Abstract
Recent years Klebsiella pneumoniae (K. pneumoniae) biofilm formation (BF) is emerging thread worldwide. For tackling this problem, we have chosen Hibiscus rosa-. pneumoniae. The HPLC purified essential oils (EOs sinensis (H. rosa-sinensis) (HRS) to inhibit the BF K) of H. rosa-sinensis was performed against BF K. pneumoniae and showed concentration dependent biofilm inhibition. At the MBIC of EOs (90 µg/ml), the biofilm inhibition was showed at 92% against selected BF K. Pneumoniae. The biofilm metabolic assay, exopolysaccharide quantification and hydrophobicity index variation results exhibited with 88%, 92% and 89% reduction at 90 μg/mL was observed respectively. In addition, the morphological modification of MBIC treated K. pneumoniae was clearly viewed by scanning electron microscope (SEM). Overall, all the invitro experiments result were confirmed that the MBIC of H. rosa-sinensis EOs was very effective against BF K. pneumonia.
Keywords: Biofilm bacteria, Medicinal plant, Essential oil, Anti-biofilm activity, Exopolysaccharide, Scanning electron microscope
1. Introduction
Recent years, biofilm forming (BF) Gram negative bacteria (GNB) has attracted more attention due to their potential risk of antimicrobial resistance and virulence factor production (Rubini et al., 2018). Biofilm formation now being considered as a serious problem and it can be developing the resistant against antiseptic agents (Doulgeraki et al., 2017, Rajivgandhi et al., 2018a). In fact, the discovery of novel antimicrobial agents for eradicate the biofilm formation is a worldwide challenge. Recently, plant essential oil has more potential for solving the problem of biofilm producing pathogens (Francesco et al., 2011, Eman et al., 2018). EO acts as an excellent antimicrobial agent due to the low viscosity, high capability, excellent surface nature, which allows them to interact with target (Norhaizan et al., 2010, Rajivgandhi et al., 2018a).
In this context, we have chosen excellent biomedical properties of HRS for inhibit the BF Klebsiella pneumoniae (K. pneumoniae). Among the various parts of HRS, the petals, flowers and seeds are frequently produced more therapeutic advances such as antibacterial, antifungal, antiviral, anticancer, larvicidal, antidiabetics, antioxidant properties (Kartinah et al., 2019, Lingesh et al., 2018, Maralit Bruan and Tianco, 2019, Elemar Gomes et al., 2010, Gandhi et al., 2019). In addition, antihypertensive, anti-inflammatory, antipyretic, antidiarrhoeic and immunomodulator properties of the HRS have been screened from various pharmaceutical industry (Begum and Younus, 2018, Abdullah et al., 2019, Malinowski et al., 2019, Eman et al., 2018, Rubini et al., 2018).
In India, the petals and roots of the Hibiscus rosa-sinensis (HRS) can be used as food and fiber (Kaleemullah et al., 2017). The calyces or flower pots are used as ingredients in various edible items jam, candy, pickles and also used in drinking items including tea, wine (Ruban and Gajalakshmi, 2012). Among this plant, red flowered HRS is mostly used in our entire world due to the production of some essential chemicals (Pillai and Mini, 2018). Among these species, 15 species of HRS have more biological activities against drug resistant pathogens were reported with some phytochemical evidences (Vijayakumar et al., 2018). Recently, the ethanopharmacological survey was reported that the HRS as a important medicinal plant that has the anti-asthmatic, detoxifier, anti-hypertensive and anti-cancer, wound healing properties. In addition, it is a traditional medicine in India for anti-diabetics and anti-oxidant activities (Ansari et al., 2020).
The seeds of the HRS are mostly used for oil production in many countries, and phytochemical compositions of the oils are applied in various biological process. In the daily meals of West Africa, the HRS leaves and powders are used frequently (Hui-Min et al., 2017). In addition, the seeds of the HRS is applied to recover high quantifies in various industries like pharmaceutical and food. The HRS oils are mainly synthesized from plastids (Elemar Gomes et al., 2010). Also, the HRS oil composed with tremendous chemical compounds including terpenes, fats and flavonoid aglycones (Gandhi et al., 2019). In addition, the more polysaccharide composition of the HRS as a format of secretory idioblasts oil or mucilage, which were associated with the parenchyma of HRS. Plant mucilages are complex polysaccharide polymers, it has high molecular weight. All the mucilages are either acidic or neutral polysaccharides (Lingesh et al., 2018). Hundred years, EOs as a natural medicine for various serious infections including multi drug resistant bacterial infections. Previously the reported Origanum majorana, Thymus zygis, Rosmarinus officinalis, Juniperus communis and Zengiber officinale Eos have excellent inhibitory effect against biofilm formation (Rihab et al., 2019). Eos has very low toxicity compared with other phytochemical compounds and also, it damaged the bacterial cell wall through hydrophobic channels. Finally, it could enter the inside of the cells and destroy the cell cycle process and cytoplasmic leakages materials (Erika et al., 2019). Therefore, the current study was initiated an attempt to assess a Chinesh medicinal plant H. rosa-sinensis EOs as a potential anti-biofilm agent against CR biofilm forming K. pneumoniae.
2. Materials and methods
2.1. Collection of samples
The BF K. pneumoniae strain was obtained from Department of Marine Science Bharathidasan University, Tiruchirappalli, Tamil Nadu, and India. All the chemicals, plates and antibiotic discs of this study were purchased from Sigma Aldrich, China.
2.2. Purification of EO and hydrosol extraction
The healthy seeds of HRS were collected from tropical environment of Bharathidasan University campus, Tamil Nadu, India. The collected plant seeds were stored at 4 °C for further use. To remove the surface contaminants, the seeds were carefully washed with double distilled water (D.DH2O) and dried at room temperature with shade for approximately 10 days. After incubation, the seeds were crushed well and maintained in hydro distillation using n-hexane (Jessica et al., 2018). In this method, 100 g of topped sample was mixed into the 1 L water of conical flask at 3–4 h distilled. After time interval, the obtained essential oil solution was maintained in sodium sulfate for drying and filtered for excluding the n-hexane under reduced pressure.
2.3. Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis of H. rosa-sinensis EO was followed by the previous report of Ruhomally et al. (2015). Briefly, 1L of the sample was mixed with 1% dichloromethane which injected with split ratio of 1:20 using EI mode of 70 eV (Agilent technologies) attached with split injector and 220 °C as an injector temperature. Continuously, 5% phenyl followed by 95% dimethylpolysiloxane with 30 m × 0.25 mm × 0.25 μm of HP-5MS was used for separate the chemical components. 1 mL/min of helium carrier gas was used for this process and 60 to 240 °C at a rate of 3 °C min−1 as a oven temperature. Finally, 1 μL of EO was used as a injected volume and diluted with chloroform (1:10).
2.4. Anti-bacterial activity
Anti-bacterial activity of the purified EO was evaluated against selected BF K. pneumoniae by agar well diffusion method (Ebani et al., 2018). Briefly, the 24 h old BF K. pneumoniae culture was spread on the muller hinton agar plate (MHA). The wells were cut by using sterile gel borer and different concentration 10, 25, 50 μL of EO was added into the well. Whereas, 10 μL of methanol and third generation cephalosporin ceftazidime was served as a positive controls and methanol added well was acted as a negative control at 37 °C for 24 h. After incubation, the zone of inhibition around the wells was noted in diameter and the experiment was conducted in triplicate.
2.5. Purification of essential oil by preparative high performance liquid chromatography (HPLC) method
Anti-bacterial effect of the EO was measured by analytical HPLC for detection of active EO fraction (AEOF). The mobile phase of acetonitrile:methanol:ammonium acetate:water (45:10:10:35) was used for purify the EOs. After, all essential oil fractions were purified separately using preparative HPLC. The agar well diffusion method was used to detect the purified EOs fraction at 37 °C for 24 h. Whereas, third generation chaphalosporin chephalosporin piperacillin/tazobactam and methanol was served as a positive and negative control respectively. Finally, active essential oil fraction (AEOF) was separated by preparative HPLC and proved by analytical HPLC followed by lyophilization at 40 °C for study. The instrument of preparatory HPLC was set up with 150 mm × 4.6 mm of C18 column, linear gradient of 4.6 µm and 1 mL/min flow rate was used. The temperature of 0–10 min with 10–90% A, 90–100% B and 100% C at 10–11 min, 11–20 min and 15–25 min were maintained in gradient elution program. The 20 µL column injected volume at 40 °C temperature was used (Rajivgandhi et al., 2018b).
2.6. Minimum biofilm inhibition concentration (MIC)
The biofilm eradication of BF K. pneumoniae at different concentration of AEOF was identified by crystal violet staining assay with some modification (Jardak et al., 2017). Briefly, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 μg/mL of EOs was added into previously filled TSB containing 24-well polystyrene plate and subsequently inoculated 100 μg/mL of 10-5 bacterial suspension at 37 °C for 24 h. After incubation, the discarded 24-well plate was rinsed thoroughly with PBS, followed by 0.5% crystal violet solution at 5 min. Next, unstained cells were washed to discard by PBS and solubilized with 2 mL of ethanol and quantified by O.D at 540 nm. Untreated bacterial culture containing well acted as a control.
All the procedure was applied three times and given formula was used to calculate the percentage of biofilm inhibition (PI),
| (1) |
2.7. Biofilm metabolic assay
The viability of bacterial cells in the presence of AEOF at various concentration was detected by 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5- carboxanilide (XTT) reduction method with the modification of Anjugam et al., 2018. Briefly, after removal of non-adherent biofilm cells, 1 mg/mL mixed XTT solution (12.5) was gently added into the 6-well plate. Subsequently, 1 μL of fresh standardized menadione acetone solution was added into each well with 100 μL of PBS. Each well were shaken gently and incubated with dark condition at 1 h at 37 °C. Whereas, without addition of AEOF in the pathogen with menadione acetone solution containing well served as control. After incubation, all the wells were calculated by using microtitre plate reader at 570 nm. The test was conducted in triplicate and the result was presented using following Eq,
| (2) |
2.8. Exopolysaccharide (EPS) quantification assay
The phenol-sulfuric acid method was adopted in this study to detect the EPS in BF K. pneumoniae by Maruthupandy et al. (2020) with some alteration. The BIC of AEOF treated or untreated K. pneumonia biofilm culture was collected and centrifuged at 5000 rpm for 10 min. For enzymatic degradation, the pellet was re-suspended in sterile saline solution, followed by 50 μL of Pronase E (Hi-Media, India) and vortexed at 30 °C for 30 min. After, 100 μL of trichloroacetic acid was inoculated into precipitate of protein at ice with 1 h. Subsequently, sample was centrifuged at 10,000 rpm for 10 min with cooling temperature. The 10 mL of cold absolute alcohol was added drop wise to precipitate the polysachaaraide quantitatively and kept at −20 °C for 12 h. After incubation, the sample was centrifuged at 5000 rpm for 20 min for collection of polysaccharides and resuspended in 1 mL of D·H2O Further, the sample was digested with 5 mL of H2SO4 (98%) into the monosaccharides with continuous vortex. After vortex, 5% phenol (1 mL) was added into recovered material and vortexed for 20 min in water bath. Samples were cooled on ice, and result was read at spectrophotometer (UV-2550, Shimadzu, Japan) at 540 nm wavelength. Dis·H2O served as a blank. In this method, glucose as a standard. The experiment was conducted in triplicate and the result was calculated in inhibition percentage of EPS with following formula
| (3) |
where Ca is t concentration of the EPS in the sample group, Cb is the concentration of the EPS in the control group.
Further, the inhibition of EPS was validated through the inoculation of CRA plate result (Rajivgandhi et al., 2018b). 0.1 mL of 24 h treated culture was directly streaked on the CRA plate at 37 °C for 24 h. After incubation, the CRA plate colonies were calculated based on color variation.
2.9. Hydrophobicity index of EO
The effect of AEOF against BF K. pneumonia hydrophobicity was evaluated by Balasubramanian et al. (2016). Briefly, the BIC of AEOF was added in to the test tube with addition of 2 mL old BF K. pneumoniae culture. Whereas, absence of AEOF was maintained as a control. Then, the sample was measured at 560 nm. Conservatively, 1 mL of misquote sample was mixed 2 min with toluene and vortex vigorously at 37 °C for 10 min to allow the phase separation. After incubation, both the phases were collected separately and aqueous phase was calculated at 560 nm using spectrophotometer and percentage of hydrophobicity was measured using following formula,
| (4) |
2.10. Morphological damage effect of EO
The shape of the BF K. pneumoniae morphology upon AEOF treatment was analyzed by SEM and TEM (Mehran et al., 2019). Shortly, the pellet of AEOF treated or untreated biofilm sample was received after centrifugation and washed thrice with 1% PBS. Required amount of suspended culture was taken and inoculated on the sterile cover glass, and the sample was fixed by 4% glutaraldehyde for 4 h. After incubation, the sample was washed two times with sterile PBS. After incubation, the fixed cells were filtered by polycarbonate membrane filters and dehydrated with 30, 40, 50, 60, 70, 80, 90 and 100% ethanol graded series. After dehydration, 1 mL of t-butanol was used and incubated at 1 h for bacterial survival. Next, the t-butanol was altered by fresh t-butanal and incubated in deep freezer with overnight. Further, the cover glass was dried at room temperature and coated with gold–palladium metal. Finally, cover glass was viewed under SEM using an accelerating voltage of 20 kV (Shimadzhu, Japan).
3. Result and discussion
3.1. Purification of essential oil
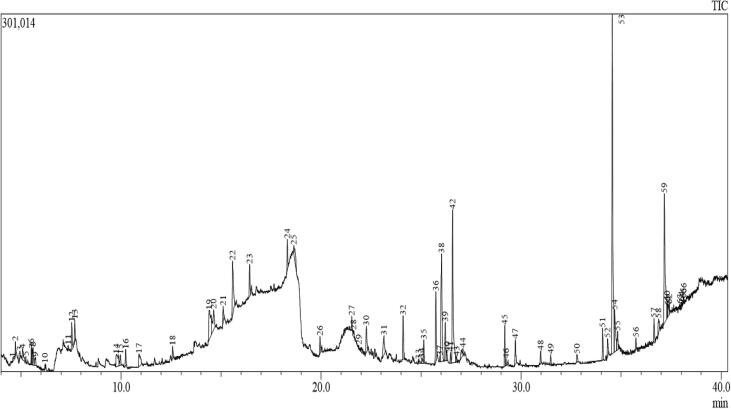
The hydrosol extraction of EO from H. rosa-sinensis more terpenes, oxygenated monoterpenes, monoterpenes hydrocarbon and sesquiterpenes composition by GC-MS (Fig. 1). The highest constituents of the EO were α-terpineol, α-terpinolene, α-pinene, β-pinene, α-terpenyl acetate, spathulenol. Significant amount of other variables were also present in the extract (Table. 1). Present result was agreed by previous report of Norhaizan et al. (2010), tropical plant H. rosa-sinensis has more oil components were determined by various genetic factors changes. Other promoter and various factors could modify chemical components including soil, fertility, pH, temperature, humidity and other stresses (Kandhare et al., 2012, Da-Costa-Rocha et al., 2014). Previously Malinowski et al., 2019, Chou et al., 2019, reported that the EO production depends on the seasonal variation and various environmental factors. It also increases the common volatile compounds natures due to the stress condition. In our study, the GC-MS report of common volatile and phytochemical compounds were also identified and reported in Table 1.
Fig. 1.
Detection of essential oil identification from H. rosa-sinensis by GC-MS analysis.
Table 1.
GC-MS analysis of essential oil form Hibiscus rosa-sinensis extract.
| Peaks | Essential Oil | RT | Molecular Formula | Total Area | Area (%) |
|---|---|---|---|---|---|
| 1 | α-Oxide pinene | 5.322 | C10H16O | ||
| 37,933 | 0.14 | ||||
| 2 | N-Nonanol | 5.475 | C9H20O | 138,413 | 0.52 |
| 3 | Octanoic acid | 5.512 | C8H16O2 | ||
| 121,073 | 0.46 | ||||
| 4 | N-Dodecane | 5.55 | C12H26 | ||
| 136,470 | 0.51 | ||||
| 5 | Iso-dihydro carveol | 5.672 | C10H18O | 122,056 | 0.46 |
| 6. | Decanoic acid | 7.498 | C10H20O2 | ||
| 288,301 | 1.09 | ||||
| 7 | β-biotol | 7.655 | C12H24O11 | ||
| 521,376 | 1.96 | ||||
| 8 | α-Atlantone | 7.787 | C15H22O | 393,782 | 1.48 |
| 9 | α-Costol | 7.903 | C15 H24 O | 285,272 | 1.07 |
| 10 | α-Cadalene | 8.045 | C15H18 | ||
| 109,955 | 0.41 | ||||
| 11 | α-Pinene | 8.364 | C10H16 | 34,370 | 0.13 |
| 12 | α-terpineol | 8.711 | C10H18O | 72,061 | 0.27 |
| 13 | Globulol | 9.745 | C15H26O | 552,913 | 2.08 |
| 14 | α-Thujene | 9.965 | C10H16 | 33,163 | 0.12 |
| 15 | β-Pinene | 13.725 | C10H16 | 47,551 | 0.18 |
| 16 | β-myrcene | 13.791 | C10H16 | 53,675 | 0.2 |
| 17 | 1,8-Cineole | 14.511 | C10H180 | 1,877,333 | 7.07 |
| 18 | Trans-p-menth-en-1-ol- | 14.775 | C10H18O | 52,819 | 0.2 |
| 19 | Cryptone | 15.571 | C9H14O | 390,658 | 11.47 |
| 20 | β-Elemene | 16.413 | C15H24 | 465,132 | 12.75 |
| 21 | Aromadendrene | 16.742 | C15H24 | 45,940 | 0.17 |
| 22 | Spathulenol | 17.477 | C15H24O | ||
| 177,889 | 0.67 | ||||
| 23 | Aromadendrene | 19.744 | C15H24 | 87,813 | 0.33 |
| 24 | Dedecanoic acid | 19.944 | C10H20O2 | 184,247 | 0.69 |
| 25 | Oleic acid | 20.190 | C18H34O2 | 726,013 | 2.74 |
| 26 | Cyclononasilodane | 20.370 | C10H30O5 | 1,765,843 | 26.65 |
| 27 | Β-Asarone | 20.565 | C12H16O3 | 2,406,180 | 19.07 |
| 28 | Triacetin | 20.625 | C9H14O6 | 465,754 | 11.75 |
| 29 | Ascaridol | 20.672 | C10H16O2 | 977,430 | 3.68 |
| 30 | Carvacrol | 20.807 | C10H14O | 4,241,491 | 15.98 |
| 31 | Isoascaridole | 20.975 | C10H16O2 | ||
| 193,562 | 14.5 | ||||
| 32 | Napthalene | 20.456 | C10H8 | ||
| 454,750 | 0.14 | ||||
| 33 | p-Cyamene | 20.670 | C10H14 | 7,126,016 | 10.50 |
3.2. Anti-bacterial activity
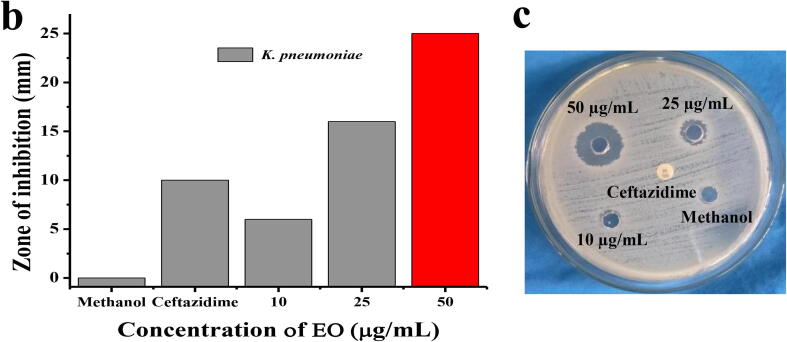
After 24 h incubation, the EO loaded discs exhibited 6, 16, 25 mm zone of inhibition against tested BF K. pneumoniae at 10, 25 and 50 µg/mL concentration were observed respectively (Fig. 2a, b). Whereas, 10 µg/mL of third generation chephalosporin antibiotic was exhibited 10 mm zone of inhibition. In addition, the positive control of methanol containing well did not produced any zone in plate. The result suggested that the H. rosa-sinensis essential oil has excellent anti-bacterial activity against BF K. pneumoniae. The anti-bacterial activity of H. rosa-sinensis EO against GPB and GNB have been described in previous studies of Da-Costa-Rocha et al., 2014, Kartinah et al., 2019, also, excellent anti-bacterial compounds were also present in the purified extract. Some reports identified the MIC of bioactive compound from plant purified compound has high concentration. In the present study, the tested range was minimum 10 µg/mL and maximum 50 µg/mL with excellent anti-bacterial activity against BF K. pneumoniae. Recently, Lingesh et al. (2018), reported that the H. rosa-sinensis essential oil has excellent anti-bacterial activity against MDR of GNB by different environmental factors such as temperature, pH, NaCL and other stresses. Previously, cineole, β-biotol, α-atlantone, α-terpiniol has excellent anti-bacterial activity when compared with bioactive compound (Gandhi et al., 2019). Mechanistically, anti-bacterial activity of some petals mediated compounds have more capacity to elevate the permeability to fat soluble compounds, while the bioactive compound constituents were also present highly in inside of the essential oil with low efficiency. They may play active role in inside of the bacterial that leads to cell wall damage, QS inhibition, enzyme alteration and biofilm inhibition activity (Rubini et al., 2018, Francesco et al., 2011).
Fig. 2.
Anti-bacterial activity differentiation of crude essential oil (a) and zone of inhibition in MHA plate (b) against CR K. pneumoniae.
3.3. Purification of active molecules from essential oil
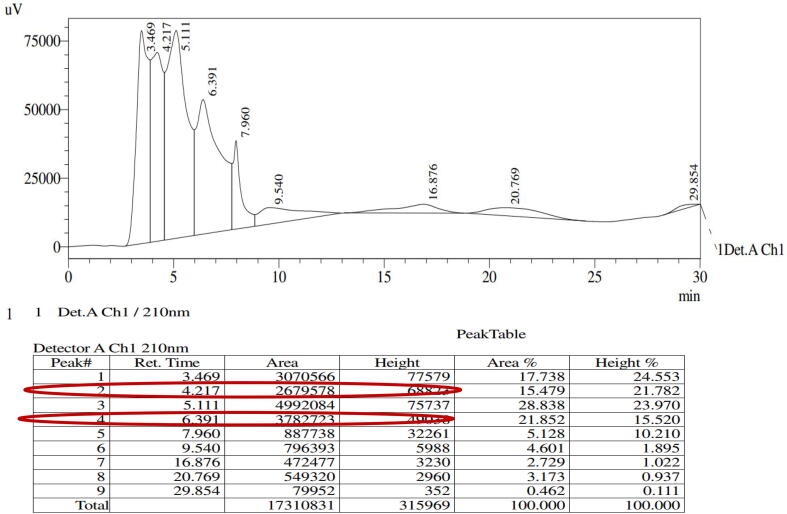
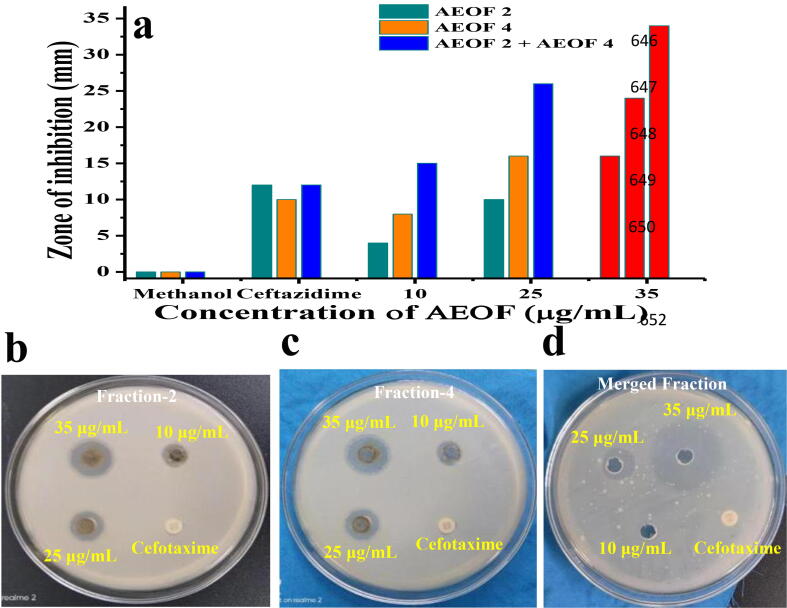
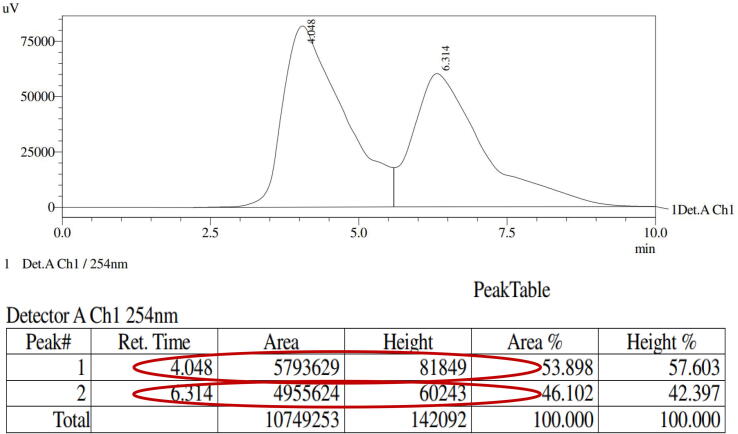
The preparative HPLC method was used to purify the active anti-bacterial EOs fraction, and the purified fraction was scanned by analytical HPLC. Based on the retention time, percentages of area and percentages of height, 9 different compound peaks (fractions) were identified from the purified essential oil. All the peaks and their retention time, occupation, height and area percentage of analytical HPLC result was inserted in table format (Fig. 3). Among the 9 AEOF, fractions 2 and 4 exhibited 16 and 24 mm zone of inhibition against CRBF K. pneumonia at 35 μg/mL was observed (Fig. 5b, c). These two fraction peaks occupied the most of the area and height percentages was 5,793,629, 4,955,624 and 15.479, 28.838 observed respectively. These were comparatively very high than other fraction peaks. These two fractions were further purified separately by preparative HPLC using same mobile phase, retention, flow rate, temperature and fraction time. It was confirmed by analytical HPLC. Both the peaks and their retention time, occupation, height and area percentage of analytical HPLC result was inserted in table format (Fig. 4). Finally, both the fractions were mixed together and exhibited 30 mm zone of inhibition at 35 μg/mL was observed (Fig. 5d). The differentiation between all the purified fractions was available in Fig. 5a. This purification result also suggests, the EO was surrounded with some bioactive compounds constituents that present inside of the essential oil. Therefore, the present result confirmed that the plant H. rosa-sinensis could play an effective role against biofilm producing CRBF K. pneumonia.
Fig. 3.
Analytical HPLC fractions of purified H. rosa-sinensis essential oil.
Fig. 5.
Evaluation of purified active fractions of essential oil against biofilm forming K. pneumoniae at various concentration (a), anti-bacterial activity of preparative HPLC fraction 2 (b), fraction 4 (c) and merged fraction (d) of essential against biofilm forming K. pneumoniae by agar well diffusion method.
Fig. 4.
Purified active anti-bacterial fraction of H. rosa-sinensis essential oil by preparative HPLC (b).
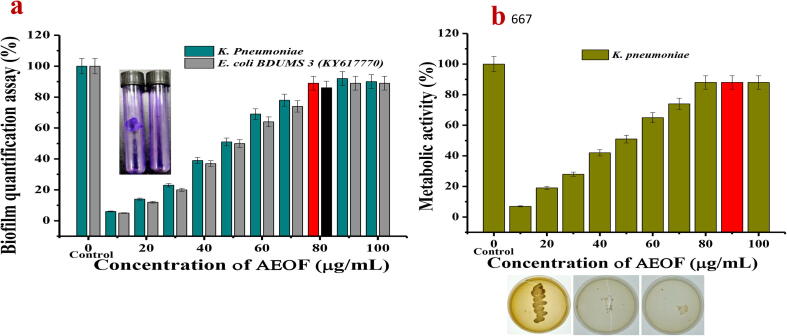
3.4. Quantification of biofilm formation
Anti-biofilm ability of AEOF was exhibited with complete biofilm inhibition against selected K. pneumonia. The result indicated that the AEOF have neither anti-bacterial nor anti-biofilm activity at very low concentration when compared to control. Among the various concentration, 90 µg/ml was exhibited 92 and 89% inhibition against CRBF K. pneumoniae and biofilm positive control E. coli BDUMS 3 (KY617770). When compared with previous report, the present result of 90 µg/ml concentration was very low against MDR pathogens (Fig. 6a). This study suggested that the AEOF was excellent bacteriostatic agent against GNB, and 90 µg/ml was fixed as a biofilm inhibition concentration (BIC). In addition, crystal violet staining assay exhibited that the rigidity of biofilm structure was degraded at the same concentration when compared with untreated control (Fig. 6a-Inset figures-1). It revealed that the adherent cells were degraded by BIC and exhibited highest inhibition result after bound with CV. The sparred cells of biofilm arrangement in the treated bacteria indicate that the AEOF has extracellular polysaccharide matrix damage ability through peptidoglycon layer and teichoic acid (Jessica et al., 2018, Ebani et al., 2018). Previously, the Rosmarinus officinalis essential oil has more inhibition ability against EPS and Hydrophobicity index (Jardak et al., 2017).
Fig. 6.
Minimum biofilm inhibition (a), biofilm metabolic activity (b) of purified active fraction of H. rosa-sinensis essential oil. The inset Fig. 1a indicates, the presence and absence of biofilm rigidity before (a) and after (b) treatment of essential oil. Inset Fig. 2 indicates the decreased viability of K. pneumoniae cells at increasing essential oil treatment in MHA plates (a, b, c).
3.5. Biofilm metabolic assay
After treatment with BIC of AEOF, the treated bacterial viability significantly decreased at increasing concentration. Bacterial viability is one of the important mechanistic approaches in biofilm inhibition (Maruthupandy et al., 2020). After 24 h incubation, the bacteria loss their antigenic characters like QS, enzyme production, gene stimulation and biofilm formation due to the arrest of formazan production (Balasubramanian et al., 2016). This evidence was compared with biofilm quantification result and suggested that the metabolic activity was reduced in the test samples. In our study, the 10 μg/mL of AEOF was released the bacteriostatic effect and extended to up to 90 μg/mL with 7 and 88% of reduction (Fig. 6b). The XTT result proved that the AEOF inhibited the bacterial viability in dose dependent manner.
Further, aliquot the evaluated result of XTT in MHA plates also proved that the AEOF has excellent bacteriostatic effect (Fig. 6b-Inset figures). The colonies of MHA plates were shown with distribution at 10 μg/mL. There was no colony at 90 μg/mL also when compared with control. Both XTT and MHA plate results proved that the AEOF has anti-biofilm activity due to the reduction of metabolic activity. Our result was agreed by Eman et al. (2018) and EO treated GNB increased the reduced viability via XTT assay due to the loss of their antigenicity. Recent report of Francesco et al. (2011), also reported that the EO disrupted the antigenicity of protein and alters the DNA replication due to the arrest of formazan production. Hence, the indirect and direct method of AEOF results were indicated that the significant reduction on metabolic activity of selected biofilm producing K. pnumoniae.
3.6. EPS quantification assay
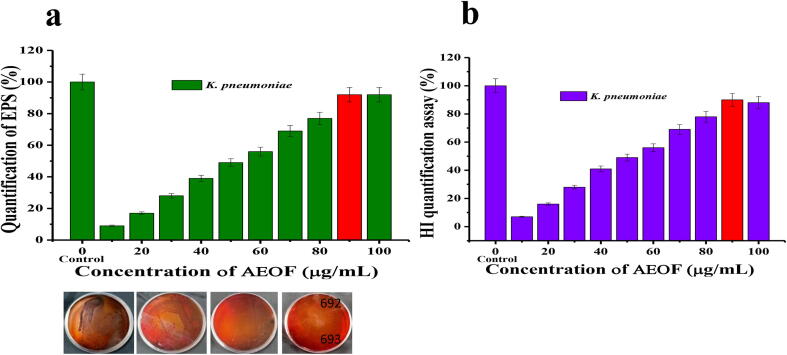
EPS is a physical important barrier for bacteria which helps to produce DNA, nucleic acid, extra cellular leakage materials for adhesion, protection and prevention of bacteria from external antibiotics (Nazzaro et al., 2013, Rajivgandhi et al., 2014, Zhang et al., 2018). It is an important virulence factor in mode anti-biofilm formation action which gives the structure and shape to bacterial biofilm lead to biofilm matrix (Lodhia et al., 2009). In our result, AEOF treated samples exhibited 92% at 90 μg/mL concentration, which indicate, adhesion, protection and expand of bacterial antigenicity was lost due to the interfere of AEOF (Fig. 7a). It may prevent the fimbriae, flagella, and other slime secreted EPS role in inside of the bacteria (Khan et al., 2009). Recent report of Maruthupandy et al. (2020), purified plant EO has the EPS ability through capsular polysachharide, which is dangerous for biofilm formation in human. The proposed mechanism was agreed by previous report of Vaillancourt et al. (2018). Inhibition of EPS was major key factor to eradicate complete biofilm formation, because it lost the production of extracellular polysachharide and damaged binding site modification (Semeniuc et al., 2017).
Fig. 7.
Decreased EPS quantification (a) and hydrophobicity inhibition (b) of active fraction of H. rosa-sinensis essential oil against CR K. pneumoniae at different concentration. Inset Fig. 7a indicates, the complete arrest of exopolysaccharide at various concentration of essential oil treated K. pneumoniae was observed in CRA plates.
Further, the CRA plate method result was also confirmed that the AEOF has excellent bacteriostatic effect. In result, control plate of untreated BF K. pneumoniae exhibited black color colonies, whereas treated plates exhibited loss of black color and no black color colonies (Fig. 7a-inset figure). At 90 μg/mL concentration, bacteria lost their complete EPS production was observed when compared with any other concentration. It indicates, the AEOF did not allow developing the bacterial virulence factors in inside of the bacteria. At half inhibition concentration of 60 μg/mL may start their function against DNA replication, nucleic acid synthesis and EPS secretion. In addition, surface charges of antigenicity were more sensitive to external antibiotics (Zhang et al., 2018). However, CRA method was evidently confirmed the invitro EPS inhibition result effectively. Also, the exhibited result consistently agreements with MBIC, XTT, EPS results, that AEOF has excellent inhibition ability against BF K. pneumoniae.
3.7. Hydrophobicity index (HI) quantification assay
The hydrophobicity is important key factor for biofilm production and involved in the initial adherence process. The rate of fimbriae role, flagella and EPS production was also essential factors for attachment of microbial cell onto the bacterial surface. It comes to enhance the increased biofilm formation in bacteria due to the influence of hydrophobicity. The hydrophobic interaction play a role in increase trends with an elevated non-polar nature of the microbial cell surface and substratum surface. In this study, the HI of AEOF treated BF K. pnemoniae exhibited with 89% at 90 μg/mL concentration. Whereas, the control result showed with no any changes on their HI (Fig. 7b). The result revealed that the hydrophobicity nature of the BF K. pneumoniae was significantly reduced when treated with AEOF. As per regulation of physico-chemical theory for bacteria (Balasubramanian et al., 2016), and the higher adhesion rate to hydrophilic surface results in dispersion of bacterial cells from hydrophobic surface.
3.8. Surface morphology damage of biofilm
Morphological damage of BIC treated bacteria was clearly depicted in SEM images Fig. 8. The surface integrity of bacteria was modified due to the effect of AEOF (Fig. 8b). Whereas, tightly packed, attached rod shaped cells were observed in untreated control cells (Fig. 8a). Cell wall of the bacteria was shown with membrane corrugation damage with belb formation (Fig. 8c). In addition, arrangement of hank-like exopolysaccharides with organization of EPS was significantly damaged and degraded due to the exposure of AEOF (Fig. 8d). The result also expressed, a stress responses of the AEOF on bacteria surface was continuously pushed and cleaved the surface, it leads to complete permeation of AEOF inside the cells and shown with irregular shape. It may leads to low electron dense in the inside of the periplasmic space and cytoplasm of the cells creates the fluid discharge (Vaillancourt et al. 2018).
Fig. 8.
Morphological analysis of active essential oil fraction untreated (a) treated (b), damage variation (c), belbing formation and shape variation (d) of CR K. pneumoniae by SEM.
4. Conclusion
In this research, the purified HPLC fraction of H. rosa-sinensis EO shows enhanced anti-biofilm activity against BF K. pneumoniae. All the invitro experiments showed that the oil exhibited excellent activity over BF K. pneumoniae. Microscopical images of SEM indicated that the EO altered the bacterial replication and internal structure due to the interference of exopolysaccharide layer damage. Also, the structural and shape arrangement was entirely modified at very low concentration. From the identified results, we confirm that the H. rosa-sinensis EO could be acted as a promising anti-biofilm agent for BF K. pneumoniae.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The work was supported by University Research Fellowship (Ref. No-05441/URF/K7/2013) Bharathidasan University, Tiruchirappalli-24 for entire of this study. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/70), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullah A., Mehmood F., Shahzadi I., Waseem S., Mirza B., Ahmed I., Waheed M.T. Chloroplast genome of Hibiscus rosa-sinensis (Malvaceae): comparative analyses and identification of mutational hotspots. Genomics. 2019;30062:S0888–S7543. doi: 10.1016/j.ygeno.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Anjugam M., Vaseeharan B., Iswarya A., Divya M., Prabhu N.M., Sankaranarayanan K. Biological synthesis of silver nanoparticles using β-1,3 glucan binding protein and their antibacterial, antibiofilm and cytotoxic potential. Microb. Pathog. 2018;115:31–40. doi: 10.1016/j.micpath.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Ansari P., Azam S., Hannan J.M.A., Flatt P.R., Abdel Wahab Y.H.A. Anti-hyperglycaemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112647. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M., Baskaralingam V., Sekar V., Raja S., Narayanan G., Ganesan S. Antibacterial and antibiofilm assessment of Momordica charantia fruit extract coated silver nanoparticle. Biocatal. Agricul. Biotechnol. 2016;8:189–196. [Google Scholar]
- Begum Z., Younus I. Hibiscus rosa sinensis mediate anxiolytic effect via modulation of ionotropic GABA-A receptors: possible mechanism of action. Metab. Brain Dis. 2018;33:823–827. doi: 10.1007/s11011-018-0188-4. [DOI] [PubMed] [Google Scholar]
- Chou C.C., Wang C.P., Chen J.H., Lin H.H. Anti-atherosclerotic effect of Hibiscus leaf polyphenols against tumor necrosis factor-alpha-induced abnormal vascular smooth muscle cell migration and proliferation. Antioxidants (Basel). 2019;8:1–13. doi: 10.3390/antiox8120620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L. – a phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Doulgeraki A., Di Ciccio P., Ianieri A., Nychas G.E. Methicillin-resistant food-related Staphylococcus aureus: a review of current knowledge and biofilm formation for future studies and applications. Res. Microbiol. 2017;168:1–15. doi: 10.1016/j.resmic.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Ebani V.V., Nardoni S., Bertelloni F., Pistelli L., Mancianti F. Antimicrobial activity of five essential oils against bacteria and fungi responsible for urinary tract infections. Molecules. 2018;23:1–12. doi: 10.3390/molecules23071668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemar Gomes, M., Rafael da Costa, H., Renato Moreira, R, Joao Antonio, P.H., Ana Lígia Lia de, P.R., Jenifer, S., 2010. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 118, 1–10.
- Eman Z., Attiaa R., Mahmoud M., Samar Y., Mahmoud H., Mohamed M., Mokhtar M., Bishrd M., Salah K. Chemical composition and antimicrobial activities of essential oils of Ruta graveolens plants treated with salicylic acid under drought stress conditions. Fut. J. Pharmaceut. Sci. 2018;4:254–264. [Google Scholar]
- Erika, Beáta, K., Anita, V., Miklós, T., Tamás, P., Csaba, V., Gyorgyi, H., Viktória, L. B., Judit, K., 2019. Anti-biofilm effect of selected essential oils and main components on mono- and polymicrobic bacterial cultures. Microorg. 7, 345. [DOI] [PMC free article] [PubMed]
- Francesco D., Mariann A., Mariarenat S., Giovann F. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Sci. Technol. 2011;44:1908–1914. [Google Scholar]
- Gandhi S.P., Lokhande K.B., Swam y.V.K., Nanda R.K., Chitlange S.S. Computational data of phytoconstituents from Hibiscus rosa-sinensis on various anti-obesity targets. Data Brief. 2019;24 doi: 10.1016/j.dib.2019.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui-Min S., Chun C., Ji-Yang J., Yi-Lin Z., Wen-Feng C., Bin Wang Z., Ling Liu T., Yuan-Hang W., Gang-Gang S. The N-butyl alcohol extract from Hibiscus rosa-sinensis L. flowers enhances healing potential on rat excisional wounds. J. Ethnopharm. 2017;198:291–301. doi: 10.1016/j.jep.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Jardak M., Elloumi-Mseddi J., Aifa S., Mnif S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Heal. Dis. 2017;16:190. doi: 10.1186/s12944-017-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessica, B.S.R., Neyrijane, T.S., Joao, O.A.S., Janaína, M.S., Myrella, C.L., Regina, C.B. Q.F., E.L. S., Marciane, M., 2018. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT. 93, 293–299.
- Kaleemullah M., Jiyauddin K., Thiban E., Rasha S., Al-Dhalli S., Budiasih S., Gamal O.E., Fadli A., Eddy Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage, Saudi. Pharm. J. 2017;25:770–779. doi: 10.1016/j.jsps.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Gosavi T.P., Badole S.L., Bodhankar S.L. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac. J. Trop. Biomed. 2012;2:337–344. doi: 10.1016/S2221-1691(12)60053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartinah, N.T., Fadilah, F., Ibrahim, E.I., Suryati, Y., 2019. The Potential of Hibiscus sabdariffa Linn in Inducing Glucagon-Like Peptide-1 via SGLT-1 and GLPR in DM Rats. Biomed. Res. Int. 8724824. [DOI] [PMC free article] [PubMed]
- Khan M.S., Zahin M., Hasan S., Husain F.M., Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009;49:354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- Lingesh A., Paul D., Naidu V., Satheeshkumar N. AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells. J. Ethnopharmacol. 2018;233:123–130. doi: 10.1016/j.jep.2018.12.039. [DOI] [PubMed] [Google Scholar]
- Lodhia M.H., Bhatt K.R., Thaker V.S. Antibacterial activity of essential oils from palmarosa, evening primrose, lavender and tuberose, Indian. J. Pharm. Sci. 2009;71:134–136. doi: 10.4103/0250-474X.54278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski D.P., Pinchak W.E., Yanker-Hansen K. Phenotypic similarities in flower characteristics between novel winter-hardy hibiscus hybrids and their tropical relatives. Front. Plant. Sci. 2019;10:1528. doi: 10.3389/fpls.2019.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maralit Bruan M.J., Tianco E.A. Efficacy and safety of 4% Hibiscus rosa-sinensis leaf extract ointment as an adjunct treatment to compression stockings on the closure of venous leg ulcers: a pilot study. Wounds. 2019;31:236–241. [PubMed] [Google Scholar]
- Maruthupandy M., Rajivgandhi G., Kadaikunnan S., Veeramanid T., Alharbi N.S., Muneeswaran T., Khaled J.M., Li W.J., Alanzi K.F. Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumonia. Carbohyd. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115646. [DOI] [PubMed] [Google Scholar]
- Mehran A., Naser K., Iraj S. phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J. Indust. Engin. Chem. 2019;72:457–473. [Google Scholar]
- Nazzaro F., Fratianni F., Martino L., Coppola R., Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norhaizan M.E., Fong S., Hern A., Ismail C., Lye Y. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chemist. 2010;122:1055–1060. [Google Scholar]
- Pillai S.S., Mini S. Attenuation of high glucose induced apoptotic and inflammatory signaling pathways in RIN-m5F pancreatic β cell lines by Hibiscus rosa sinensis L. petals and its phytoconstituents. J. Ethnopharmacol. 2018;227:8–17. doi: 10.1016/j.jep.2018.08.022. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G., Vijayarani J., Kannan M., Murugan A., Vijayan R., Manoharan N. Isolation and identification of biofilm forming uropathogens from urinary tract infection and its antimicrobial susceptibility pattern. Int. J. Adv. Lif. Sci. 2014;7:352–363. [Google Scholar]
- Rajivgandhi G., Senthil R., Ramachandran G., Maruthupandy M., Manoharan N. Antibiofilm activity of marine endophytic actinomycetes compound isolated from mangrove plant Rhizophora mucronata, Muthupet Mangrove Region, Tamil Nadu, India. J. Terr. Mar. Res. 2018;2:1–7. [Google Scholar]
- Rajivgandhi G., Vijayan R., Maruthupandy M., Vaseeharan B., Manoharan N. Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs. Microb. Pathog. 2018;118:190–198. doi: 10.1016/j.micpath.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Rihab, L., Fethi Ben, A., Badriah Osama, AL. S., Yassin, Al. S., 2019. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 24, 1161. [DOI] [PMC free article] [PubMed]
- Ruban P., Gajalakshmi K. In vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens. Asian Pacif. J. Trop. Biomed. 2012;24:399–403. doi: 10.1016/S2221-1691(12)60064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini D., Banu S.F., Nisha P., Murugan R., Thamotharan S., Percino M.J., Subramani P., Nithyanand P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb Pathog. 2018;122:162–173. doi: 10.1016/j.micpath.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Ruhomally Z., Somanah J., Bahorun T., Neergheen-Bhujun V.S. Morinda citrifolia L. fruit extracts modulates H2O2-induced oxidative stress in human liposarcoma SW872 cells. J. Tradit. Complement. Med. 2015;6:299–304. doi: 10.1016/j.jtcme.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeniuc C.A., Pop C.R., Rotar A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug. Anal. 2017;25:403–408. doi: 10.1016/j.jfda.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt K., LeBel G., Yi L., Grenier D. In vitro antibacterial activity of plant essential oils against Staphylococcus hyicus and Staphylococcus aureus, the causative agents of exudative epidermitis in pigs. Arch. Microbiol. 2018;200:1001–1007. doi: 10.1007/s00203-018-1512-4. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S., Morvin Yabesh J.E., Arulmozhi P., Praseetha P.K. Identification and isolation of antimicrobial compounds from the flower extract of Hibiscus rosa-sinensis L: In silico and in vitro approaches. Microb. Pathog. 2018;123:527–535. doi: 10.1016/j.micpath.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kong J., Xie Y., Guo Y., Cheng Y., Qian H., Yao W. Essential oil components inhibit biofilm formation in Erwinia carotovora and Pseudomonas fluorescens via anti-quorum sensing activity. LWT. 2018;92:133–139. [Google Scholar]
Further Reading
- Rajivgandhi G., Ramachandran G., Maruthupandy M., Vaseeharan B., Manoharan N. Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) and its antibacterial efficacy against isolated ESBL producing bacteria. Microb. Pthog. 2019;126:138–148. doi: 10.1016/j.micpath.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Ramachandran G., Rajivgandhi G., Maruthupandy M., Manoharan N. Isolation and identification of antibacterial compound from marine endophytic actinomycetes against multi drug resistant bacteria. Ann. Microbiol. Immunol. 2018;1:1003. [Google Scholar]