Abstract
Background
Within the context of Canada’s opioid crisis, medical complications associated with intravenous drug use (IVDU) are increasing. Infective endocarditis (IE) is a serious complication of IVDU, and understanding the characteristics of these patients could aid health systems, clinicians, and patients in the optimization of treatment and prevention of IVDU-IE.
Methods
At a tertiary care hospital in southern New Brunswick, we conducted a retrospective chart review to identify patients with IVDU-IE admitted between January 1, 2013, and December 31, 2017. We collected data related to the epidemiology, microbiology, clinical manifestations, echocardiography, complications during hospital admission, and outcomes.
Results
Forty-two cases of IVDU-IE met inclusion criteria. The rate of IVDU-IE increased from 2.28 per 100,000 population in 2014 to 4.00 in 2017, which, although not statistically significant, reflects patterns in other jurisdictions. Most patients (72.4%) were male, and the mean age was 38.3 (±11.5) years. Most patients (79.3%) injected opioids. The most common clinical sign was fever (90.5%), and Staphylococcus aureus (61.9%) was the most common microorganism. The tricuspid valve was most commonly infected (58.5%), 50% of cases had heart failure as a complication during admission, and 45.2% of cases required valve replacement or repair. The 2-year survival rate after admission for initial IVDU-IE episode was 62.0% (95% confidence interval: 36.5-79.7).
Conclusion
IVDU-IE is common in New Brunswick and may be increasing. Despite the relatively young age of this patient population, IVDU-IE is associated with significant morbidity and mortality. Expanding effective harm reduction and addiction treatment strategies for this cohort is recommended.
Résumé
Contexte
Dans le contexte de la crise des opioïdes au Canada, les complications médicales liées à l'utilisation de drogues par voie intraveineuse (UDIV) sont en augmentation. L'endocardite infectieuse (EI) est une complication grave de l'UDIV, et la compréhension des caractéristiques de ces patients pourrait aider les systèmes de santé, les cliniciens et les patients à optimiser le traitement et la prévention de l’EI liée à l’UDIV (EI-UDIV).
Méthodes
Dans un hôpital de soins tertiaires du sud du Nouveau-Brunswick, nous avons effectué un examen rétrospectif des dossiers afin d'identifier les patients atteints de l’EI-UDIV admis entre le 1er janvier 2013 et le 31 décembre 2017. Nous avons recueilli des données relatives à l'épidémiologie, la microbiologie, les manifestations cliniques, l'échocardiographie, les complications lors de l'admission à l'hôpital et les bilans.
Résultats
Quarante-deux cas d'EI-UDIV ont répondu aux critères d'inclusion. Le taux d'EI-UDIV est passé de 2,28 pour 100 000 habitants en 2014 à 4,00 en 2017, ce qui, bien que non significatif statistiquement, reflète les tendances observées dans d'autres juridictions. La plupart des patients (72,4 %) étaient des hommes, et l'âge moyen était de 38,3 ans (±11,5). La plupart des patients (79,3 %) s'injectaient des opioïdes. Le signe clinique le plus fréquent était la fièvre (90,5 %), et le Staphylococcus aureus (61,9 %) était le micro-organisme le plus couramment observé. La valve tricuspide était le plus souvent infectée (58,5 %), 50 % des cas avaient une insuffisance cardiaque en tant que complication lors de l'admission, et 45,2 % des cas ont nécessité un remplacement ou une réparation de la valve. Le taux de survie à deux ans après l'admission pour l'épisode initial d'EI-UDIV était de 62,0 % (intervalle de confiance à 95 % : 36,5-79,7).
Conclusion
L'EI-UDIV est fréquent au Nouveau-Brunswick et pourrait être en augmentation. Malgré l'âge relativement jeune de cette population de patients, l'UDIV-IE est associée à une morbidité et une mortalité importantes. Il est recommandé d'étendre les stratégies efficaces de réduction des risques et de traitement des dépendances pour cette cohorte.
An epidemic of nonmedical opioid use is sweeping across Canada, including intravenous drug use (IVDU).1 An estimated 172,000 Canadians engage in IVDU,2,3 and New Brunswick is thought to have the second-highest rates of IVDU in Canada at around 1% of the population.3 IVDU carries an increased risk of serious medical complications, including infective endocarditis (IE).4 It has been reported as a risk factor in approximately 10.0%5 to 60.0%6 of IE cases, and rising incidence of IVDU-associated infective endocarditis (IVDU-IE) has been described in Ontario,7,8 North Carolina,6,9 Virginia,10 Kentucky,11 national US data,12, 13, 14, 15 and national UK data.16 There is an urgent need to better understand the characteristics and care needs of this population.
IVDU-IE is more frequently seen in males and also affects younger patients when compared with IE in non-IVDU populations.17 Despite their younger age and fewer comorbidities, mortality rates are similar or higher18 and postoperative complication rates are higher19,20 among patients with IVDU-IE compared with non-IVDU-IE. If IVDU continues, these individuals have greater risk of recurrent IE.17
In this study, our objective was to describe the epidemiology, microbiology, clinical manifestations, echocardiography, and outcomes among patients with IVDU-IE admitted to the Saint John Regional Hospital (SJRH) in Saint John, New Brunswick. We aimed to estimate the incidence of IVDU-IE in this region, as well as the rates of complications and mortality among these patients, enabling a better understanding of the health care needs of this population.
Methods
Study design and inclusion criteria
Research ethics approval was obtained through the Horizon Health Network Research Ethics Board. We conducted a retrospective chart review identifying patients who met the following 3 criteria: (1) International Classification of Diseases, Tenth Revision (ICD-10) codes with a discharge diagnosis potentially consistent with IE (Supplemental Table S1) and confirmed as “possible” or “definite” IE as per the modified Duke criteria,21 (2) active injection drug use (defined as injection drug use within 3 months before the IE diagnosis22), and (3) admitted to the SJRH in Saint John, New Brunswick, between January 1, 2013, and December 31, 2017. The SJRH is home to the New Brunswick Heart Centre and receives patients from across New Brunswick.
Data collection
We collected data in relation to 6 prespecified categories during the structured chart review: epidemiology (age, gender, comorbidities, type of injection drug used), microbiology (the infecting microorganism), clinical manifestations (fever, chest pain, murmur, vascular phenomena, immunologic phenomena), echocardiography (the valve[s] involved, native valve, prosthetic valve, vegetation, or abscess present), noted complications (cardiac, pulmonary, lymphatic, neurologic, bone/joint, or other complications), and outcomes (valve replacement or repair, type of antibiotic and duration, recurrence of IVDU-IE, length of admission, all-cause mortality, attendance at follow-up appointment). Each included case of IVDU-IE was classified according to the modified Duke criteria.21
Statistical analysis
Patient and clinical characteristics of IVDU-IE cases were presented as means and proportions, as appropriate. In patients who had more than 1 episode of IVDU-IE during the study period, patient characteristics were collected at the time of the first IVDU-IE episode. To assess 24-month all-cause mortality, a Kaplan-Meier survival curve was produced with the date of admission to hospital for the first occurrence of IVDU-IE representing time “0.” Individuals were censored if they were lost to follow-up, were still alive at 24 months after the first occurrence of IVDU-IE in the study period, or alive as of the end of the outcome follow-up period (March 31, 2018) for individuals who had not yet reached 24 months after admission. The local incidence of IVDU-IE (numerator and denominator) was calculated using only patients who resided within the southern New Brunswick area (Health Region 2) at the time of diagnosis; all cases of IVDU-IE from within southern New Brunswick are treated at the SJRH, whereas all cases of IVDU-IE from outside southern New Brunswick will not necessarily be transferred to the SJRH for care. Confidence intervals (CIs) for incidence rates were calculated using Fisher’s exact. Health Region 2 population was extracted from the Statistics Canada 2016 Census.23
Results
A total of 272 cases with a discharge diagnosis of IE admitted to the SJRH between January 1, 2013, and December 31, 2017, were identified through the relevant ICD-10 codes and confirmed via chart review. Of these episodes of IE, 42 (15%) met our inclusion criteria for IVDU-IE. Twenty-nine patients accounted for these 42 episodes, with 5 patients experiencing 2 episodes of IVDU-IE and 4 patients experiencing 3 episodes of IVDU-IE during the study period. For 41 (98%) of the episodes, the patient resided in New Brunswick at the time of hospital admission; for 1 episode, the patient was residing elsewhere in Canada and was transferred to the patient’s home province of New Brunswick during hospitalization. Before the study period, 20.7% of patients with IVDU-IE had 1 or more other episodes of endocarditis. As per the modified Duke criteria, 33 episodes met criteria for “definite” IE, whereas 9 episodes met criteria for “possible” IE. No patients were lost to follow-up. Of patients who were alive as of the study end date of March 31, 2018 (n = 20), 70% (n = 14) had a full 2 years of follow-up after their first admission for IVDU-IE available for analysis. The average follow-up time available for the remaining 30% (n = 6) was 477 days (range, 331-604 days).
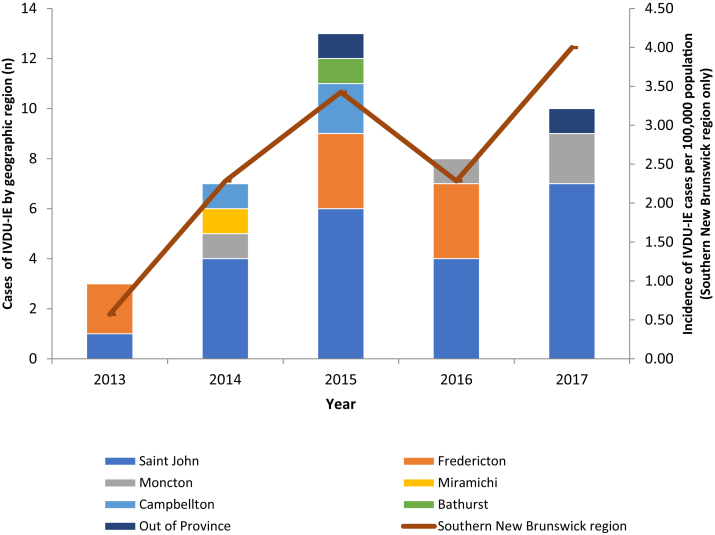
Patient characteristics including demographics and comorbidities are presented in Table 1. For the 41 cases residing in New Brunswick at the time of hospital admission, Figure 1 displays the number of IVDU-IE cases by geographic region and the rate of IVDU-IE per 100,000 population in southern New Brunswick only. The rate of IVDU-IE in southern New Brunswick increased from 2.28 per 100,000 population (95% CI: 0.62-5.85) in 2014 to 4.00 per 100,000 population (95% CI: 1.61-8.24) in 2017. The majority of patients were male (72.4%), and the mean age of the patient cohort was 38.3 (±11.5) years. All patients smoked tobacco, and the most common comorbidity was hepatitis C infection (69.0%). Participants reported opioid use most frequently (79.3%), followed by cocaine (65.5%) and amphetamines (17.2%). The most common clinical signs were fever (90.5%) and murmur noted on auscultation (83%).
Table 1.
IVDU-associated infective endocarditis patient characteristics (n = 29)
| Male, % (n) | 72.4 (21) |
| Age (y), mean (SD)∗ | 38.3 (11.5) |
| Active smoker, % (n) | 100.0 (29) |
| Body mass index > 30, % (n) | 13.8 (4) |
| Diabetes, % (n) | 6.9 (2) |
| HIV, % (n) | 0.0 (0) |
| Hepatitis C, % (n) | 69.0 (20) |
| Prior endocarditis (pre-study), % (n) | 20.7 (6) |
| Hypertension, % (n) | 13.8 (4) |
| Previous myocardial infarction, % (n) | 10.3 (3) |
| Pacemaker, % (n) | 6.9 (2) |
| Valvular disease, % (n)† | 6.9 (2) |
IE, infective endocarditis; IVDU, intravenous drug use; SD, standard deviation.
Age is calculated using the age at the time of the first episode of IE within the study period.
Valvular disease is based on stenosis or regurgitation on prior echocardiography.
Figure 1.
Number of cases of IVDU-IE by the New Brunswick geographic region per year (left Y axis) and incidence of IVDU-IE for southern New Brunswick (Saint John area, Health Region 2) per 100,000 population (right Y axis). IVDU-IE, intravenous drug use–associated infective endocarditis.
As shown in Table 2, the most common organism isolated from blood cultures was Staphylococcus aureus (61.9%), the majority of which were methicillin-sensitive (88.5%) (Table 2). Table 3 presents the echocardiographic findings. The tricuspid valve (57.1%) was the most frequently infected heart valve. Also noted in Table 3 is the percentage of cases found to have native valve endocarditis (69.0%) vs prosthetic valve endocarditis (31.0%). Complications noted during the hospital admission are outlined in Table 4. The 2 most common complications of IVDU-IE in this cohort were heart failure (50%) and septic pulmonary emboli (40.5%).
Table 2.
Causative microorganisms among cases of IVDU-IE
| Organism(s) isolated | % (n) |
|---|---|
| Staphylococcus aureus | 61.9 (26) |
| Methicillin-sensitive | 88.5 (23) |
| Methicillin-resistant | 11.5 (3) |
| Streptococcus viridans group | 21.4 (9) |
| Enterococcus faecalis | 7.1 (3) |
| Coagulase-negative Staphylococcus | 4.8 (2) |
| Beta-hemolytic group B Streptococcus | 4.8 (2) |
| Beta-hemolytic group G Streptococcus | 2.4 (1) |
| Enterobacter cloacae | 2.4 (1) |
| Candida albicans | 2.4 (1) |
| Klebsiella pneumonia | 2.4 (1) |
| Culture-negative | 4.8 (2) |
| > 1 organism isolated∗,†,‡,§ | 9.5 (4) |
IVDU-IE, intravenous drug use–associated infective endocarditis.
In 4 patients with > 1 organism isolated, these organisms included:
Methicillin-sensitive Staphylococcus aureus, Streptococcus viridans group, Enterobacter cloacae, and Candida albicans.
Methicillin-sensitive Staphylococcus aureus and Coagulase-negative Staphylococcus.
Methicillin-sensitive Staphylococcus aureus and Enterococcus faecalis.
Methicillin-sensitive Staphylococcus aureus and Klebsiella pneumonia.
Table 3.
Echocardiographic findings among IVDU-IE cases
| Echocardiographic findings | % (n) |
|---|---|
| Valve(s) affected∗ | |
| Aortic | 45.2 (19) |
| Vegetation | 31.0 (13) |
| Abscess | 9.5 (4) |
| Moderate regurgitation | 2.4 (1) |
| Severe regurgitation | 28.6 (12) |
| Mitral | 21.4 (9) |
| Vegetation | 11.9 (5) |
| Abscess | 0.0 (0) |
| Moderate regurgitation | 9.5 (4) |
| Severe regurgitation | 9.5 (4) |
| Tricuspid | 57.1 (24) |
| Vegetation | 45.2 (19) |
| Abscess | 2.4 (1) |
| Moderate regurgitation | 14.3 (6) |
| Severe regurgitation | 19.0 (8) |
| Pulmonic | 0.0 (0) |
| Type of valve(s) involved | |
| Native | 69.0 (29) |
| Prosthetic | 31.0 (13) |
| Large vegetation (> 20 mm) | 19.0 (8) |
| Any abscess | 11.9 (5) |
IVDU-IE, intravenous drug use–associated infective endocarditis.
10 cases had 2 valves with abnormal findings.
Table 4.
Clinical complications among IVDU-IE cases
| Complication | % (n) |
|---|---|
| Cardiac | 54.8 (23) |
| Heart failure | 50.0 (21) |
| Heart block | 16.7 (7) |
| Pericardial effusion | 2.4 (1) |
| Myocardial infarction | 4.8 (2) |
| Myocardial/perivalvular abscess | 14.3 (6) |
| Pulmonary | 40.5 (17) |
| Septic pulmonary emboli | 40.5 (17) |
| Empyema | 2.4 (1) |
| Lymphatic | 16.7 (7) |
| Splenic infarct | 11.9 (5) |
| Splenic abscess | 7.1 (3) |
| Neurologic | 19.1 (8) |
| Stroke | 14.3 (6) |
| Cerebral hemorrhage | 9.5 (4) |
| Cerebral abscess | 7.3 (3) |
| Meningitis | 2.4 (1) |
| Bone/joint | 26.2 (11) |
| Osteomyelitis | 7.1 (3) |
| Septic arthritis | 21.4 (9) |
| Other | |
| Non-splenic infarct | 11.9 (5) |
| Other metastatic abscess | 14.3 (6) |
| Sepsis | 31.0 (13) |
| Glomerulonephritis | 38.1 (16) |
IVDU-IE, intravenous drug use–associated infective endocarditis.
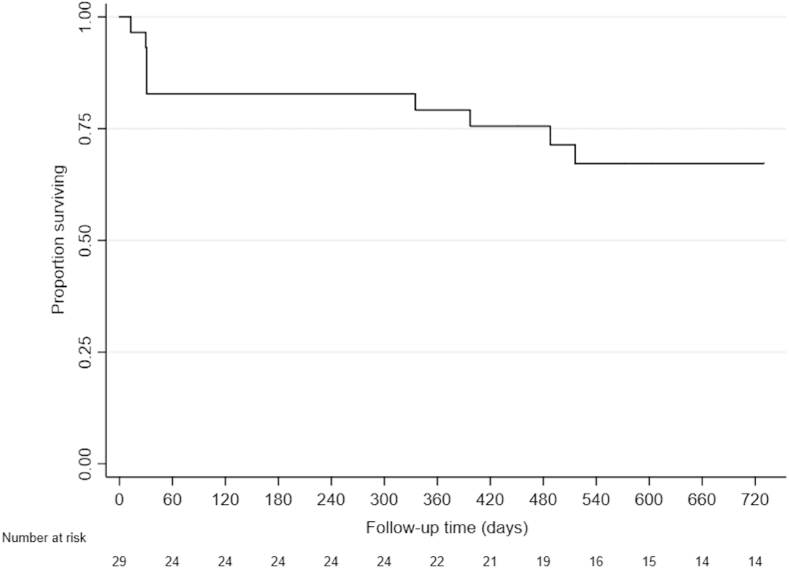
Outcomes are displayed in Table 5. The most common surgical intervention was valve replacement (45.2%). Twenty-three patients (57%) had valve surgery; 3 of these 23 patients (13%) died within 30 days of valve surgery. All patients who completed antibiotic therapy received antibiotics in accordance with current guidelines. The duration of antibiotic therapy ranged from 2 to 84 days (median, 42 days). The overall survival rate at 1-year after admission for the first episode of IE was 82.8% (95% CI: 63.4%-92.4%). The survival rate dropped to 62.0% (95% CI: 36.5%-79.7%) at 2 years, as demonstrated in the Kaplan-Meier survival curve shown in Figure 2. A total of 9 patients (31.0%) died during the study period, and in each case, death was a direct result of IE.
Table 5.
Outcomes of IVDU-IE cases (n = 42)
| Died∗, % (n) | 31.0 (9) |
|---|---|
| Surgical intervention, % (n) | |
| Pacemaker insertion | 11.9 (5) |
| Valvuloplasty | 11.9 (5) |
| Aortic valve replacement | 31.0 (13) |
| Tricuspid valve replacement | 23.8 (10) |
| Mitral valve replacement | 9.5 (4) |
| Replacement of ascending aorta | 4.8 (2) |
| Debridement of aortic root | 9.5 (4) |
| Repair of left ventricular outflow tract | 2.4 (1) |
| Closure of atrial-septal defect | 4.8 (2) |
| Closure of patent foramen ovale | 4.8 (2) |
| Reasons for surgical intervention | |
| Heart failure related to left-sided valve IE with severe regurgitation | 14.3 (6) |
| Uncontrolled infection | 7.1 (3) |
| Left-sided valve IE with vegetations > 10 mm and an embolic episode despite appropriate antibiotic therapy, or predictors of a complicated course | 16.7 (7) |
| Large left-sided vegetation > 15 mm | 11.9 (5) |
| Tricuspid valve IV with severe or worsening regurgitation | 9.5 (4) |
| Intensive care unit (ICU) admission; other than postoperative cardiac surgery ICU admit, % (n) | 9.5 (4) |
| Ongoing moderate-severe valvular regurgitation at discharge, % (n) | 19.0 (8) |
| Median length of stay (range) (d) | 19 (2-74) |
| Median antibiotic duration (range) (d) | 42 (2-84) |
IE, infective endocarditis; IV, intravenous; IVDU-IE, intravenous drug use–associated infective endocarditis.
Based on 29 patients who experienced 42 IE episodes during the study period.
Figure 2.
Survival (all-cause mortality) after hospital admission for the first episode of IVDU-IE during the study period. IVDU-IE, intravenous drug use–associated infective endocarditis.
Discussion
In this retrospective cohort study of patients admitted to a tertiary care centre in southern New Brunswick, we found that IVDU-IE is common, is associated with high mortality, and may be increasing over time. We described the most common clinical, microbiological, and echocardiographic characteristics.
It has been noted that the incidence of endocarditis overall is increasing over time,15 and increasing rates of IVDU-IE are a major driving factor.10 Repeated exposure to particulate matter in the bloodstream can damage cardiac valves, which then serve as a nidus for infection during bacteremia episodes associated with IVDU.4,17 IE was initially recognized to be a complication of injection drug use in the 1950s;24 the IVDU-IE cohort is unique and possesses specific clinical manifestations and complicating factors. Knowledge of these factors is relevant to ensuring appropriate diagnostics, targeted therapy, and supportive management to prevent short- and long-term complications.
Our findings are consistent with earlier studies of IVDU-IE in other regions, in that the majority of patients in the cohort were male and of younger age than the typical cardiac patient,17 and the majority of these patients had few cardiac-related comorbidities or risk factors for IE,20 other than IVDU. Although none of our patients had human immunodeficiency virus infection, more than half had hepatitis C infection. Demographics and characteristics of this population are important to understand to tailor interventions and harm reduction strategies to best suit patient needs.
We found that the most common organism responsible for IVDU-IE was methicillin-sensitive S. aureus. Previous studies have found that S. aureus accounts for up to 60.8% of cases of IVDU-IE,25 and patients with IE due to S. aureus may be more likely to develop large vegetations (> 15 mm).26 Knowledge of the common causative microorganisms of IE in a particular region is important to guide empiric therapy and to predict potential complications.
In the context of the current opioid crisis in Canada,1 opioids were the most common illicit drug used by patients in this study. We observed that the rate of IVDU-IE in the Saint John municipal area nearly doubled from 2014 to 2017, which, although not statistically significant, does reflect patterns observed in multiple other jurisdictions, including Ontario,7,8 US,12,15 and UK16 cohorts. Reasons for this remain unclear, but may be related to increasing rates of injection drug use, increasing frequency of injection, changes in drug supply and formulation,8,27, 28, 29 or social marginalization and exclusion.30 Some increased risk for bacterial and fungal infections has been attributed to the rise in fentanyl use,31 but New Brunswick has not seen the influx of fentanyl as in other parts of Canada. A recent modelling study estimates that the number of people who inject drugs in New Brunswick increased by 24% between 2011 and 2016.3 A better understanding of risk and prevention of serious bacterial infections among people who inject drugs is urgently needed.
It has been noted that a coordinated, multidisciplinary “endocarditis team” responsible for all inpatients with IE will improve care.32 Multidisciplinary endocarditis teams also provide important opportunities to offer addiction medicine care to every patient with IE who injects drugs and coordinate this as part of the care plan.33 Inpatient addiction medicine consultation is associated with increased treatment completion and decreased rates of IE recurrence and readmission34 as well as decreased mortality.35 Opioid agonist therapy and harm reduction strategies are the standard of care for moderate-severe opioid use disorder and can be offered during hospital admissions to help facilitate the overall care plan.
Recurrence of IE is more common in the IVDU-IE cohort. The International Collaboration on Endocarditis—Prospective Cohort Study investigators have noted that among 1874 enrolled patients, IVDU is the strongest independent risk factor for repeat IE (odds ratio: 2.9; 95% CI: 1.6-5.4).36 However, this study and others of recurrent IE in patients who inject drugs have not assessed whether patients were offered treatment for addiction. Our data support the need for increased focus on harm reduction and addiction treatment strategies for this population, not only in larger centres in Canada but also in smaller cities and rural areas where illicit opioid use is a major public health issue.
Nonetheless, health care systems have been slow to develop dedicated inpatient programs and provide comprehensive addiction care. A recent retrospective review of patients with IVDU-IE hospitalized at a large academic tertiary care centre in Boston, Massachusetts,22 found high rates of readmission (49%), recurrent IE (13.7%), and death (25.5%) in the cohort. Only 7.8% of patients had a plan for addiction treatment on discharge from their sentinel admission.
The common clinical signs and symptoms of IE seen in this study are important for clinicians to be aware of in order to accurately diagnose and treat IE early in the disease course so as to optimize outcomes. Both overdiagnosis and underdiagnosis of IE can prove problematic: a missed diagnosis of IE can be fatal, and overdiagnosis can lead to unnecessary antibiotic treatment.4,37 The most common clinical manifestations of IE in this study were fever, murmur, chest pain, and splenomegaly. Janeway lesions were the most common peripheral stigmata of endocarditis.
The echocardiographic findings in our study were also consistent with similar studies17 in that the tricuspid valve was the most common valve infected. Right-sided valve involvement has been found to be more common in IVDUs as compared with non-IVDUs.19 Right-sided valve involvement also carries a greater risk of septic pulmonary embolism and associated respiratory complications that cause significant morbidity and mortality and prolong the hospital stay.19 The 2 most common complications that arose for our patients throughout their hospital admissions were heart failure and septic pulmonary emboli, findings consistent with a 2015 Norwegian study of IE in patients with IVDU.38
Most patients underwent surgical interventions including valve replacement, valvuloplasty, grafting, repair of atrial septal defect, or closure of cardiac fistulas. The high rate of surgical management demonstrates significant burden of disease among this cohort and large costs to the health care system. In-hospital mortality for patients with first-episode IVDU-IE in this study was significant at 20.7%. This highlights the need for addiction treatment, harm reduction, and education for these patients to assist in minimizing high-risk behaviours associated with recurrent endocarditis.
Limitations
The limitations of this study include its retrospective design in which we used ICD-10 codes to identify patients over the 5-year study period. Although there may have been cases of IVDU-IE that were not included in the selected codes, we included a wider group of diagnostic codes to capture more potential cases. Furthermore, we manually reviewed the IE charts to identify IVDU, and cases may have been missed if the patients did not disclose their IVDU or if the clinician did not clearly document IVDU. Despite the potential of some missed cases, we identified that IVDU-IE is an increasingly common problem in New Brunswick. It is possible that patients who reside in southern New Brunswick (Health Region 2) could have been diagnosed with and treated for IVDU-IE in another region, which would cause our estimate of incidence for the region to be falsely low; however, given that the SJRH is the referral centre for cardiac surgery in New Brunswick, as well as cardiology and infectious diseases consultations in southern New Brunswick, the likelihood of this is low. Lastly, the data on substance use, symptoms, and signs are as accurate as the patient’s symptom reporting and the clinician’s physical examination and documentation. For example, we were unable to reliably capture patients’ duration of IVDU as this was not always documented in the medical chart. Prospective cohort studies of patients with suspected IE could address these limitations.
Conclusions
In this study, we described the epidemiology, microbiology, clinical manifestations, echocardiography, and outcomes of patients with IVDU-IE admitted to the SJRH in Saint John, New Brunswick. We found that IVDU-IE is common, associated with poor outcomes, and may be increasing in incidence. Despite the relatively young age of this patient population, IVDU-IE is a disease associated with significant morbidity and mortality. Cardiac surgical intervention is frequently required, and implementing effective harm reduction strategies is critical in the care of this at-risk cohort.35,36,39 This descriptive study allows a better understanding of the health care needs of this population to assist with provision of high-quality health care as well as identify other areas of research that may further improve the health care delivered to this vulnerable population.
Funding Sources
This study was supported by Kimiko Mosseler's RIM studentship funding from the DMRF (Dalhousie Medical Research Foundation) Katelyn Robarts Studentship. Thomas D. Brothers was supported by the Ross Stewart Smith Memorial Fellowship in Medical Research, from Dalhousie University.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Research ethics approval was obtained through the Horizon Health Network Research Ethics Board.
See page 384 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.05.002.
Supplementary Material
References
- 1.Health Canada Opioid-related harms and deaths in Canada. 2017. https://www.canada.ca/en/health-canada/services/substance-use/problematic-prescription-drug-use/opioids/data-surveillance-research/harms-deaths.html Available at:
- 2.Roy É., Arruda N., Bruneau J., Jutras-Aswad D. Epidemiology of injection drug use. Can J Psychiatry. 2016;61:136–144. doi: 10.1177/0706743716632503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacka B., Larney S., Degenhardt L. Prevalence of injecting drug use and coverage of interventions to prevent HIV and hepatitis C virus infection among people who inject drugs in Canada. Am J Public Health. 2019;14:e1–6. doi: 10.2105/AJPH.2019.305379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuny F., Grisoli D., Cautela J. Infective endocarditis: prevention, diagnosis, and management. Can J Cardiol. 2014;30:1046–1057. doi: 10.1016/j.cjca.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Mostaghim A.S., Lo H.Y.A., Khardori N. A retrospective epidemiologic study to define risk factors, microbiology, and clinical outcomes of infective endocarditis in a large tertiary-care teaching hospital. SAGE Open Med. 2017;5 doi: 10.1177/2050312117741772. 2050312117741772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman L., Barnes E., Bachmann L. Opiate injection-associated infective endocarditis in the southeastern United States. Am J Med Sci. 2016;352:603–608. doi: 10.1016/j.amjms.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coverdale N., Brogly S., Payne D. Rates of infective endocarditis in substance use disorder and associated costs in Ontario. Can J Addict. 2019;10:36–43. [Google Scholar]
- 8.Weir M.A., Slater J., Jandoc R. The risk of infective endocarditis among people who inject drugs: a retrospective, population-based time series analysis. CMAJ. 2019;191:E93–E99. doi: 10.1503/cmaj.180694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schranz A.J., Fleischauer A., Chu V.H., Wu L.-T., Rosen D.L. Trends in drug use–associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019;170:31. doi: 10.7326/M18-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray M.E., Rogawski McQuade E.T., Scheld W.M., Dillingham R.A. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis. 2018;18:532. doi: 10.1186/s12879-018-3408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin C.A., Burgess D.R., Wallace K.L. Trends in infective endocarditis during the substance use disorder epidemic at an academic medical center. Open Forum Infect Dis. 2018;5(Suppl 1):S306. [Google Scholar]
- 12.Wurcel A.G., Anderson J.E., Chui K.K.H. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3:ofw157. doi: 10.1093/ofid/ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadri A.N., Wilner B., Hernandez A.V. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudasill S.E., Sanaiha Y., Mardock A.L. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol. 2019;73:559–570. doi: 10.1016/j.jacc.2018.10.082. [DOI] [PubMed] [Google Scholar]
- 15.Deo S.V., Raza S., Kalra A. Admissions for infective endocarditis in intravenous drug users. J Am Coll Cardiol. 2018;71:1596–1597. doi: 10.1016/j.jacc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Lewer D., Harris M., Hope V. Opiate injection-associated skin, soft tissue, and vascular infections, England, UK, 1997-2016. Emerg Infect Dis. 2017;23:1400–1403. doi: 10.3201/eid2308.170439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colville T., Sharma V., Albouaini K. Infective endocarditis in intravenous drug users: a review article. Postgrad Med J. 2016;92:105–111. doi: 10.1136/postgradmedj-2015-133648. [DOI] [PubMed] [Google Scholar]
- 18.Wurcel A.G., Boll G., Burke D. Impact of substance use disorder on midterm mortality after valve surgery for endocarditis. Ann Thorac Surg. 2020;109:1426–1432. doi: 10.1016/j.athoracsur.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J.B., Ejiofor J.I., Yammine M. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152:832–841.e1. doi: 10.1016/j.jtcvs.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Lemaire A., Dombrovskiy V., Saadat S. Patients with infectious endocarditis and drug dependence have worse clinical outcomes after valvular surgery. Surg Infect. 2017;18:299–302. doi: 10.1089/sur.2016.029. [DOI] [PubMed] [Google Scholar]
- 21.Li J.S., Sexton D.J., Mick N. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal E.S., Karchmer A.W., Theisen-Toupal J., Castillo R.A., Rowley C.F. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129:481–485. doi: 10.1016/j.amjmed.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 23.New Brunswick Census Profile, 2016 Census—Zone 2 (Saint John area) [Health region, December 2017] https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=HR&Code1=1302&Geo2=PR&Code2=13&SearchText=Zone%202%20%5bSaint%20John%20area%5d&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=1302&TABID=1&type=0 Available at: Accessed November 6, 2019.
- 24.Hussey H.H., Katz S. Infections resulting from narcotic addiction: report of 102 cases. Am J Med. 1950;9:186–193. doi: 10.1016/0002-9343(50)90021-0. [DOI] [PubMed] [Google Scholar]
- 25.Brown P.D., Levine D.P. Infective endocarditis in the injection drug user. Infect Dis Clin North Am. 2002;16:645–665. doi: 10.1016/s0891-5520(02)00019-3. viii-ix. [DOI] [PubMed] [Google Scholar]
- 26.Trifunovic D., Vujisic-Tesic B., Obrenovic-Kircanski B. The relationship between causative microorganisms and cardiac lesions caused by infective endocarditis: new perspectives from the contemporary cohort of patients. J Cardiol. 2018;71:291–298. doi: 10.1016/j.jjcc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Kasper K.J., Manoharan I., Hallam B. A controlled-release oral opioid supports S. aureus survival in injection drug preparation equipment and may increase bacteremia and endocarditis risk. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateu-Gelabert P., Guarino H. The opioid epidemic and injection drug use: MIPIE and health harms related to the injection of prescription opioids. Int J Drug Policy. 2018;57:130–132. doi: 10.1016/j.drugpo.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy É., Arruda N., Bourgois P. The growing popularity of prescription opioid injection in Downtown Montréal: new challenges for harm reduction. Subst Use Misuse. 2011;46:1142–1150. doi: 10.3109/10826084.2011.552932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wurcel A.G. Drug-associated infective endocarditis trends: what’s all the buzz about? Ann Intern Med. 2019;170:68. doi: 10.7326/M18-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambdin B.H., Bluthenthal R.N., Zibbell J.E. Associations between perceived illicit fentanyl use and infectious disease risks among people who inject drugs. Int J Drug Policy. 2019;74:299–304. doi: 10.1016/j.drugpo.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaura A., Byrne J., Fife A. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4 doi: 10.1136/openhrt-2017-000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brothers TD, Webster D. Multidisciplinary infective endocarditis care teams should address substance use disorders and harm reduction services [e-pub ahead of print]. Open Heart. Available at: https://openheart.bmj.com/content/4/2/e000699.responses#multidisciplinary-infective-endocarditis-care-teams-should-address-substance-use-disorders-and-harm-reduction-services.
- 34.Marks L.R., Munigala S., Warren D.K. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68:1935–1937. doi: 10.1093/cid/ciy924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodger L., Glockler-Lauf S.D., Shojaei E. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alagna L., Park L.P., Nicholson B.P. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis – Prospective Cohort Study. Clin Microbiol Infect. 2014;20:566–575. doi: 10.1111/1469-0691.12395. [DOI] [PubMed] [Google Scholar]
- 37.Pierce D., Calkins B.C., Thornton K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician. 2012;85:6. [PubMed] [Google Scholar]
- 38.Østerdal O.B., Salminen P.-R., Jordal S. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22:633–640. doi: 10.1093/icvts/ivv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchanan N.E., Lennox R.B., Whitlock R., Belley-Côté E., O’Shea T. Making the cut: perspectives on the surgical management of infective endocarditis among people who use intravenous drugs. Can J Cardiol. 2019;35:1416–1418. doi: 10.1016/j.cjca.2019.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.