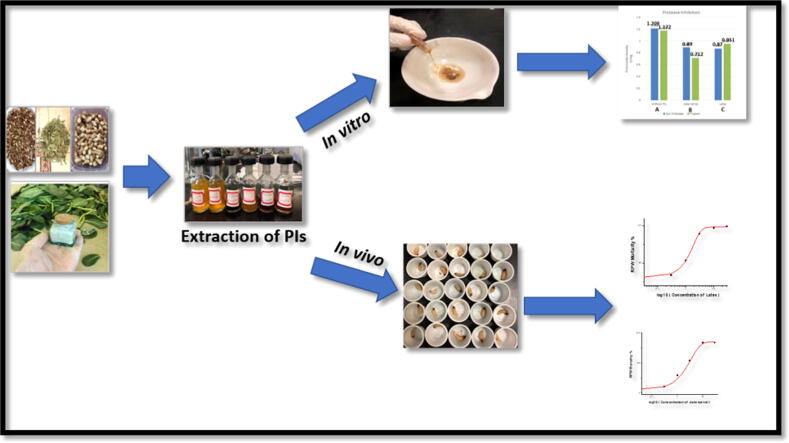
Graphical abstract
Keywords: Red palm weevil, Rhynchophorus ferrugineus, Protease inhibitors, Date’s kernel, Calotropis latex
Abstract
Serine proteases are essential metabolic enzymes in the midgut of many pests, including the red palm weevil (RPW), Rhynchophorus ferrugineus Olivier, which has a significant impact economically, environmentally and socially worldwide especially in the middle east. Some methods have been used to manage this pest such as trapping of RPW with pheromones, chemicals, and X-rays. However, these methods are costly, not effective and negatively impact the human. The main objective of this study is to contribute to the discovery of an eco-friendly pesticide to eradicate this infection by using serine protease inhibitors (SPIs) extracted from different parts of plant resources. In this research, both in vitro and in vivo effects of SPIs activity against RPW were examined. The protease inhibitors (PIs) activity was recorded in the crude extract that was isolated from the date’s kernel (DKE), host and Calotropis latex (CLE), non-host. These PIs were partially purified by ammonium sulfate precipitation. The midgut tissue of RPW was extracted and analyzed for protases activity assay. PIs assays were consistent with the increased in the inhibitory activity against the midgut proteases after treatment with a DKE and CLE. The reduction of gut proteases by DKE solution and CLE was 39%, 18%, respectively. Partially purified DKE showed the most prominent inhibition pattern of protease activity of the gut extract. While, latex exhibited acute toxicity, imparting the least LC50 (5.132 mg/mL) against RPW larvae. Taken together, these findings provide evidence for the hypothesis that SPIs activity may play an important role in enhancing the mortality of RPW and relieving the toxicity of insecticide in palm trees.
1. Introduction
RPW, commonly known as red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) is documented as tremendously antagonistic palm pests with known broad economic and environmental impacts when appearing in a different geographical region (Zhang H et al., 2020). RPW has a harmful social influence in the Middle-East region, where the palm tree is connected with culture and religion (El-Fattah AYA et al., 2020). It is well-known as the utmost significant pest on date palm in the globe and dangerous pests for Cocos nucifera (coconut) (Mohamed MA et al., 2020). RPW has demonstrated as the damaging pests in numerous locations in the Mediterranean; Saudi Arabia, United Arab Emirates, and Egypt in the middle-east (Azmi WA et al., 2017 : Ishak I et al., 2020). All life-cycles of RPW might devote to the host palm, which credits 200–300 eggs in an isolation cavity. Formed eggs crosshatch in 2–5 days, and larvae holes in inner host palm nourishing at soft succulent tissues, abandoning total fibrous materials (Muhammad A et al., 2019). According to host species and temperatures, the range of the larval period is between 36 and 78 days (average range 55 days). Inside the coconut and RPW of larvae pupate arises from 2 to 3 weeks afterward, pupation and completion of life-phase in 4-months (Jia S et al., 2013). The larval stage is the most destructive and responsible for damaging the palm. The concealed behavior of the pest makes it hard to spot the infestation at an early stage; therefore, practical methods for the treatment of this pest have difficulties to be developed (Jian F, 2019).
Serine is a protease enzyme that hydrolysis peptide bond by using serine residue, which includes trypsin, chymotrypsin, thrombin, and plasmin (Neitzel J, 2010). It’s crucial for mammal’s life by playing the central role in many biological functions such as anticoagulation, digestion, and immune system. It’s found in organisms like bacteria which help them to digest material (Neitzel J, 2010 : Rose T, Di Cera EJ, 2002) and extracted from different part of plant (Antão CM, Malcata FX, 2005). Also, those enzymes founded in the midgut of the insect, which secreted by two mechanisms; the first one is by a direct effect of the food ingredients on the epithelial cell of the midgut; the second one is by food consumption that triggers some hormonal effect (Jamal F et al., 2013 : Bhagwat MP, 2014). Protease inhibitors (PIs) interact with the protease activity of those enzymes and inhibit them, which results in a reduction of protein that can be digested. Then, the hyperproduction of the digestive enzyme leads to further loss of sulfur amino-acid, as a result of that insect weaken, which ultimately demonstrated as inhibition of growth and death (Jamal F et al., 2013 : Shukle R, Murdock L, 1983). PIs can be extracted from various plant parts and its related materials especially seeds and latex (Gomes et al., 2005, Zhou et al., 2008), such as chickpea seeds, smooth crotalaria seeds, kalkora mimosa seeds as well as soybeans. In addition to seeds of barley, rice, the latex of dandelion, roots of sweet potato, leaves of tobacco, and fruits of melon. It is essential to consider PIs in non-host plants because host PIs are evolutionary dynamic and codependent on other resistance mechanisms and interaction of other pests; also, insects might have coevolved and adapted instantaneously (Harsulkar AM et al., 1999 : Qu L-J et al., 2003). Farrukh et al (Jamal F et al., 2019) study showed the efficacy of PIs extracted from different parts of different plants against pest's gut protease enzymes by slowing down the metabolism of essential nutrients for pest life. Thus, considering the usage of PIs as one of the most potent and effective methods to eradicate many environmental and economically harmful threatening insects.
Another study was aiming for protein profiling of RPW digestive enzymes based on feeding the larvae three various diets (sago stem, coconut cabbage, and oil palm cabbage), putting into consideration food consumption and developmental time. This study showed that cationic trypsin was the most dominant protein and the most dominant enzyme was aminopeptidase N. These findings can lead to developmental strategies for pest management based on the antinutritional PIs as potential bioagents for pest control (Zulkifli AN et al., 2018).
Based on previous literatures, serine protease inhibitors (SPIs) activity may play an indirect role in the mortality of insecticide in RPW since it’s essential for their life as it’s found in the midgut of them (Jabr AA et al., 2008: Wijanarko A et al., 2017). In this context, the aim of the present study was to develop an effective method to manage the infection of RPW, and to decrease its damaging effect on environment and economics by using biologically safe products on both palm trees and human. Palm leaves, dates, and date’s kernel (DKE) were used as the host source of PIs, while Calotropis (Calotrapos gigantean) latex (CLE) was used as the non– host PIs source (Qu L-J et al., 2003).
2. Materials and methods
2.1. Extraction of protease inhibitor using the blending method
2.1.1. From host plant aerial parts
Selected leaves, seeds, fruits, and barks of the palm tree were collected in August-2018 from King Saud University botanical garden, Riyadh. The collected material was rinsed, air-dried at ambient temperatures, and milled. A phosphate buffer (pH 7) 0.05 M HCl, 0.2% NaOH, 15% NaCl, 0.1% PBS and distilled water (1:1) were added into each sample. The extraction process was continued by blending and filtering with nylon filter steps. The samples were then centrifuged at 200 rpm for 2-hours. Consequently, filtration and centrifugation were performed at 12000 rpm for 15 mins. PIs activity was assessed for resultant insoluble crude extracts (Alencar N et al., 2004).
2.1.2. From non-host plant latex
Freshly, crude latex was collected from parts of Calotropis gigantea; grows in the botanical garden at KSU, Riyadh during March-April 2018. The youngest leaves petioles of plant branches were cut, latex was collected and placed in 250 ml sterile Eppendorf tubes, purified distilled water was added. During the collection, latex was gently stirred due to its tendency for coagulation (Alencar N, et al., 2004: Shukla P, et al., 2012). In the laboratory, collected latex was placed at 4 °C. Centrifugation of the mixture was performed at 10 °C for 10 mins at 5000 g. After centrifugation, rubber-like precipitates were removed, and the soluble phase of latex supernatant was dialyzed using distilled water at 8 °C using membranes of 8000 molecular mass cut-off. Thrice, dialysis water was improved on a daily basis and precipitate, encompassing rubber was extracted at 8 °C for 60-hours. The almost pure soluble phase, contains almost all rubber-free supernatants soluble-latex proteins was freeze-dried at 25 °C. The separated rubber fraction precipitated materials, produced after every centrifugation, was washed thrice using distilled water and suspended to be assayed in repellent assays. Purified, rubber-free supernatants, laticifer proteins (LP) were lyophilized and dissolved in appropriate solutions for the following use in experiments (Wijanarko A et al, 2017).
2.1.3. Enzymatic activity determination
Protease activity can be defined as the production of 1 μg tyrosine by hydrolyzing casein at a certain temperature. The following steps were used to determine protease activity. Both 1 ml of crude extract and 1 ml phosphate buffer (pH 7) were mixed and then the solution was incubated for 5 min at 37 °C. Next, 1 ml of 2% casein was added to 1 ml phosphate buffer 0.05 M (pH 7), then the solution was incubated for 10 min at 37 °C. The assay was stopped by adding 2 ml of 0.4 M TCA (Qiao et al., 2009). The color reaction was observed by pouring 1 ml of the extracted casein-TCA solution with 6 ml of biuret and 0.3 ml of Folin-Ciocalteu reagent. Then, the solution for a control group was used to adjust the extinction, and the absorbance at 750 nm was measured. The free tyrosine standard curve was used to compare the absorbance at 20, 40, 60, 80, and 100 ppm. The formula of protease activity calculation is as follows:
Where Ct is the concentration of free tyrosine in crude extract (ppm), At is the crude enzyme absorbance, Cstd is the concentration of free tyrosine in standard curve (ppm), and Astd is the tyrosine standard absorbance (Wijanarko A et al, 2017).
2.2. Partial extraction of PIs from DK
DK was used for crushing in blender. Lyophilized dried kernel was mixed with 10 times of extraction buffer (pH-6.8) and incubated complete night. Centrifugation at 10,000 rpm at 35 mins was performed and the supernatant was stored at 4 °C. The protein concentration of DK was estimated using Bradford’s method, in which bovine serum albumin was used. This is known as sensitive and rapid methods for quantifying micro-organisms using protein quantities utilizing the principle of protein–dye binding. Saturated solution of (NH4)2SO4 was mixed with the crude extract of supernatant for obtaining 60–90% of saturation. The pellet was dissolved in the minimum volume of extraction buffer and dialyzed in a similar extraction buffer (Bradford MMJAb, 1976). Both the protein content and inhibitory activity of proteinase were estimated and viewing a high level of inhibitory activity towards trypsin. This methodology was completed at 4 °C.
2.3. Insects
The RPW was collected in April 2019 from the infested orchards (with no pesticide sprays in the last 90 days) in Al Kharj region, Saudi Arabia. Sugarcane (Saccharum officinarum L.) stem pieces were used for feeding. The culture was established in the rearing room at 27 ± 2 °C and 50–60% comparative humidity through a photoperiod of 14:12 (Light: Dark) hours and larvae were checked daily for pupation.
2.4. Extraction of RPW gut proteases
Isolated gut tissue from RPW was instantly stored at −20 °C. The midgut tissue was mixed, homogenized using an extraction buffer and placed for 30 mins. Centrifugation was performed at 10,000 rpm for 10 mins towards the removal of gut luminal content. The formed supernatant was used for the assay of protease activity.
2.5. Proteinase and PIs assay
RPW-gut protease inhibitor (RGPI) was measured using azocaseinolytic assay (Kunitz MtJTJoGP, 1947). This assay involves 60 ml of diluted enzyme was mixed to 200 ml of 1% azocasein (0.2 M glycine-NaOH; pH-10) and placed at 37 °C for 30 mins. This reaction was terminated by adding 300 ml of 5% trichloroacetic acid. A similar volume of 1 M NaOH was added to supernatant after centrifuging at 12000 rpm for 10 mins and absorbance was measured at 450 nm. The inhibition assay was carried out with a suitable volume of seed extract, added gut proteinase extract and incubated for 15 mins at 27 °C. The enduring proteinase activity was estimated for each and every assay as follows, a proper control (blank) contains only the reagent, a sample containing gut protease to evaluate complete gut protease activity, a sample containing gut protease + proteinase inhibitor samples DKE for one sample and (CLE) for other samples to estimate the activity of protease in the samples.
2.6. Diet preparation and insect bioassay
About 18 newly emerged RPW larvae were raised on an artificial diet to test the effect of PIs on the mortality and growth of RPW. Artificial diet was prepared using wheat flour (20%), yeast extract (6.5%) sucrose (2.5%), wheat starch (25%), casein (10.5%), oil (2.5%) (w/v) and water (40%) (v/w). Three experimental groups CLE, DKE, and control were set under the same conditions at (27 ± 2 °C and 50–60% relative humidity, with a photoperiod of 14:12 ((Light: Dark) h) in culture media. In each group, 6 newly molted 3rd instar larvae were used for setting the bioassay. The autoclaved (20 min at 120° C) media was cooled then mixed with CLE (first group) to prepare treated mixture ratios of 2.50, 5, 10 and 20 mg ml -1 of CLE. The settled media with specific concentrations of latex and were then cut into 20 g cubes and placed inside round wide-mouthed bottles (5 cm diameter × 3 cm height) with screw-cap equipped with air-holes (Fig. 1). The same ratios were prepared for DKE treated mixture (second group). The media with specific concentrations of DKE were then cut into 20 g cubes and placed inside bottles as described previously. The third group were the pure media without treatment. Healthy, untreated, starved larvae were added to each group of bottles individually. After 24 h, body weight of larvae that remain alive was weighed in both control and treated groups. The experiment was conducted for 10 days. The number of dead insects was recorded every day, and the median (50%) lethal concentration (LC50) was calculated.
Fig. 1.
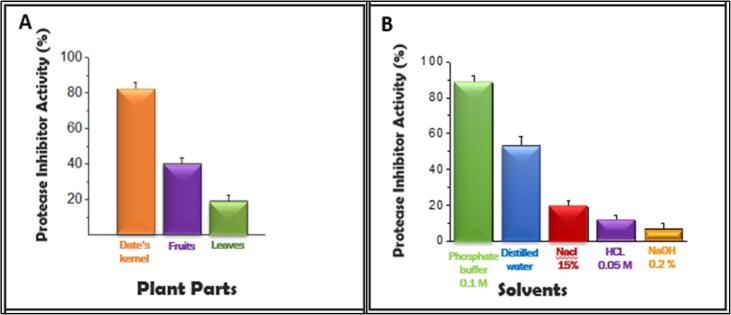
a. Distribution of protease inhibitors (PIs) in diverse part of palm tree. b. Extraction of PIs using numerous solvents.
2.7. Statistical analysis
Data was displayed as a mean ± standard deviation for triplets. Data was represented as independent experiments. Origin software was conducted to determine LC50, LC90 using the equation for sigmoidal dose–response that represents the concentrations that caused 50% and 90% mortality, respectively (Lenovo, Thinkpad, Riyadh, Kingdom of Saudi Arabia).
Statistical tools like t-tests and one-way ANOVA analysis was performed using Openepi software (Khan IA,et al, 2019).
3. Results and discussion
SPs are leading proteolytic enzymes in an insect midgut. They are prevailing in all phylogenetic kingdom including viruses and are participated in various physiological functions. Essentially, the trypsin and chymotrypsin have a significant role in food digestion in insect, zymogen activation, and immune system (Rose T, Di Cera EJ, 2002). This study is the initial report on using DKE, host source of PIs to develop an effective, economic and eco-friendly method to manage RPW infection.
3.1. Screening of PIs through various parts of palm tree
Screening for PIs from several portions of leaves, seeds, fruits, and stems of palm tree. PIs are tiny proteins in general that primarily exist in storage tissues, for example, seeds; nevertheless, they appear in the aerial portions of plants as well (Ghodke A et al., 2013). In response to an attack either by insects or injury PIs are induced (Pichare M, 1996). They play an important role as anti-metabolic proteins triggering the inhibition of the digestive activity of insects (Shukla P et al, 2012). Date palm (Phoenix dactylifera) is a well-known source of numerous bioactive elements with industrial and pharmaceutical uses. It is certainly not labeled as a source of any protein inhibitors. In the current study, it was noticed that amongst the various segments of the palm trees, DK (82 ± 4.2% inhibition) revealed the uppermost levels of inhibition against trypsin activity. Unlike fruits (40 ± 3.3% inhibition), leaves (19 ± 3.5% inhibition), and stems (11 ± 3.1% inhibition) which revealed less quantity of trypsin inhibitor activity (Fig. 1a). As presented in (Fig. 1b) the uppermost PIs activity was noted in the crude extract prepared in 0.1 M phosphate buffer (pH 7.6) (88 ± 4.1% inhibition) trailed by the one prepared in distilled water (53 ± 5.6% inhibition). Equivalent outcomes were testified for the highest extraction of proteins from Cajanus cajan seeds (Shukla P et al, 2012). Therefore, DKE was an abundant source of PIs was taken into consideration for additional studies.
3.2. In vitro study
3.2.1. Protease activity of red palm weevil gut protases (RGP)
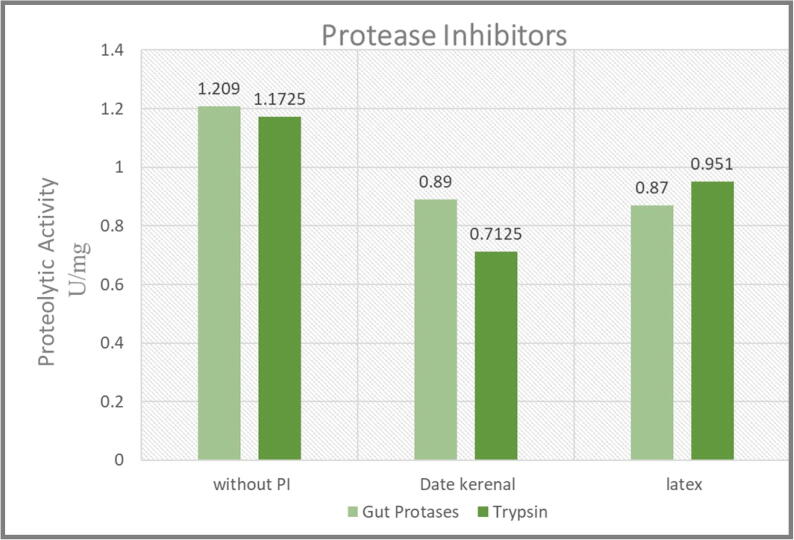
By using trypsin-specific synthetic substrate, trypsin-like activity of the crude gut extract was estimated. The Red palm weevil gut protases (RGP) extract shows the specific activity of 1.2 U/mg of proteins exist in the RGP extract (Fig. 2). The larvae midgut was dissected to assess potential effects of DKE and CLE PIs on the digestive proteinases of RPW larvae. The presence of serine proteinases (trypsin) was detected in midgut extracts of RPW by using Azocasein as synthetic substrates. Inhibitory assays using both DKE PIs and CLE PIs showed high inhibitory activity against total gut proteolytic enzymes activity (Fig. 2). The highest inhibition against standard trypsin was found (Fig. 2). It shows the DKE PIs that are more effective against trypsin enzyme than the CLE PIs. The standard trypsin inhibition by the DKE was almost two-fold as compared to the inhibition of trypsin by the CLE PIs (Fig. 2).
Fig. 2.
Proteolytic activity assay of date kernel extracts (DKE) and Calotropis latex extracts (CLE) protease inhibitors (PIs) of 450 nm.
According to the readings obtained from (Spectro UV–VIS double beam PC and scanning auto cell) (KSU, Saudi Arabia, Riyadh), the gut absorbance was 1.1 (Fig. 3a), gut + DKE was 0.85 (Fig. 3b), and gut + CLE was 0.9 (Fig. 3c) at 450 nm wave length. Accordingly, we confirmed that DKE PIs and CLE PIs showed inhibitory activity against total gut proteolytic enzymes. Moreover, the inhibition of partially purified DKE PIs is the highest than any other solutions and was carried further to analyze the inhibitory activity toward RPW. Actually, the in vitro determination of PI’s activities provided some evidences for our hypothesis, but besides we determined the in vivo responses of PI’s toxicities.
Fig. 3.
Absorbance performed using UV–VIS double beam spectrophotometry; a: Absorbance of gut protease without inhibitor; b: Absorbance of gut protease + date kernel extracts (DKE) and c: Absorbance of gut protease + Calotropis latex extracts (CLE).
3.3. In vivo study
3.3.1. Effect of protease inhibitors on RPW weight
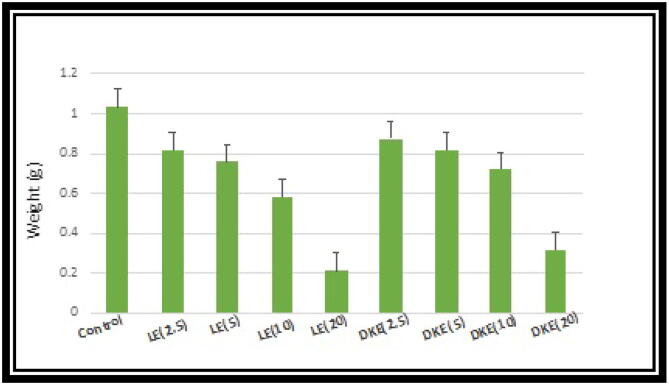
The incorporation of PIs CLE (2.5, 5, 10 and 20 mg/ml), and DKE (2.5, 5, 10 and 20 mg/ml) into diets resulted in significant reduction of weight when compared with that of control fed insects. Insect weight when fed on DKE (2.5 mg ml -1) was also significantly decreased and was enormously less than the control. The weight in control was more than 9 mg; whilst weight in CLE (both 2.5, 5, 10 and 20 mg ml -1 doses) was less than 8 mg. When the insects were fed with CLE and DKE containing diets, severe impacts on overall larval morphology were observed.
3.3.2. Dose mortality response
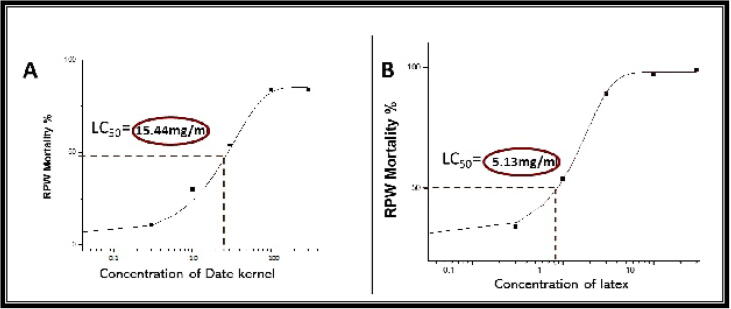
The mortality rate of RPW in the PIs treatments was significantly higher than that of the control group. CLE caused 100% mortality with the least concentration value after 24 h (5.13 mg/ml); and found to be the most toxic PIs in the current study (Fig. 4b). Comparatively, PIs at all tested doses (5.13 and 7.28 mg/ml) within 24 h imparted significantly higher mortality (Fig. 4b). In our toxicity analysis, DKE PIs showed some reduction in RPW control imparted 50% mortality and their LC50 values calculated after 24 h. The values were between 15.13 and 18.3 mg/ml (Fig. 4a). Dietary laboratory experimentation toward RPW showed different biological impacts from low to high degrees.
Fig. 4.
Dose mortality response; 4a: concentration of date kernel extracts (DKE) in mg/ml and 4b: concentration of Calotropis latex extracts (CLE) in mg.ml.
The LC50 of CLE after 6 h was 14.71 mg/ml, while after 12 h it was 10.82 mg/ml that was 38.57% decrease in LC50 value compared to 6 h LC50 value. Furthermore, LC50 value after 24 h was 5.13 mg/ml that was 90.23% decrease in LC50 value compared to 6 h LC50 value. Among all the tested compounds, artificial diet with both CLE and DKE PIs exhibited important toxicity, reporting small LC50 values against RPW larvae. Results of experiments indicate that there was some difference between the in vitro and in vivo outcomes. Many new studies have revealed the disparity between the results obtained from in vivo and those from in vitro. As PIs need a very specific environment, we recommend that further in vivo experiments should be conducted in different environments until revealing similar results.
4. Conclusion
DKE and CLE PIs inhibit certain types of digestive protease enzymes that present in the midgut of the RPW. PIs assays were consistent with the increase in the inhibitory activity against the midgut proteases after treatment with DKE and CLE. The reduction of gut proteases by DKE and CLE was 39%, 18%, respectively. While feeding RPW larvae on PIs CLE and DKE tremendously reduced their survival. These characteristics suggest that host and non-host PIs as a bioactive valuable source for future eco-friendly bio-pesticides with less cost and a low chance to develop insect resistance without damaging ecological balance.
Author contributions
Conceptualization: R.O. and R.S., methodology: A.S., H.A., S.G., and N.Z.; PIs Isolation: M.H.; insect experiment: M.A.; formal analysis: R.O. and S.P.; investigation: N.M. and K.A.; writing the manuscript and proof reading: R.O. and R.S.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Student`s Research Support Program, Project no. (URSP-4-19-17).The authors are highly grateful to chair of date palm research (Prof. Mohammad Al-Saleh) and chair of protein research (Prof. Majed Alokail) at King Saud University for their support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Raha Orfali, Email: rorfali@ksu.edu.sa.
Shagufta Perveen, Email: shakhan@ksu.edu.sa.
Najwa Majrashi, Email: nmajrashi@kacst.edu.sa.
Khulud Alluhayb, Email: khalluhayb@kacst.edu.sa.
Razan S. Orfali, Email: Orfali101@mail.chapman.edu.
References
- Alencar N., Figueiredo I., Vale M., Bitencurt F., Oliveira J., Ribeiro R. Anti-inflammatory effect of the latex from Calotropis procera in three different experimental models: peritonitis. paw edema and hemorrhagic cystitis. 2004;70:1144–1149. doi: 10.1055/s-2004-835842. [DOI] [PubMed] [Google Scholar]
- Antão C.M., Malcata F.X. Biochemistry. Plant serine proteases: biochemical, physiological and molecular features. 2005;43:637–650. doi: 10.1016/j.plaphy.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Azmi W.A., Lian C.J., Zakeri H.A. The red palm weevil. Rhynchophorus ferrugineus: current issues and challenges in Malaysia. 2017;74:17–24. [Google Scholar]
- Bhagwat MP. utilization of helicoverpa gut protease inhibitor proteins in strengthening host plant defense mechanism. 2014. DOI: 10.1093/ee/12.3.787
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical. Biochemistry Journal. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- El-Fattah A.Y.A., El-Wahab A.S.A., Jamal Z.A., El-Helaly A.A. Histopathological studies of red palm weevil Rhynchophorus ferrugineus, (Olivier) larvae and adults to evaluate certain nano pesticides. Braz. J. Biol. 2020 doi: 10.1590/1519-6984.22762. [DOI] [PubMed] [Google Scholar]
- Ghodke A., Chavan S., Sonawane B., Bharose A.J. Isolation and in vitro identification of proteinase inhibitors from soybean seeds inhibiting Helicoverpa gut proteases. J. Plant Interact. 2013;8:170–178. [Google Scholar]
- Gomes CE, Barbosa AE, Macedo LL, Pitanga JC, Moura FT, Oliveira AS, et al. Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). 2005;43:1095-102. [DOI] [PubMed]
- Harsulkar AM, Giri AP, Patankar AG, Gupta VS, Sainani MN, Ranjekar PK, et al. Successive use of non-host plant proteinase inhibitors required for effective inhibition of Helicoverpa armigera gut proteinases and larval growth. 1999;121:497-506. [DOI] [PMC free article] [PubMed]
- Ishak I., Ng L., Haris-Hussain M., Jalinas J., Idris A., Azlina Z. Pathogenicity of an Indigenous Strain of the Entomopathogenic Fungus Metarhizium anisopliae (Hypocreales: Clavicipitaceae)(MET-GRA4 Strain) as a Potential Biological Control Agent Against the Red Palm Weevil (Coleoptera: Dryophthoridae) J. Econ. Entomol. 2020;113:43–49. doi: 10.1093/jee/toz233. [DOI] [PubMed] [Google Scholar]
- Jabr A.A. Saad MAJ A putative serine protease from larval midgut of red palm weevil Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae): partial purification and biochemical characterization. American Journal of Environmental Sciences. 2008;4:595. doi: 10.3844/ajessp.2008.595.601.pdf. [DOI] [Google Scholar]
- Jamal F., Pandey P.K., Singh D., Khan M. Serine protease inhibitors in plants: nature’s arsenal crafted for insect predators. FAO of the united nations. 2013;12:1–34. https://agris.fao.org/agris-search/search.do?recordID=US201400054822 URL. [Google Scholar]
- Jamal F., Singh S., Pandey P.K., Singh R. Proteinaceous Trypsin Inhibitors from Plants in Disarming the Insect Pest. Biocatalysis Enzymatic Basics and Applications. 2019:309–331. [Google Scholar]
- Jia S., Zhang X., Zhang G., Yin A., Zhang S., Li F. Seasonally variable intestinal metagenomes of the red palm weevil (Rhynchophorus ferrugineus) Environ. Microbiol. 2013;15:3020–3029. doi: 10.1111/1462-2920.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian F. Influences of stored product insect movements on integrated pest management decisions. J. Insects. 2019;10:100. doi: 10.3390/insects10040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab Syndr. 2019;13:688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Kunitz M.t.J.T. Crystalline soybean trypsin inhibitor: II. General properties. J. Gen. Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.A., Shaalan S., Ghazy A.M., Ali A.A., Abd-Elaziz A.M., Ghanem M.M.E. Purification and characterization of acetylcholinesterase in Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Int J Biol Macromol. 2020;147:1029–1040. doi: 10.1016/j.ijbiomac.2019.10.071. [DOI] [PubMed] [Google Scholar]
- Muhammad A., Habineza P., Ji T., Hou Y., Shi Z. Intestinal Microbiota Confer Protection by Priming the Immune System of Red Palm Weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) Front. Physiol. 2019;10:1303. doi: 10.3389/fphys.2019.01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel J. Enzyme catalysis: the serine proteases.2010;3:21. URL: https://www.nature.com/scitable/topicpage/enzyme-catalysis-the-serine-proteases-nbsp-14398894/
- Pichare M., Kachole M. Protease inhibitors of pigeonpea (Cajanus cajan) and its wild relatives. Physiol. Plant. 1996;98:845–851. [Google Scholar]
- Qu L-J, Chen J, Liu M, Pan N, Okamoto H, Lin Z, et al.Molecular cloning and functional analysis of a novel type of Bowman-Birk inhibitor gene family in rice. 2003;133:560-70. [DOI] [PMC free article] [PubMed]
- Rose T, Di Cera EJ . Substrate recognition drives the evolution of serine proteases. 2002;277:19243-6. [DOI] [PubMed]
- Shukla P., Vidyasagar P., Aldosari S.A., Abdel-Azim M. Antifeedant activity of three essential oils against the red palm weevil. Rhynchophorus ferrugineus. Bulletin of Insectology Journal. 2012;65:71–76. [Google Scholar]
- Shukle R., Murdock L. Lipoxygenase trypsin inhibitor, and lectin from soybeans: effects on larval growth of Manduca sexta (Lepidoptera: Sphingidae) Environ entomol. 1983;12:787–791. [Google Scholar]
- Wijanarko A., Nur D.F., Sahlan M., Afnan N.T., Utami T.S., Hermansyah H.J. Production of a Biopesticide Based on a Cysteine Protease Enzyme from Latex and Papaya (Carica papaya) for Spodoptera Litura in Red Chili Peppers (Capsicum annuum) International Journal of Technology. 2017;8:1455–1461. [Google Scholar]
- Zhang H., Bai J., Huang S., Liu H., Lin J., Hou Y. Neuropeptides and G-Protein Coupled Receptors (GPCRs) in the Red Palm Weevil Rhynchophorus ferrugineus. Olivier (Coleoptera: Dryophthoridae) 2020;11:159. doi: 10.3389/fphys.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-Y, Liao H, Zhang N-H, Tang L, Xu Y, Chen F. Identification of a Kunitz inhibitor from Albizzia kalkora and its inhibitory effect against pest midgut proteases. 2008;30:1495-9. [DOI] [PubMed]
- Zulkifli A.N., Zakeri H.A., Azmi W.A.J. Food Consumption, Developmental Time, and Protein Profile of the Digestive System of the Red Palm Weevil, Rhynchophorus ferrugineus (Coleptera: Dryophthoridae) Larvae Reared on Three Different Diets. J. Insect Sci. 2018;18:10. doi: 10.1093/jisesa/iey093. [DOI] [PMC free article] [PubMed] [Google Scholar]