Abstract
Statement of Problem
Acrylic plastics are used for over 80 years for the manufacture of prostheses. This kind of material has some limitations, one of them is a residual monomer, that remains after the polymerization has been terminated, which can influence the biological properties of the final medical device.
The purpose
The aim of this investigations was a comparison of the residual monomer concentration and cytotoxic effect of three various acrylic materials which differ in the polymerization method (hot-cured, polymerized under pressure and at lower temperatures).
Material and methods
The cytotoxicity of three different acrylic resins from the same producer were tested on the in vitro model (VERO CCL-81) by MTT assay. The residual monomer of acrylic materials was detected by gas chromatography.
Results
The Superacryl Plus material polymerized in hot water has a residual monomer concentration of 0.67 ± 0.05%, Superpont C + B hardened under pressure of 2.61 ± 0.208%, and Premacryl Plus after cold curing process has 3.53 ± 0.27% of uncured MMA. The results revealed that the least cytotoxic effect were observed in case of a thermally polymerized material.
Conclusion
Material polymerized in high temperatures has lower residual monomer concentration and not affect cell cultures. Self-curing materials polymerized in lower temperature have a higher concentration of residual monomer, which reduces the number of living cells by 20%, which can cause allergic reaction shortly after new denture was prepared.
Keywords: Acrylic resins, Hot curing, Cold curing, Cytotoxicity, Residual monomer concentration
1. Introduction
Currently, despite the development of technology, acrylic dentures are still one of the most frequently performed prosthetic restorations, as full or partial removable dentures. This is mainly since the process of producing this kind of restoratives is relatively simple, not requiring a large financial outlay. Besides, acrylics have a long clinical history of use, sufficient mechanical properties, are easily polishing and have relatively good biocompatibility (Jadhav et al., 2018, Rashid et al., 2015). Sometimes, after using acrylic dentures, in some patients, however, an allergic reaction may occur, which is characterized by changes in the mucous membrane in the oral cavity in direct contact with the denture base (Jorge et al., 2003, Goiato et al., 2015).
For this type of allergic reactions, the residual monomer is mainly responsible, which remains uncured in the interior of the material after polymerization. The concentration of unpolymerized monomer is reduced under the influence of time (Jadhav et al., 2018, Rashid et al., 2015, Qaisar et al., 2017, Ayaz et al., 2014). Acrylic resins for the manufacture of acrylic dentures, they consist of two main components: a fluid based on methyl methacrylate (MMA) and ethylene glycol dimethacrylate (EGDMA), and a powder which is a polymethyl methacrylate with low dibenzoyl peroxide (BPO) concentration like a catalyst. After mixing these two components, the liquid gradually absorbs through the powder. Then the acrylic materials can be cured in two ways. Through thermal curing - boiling in hot water, or at room temperature. Materials polymerized at room temperature also contain in their composition N, N dimethyl-toluidine in the liquid and a larger amount of dibenzoyl peroxide in the powder. Polymerization in hot water occurs over a longer period from 1 to 7 h and materials of this type contain a relatively small content of the residual monomer. Room temperature polymerization leaves in the acrylic much higher concentration of this substance. As this process is carried out at temperatures from 23 °C to 60 °C for 15–30 min. Then the concentration of free methacrylate can be from 3 to 6%. For comparison, after polymerization, the boiling water in the material remains below 1.5% of free MMA (Rashid et al., 2015, Jorge et al., 2003, Goiato et al., 2015). In the literature it is possible to find a lot of information about cytotoxicity, irritancy properties associated with the free monomer in acrylic dentures (Rashid et al., 2015, Qaisar et al., 2017, Ayaz et al., 2014, Zissis et al., 2008). The other authors quite extensively presented the works on the subject of residual monomer and its concentration in various materials. There are only a few studies that would compare the same materials in which the whole concentration of residual monomer was tested according to the ISO standard and their cytotoxicity.
On the other hand, each producer of acrylic resins uses different raw materials and dyes that can significantly affect the biocompatibility of the final product.
Therefore, the aim of this work was to test 3 different acrylic materials from the same manufacture polymerized in different ways, on the concentration of the total residual monomer, using gas chromatography. In the second part of the study, the same materials have been tested for cytotoxicity using MTT cytotoxicity test in direct 24 h contact with cured material.
2. Material and methods
2.1. Acrylic materials
Three acrylic resins manufactured by SpofaDental (Czech Republic, Jicin) were used as the test material: Superacryl Plus (hot curing denture base resin), Superpont C + B (pressure curing for temporary crown and bridges) and Premacryl Plus (cold curing, for removable orthodontic apparatus). Detailed information on the materials and their compositions are presented in Table 1.
Table 1.
Materials and their main composition used for biovalidation.
| Material | Batch number | Composition |
|---|---|---|
| Superacryl Plus powder(SpofaDental, Jicin Czech Rep.) | 6,977,651 | PMMA, pigments, BPO |
| Superacryl Plus liquid (SpofaDental, Jicin, Czech Rep.) | 5,594,241 | MMA, EGDMA |
| Superpont C + B powder (SpofaDental, Jicin, Czech Rep.) color D2 dentin | 6,789,991 | PMMA, pigments, BPO |
| Superpont C + B liquid (SpofaDental, Jicin, Czech Rep.) | 5,967,779 | MMA, triethylene glycol dimethacrylate |
| Premacryl Plus powder (SpofaDental, Jicin, Czech Rep) | 6,198,981 | PMMA, pigments, BPO |
| Premacryl Plus liquid (SpofaDental, Jicin, Czech Rep) | 5,635,530 | MMA, EGDMA, DMPT |
| Convertin Hard (SpofaDental, Jicin Czech Rep) | 6,598,805 | Calcium sulfate hemihydrate pigments |
Superacryl Plus was prepared according to the manufacturer's recommendations, mixing 2.2 g of powder with 1 g of monomer. Totally 22 g of powder and 10 g of liquid were prepared to obtain 5 discs which are necessary for residual monomer detection according the ISO standard. The material, after 15 min in the phase of the soft dough, was applied to a metal mold with a diameter of 50 mm and a thickness of 0.5 mm, which was placed in a polymerization flask and covered with class IV gypsum (Convertin Hard). In this way, 9 samples for testing the residual monomer were prepared. In general, 21 samples of Superacryl Plus- hot curing acrylic resins were prepared. Example of pressure curing resin it was used Superpont C + B, which is material for preparation crowns and bridges. The material was prepared to mix 2 g of powder with 1 g of monomer (20 g powder and 10 g of liquid) and allowed to stand for 20 min at room temperature until the material reached the soft dough phase. Samples for residual monomer and cytotoxicity were the same dimensions as for Superacryl Plus. After being applied dough to the forms, the altogether was polymerized in a pressure equipment (Zhermapol) at a pressure of 3 bar and a temperature of 92 °C for a period of 20 min.
The third resin used for the tests was the low-temperature polymerized material, Premacryl Plus for making removable orthodontic appliances. The samples were prepared by combining powder and liquid with a proportion of 2 g of powder and 1 g of liquid (20 g of powder.10 g of liquid). The material before being applied to the molds was stored at room temperature for 6 min, when it did not stick to the walls of the vessel. Premacryl Plus was cured in a pressure curing unit (Zhermapol- Warsaw, Poland) for 15 min, at a pressure of 0.2 bar and a temperature of 60 °C. Totally 21 samples of Premacryl Plus were prepared. All samples of acrylic resins removed from the mold were stored at 23 °C in a dark place, before the tests. A total of 66 samples from 3 acrylic resins were prepared for the tests.
2.2. Gas chromatography for residual monomer detection
Samples of dimension 50 × 0.5 mm were crushed in to small pieces and placed in to a closable 10 ml volumetric glass flask, by weighing approximately 650 mg from the specimen disc. Resins were dissolved in a pure acetone (99%, Merck Millipore, US). To determine the residual monomer, the methodology described in the ISO 20795-1:2013, standard was followed (International Organization for Standardization ISO, 2013). As an internal standard, n- butanol (99% Merck Millipore, US) was used. The standard curve was prepared using known concentration of methacrylate in acetone, from 6, 60, 150, 300 until 400 mg of MMA. The detection device was a Shimadzu GC-7 gas chromatograph (Shimadzu, Japan) equipped with a flame ionization detector (FID) system. Nitrogen was used as the inert gas carrier, which had a flow rate of approximately 1 ml/min. The detection of residual monomer was carried out by a flame ionization detector with the temperature of 200 °C and the ratio of H2/air flow at 45/450 ml/min.
From a scatter plot, a regression line between ′Amma/A internal standard. and concentration of MMA was drawn. The following equation (1) for the regression line was used:
| (1) |
For R = 0.995
Determination of residual monomer in sample solutions
The peak areas of MMA and I.S. of each sample solution were recorded by the software of gas chromatography machine. The concentration of MMA (µg/ml) or CMMA in the sample solution was calculated using equation o 1, to know the peak field. CMMA was used to calculate the total amount of MMA in the sample solution (m MMA) in micrograms (µg) using the equation (2).
| (2) |
At the end, mMMA was used to calculate the percentage of total residual monomer in the sample by the following equation (3).
| (3) |
Mean residual monomer value of each material was then calculated from 9 repetitions.
2.3. Cell culture
There was used Vero CCL-81 (ATCC®, UK) cell line for cytotoxicity studies. Vero cells are normal epithelial cells and derive from the kidney of an African green monkey (Cercopithecus aethiops) (Khan et al., 2017, Decha et al., 2019). This in vitro model is commonly used for cytotoxic and biocompatibility studies of various dental materials. Cells were maintained in MEM (Gibco, Germany) medium containing 4% FBS (Gibco, Germany) in 37 °C in humified atmosphere enriched by 5% CO2. Before experiment cells were trypsinized (Trypsin-EDTA, Simga-Aldich) and resuspended for the appropriate cell culture dishes.
Tested article preparation for cytotoxicity studies
The test article extract was prepared in 1x MEM cell growth medium (MEM supplemented with 10% fetal bovine serum extract) at the sample to extraction medium ratio of 6.0 cm2/mL and extracted at 37 ± 1 °C for 72 ± 2 h. The sample was unchanged by the extraction procedure and the extract was found to be clear and free of particles.
2.4. Cytotoxicity assay test – direct contact
For the cytotoxicity studies, 12 samples with a diameter of 5 mm and a thickness of 1 mm were prepared, using a metal form. The material was polymerized gradually by raising the temperature of water for 30 min to 70 °C then in warm water at 70 °C for a further 30 min and in 30 min in boiling water. The acrylic disc test articles were evaluated using the MTT (methyl thiazolyl diphenyltetrazolium bromide, Sigma Aldrich). Before exposition Vero cells were suspended on 96-well plate in density 1 × 104 cells/100 μl in MEM/well. The cells were cultivated for 24 h to obtained 80% of confluency, and then were exposed to tested samples, positive and negative controls during 24 h. Cell medium was removed and 6x 100 μl of the sample, positive control, negative control, blank samples were added to individual wells. After the incubation time with samples, MTT test was applied using in final step 2-isopropanol (100 μg/l/well, Sigma Aldrich, Poland) with simultaneous shaking. The absorbance was detected at 570 nm after 24 h incubation with tested articles. According the obtained results the following data analysis was performed. The results obtained from spectrophotometric measurements defined as the viability were calculated according to the formula (4):
| (4) |
In the equation were used mean values of all measured optical densities at 570 nm of respective samples.
3. Statistical analysis
All experiments were performed in triplicate for each parameter, which gives 9 repetitions for each parameter. Data were represented as mean ± standard error of the mean. Data were analyzed by two-way ANOVA (in GraphPad Software Inc., San Diego, CA, USA), with p-value < 0.05 as a statistically significant.
4. Results
4.1. Residual monomer concentration
The obtained results and final concentration of residual monomer for Superacryl Plus, Superpont C + B and Premacryl Plus are shown in Table 2. The lowest concentration of residual monomer was observed for the samples of Superacryl Plus, which is a material polymerized in hot curing method. The result of free MMA was 0.67%. In case of samples of Premacryl Plus which were cured in the lower temperature and shorter period of time had the free methyl methacrylate concentration about 3.53%.
Table 2.
Residual monomer concentration of tested samples.
| Superacryl | Superpont C + B | Premacryl Plus | |
|---|---|---|---|
| sample [mg] | 657.11 ± 13.11 | 657.22 ± 14.85 | 656.22 ± 22.89 |
| Amma/A | 58.87 ± 4.41 | 228.18 ± 16.03 | 309.48 ± 29.974 |
| C MMA [mg] | 0.09 ± 0.007 | 0.34 ± 0.024 | 0.46 ± 0.045 |
| M MMA [mg] | 4.41 ± 0.33 | 17.11 ± 1.203 | 23.21 ± 2.25 |
| residual monomer [%] | 0.67 ± 0.05 | 2.61 ± 0.208 | 3.53 ± 0.27 |
4.2. MTT cytotoxicity
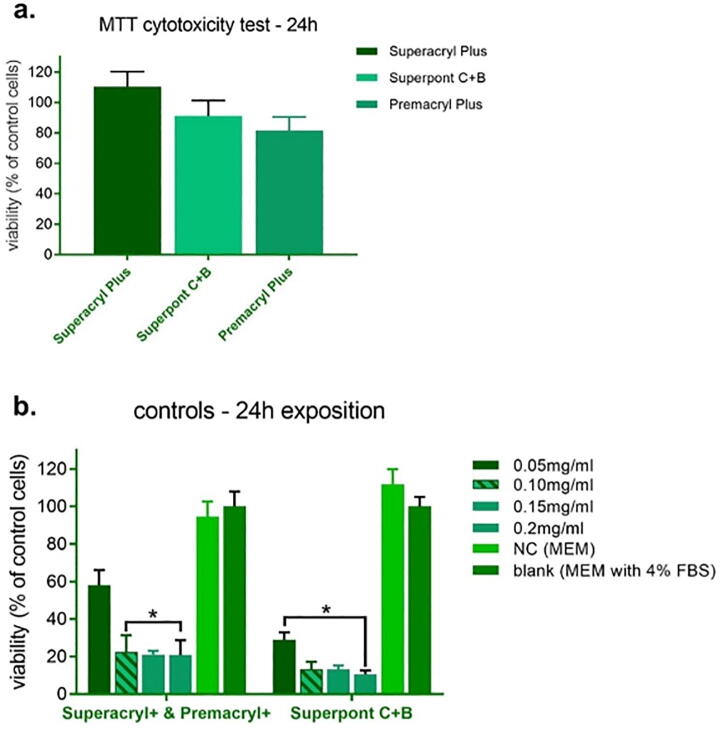
The basic interpretation is based on the following observation, the lower the viability % value, the higher the cytotoxic potential of the test sample. If viability is reduced to < 70% of the blank, it has a cytotoxic potential. The obtained results are shown in a for Superacryl, Premacryl and Superpont C + B and in Fig. 1b for control samples. The negative control (MEM) and blank sample (MEM with 4% FBS) both demonstrated no cytotoxic effect, thus cell oxidoreductive potential was undisturbed. The positive control (SLS, Sodium lauryl sulphate, Sigma Aldrich, Poland) demonstrated significant cytotoxic impact. Cell viability decreased with the increasing PC concentration. After 24 h exposition to Superacryl and Premacryl the viability was on the level of 20%, in case of Superpont C + B cell viability reached<10%. The verified articles not significantly affected mitochondrial activity. After 24 h the cell viability reached 110.31% for Superacryl of control cells, for Superpont C + B 91.21% of control cells, and the self-curing resin Premacryl caused that cell viability 81.5%.
Fig. 1.
The Vero cells viability results after 24 h of exposition to (a) Superacryl Plus, Superpont C + B and Premacryl Plus, and (b) positive (PC) and negative controls (NC), PC in various concentrations. * p ≤ 0.05.
The viability of the cells after 24 h exposition to Superacryl Plus, Premacryl Plus and Superpont C + B reached > 70% of control cells. The cytotoxic effect of positive control has been detected in all concentrations. Negative control proved no cytotoxic potential.
The results obtained from MTT cytotoxicity test were in correlation with the residual monomer concentration, for the Superacryl Plus material the cell survival is 110.31% and for the self-curing resin these values are much lower and amount to 81.56%.
5. Discussion
There is commonly known, that unpolymerized methyl methacrylate adversely affects physical properties (color stability, polishing ability, resistance to bending) and biocompatibility of acrylic dentures (Jadhav et al., 2018, Rashid et al., 2015, Catunda et al., 2017, Huang et al., 2001).
Unpolymerized MMA can be responsible for mucosal irritation and sensitization of tissues. Different cell cultures may react differently to the residual monomer released from acrylic dentures. For example big difference was observed by Huang et al. for a human oral epithelial KB cell line and primary human oral fibroblasts derived from buccal mucosa (Qaisar et al., 2017). Particularly noteworthy is the study of Masayuki et al., who tested the influence of 4 different mammalian fibroblasts on MMA. They observed, that L929 were marked the largest IC50 value (the amount of MMA that caused 50% cell death) (34 mM/L), and for comparison Balb/3T3 clone A31 had the least value (1 mM/L); for cells C3H10T1/2 and MC3T3-E1 these values were 25 mM/L and 16 mM/L, respectively (Masayuki et al., 2000).
In some cases, when patients are allergic to substitutes that can be eluted out of acrylic resins, there is the possibility of using hypoallergenic materials that do not have MMA in their composition (Pfeiffer and Rosenbauer, 2004, Spartalis et al., 2017). There are many ways to reduce the content of this disadvantageous substance in acrylic dentures, such as long polymerization time, storage of dentures after curing in water, polymerization by means of microwave radiation, freezing of acrylic cake before polymerization or using chemical agents based on hydrogen peroxide (Thaitammayanon et al., 2018, Wonglamsam et al., 2016, Charasseangpaisarn et al., 2016, Gonçalves et al., 2008, Kurata et al., 2012).
In literature it is possible to find few works about concentrated of eluted monomer from acrylic resins and the cytotoxicity. However, the authors of the study measure only the amount of monomer released into the water and not the total monomer contained in the material. At the same time they are observing that MMA can be washed out of the material for a longer period of time. The second important point is the fact that methyl methacrylate is not well soluble in water and people using prostheses also drink alcohol, fat and carbonate, in which the residual monomer dissolves better. Jorge et al. described in their research that the additional placement of polymerized acrylic at 55 °C for 60 min reduced its cytotoxicity in L929 cells by means of a test H-thymidine, which measures the number of cells synthesizing DNA. For comparison, samples of Lucitone and QC20 were post cured by the microwave energy for 3 min at 500 W. Such treatment has not changed the cytotoxicity of heat curing resins (Jorge et al., 2006).
Bural et al. (2011) compared the effect of the acryl polymerization method on the amount of acrylic monomer released and the survival of cells. This test applies to the material cured by long time polymerization for 7 h at 70 °C and 3 h at 100 °C. The concentration of released MMA validated during 1–7 days was tested using high-performance liquid chromatography. The cytotoxicity was determined on L-929 fibroblast model XTT proliferation assay. Authors stated, that samples that were not cured in boiling water adversely affect cell cultures, reducing their proliferation values from 67 to 73%, which indicated that these resins have cytotoxic properties. There was noted that samples boiled in hot water showed a cell survival rate of 84%. In the first two cases, the concentration of residual monomer in the samples was 6.45% and 2.29% and in the third 0.92%. The obtained results indicate that the materials hardened in various methods were characterized by slight cytotoxicity for a period of 7 days (Bural et al., 2011).
In the other study there was examined the influence of acrylic heat-resistant acrylic materials and resins for making mobile orthodontic devices on the released monomers of methyl methacrylate and urethan dimethacrylate (UDMA), on cellular cytotoxicity using the method Mosmann's proliferation-inhibition test with an established culture of fibroblasts (MTT test). The lowest amount of monomer released was observed for thermally polymerizable materials, which translated into no effect on cell cultures. The orthodontic material polished at lower temperatures was characterized by slight cytotoxicity. The material Triad, which is acrylate hardened with the help of a light and contains UDMA, has cytotoxic properties, if the non-polymerized layer of oxygen inhibition is not removed from it (Rose et al., 2000).
The results obtained by these authors (Jorge et al., 2006, Bural et al., 2011, Rose et al., 2000, Bural et al., 2011) are consistent with the results obtained in our studies. We observed, that the content of residual monomer in polymerized samples polymerized at lower temperatures is higher (3.5% for Premacryl and 2.6% for Superpont C + B) with the simultaneous lower survival of cell culture. But, for the Superacryl Plus resin, the survival rate was very high, since the residual monomer concentration is 0.69%.
In the case of other studies, where have been observed cytotoxicity, and our research, the main difference may be in the composition of the materials used for research. If the product contains in its composition more or less benzoyl peroxide as a catalyst, it may be cured in more efficient way and therefore not exhibit cytotoxic properties. The same statement can be used for self-cure materials, where the higher content of BPO and DMPT also helps the curing and lower content of residual monomer to a greater extent (Rose et al., 2000).
Materials polymerized in hot water do not affect the inhibition of cell cultures. Too high residual monomer concentration affects the growth of cell cultures, which has been confirmed for self-curing and polymerized materials in which pressure periods (Rose et al., 2000, Bural et al., 2011).
Materials produced by the same manufacturer, containing the same dyes and raw materials from the same suppliers, may have different biocompatibility depending on the polymerization method and the amount of powder and fluid used to prepare the prosthesis. For example, Material Spofacryl Plus, which has a 2.2/1 (powder/liquid) ratio, does not inhibit the growth of cell cultures as opposed to the Premacryl material. In this resin, the mixing ratio between powder and liquid is 2.0/1 g.
6. Limitation of the study
The main limitation of the study is the composition of individual acrylic resins, which may differ from each other. Therefore, it is necessary to optimize the composition due to obtain material which could be cured in higher temperatures and with low concentration of residual monomer.
7. Clinical conclusion
Acrylic materials for the performance of settling prostheses should be cured in a process recommended by the manufacturer of the material. In patients who have suspicions for allergic reactions, the recommendation should be to use thermally polymerized materials for preparation of removable dentures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Jadhav S.S., Mahajan N., Sethuraman R. Comparative evaluation of the amount of the residual monomer in conventional and deep-frozen heat cure polymethylmethacrylate acrylic resin: an in vitro study. J. Indian Prosthodont. 2018;18:147–153. doi: 10.4103/jips.jips_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid H., Sheikh Z., Vohra F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur. J. Dent. 2015;9:614–619. doi: 10.4103/1305-7456.172621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge J.H., Giampaolo E.T., Machado L., Vergani C.E. Cytotoxicity of denture base acrylic resins: a literature review. J. Prost. Dent. 2003;90:190–193. doi: 10.1016/s0022-3913(03)00349-4. [DOI] [PubMed] [Google Scholar]
- Goiato M.C., Freitas E., dos Santos D. Acrylic resin cytotoxicity for denture base – literature review. Adv. Clin. Exp. Med. 2015;24:679–686. doi: 10.17219/acem/33009. [DOI] [PubMed] [Google Scholar]
- Qaisar A., Jatala U.W., Fareed M.A., Yazdanie N. Measurement of residual monomer in auto polymerized acrylic resins by high pressure liquid chromatography. Biomedica. 2017;33:211–215. [Google Scholar]
- Ayaz E.A., Durkan R., Koroglu A., Bagis B. Comparative effect of different polymerization techniques on residual monomer and hardness properties of PMMA-based denture resins. J. Appl. Biomater. Funct. Mater. 2014;30:228–233. doi: 10.5301/jabfm.5000199. [DOI] [PubMed] [Google Scholar]
- Zissis A., Yannikakis S., Polyzois G., Harrison A. A long term study on residual monomer release from denture materials. Eur. J. Prosthodont. Restor. Dent. 2008;16:81–84. [PubMed] [Google Scholar]
- International Organization for Standardization ISO 20795-1:2013, Dentistry — Base polymers — Part 1 Denture base polymers, point 8.8.
- Khan M.A., Ahmad R., Srivastava A.N. Effect of ethyl acetate aroma on viability of human breast cancer and normal kidney epithelial cells in vitro. Integr. Med. Res. 2017;6:47–59. doi: 10.1016/j.imr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decha N., Talungchit S., Iawsipo P., Pikulngam A., Saiprasert P., Tansakul C. Synthesis and characterization of new hydrolytic-resistant dental resin adhesive monomer HMTAF. Des. Monomers Polym. 2019;22:106–113. doi: 10.1080/15685551.2019.1615789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catunda R.Q., Cardoso Vieira J.R., de Oliveira E.B., da Silva E.C., Brasil V.L.M., da Cruz D.E. Cytotoxicity evaluation of three dental adhesives on Vero cells in vitro. J. Clin. Exp. Dent. 2017;9:e61–e66. doi: 10.4317/jced.53039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.M., Tai K.W., Hu Ch.Ch., Chang Y.Ch. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblasts. In Vitro J. 2001;14:439–443. [PubMed] [Google Scholar]
- Masayuki T., Hiroyuki N., Takuya M., Junzo T. Cytotoxic effect of methyl methacrylate on 4 cultured fibroblasts. Int. J. Prosth. 2000;13:311–315. [PubMed] [Google Scholar]
- Pfeiffer P., Rosenbauer E.U. Residual methyl methacrylate monomer, water sorption, and water solubility of hypoallergenic denture base materials. J. Prosthet. Dent. 2004;92:72–78. doi: 10.1016/j.prosdent.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Spartalis G.K., Cappelletti L.K., Schoeffel A.C., Michel M.D., Pegoraro T.A., Arrais C.A., Neppelenbroek K.H., Urban V.M. Effect of conventional water-bath and experimental microwave polymerization cycles on the flexural proper-ties of denture base acrylic resins. Biomedica. 2017;33:211–215. doi: 10.4012/dmj.2015-047. [DOI] [PubMed] [Google Scholar]
- Thaitammayanon P., Sirichompun Ch., Wiwatwarrapan Ch. Ultrasonic treatment reduced residual monomer in methyl methacrylate-based orthodontic base-plate materials. Dent. Oral Craniofac. 2018;4:1–5. [Google Scholar]
- Wonglamsam A., Kaewkornpradit W., Nagaviroj N., Kanchanavasita W. Effect of processing and curing procedures on residual monomer levels of denture base materials. Mater. Dent. 2016 J.36,145–154. [Google Scholar]
- Charasseangpaisarn T., Wiwatwarrapan C., Leklerssiriwong N. Ultrasonic cleaning reduces the residual monomer in acrylic resins. J. Dent. Sci. 2016;11:443–448. doi: 10.1016/j.jds.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves T.S., de Menezes L.M., Silva L.E. Residual monomer of autopolymerized acrylic resin according to different manipulation and polishing methods. An in situ evaluation. Angle Orthod. 2008;78:722–727. doi: 10.2319/0003-3219(2008)078[0722:RMOAAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kurata S., Morishita K., Kawase T., Umemoto K. Cytotoxic effects of acrylic acid, methacrylic acid, their corresponding saturated carboxylic acids, HEMA, and hydro-quinone on fibroblasts derived from human pulp. Dent. Mater. J. 2012;31:219–225. doi: 10.4012/dmj.2011-085. [DOI] [PubMed] [Google Scholar]
- Jorge J.H., Giampaolo E.T., Vergani C.E., Machadoa L., Pavarina A.C., Carlos I.Z. Effect of post-polymerization heat treatments on the cytotoxicity of two denture base acrylic resins. J. Appl. Oral Sci. 2006;14:203–207. doi: 10.1590/S1678-77572006000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bural C., Deniz G., Bayraktar G. Effect of leaching residual methyl methacrylate concentrations on in vitro cytotoxicity of heat polymerized denture base acrylic resin processed with different polymerization cycles. J. Appl. Oral Sci. 2011;19:307–311. doi: 10.1590/S1678-77572011005000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E.C., Bumann J., Jonas I.E., Kappert H.F. Contribution to the biological assessment of orthodontic acrylic materials measurement of their residual monomer output and cytotoxicity. J. Orofacial Orthopedics/ Fortschritte Kieferorthopädie. 2000;61:246–257. doi: 10.1007/s000560050010. [DOI] [PubMed] [Google Scholar]
- Bural C., Deniz G., Ünlüçerçi Y., Kızılc N., Bayraktara G. Effect of post-polymerization heat-treatments on degree of conversion, leaching residual MMA and in vitro cytotoxicity of autopolymerizing acrylic repair resin. Dent. Mater. J. 2011;27:1135–1143. doi: 10.1016/j.dental.2011.08.007. [DOI] [PubMed] [Google Scholar]