enhancement and maturation of the host’s immunity; (4) degradation of non-digestible plant polysaccharides; and (5) production of metabolites such as short chain fatty acids (SCFAs) and vitamins (Al-Asmakh and Zadali, 2015, Sanchez, 2017).
In the recent past, tremendous interest in poultry production has been generated. Chicken meat and eggs are optimal sources of quality protein along with crucial vitamins and minerals. Broiler chickens’ outstanding performance in terms of feed efficiency and feed-to-meat conversion (Vandehaar et al., 2016) within 6 weeks has been found to be linked to intestinal microbiota, as well as various other factors, including bird management, environment, vaccines, and disease control (Kiarie et al., 2013). Modulating the gut ecosystem and functions of farm animals with dietary variable is an effective strategy for achieving desired results in poultry (Madal et al., 2015). Therefore, growth promoters are incorporated into poultry feed to improve chickens’ health and establish a stable gut ecosystem.
Natural growth promoters (NGPs) are real, toxin-free, and less residual; thus, they are considered quintessential additives in poultry feeds. Among these alternatives, organic acids and phytogenic feed additives modulate the host immune system through generating antioxidant and anti-inflammatory responses in the gut, which enhance maximum nutrient absorption (Liu et al., 2014, Mueller et al., 2012).
The primary aim in rearing broiler chickens is to increase their body weight in the shortest possible time. In this domain, gut microbiota are key players because they maintain beneficial interactions with the host (Turnbaugh et al., 2009, Dahiya et al., 2017). In the gut, the cecum is densely populated with a variety of bacteria that are responsible for fermentation, which prevents pathogenic bacterial colonization; by contrast, the ileum is the main site for the absorption and digestion of nutrients (Lee et al., 2017). The chicken cecum might harbor obligate anaerobic pathogenic bacteria (Clostridium spp., Campylobacter spp.), whereas microaerophilic bacteria (Lactobacillus spp., Enterococcus spp.) are predominant in the ileum (Yin et al., 2010, Boguslawska-Tryk et al., 2015). Gut bacteria were suggested to be positively or negatively correlated with animal performance (Yin et al., 2018). However, investigations of the relationship between performance-related gut microbiota in poultry based on dietary modulation are scarce. In this context, an effective strategy for improved body weight gain is required to identify and modulate relevant gut bacteria (Han et al., 2016).
Prebiotics and probiotics added to poultry feed amplify the number of performance-related bacteria (Murshed and Abudabos, 2015), but the same has not yet been confirmed for organic acids and phytogenic feed additives. Thus, the objective of this study was to assess the influence of phytogenic feed additives and organic acids on animal performance, carcass characteristics, biochemical indices, caecal and ileal microbial populations, and host–microbe interactions of broiler chickens in the quest for alternatives to antibiotics in poultry.
2. Methods
2.1. Bird management
This trial was conducted at Ideal feeds and experimental units, Karachi, Pakistan under standard operating procedures for broiler housing management after approval of the Ethical Review Board (DG/AA-089) of the Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi, in correspondence with the standard protocol from the guidelines “Care and use of agricultural animals in research” (Mcglone, 2010). Two-hundred-fifty-day-old unsexed, healthy, disease-free, Hubbard-strain chicks were purchased from a commercial hatchery and reared from 42 days. The birds were nurtured on the commercial starter (days 1–21) and finisher (days 22–42) diets. The management and feeding practices (vaccination, temperature, humidity, watering, feeding, and lighting) were similar for all chicks as described in the Hubbard Management guide (Aviagen, 2014). The diet was provided in mash form and the birds’ access to feeders and water were ad libitum. The feed and water were provided in cylindrical hanging feeders and drinkers, respectively. The provision of light during the trial was for 24 h for first 3 days, and subsequently for 23 h/day with 1 h of darkness. Table 1 presents the chemical composition and formula of the experimental diet. Each diet was analyzed for proximate composition on the basis of the Association of Official Analytical Chemists Procedures (AOAC, 2005). The supplementation of the basal diet was in iso-caloric and iso-nitrogenous forms with the addition of feed additives.
Table 1.
Feed ingredients and chemical composition of experimental diet.
| Ingredients | Composition |
|
|---|---|---|
| Starter (%) | Finisher (%) | |
| Corn | 60.1 | 66.2 |
| Soya bean meal (46%) | 21.3 | 22.4 |
| Canola meal | 5.33 | 0.00 |
| Fish Meal (48%) | 0.00 | 4.75 |
| Corn Gluten (60%) | 5.01 | 0.00 |
| APCa (50%) | 5.03 | 4.89 |
| Limestone | 1.60 | 1.22 |
| Oil | 0.00 | 0.44 |
| DL-Methionine | 0.19 | 0.14 |
| Lysine HCl | 0.405 | 0.223 |
| Vitamin premixb | 0.053 | 0.056 |
| Mineral premixc | 0.057 | 0.051 |
| L-Threonine | 0.071 | 0.023 |
| Salt (NaCl) | 0.200 | 0.200 |
| Sodium -bi- carbonate | 0.205 | 0.159 |
| Anti –coccidiald | 0.022 | 0.029 |
| DCP 2e | 1.12 | 0.658 |
| Choline chloride (60%) | 0.100 | 0.100 |
| Calculated chemical composition (%) | ||
| Metabolize energyf (MJ/kg) | 12.1 | 12.9 |
| Crude Protein (%) | 21.5 | 19.1 |
| Calcium (%) | 1.00 | 0.859 |
| Available Phosphorous (%) | 0.400 | 0.395 |
| Dig. Lysine (%) | 1.19 | 1.06 |
| Dig. Methionine (%) | 0.559 | 0.454 |
| Dig. Methionine + Cysteine (%) | 0.890 | 0.743 |
| Dig. Tryptophan (%) | 0.284 | 0.191 |
| Dig. L-Threonine (%) | 0.846 | 0.723 |
Animal protein concentrate (Feather meal).
Vitamin premix composition per kg (Vitamin A 20,000 KIU/kg, Vitamin D3 5,400 KIU/kg, Vitamin E 48,000 mg/kg; Vitamin K3 4,000 mg/kg, Vitamin B1 4,000 mg/kg, Vitamin B2 9,000 mg/kg, Vitamin B6 7,600 mg/kg, Vitamin B12 20 mg/kg, Niacin 60,000 mg/kg, Folic acids 1,600 mg/kg, Pantothenic acid 20.000 mg/kg, Biotin 200 mg/kg).
Mineral premix composition per kg (Iron 60.000 mg/kg, Zinc 120,000 mg/kg, Manganese 130.000 mg/kg, Copper 10,000 mg/kg, Iodine 1,800 mg/kg, Selenium 360 mg /kg, Cobalt 400 mg/kg).
Anti –coccidial (Diclazuril 0.5%).
DCP 21(MDCP) Phosphorous 21%, Calcium 17%.
ME (MJ/Kg) = gross energy fed in diet to birds (MJ) – gross energy of excreta collected (MJ) feed intake of diet to each bird (DM based).
2.2. Experimental design and diet
In this experiment, the birds were randomly distributed to five treatments with five replicates per treatment, and a total of 10 birds per replicate were placed in a clean cage (4 X 8 ft.). Experimental groups received a commercial corn-soybean basal diet prepared with the following variations:
-
(i)
Control (CON): basal diet only;
-
(ii)
Antibiotic growth promoter (AB): basal diet + 40 g/tons Enramycin®
-
(iii)
Organic acids (ORG): basal diet + 3 kg/tons Acidifiers
The Acidifiers used in the study were supplied by Kemira Pro GIT SF3 (Shanghai, China) and comprised of 26.5% formic acid; 16% lactic acid; 5% citric acid; 13.1% MCFA (lauric acid-based); and 3.5% mono-, di-, and triglycerides of MCFAs.
-
(iv)
Phytogenic feed additives (PHY): basal diet + 3 kg/tons phytogenics
The phytogenic feed additives contained a mixture of dried powders of Allium sativa (garlic) and Cinnamomum verum (cinnamon) (10%), dried leaves of Mentha piperita (peppermint) and Camellia sinensis (green tea) (10%), and seeds of Nigella sativa (black cumin) (15%).
-
(v)
Combination (COM): basal diet + 3 kg/tons organic acids + phytogenic feed additives.
2.3. Performance parameters
Performance analyses were executed on days 21 and 42, including a daily feed intake (FI) estimation through providing a known amount of feed and measuring the remainder. Additionally, body weight gain (BWG) was recorded daily and weekly for individual birds and per cage, respectively. The feed conversion ratio (FCR) was computed as follows:
2.4. Carcass characteristics
At the end of the experiment, the chicks were starved for 8 h prior to slaughtering. Three birds from each replicate with a body weight under one standard deviation of the average treatment weight (15 birds/treatment or 75 birds in total) were randomly selected for estimating biochemical indices, carcass characteristics, and gut microflora count. Birds were slaughtered by having their throats cut with a sharp knife. Carcass characteristics after slaughtering were calculated followed by evisceration. The dressing percentage along with percentages of breast meat, giblets (gizzard, liver, and heart), and abdominal fat were measured as percentages of body weight (cm or g/100 g of body weight).
2.5. Biochemical parameters
For estimating biochemical indices, 2 ml of blood in sterile tubes was collected from the brachial vein using sterile needles and syringes. The blood-containing tubes were placed at room temperature for 6 h in a slanted position and incubated overnight at 4 °C for serum collection. Serum samples were maintained at −20 °C before biochemical analysis was performed. Later, cholesterol, globulin, and albumin levels as well as total protein and albumin/globulin (A/G) ratio were calculated.
2.6. Caecal and ileal sample processing
Aseptically whole gastrointestinal tracts (GITs) were removed and the caecum and ileum regions were washed with phosphate-buffered saline (PBS). Later, caecum and ileum contents were squeezed from one end and collected in sterile tubes filled with cryoprotective broth (pre-autoclaved 50 ml of brain heart infusion broth +20% glycerol v/v). Samples were immediately stored at −80 °C for further analyses (Ballongue, 1997).
2.7. Quantitative bacterial analysis
Microbial enumeration: For microbial enumeration, deep frozen cecum and ileum sample per bird were thawed for 20 min; 1 g of sample was macerated in 9 ml of sterilized PBS, of which 1 ml was transferred to 9 ml of sterilized PBS. Later, samples were serially diluted from 10−2 to 10−6, and 0.1 ml of each diluted sample was plated in triplicates on appropriate agar plates for a bacterial count of targeted organisms using the spread plate technique. All bacterial counts were denoted as colony-forming units (log10/g of wet digestion) based on the following calculation (Andrews et al., 2014):
where
N = number of colonies (cfu /g)
∑C = sum of colonies on all counted plates
n = number of plates from dilution counted
d = dilution factor
Bacterial growth media: Gut microflora was identified using the respective growth media. Total aerobes and anaerobes were enumerated on plate count agar (Oxoid CM0325); Coliforms on MacConkey agar (Oxoid CM0505); Lactobacillus spp. on De Man, Rogosa, and Sharpe (MRS) agar (Oxoid CM0359); Enterococcus spp. on bile esculin azide agar (Oxoid CM0888); Escherichia coli on eosin methylene blue agar (Oxoid 0069); Campylobacter spp. on Campylobacter selective agar (Oxoid CM0689) after the addition of laked horse blood (Oxoid SR0048) and Campylobacter selective supplements (Oxoid SR0117); Salmonella spp. on Hektoen enteric agar (Merck 111681), xylose lysine deoxycholate agar (Oxoid 0469), and bismuth sulphite agar (Oxoid 0201). The plates were incubated at 37 °C for 24–48 h aerobically (plate count, MacConkey, eosin methylene blue, Hektoen enteric, xylose lysine deoxycholate, and bismuth sulphite agar) or 48–72 h anaerobically (plate count, MRS, Campylobacter selective agar) and the anaerobic environment was created using an appropriate catalyst (OxoidTM AnaeroGenTM AN0025A) and an anaerobic gas jar (Oxoid, AG0025A).
Phenotypic characterization: The targeted bacterial genera (Lactobacillus, Enterococcus, Escherichia, and Campylobacter) were phenotypically characterized based on morphological and biochemical parameters. In the morphological analysis, typical colonies on selective growth media were further confirmed by Gram staining, microscopic appearance, and colonial characteristics. For biochemical testing, oxidase, catalase, and indole tests were performed. For Enterococcus spp. identification, a 6.5% NaCl test was performed to discriminate between group D streptococci and Enterococcus spp.
Salmonella spp. counts: For Salmonella spp. identification, 1 g of sample was pre-enriched in lactose broth for 24 h. The pre-enriched sample (1 ml) was transferred to Rappaport–Vassiliadis broth (Oxoid CM0669) for the selective growth of Salmonella spp.; tubes were aerobically incubated at 37 °C for 24 h. Subsequently, 0.1 ml of diluted samples was plated onto selective growth media followed by aerobic incubation for 24 h at 37 °C. The appearance of a typical Salmonella colonies on all growth plates was observed (Andrews et al., 2014).
2.8. Molecular characterization of Salmonella spp.
Because of the importance of Salmonella spp. as a potential poultry pathogen, molecular analysis of samples was conducted to confirm it. Initially, samples were pre-enriched overnight in Rappaport–Vassiliadis broth. Later, DNA extraction was performed using a DNA extraction kit (Promega, USA). Primer sequences of Salmonella inv A gene Salm 4 (5′-TCCCGGCAGAGTTCCCATT-3′), Salm 3 (5′-GCTGCGCGCGAACGGCGAAG-3′) were used for identification. Polymerase chain reaction was performed with an initial denaturation for 5 min (95 °C), followed by 35 cycles of denaturation at 95 °C (90 s), annealing at 62 °C (60 s), extension at 72 °C (90 s), followed by a final extension at 72 °C (7 min) in a Thermal Cycler Bio-Rad (Hercules, California, USA). Amplified products (389 bp) were separated on 2.0% agarose gel and bands were visualized with ultraviolet trans illumination.
2.9. Statistical analyses
Data analysis was performed using a one-way analysis of variance through SPSS, version 17.0 (IBM, Armonk, NY, USA). Multiple comparison of means was performed using post-hoc analysis with Tukey’s HSD test; significance was assumed at p < 0.05. The relationship between host weight and targeted bacterial genera was assessed using Pearson’s correlation coefficient (r) and P values through a simple linear regression analysis.
3. Results
3.1. Performance analysis
Group-wise analyses of performance parameters were recorded during the experiment (Table 2). At 21 days, non-significant differences (p > 0.05) in body weight, FI, and FCR were found in all groups; by contrast, at 42 days, significant differences in body weight, FI, and FCR (p < 0.05) were noted. Higher FI and FCR were observed in CON among all study groups. At 21 and 42 days, BWG (p < 0.05) was remarkably decreased in CON compared with treatment groups. At 42 days, significantly higher BWG (p < 0.05) and reduced FCR (1.89) were recorded in PHY among the study groups. In the case of FCR, non-significant differences were noted between ORG and COM groups (p > 0.05) at 42 days.
Table 2.
Effect of dietary treatments on performance parameters of broilers at 21 and 42 days.
| Traits | Groups* |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AB | ORG | PHY | COM | |||
| Initial body weight (g) | 42.3 | 42.4 | 42.6 | 42.5 | 42.8 | 0.012 | 0.970 |
| 0–21 days | |||||||
| Feed intake (g) | 1040b | 1057ab | 1056ab | 1086a | 1079a | 6.55 | 0.132 |
| Body weight (g) | 654b | 679ab | 690a | 704a | 695a | 6.02 | 0.068 |
| FCR (g:g) | 1.66a | 1.54a | 1.52a | 1.53a | 1.55a | 0.014 | 0.495 |
| 22–42 days | |||||||
| Feed intake (g) | 3940a | 3890ab | 3835c | 3807c | 3843bc | 13.1 | <0.001 |
| Body weight (g) | 1870c | 1960ab | 1940b | 2013a | 1991ab | 14.0 | 0.001 |
| FCR (g:g) | 2.12a | 1.99b | 1.95b | 1.82c | 1.90bc | 0.013 | <0.001 |
Values are mean of 50 birds/treatment.
SEM: standard error of mean.
Means with different supersript in the same row differ significantly at p < 0.05.
Groups; CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination.
3.2. Carcass characteristics
The addition of NGPs in diets significantly enhanced carcass characteristics (p < 0.05) in the treatment groups (Table 3). The highest dressing percentage of all groups were noted in the PHY group. All other carcass parameters (liver, heart, gizzard, abdominal fat, and breast meat) remained unaffected (p > 0.05) by the dietary changes. Furthermore, the inclusion of phytogenic feed additives alone and combined with organic acids increased carcass and breast meat percentages.
Table 3.
Effect of dietary treatments on carcass characteristics of broilers at 42 days.
| Traits† (%) | Groups* |
SEM | P-values | ||||
|---|---|---|---|---|---|---|---|
| CON | AB | ORG | PHY | COM | |||
| Dressing | 61.0d | 62.6cd | 63.5bc | 66.3a | 64.9ab | 0.533 | 0.004 |
| Liver | 1.92c | 2.23b | 2.35b | 2.51a | 2.56a | 0.050 | 0.212 |
| Heart | 0.433b | 0.443b | 0.523a | 0.576a | 0.577a | 0.018 | 0.620 |
| Gizzard | 2.09c | 2.35b | 2.44b | 2.82a | 2.61a | 0.077 | 0.439 |
| Breast meat | 20.2d | 22.4c | 22.6c | 24.8a | 23.3b | 0.377 | 0.163 |
| Abdominal fat | 1.34a | 1.38a | 1.35a | 1.42a | 1.41a | 0.015 | 0.867 |
SEM: standard error of mean.
These are mean values of five replicates (cages).
Groups; CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination.
Means with different superscript in the same row differ significantly at p < 0.05.
3.3. Biochemical parameters
Serum biochemical analysis of dietary changes revealed significant differences in cholesterol and the A/G ratio (p < 0.05) for all groups (Table 4). High cholesterol levels were noted in the CON group. The phytogenic feed additive and combination groups showed significantly minimized cholesterol levels (p < 0.05) in blood. A/G ratio was significantly higher in the treatment groups (p < 0.05) compared with CON. Among the groups, non-significant interactions linked with diet and blood parameters (p > 0.05) were observed for total protein, serum albumin, and globulin levels.
Table 4.
Effect of dietary treatments on biochemical parameters of broilers at 42 days.
| Traits (mg/dl)† | Groups* |
SEM | P-values | ||||
|---|---|---|---|---|---|---|---|
| CON | AB | ORG | PHY | COM | |||
| Cholesterol | 76.1a | 72.4b | 73.2b | 69.0c | 68.7c | 0.799 | 0.033 |
| Total protein | 3.55a | 3.22a | 3.37a | 3.55a | 3.68a | 0.298 | 0.221 |
| Serum albumin | 1.67b | 1.53c | 1.56c | 1.66b | 1.75a | 0.010 | 0.241 |
| Serum globulin | 1.60a | 1.35c | 1.27d | 1.48b | 1.34c | 0.047 | 0.103 |
| **A/G ratio | 0.91c | 1.13b | 1.20a | 1.15b | 1.26a | 0.034 | 0.021 |
SEM: standard error of mean.
The are mean values of five replicates (cages).
Means with different superscript in the same row differ significantly at p < 0.05.
Groups; CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination.
A/G ratio: albumin/globulin ratio.
3.4. Quantitative bacterial analyses
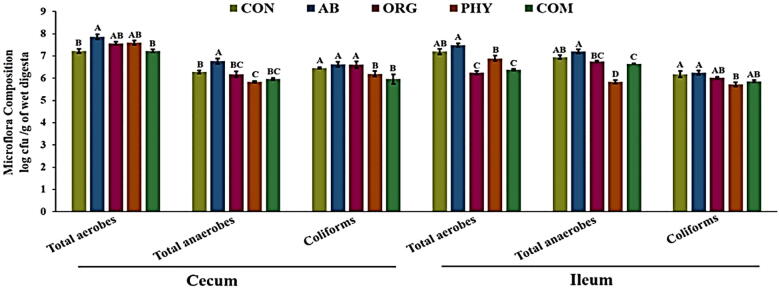
Microflora composition: The microbial counts result revealed a higher proportion of total aerobes in the CON and AB groups (p < 0.05) in both the caecum and ileum (Fig. 1). Similarly, in the caecum, an eminent number of total anaerobes were noticed in the CON and AB groups (log cfu/g 6.72 and 6.78, respectively), whereas lower counts of total anaerobes were observed in the ORG, PHY, and COM (log cfu/g 6.18, 5.92 and 5.96 respectively) groups. In the ileum, a significantly lower total anaerobe count (p < 0.05) was noticed only in the PHY group (log cfu/g 5.84). Coliforms, gram-negative bacteria, indicate a possible presence of harmful, disease-causing bacteria in the gut. In this study, the PHY group had lower coliform counts in the ileum region. Moreover, significantly lower coliform counts in the caecum COM group (p < 0.05) indicated the synergistic effects of organic acids and phytogenic feed additives in decreasing gut pathogens.
Fig. 1.
Microflora composition (log cfu/g) of study groups in broiler cecum and ileum regions at 42 days. Groups: CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination. A,B,C Bars (Means ± standard error of mean) with different letters within the same microbial group differ significantly at p < 0.05.
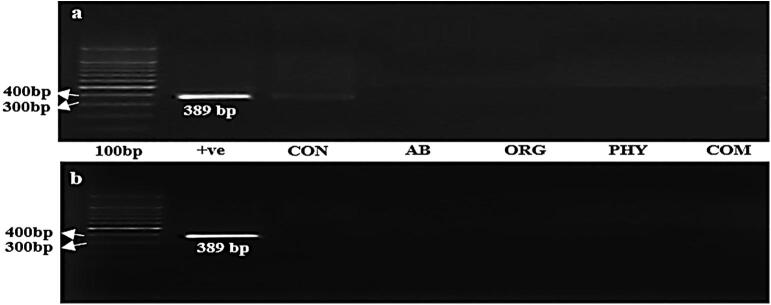
In addition, targeted beneficial and pathogenic gut bacterial counts of the study groups were performed in the caecum and ileum samples (Table 5). Lactobacillus spp. and Enterococcus spp. exhibited pronounced increases (p < 0.05) in the AB, ORG, PHY, and COM groups compared with the CON group in the caecum. In the ileum, increased counts in the treatment groups were noticed for Enterococcus spp., although no significant differences for Lactobacillus spp. (p < 0.05) were found among all student groups. An increased E. coli count was observed in the AB and ORG groups along with CON in both the caecum and ileum. By contrast, Campylobacter spp. was decreased significantly in the treatment groups (p < 0.05) compared with the CON group. Notably, in the caecum, significantly lower (p < 0.05) E. coli counts were obtained in the PHY group; however, no E. coli populations were detected in the ileum region. In addition, Campylobacter spp. was absent in both the PHY and COM groups. Notably, Salmonella spp. was not detected through conventional methods in either region, which was then validated through molecular analysis for Salmonella spp. detection. A caecum sample gel image (Fig. 2a) showed the presence of Salmonella spp. in the CON group with a lower band intensity. In the ileum (Fig. 2b), however, all samples were negative for Salmonella spp. in all groups. The lower band intensity in the CON sample compared with the positive control (Salmonella typhimurium ATCC 14028) indicated a healthy chicken gut.
Table 5.
Effect of dietary treatments on targeted bacterial genera composition (log cfu/g) in broiler at 42 days.
| Targeted bacteria | Groups* |
Statistics |
|||||
|---|---|---|---|---|---|---|---|
| CON | AB | ORG | PHY | COM | SEM | P-values | |
| Lactobacillus spp. | |||||||
| Cecum | 6.49b | 6.52b | 6.77ab | 6.91ab | 7.13a | 0.077 | 0.019 |
| Ileum | 6.03a | 6.65b | 6.31b | 6.80b | 6.47b | 0.095 | NS |
| Enterococcus spp. | |||||||
| Cecum | 6.51b | 7.21a | 7.21a | 7.30a | 7.37a | 0.096 | 0.015 |
| Ileum | 5.83b | 6.61a | 6.52a | 6.48a | 6.58a | 0.094 | 0.015 |
| Escherichia coli | |||||||
| Cecum | 6.49b | 7.13a | 7.14a | 6.31b | 6.38b | 0.114 | 0.013 |
| Ileum | 6.57a | 6.25b | 6.12bc | ND | 6.01c | 0.665 | <0.001 |
| Campylobacter spp. | |||||||
| Cecum | 6.43a | 6.79a | 6.54a | 6.48a | 6.03b | 0.073 | 0.012 |
| Ileum | 6.85a | 6.95a | 5.66b | ND | ND | 0.854 | <0.001 |
SEM: standard error of mean.
ND: Not detected.
Means with different superscript in the same row differ significantly at p < 0.05.
Groups; CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination.
Fig. 2.
Gel images of Salmonella spp. detection in cecum (a) and ileum (b) regions of broiler chickens. Groups: CON: control, AB: antibiotic growth promoter, ORG: organic acid, PHY: phytogenic feed additives, COM: combination. “+ve” = Positive control (Salmonella typhimurium, ATCC 14028).
Phenotypic characterization of gut bacteria: The targeted bacterial genera in this study were also phenotypically characterized (Table 6). Lactobacillus spp. colonies appeared round and opaque on MRS agar. E. coli was observed as characteristic metallic green sheen colonies, indicating lactose fermentation on eosin methylene blue agar. Campylobacter spp. Showed gray, watery colonies on Campylobacter selective agar. The microscopic analysis confirmed phenotypic identification to be gram-positive (Enterococcus spp. and Lactobacillus spp.) and gram-negative (E. coli and Campylobacter spp.). Biochemical analysis revealed the growth of Enterococcus spp. in 6.5% NaCl. Furthermore, E. coli produced catalase enzyme, which lacks cytochrome c oxidase, whereas Campylobacter spp. were positive for both enzyme production, respectively. E. coli has the ability to break down amino acid tryptophan to form indole, which after 24 h and the addition of Kovac’s reagent turns a pink colour, indicative of a positive result.
Table 6.
Phenotypic characteristics of targeted bacterial genera.
| Targeted bacteria | Morphological identification |
Biochemical identification |
|||||
|---|---|---|---|---|---|---|---|
| Gram stain | Morphology | Macroscopic appearance | Growth with 6.5% NaCl | Catalase test | Oxidase test | Indole test | |
| Lactobacillus spp. | + | Slender rods | Round, white | + | − | − | − |
| Enterococcus spp. | + | Spherical cocci | Smooth, black | + | − | − | − |
| Escherichia coli | − | Short rods | Round, metallic green | − | + | − | + |
| Campylobacter spp. | − | Curved rods | Flat, mucoid, irregular edges | − | + | + | − |
“+” = Growth/positive, “− “= No growth/negative.
3.5. Relationship between bacterial genera and body weight in chickens
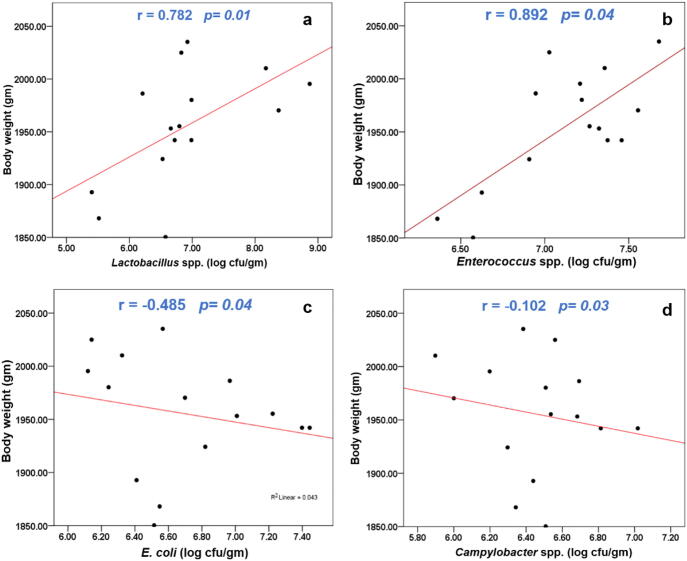
To elucidate the relationship between targeted bacterial genera and host weight gain, a simple linear regression analysis was performed (Fig. 3). Gut bacteria count either positively or negatively affected host weight gain. In particular, the beneficial bacteria Lactobacillus spp. (r = 0.782, p = 0.01) and Enterococcus spp. (r = 0.892, p = 0.04) were correlated positively with BWG (Fig. 3a and b), whereas the pathogenic bacteria, E. coli (r = − 0.585, p = 0.04) and Campylobacter spp. (r = − 0.102, p = 0.03) were negatively correlated with BWG in hosts (Fig. 3c and d).
Fig. 3.
The relationship between body weight and targeted bacteria (a) Lactobacillus spp., (b) Enterococcus spp., (c) E. coli, and (d) Campylobacter spp. in chickens. Simple linear regression was performed to assess relationship based on Pearson’s correlation coefficient (r) and P values.
4. Discussion
The results obtained in the current study illustrated that the addition of NGPs (organic acids and phytogenic feed additives) in the diets of chickens significantly enhanced BWG, decreased pathogenic bacterial load, maintained blood cholesterol levels and A/G ratio, and promoted the growth of beneficial bacteria, which was positively correlated with enhanced host weight gains without affecting the carcass yield.
Indeed, the profound role of dietary growth promoters on gut bacteria for enhancing broiler weight gain is the subject of intensive research. It is claimed that AGPs are being used in poultry to improve feed efficiency, body weight, and carcass yield; reduce mortality; and eventually enhance growth (Kumar et al., 2019). Similarly, AGPs tended to increase the FCR and body weight in broilers compared with a control group (Kumar et al., 2019). Interactions with gut microbial populations provide the basis for antibiotics to exert growth-promoting effects on bird health (Gadde et al., 2018). A germfree approach employing animal models suggested that antibiotics may alter the divergence and composition of microbial ecosystems in the gut, resulting in stable and balanced microbiota, which in turn improves growth performance (Lin, 2011). After the EU ban on in-feed antibiotics, various strategies have been considered as alternatives over the last decade. To achieve healthier and more economical poultry meat, equivalent results have been achieved using phytogenic feed additives and organic acids as growth promoters in broiler chicken diets (Ghaly et al., 2017, Karangiya et al., 2016).
Phytogenics are still in their infancy in terms of being used as feed additives, but we attempted to unravel their effects on maintaining gut microbiota composition and improving animal performance. This study revealed that phytogenic feed additives selectively inhibited E. coli and Campylobacter spp. with increased Lactobacillus spp. One possible mode of action of phytogenic feed additives is modulating the immune and oxidative defense systems along with promoting digestive enzyme secretion (amylase and proteases) to improve animal health (Kaschubek et al., 2018, Brenes and Roura, 2010). Hashemi and Davoodi (2010) reported that supplementation of phytogenic feed additives improved body weight, increased carcass response, and maintained feed efficiency in broilers. Najafi and Taherpour (Najafi and Taherpour, 2014) asserted that the immunomodulatory effects of cinnamon in broiler chickens on dietary inclusion levels of 0.4% (4 g/kg) and 0.8% (8 g/kg) improved FCR as well as enhanced hemoglobin concentration and lymphocyte proportions in the blood. Phytogenic feed additives increase the hydrophobicity of pathogenic bacterial species through modulating their cellular membrane, consequently affecting the surface properties of microbial cells. In this manner, they not only affect the virulence properties of harmful bacteria, but also play a crucial role in bacterial adhesion to mucosal cells, which is dependent on the hydrophobicity of microbial surface cells (Mohiti-Asli and Ghanaatparast-Rashti, 2018). The present study used a blend of different phytogenics, which were recognized to deferentially inhibit pathogenic bacteria and promote beneficial bacterial growth in broiler gut (Munir, 2015). Additionally, phytogenic feed additives prevent host gut inflammation, which might lead to reduced animal performance as well as economic losses. A blend of phytogenics (cinnamaldehyde, carvacrol, and capsicum) modified the Nrf2 and NF-κB pathways, provided protection against oxidative stress, and reduced inflammation, which in turn improved host health and growth performance (Yang et al., 2015).
Organic acids (also known as acidifiers) are weak acids with lower dissociation constants. Blends of organic acids have been used as feed additives in poultry as an alternative to antibiotics for a long time. They have distinct roles in enhancing immunity and nutrient digestibility, sustaining the gastrointestinal tract, and improving animal performance in broilers (Rodriguez-Lecompte et al., 2012, Brzoska et al., 2013). In this study, the ORG group exhibited inhibition of E. coli and Campylobacter spp. in the caecum and ileum. In their non-dissociated form (MCFA; e.g., lauric acids), they penetrate bacterial cell walls and disrupt usual physiological processes in the gut (Dhama et al., 2014). Mostly, the pathogenic bacteria E. coli, Campylobacter, and Salmonella spp. Is pH-sensitive; they are unable to tolerate pH gradients (internal and external), and hence, including organic acids in diets cause a reduction in pathogenic colonization in the gut and increases beneficial bacteria (Khan and Iqbal, 2016). The combination of acidifier complexes used in this study was highly enriched with lauric acid (C12) and contained both SCFAs and MCFA. MCFA exerts potential antibacterial effects in the gut against gram-positive bacteria (Clostridium spp.), unlike SCFAs, which mainly control gram-negative bacteria. The addition of SCFAs complements the activity of MCFA by creating pores in the cell membranes of gram-positive bacteria, which facilitates the penetration of MCFA (Ding et al., 2017). These findings are also related to the fact that the predominant bacterial genus in the ileum is Lactobacillus spp. Lactobacillus spp. was proven to dominate Campylobacter spp. in the ileum as a consequence of the production of inhibitory organic acids (Andreopoulou et al., 2014). In the gut, phytogenic feed additives and organic acids tend to increase the Lactobacillus spp. count and decrease E. coli and Campylobacter spp.
Regarding the relationship of body weight and gut microbiota, studies are limited; hence, the correlation between them (positive or negative) is ambiguous (Clarke et al., 2014, Delzenne and Cani, 2011). In broiler chickens, several bacterial genera are identified as performance-related bacteria based on dietary modulation (Torok et al., 2011). The findings of the present study attribute to the property of Lactobacillus spp. of producing SCFAs as a result of bacterial fermentation (Meimandipour et al., 2010). These SCFAs are involved in (1) lowering the pH, which changes the gut microbiota composition; and (2) preventing pH-sensitive pathogenic bacteria. These variations in the gut are significantly linked to increased host weight gain (He et al., 2019). In addition, Enterococcus spp. is positively correlated with increased BWG because it prevents pathogenic bacterial adhesion to mucosal walls through the “competitive exclusion” phenomenon. Campylobacter spp. is a well-known pathogen causing campylobacteriosis in broilers. In humans, it can cause food-borne illnesses; infected poultry has been shown to be the principal source of this zoonosis (Kashoma et al., 2019). The present study observed that Campylobacter spp. and E. Cole were negatively correlated with host weight gain along with variation in the feed.
5. Conclusion
In short, microbial diversity in the caecum and ileum regions of broiler chicken’s gut is positively responsive to changes in diet. Growth performance, carcass characteristics, and biochemical and quantitative bacterial analyses revealed that variation in diet enhanced performance-related bacteria in the gut. In chicken guts, phytogenic feed additives and organic acids tend to increase the Lactobacillus spp. count and decrease E. coli and Campylobacter spp. Decreased targeted pathogenic bacterial growth in the NGP (ORG, PHY, and COM) groups together with an increased host weight gain with increased feed efficiency. Hence, safe, healthier, and economically broiler production can be achieved using these feed additives without compromising body weight. However, the underlying relationships of these additives with increased beneficial gut bacteria remain a subject for elucidative research in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors like to acknowledge Ideal feeds and experimental units, superhighway, Karachi for providing the animal house facility. The authors (SM and KAAG) express their sincere appreciation to the Research Supporting Project No. RSP-2020-93 the King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shahid Mahboob, Email: mushahid@ksu.edu.sa.
Saddia Galani, Email: sadia.galani@kibge.edu.pk.
References
- Al-Asmakh M., Zadali F. Use of germ-free animal models in microbiota-related research. J. Microbiol. 2015;25:1583–1588. doi: 10.4014/jmb.1501.01039. [DOI] [PubMed] [Google Scholar]
- Andreopoulou M.V., Tsiouris I., Georogopoulou I. Effects of organic acids on the gut ecosystem and on the performance of broiler chickens. J. Hellenic Veterinary Med. Soc. 2014;65:289–302. doi: 10.1111/jpn.12858. [DOI] [Google Scholar]
- Andrews, W., Jacobson, A., Hammack, T., 2014. Salmonella. In: Bacteriological Analytical Manual (BAM), 8th ed. Center for Food Safety and Applied Nutrition, U.S. FDA, College Park, MD.
- AOAC, 2005. Official Methods of Analysis. 18th rev. ed. Association of Official Analytical Chemists International. Gaithersburg, MD, USA.
- Aviagen . Aviagen; Midlothian, Scotland: 2014. Ross 308 – Broiler Nutrition Specification. [Google Scholar]
- Ballongue J. Technical problems related to in vitro study of colon flora. Scand. J. Gastroenterol. 1997;32(sup222):14–16. doi: 10.1080/00365521.1997.11720710. [DOI] [PubMed] [Google Scholar]
- Boguslawska-Tryk M., Szymeczko R., Piotrowska A., Burlikowska K., Ślizewska K. Ileal and cecal microbial population and short-chain fatty acid profile in broiler chickens fed diets supplemented with Lignocellulose. Pakistan Veterinary J. 2015;35:212–216. [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. doi: 10.1016/j.anifeedsci.2010.03.007. [DOI] [Google Scholar]
- Brzoska F., Śliwinski B., Michalik-Rutowska O. Effect of dietary acidifier on growth, mortality, post-slaughter parameters and meat composition of broiler chickens/Wpływ zakwaszacza diety na masę ciała, śmiertelność, wydajność rzeźną i skład mięsa kurcząt rzeźnych. Ann. Anim. Sci. 2013;13:85–96. doi: 10.2478/v10220-012-0061-z. Open access. [DOI] [Google Scholar]
- Clarke S.F., Murphy E.F., O’sullivan O., Lucey A.J., Humpherys M., Hogan A., Hayes P., Oreilly M., Jeffery I.B., Wood-Martin R. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- Dahiya D.K., Reuka M.P., Shandilya U.K., Dhewa T., Kumar N., Kumar S., Puniya A.K., Shukla P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front. Microbiol. 2017;8:563. doi: 10.3389/fmicb.2017.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzenne N.M., Cani P.D. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu. Rev. Nutr. 2011;31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- Dhama K., Tiwari R., Khan R.U., Chakaraborty S., Gopi M., Karthik K., Saminathan M., Desingu P.A., Sunkara L.T. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trends and advances-a review. Int. J. Pharmarmacol. 2014;10:129–159. doi: 10.3923/ijp.2014.129.159. [DOI] [Google Scholar]
- Ding X., Yang C.W., Yang Z.B., Yang W.R., Jiang S.Z., Wen K.E. Effects of feed acidifiers on growth performance, caecum microflora and nutrient and energy utilisation in broilers. Eur. Poultry Sci. 2017;81:1–13. doi: 10.1399/eps.2017.180. [DOI] [Google Scholar]
- Gadde U.D., Oh S., Lillhoj H.S., Lillehoj E.P. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci. Rep. 2018;8:3592. doi: 10.1038/s41598-018-22004-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ghaly M.H., Elghoneimy A.A., Mohamed H.K., Ali M.F. Biochemical and histopathological effects of dietary supplementation of Nigella sativa and Mentha piperita oils to broilers. J. Adv. Vet. Res. 2017;7:7–15. [Google Scholar]
- Han G.G., Kim E.B., Lee J., Lee J.Y., Jin G., Park J., Huh C.S., Kwon I.K., Kil D.Y., Choi Y.J., Kong C. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. SpringerPlus. 2016;5:911. doi: 10.1186/s40064-016-2604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S., Davoodi H. Phytogenics as new class of feed additive in poultry industry. J. Anim. Veterinary Adv. 2010;9:2295–2304. doi: 10.3923/javaa.2010.2295.2304. [DOI] [Google Scholar]
- He T., Zhu Y.H., Yu J., Xia B., Liu X., Yang G.Y., Su J.H., Guo L., Wang M.L., Wang J.F. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 2019;230:187–194. doi: 10.1016/j.vetmic.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Karangiya V.K., Savsani H.H., Patil S.S., Garg D.D., Murthy K.S., Ribadiya N.K., Vekariya S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Veterinary World. 2016;9:245–250. doi: 10.14202/vetworld.2016.245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschubek T., Mayer E., Rzesink S., Grenter B., Bachinger D., Schieder C., Kong J., Teichmann K. Effects of phytogenic feed additives on cellular oxidative stress and inflammatory reactions in intestinal porcine epithelial cells. J. Anim. Sci. 2018;96:3657–3669. doi: 10.1093/jas/sky263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashoma, I.P., Srivatava V., Rajshekara G., 2019. Advances in vaccines for controlling Campylobacter in poultry. In: Food Safety in Poultry Meat Production. Springer, Cham. pp. 191–210.
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. doi: 10.1080/09712119.2015.1079527. [DOI] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutrit. Res. Rev. 2013;26:71–88. doi: 10.1017/S095442241300004. [DOI] [PubMed] [Google Scholar]
- Kumar D., Pornsukarom S., Thakur S. Food Safety in Poultry Meat Production. Springer; Cham.: 2019. Antibiotic usage in poultry production and antimicrobial-resistant Salmonella in poultry; pp. 47–66. [Google Scholar]
- Lee S.A., Apjalhti J., Vienola K., Gonzalez-Ortiz G., Fontes C., Bedford M. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim. Feed Sci. Technol. 2017;234:29–42. doi: 10.1016/j.anifeedsci.2017.07.017. [DOI] [Google Scholar]
- Lin J. Effect of antibiotic growth promoters on intestinal microbiota in food animals: a novel model for studying the relationship between gut microbiota and human obesity? Front. Microbiol. 2011;2:53. doi: 10.3389/fmicb.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T.M., Bravo D., Maddox C.W., Pettigrew J.E. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Anim. Sci. 2014;92:3426–3440. doi: 10.2527/jas.2013-6496. [DOI] [PubMed] [Google Scholar]
- Madal R.S., Saha S., Das S. Metagenomic surveys of gut microbiota. Genome Proteom. Bioinform. 2015;13:148–158. doi: 10.1016/j.gpb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcglone J. third ed. Federation of Animal Science Societies; 2010. Guide for the Care and Use of Agricultural Animals in Research and Teaching. [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A., Azhar K., Hair-Bejo M., Kabeir B., Javanamard A., Anas O.M., Yazid A. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Comparing the effects of a combined phytogenic feed additive with an individual essential oil of oregano on intestinal morphology and microflora in broilers. J. Appl. Anim. Res. 2018;46:184–189. doi: 10.1080/09712119.2017.1284074. [DOI] [Google Scholar]
- Mueller K., Blum N.M., Kluge H., Muller A.S. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic-and antioxidant enzymes in broiler chickens. Br. J. Nutr. 2012;108:588–602. doi: 10.1017/S0007114511005873. [DOI] [PubMed] [Google Scholar]
- Munir M.T. Effect of garlic on the health and performance of broilers. Veterinaria. 2015;3:32–39. doi: 10.3923/ijps.2017.515.521. [DOI] [Google Scholar]
- Murshed M., Abudabos A. Effects of the dietary inclusion of a probiotic, a prebiotic or their combinations on the growth performance of broiler chickens. Brazilian J. Poultry Sci. 2015;17:99–103. doi: 10.1590/1516-635XSPECIALISSUENutrition-PoultryFeedingAdditives099-104. [DOI] [Google Scholar]
- Najafi S., Taherpour K. Effects of dietary ginger (Zingiber Ofjicinale), cinnamon (Cinnamomum), synbiotic and antibiotic supplementation on performance of broilers. J. Anim. Sci. Adv. 2014;4:658–667. [Google Scholar]
- Rodriguez-Lecompte J.C., Yitbarek A., Brady J., Sharif S., Cavnagh M., Cow G., Gunter W., House J., Camelo-Jamies G. The effect of microbial-nutrient interaction on the immune system of young chicks after early probiotic and organic acid administration. J. Anim. Sci. 2012;90:2246–2254. doi: 10.2527/jas.2011-4184. [DOI] [PubMed] [Google Scholar]
- Sanchez B., Delgado S., Blanco-Miuguez A., Lourenco A., Gueimonde M., Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mole. Nutrit. Food Res. 2017;61(1) doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- Sommer F., Backhed F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Hughes R.J., Mikkelsen L.L., Perez-Maldonado R., Balding K., Macalpine R., Percy N.J., Ophel-Keller K. Identification and characterization of potential performance related gut microbiota in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011;77:5868–5878. doi: 10.1128/AEM.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009;11;1(6):6ra14. doi: 10.1126/scitranslmed.3000322.U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandehaar M.J., Armentano L.E., Weigel K., Spurlock D.M., Tempelman R.J., Veerkamp R. Harnessing the genetics of the modern dairy cow to continue improvements in feed efficiency. J. Dairy Sci. 2016;99:4941–4954. doi: 10.3168/jds.2015-10352. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M.A., Huo Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Yin X., Wang X., Lei Z., Wang M., Guo Y., Aggrey S.E., Nie W., Yuan J. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018;12(9):24. doi: 10.1186/s40104-018-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Lei F., Zhu L., Li S., Wu Z., Zhang R., Gao G.F., Zhu B., Wang X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010;4(3):367–376. doi: 10.1038/ismej.2009.128. [DOI] [PubMed] [Google Scholar]