Abstract
Many Plant extracts had proved a potential antifungal activity against a wide range of phytopathogenic fungi. The aim of this study was to evaluate the antifungal activity of the aqueous extracts of Rumex vesicarius L. and Ziziphus spina-christi (L) Desf. against some fungal species. The effect on growth inhibition, conidia germination, sporogenesis, morphological, and ultrastructural characterizations of fungal growth by scanning and transmission electron microscopes, have been investigated. Both plant extracts exhibited an antifungal activity against Fusarium, Helminthosporium, Alternaria, and Rhizoctonia species, besides, the sporogenesis of Alternaria and Fusarium species was suppressed. Both plants induced severe morphological changes in the hyphal shape and surface. We concluded that the aqueous extracts of these plants had strong antifungal activities. More investigations should be performed to evaluate the possible applications in agriculture and in vivo.
Keywords: Rumex vesicarius L., Ziziphus spina-christi (L) Desf., Scanning and Transmission electron microscopy, Plant pathogenic fungi
1. Introduction
Annually, one-third of global agricultural production is lost due to various pests and diseases. The impacts of plant pests and diseases on crop production play a key role in agricultural simulation modeling (Whish et al., 2015). Harmful plant fungi represent about 20–40% of all known pathogenic diseases (McDonald and Stukenbrock, 2016). Pesticides are the major control measure to avoid the excessive loss of yield or quality due to fungal infection. No doubt the use of chemicals is effective in controlling these diseases, but some major problems threaten to limit the continued use of these chemical fungicides (Abouziena and Haggag, 2016). Besides, as most commercial plants are genetically modified, this develops a resistance to the broad-spectrum herbicides which have high concentrations glyphosate and glufosinate (Schütte et al., 2017). In general, pesticides are known for their high persistence and pervasiveness in the environment, and along with products of their biotransformation, they might remain in and interact with the environment and living organisms in multiple ways (Lushchak et al., 2018). Moreover, the chemical fungicides may develop a fatal medical complication associated including cancer which suggested the development of safe antifungal materials of natural origin as an alternative method for controlling plant diseases; this, in turn, has stimulated research on the occurrence of natural botanical pesticides and the potential for commercialization of these materials (Da et al., 2019).
Many saprophytic soil-borne fungi, including Rhizoctonia solani, Fusarium solani, and Fusarium oxysporum infect plants causing damping-off and wilt diseases and such diseases cause a great decrease in the yield (Lamichhane et al., 2017). Moreover, Some Alternaria spp. cause early blights which are serious destroyers that inflict serious damage to vegetables (Roy et al., 2019). Besides, different types of Fusarium sp. cause many wilting diseases in several crops such as lentil, tomato, and banana. It was reported that Alternaria alternata might cause leaf spot and other diseases on more than 400 host species. It is an opportunistic pathogen on numerous hosts causing leaf spots, rots and blights on many plant parts (Seo and Kim, 2017).
The use of plant extracts and natural products is gaining a lot of attention, as these products cause no health hazard or pollution. Besides, these products are cheaper than chemicals with minimal or practically no adverse side effects on hosts. Plant extracts by-pass the undesirable attribution of chemical pesticides such as high and acute toxicity, long degradation periods, and accumulation in the food chain and an extension of their power to destroy both useful and harmful pests (Sales et al., 2016). Although, many studies have been proved the potential activity of various plant extracts against phytopathogenic fungi and conducted to understand the mechanism of action of plant extracts, however, it is still unclear.
Therefore, this research aims to study the antifungal activity of certain wild plant extracts Rumex vesicarius L. and Ziziphus spina-christi, which are grown mainly in the desert of Saudi Arabia, towards developing of eco-friendly fungicides for controlling fungal plant diseases.
2. Materials and methods
2.1. Fungal strains collection and identification
In the current study, twelve local saprophytic and phytopathogenic fungal strains were used. The fungi were obtained from either the National Center for Research on Agriculture and Livestock, Riyadh, Saudi Arabia or the Department of Plant Protection, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia. The fungal species used were as follows: Fusarium sp., Alternaria spp., Trichoderma sp., Colletotrichum sp., Drechslera sp., Fusarium oxysporum (Tomato), Helminthosporium sp., Rhizoctonia solani (Potato), Macrophomina phaseolina (Faba bean), and Rhizoctonia solani (Faba bean). The certain fungal species were identified by the genetic analysis platforms in MACROGEN laboratory (MACROGEN, Seoul, South Korea) which were evaluated by 16S rRNA gene sequencing using the 3730XL automated DNA sequencing system (Applied BioSystems, Foster City, CA, USA), as described elsewhere (Johnson et al. 2019).
2.2. Plants collection and preparation of aqueous extract
Healthy whole plants, Rumex sp. and Ziziphus sp. were collected from Riyadh region, Kingdom of Saudi Arabia. The fresh aerial parts (shoot) from Rumex sp. and the leaves from Ziziphus sp. were cleaned, shade dried, crushed and grounded to a coarse powder, and then stored at room temperature in an airtight container for the experimental use. The aqueous extract was prepared as described before (Beddou et al., 2015). Briefly, 25 gm of each plant powder was soaked in 300 ml of distilled water for 24 h followed by vortexing on a rotary shaker for another 24 h. The crude aqueous extracts were filtered and sterilized by a two-step filtration process through sterile bacteriological filters with pore sizes of 0.45 μm and 0.22 μm, MF-Millipore™ Membrane Filters (Millipore, MA, USA). The supernatants were dried overnight at room temperature (25 °C), collected into a clean glass container, and stored into 4 °C.
2.3. Growth inhibition assay
The inhibitory effect of the plant extracts on the radial mycelial growth of the selected fungi was assessed by the agar dilution method, as described before (Balouiri et al., 2016). Briefly, the sterile aqueous extracts were mixed, equally, with molted cooled (40–45 °C) Czapak Dox agar medium (Sigma-Aldrich, Saint Louis, Mo, USA) and different working solution (0, 10, 25 or 50 mg/ml) were prepared. The mixtures were poured into sterile Petri dishes and left for solidification. Later, a disc of 6 mm was isolated from the edge of the active growing colonies of each fungus, placed in the center of the prepared agar plates, and then incubated for 7 days at 25 ± 2 °C incubators; each assay was performed in triplicates. The radical mycelial growth was evaluated by calculating the mean of two perpendicular colony diameters for each replicate after the incubation time. The percentage of mycelial growth inhibition was calculated according to the following formula:
where DC and DT are the diameter of the control and treated colonies, respectively.
2.4. Conidia germination percentage
The conidial concentrations were determined using a hemocytometer (Thermo Fischer Scientific, Waltham, MA, USA) as described before (Oliveira et al., 2015). Briefly, the different fungi were cultured into Czapak Dox agar, as mentioned above, then 10 mm agar/spore discs taken from each petri dish and added to 9 ml of sterile deionized water + 9 ml of aqueous plant extracts at different doses. The mixtures were vortexed to get homogenized suspensions, approximately, with 106 conidia/ml. 0.5 ml of each suspension was added to a sterile slide connected to a U-shaped glass rod with a filter paper and placed in a sterile petri dish while they were incubated at 25 °C. The germinated spores were observed at different time intervals using a light microscope. The percentage of conidia germination was calculated for all spores versus the control at different time intervals until the 50% growth confluency of the untreated plates. The measurements were performed in triplicates.
2.5. Scanning electron microscope (SEM)
The Czapak Dox agar/spores’ dishes with different concentrations of plant extracts were prepared as mentioned above. The electron microscopy facility in the Central Laboratory, at Women Students’ Medical Studies and Sciences Sections, King Saud University was used to determine the effect of the plant extracts on the morphology and ultrastructure of studied fungi, according to the standard protocols. In brief outlines, the agar was cut into pieces of 5 mm × 5 mm size included the growth colony, placed into petri dishes, fixed by 2.5% buffered glutaraldehyde, and incubated overnight at 4 °C. On the next day, the slides were washed twice with 1X phosphate-buffered saline (PBS) for 20 min and re-fixed with 1% buffered osmium tetroxide (OsO4) for additional overnight incubation at 4 °C. Later, the petri dishes with slightly open cover were dehydrated by serial dilutions of ethanol and then by hexamethyldisilazane (HMDS) for 30 min. Later, the specimens were left in a desiccator to be air-dried at room temperature. After dryness, the specimens were directly, mounted on the specimen stub (holder) using adhesive carbon carrier tapes with brass shims. The specimens were then coated with a thin layer of gold (20–30 nm) using a sputter coater. The specimens were examined by the JSM-6060LV scanning electron microscope (JEOL Ltd. Inc., Tokyo, Japan) with an accelerating voltage of 15 kV.
2.6. Transmission electron microscopy (TEM)
The slides were prepared, as mentioned above, to be examined by the transmission electron microscope (TEM), as well. Briefly, after the second fixation with OsO4, the slides were embedded by a resin mixture using the SPI-Pon™ -Araldite® Epoxy Embedding Kit (Structure Probe, Inc. and SPI Supplies, West Chester, PA, USA). Later, the specimen blocks well were cut into ultrathin sections (70–80 mm) by Leica EM UC6 ultra-microtome (Leica Microsystems, Wetzlar, Germany) and loaded on carbon-coated copper TEM grids. Then, the ultrathin sections were stained by Uranyl Acetate (UA) and Lead Citrate (LC) and examined by JEM-1011 transmission electron microscope (JEOL Ltd. Inc., Tokyo, Japan) at different magnification powers.
2.7. Statistical analysis
The resulted data were analyzed by STATISTIX software version 10.1. One-way ANOVA and the incorporated Duncan test were used to assess the significance of data (where P values < 0.05 were considered significant). The means were calculated according to the values of the Least Significant Difference (LSD) at the 0.05 probability level and were represented as mean ± standard error (SE).
3. Results and discussion
3.1. Screening of certain plant pathogenic fungi (Myco-Phytopatogens)
Fungal pathogens have become more frequent due to the wide differentiation and quick ubiquitousness in the environment. Conventionally, plant fungal diseases are controlled using synthetic fungicides, which increase agricultural costs and environmental contamination, as well. A recent possible alternative strategy is the use of plants that are, hypothetically or practically, able to produce antifungal substances in the treatment or protection of crops from phytopathogens induced by fungi (Shuping and Eloff, 2017). In the current study, we used twelve local saprophytic and phytopathogenic fungal strains and from their taxonomic classification, they belong to seven fungal genera, Alternaria spp., Colletotrichum sp., Drechslera sp., Fusarium sp., Fusarium oxysporum, Helminthosporium sp., Macrophomina phaseolina, and Rhizoctonia solani.
The antifungal compounds are present, naturally, in some higher plants which have key roles in self-resistance of these plants against certain fungal pathogens (Shuping and Eloff, 2017). The variable effects of some plant extracts against some fungal diseases revealed that they might either stimulate or inhibit the growth of some fungi species (Cheng and Cheng, 2015). The antifungal activity of the plant aqueous extracts of Ziziphus sp. and Rumex sp. were evaluated against these fungal species which varied among these two plants and according to the tested fungal strains, as shown in [Table 1]. The findings showed that the fungal growth was inversely proportional to the increment of plant extract concentrations of Fusarium oxysporum, Helminthosporium sp., Alternaria spp. and Rhizoctonia solani, despite there were no reported-inhibitory effects on the other species.
Table 1.
In vitro antifungal activity of Rumex sp. and Ziziphus sp. aqueous extracts at various concentrations (mg/ml) against the plant pathogenic fungi under study.
| Fungal strains |
R. vesicarius extract conc. (mg/ml) |
Z. spina-christi extract conc. (mg/ml) |
||||
|---|---|---|---|---|---|---|
| 1 | 10 | 50 | 1 | 10 | 50 | |
| Trichoderma sp. | – | – | – | – | – | – |
| Colletotrichum sp. | – | – | – | – | – | – |
| Drechslera sp. | – | – | – | – | – | – |
| Fusarium oxysporum | – | + | + | – | – | + |
| Fusarium sp. | – | – | – | – | – | – |
| Helminthosporium sp. | – | + | + | – | + | + |
| Alternaria spp. | – | + | + | – | + | + |
| Rhizoctonia solani | – | – | – | – | – | – |
| Macrophomina phaseolina | – | – | – | – | – | – |
| Rhizoctonia solani | – | – | + | – | – | + |
(−) no inhibition induced, (+) inhibition induction of fungal growth.
Moreover, the antifungal activity of Rumex sp. and Ziziphus sp. plant extracts revealed the discrepancy between various strains of the same fungal genera. These data were considered a preliminary screening assessment for selecting the most fungal strains sensitive and susceptible to plant extracts effect. Several earlier studies about the effect of some medicinal plants and their antifungal activity shown variable inhibitory effects against Aspergillus niger, Penicillium janthinellum, Fusarium oxysporum, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans and other fungi species due to their different chemical compositions (Mahlo et al., 2016, Suurbaar et al., 2017). A previous study showed that methanol extract of Ziziphus sp. stem bark showed an antifungal activity against fungal species, including Aspergillus fumigatus, Syncephalastrum racemosum and Geotricum candidum (Ads et al., 2017) besides, the ethanol extracts of the Ziziphus sp. fruits were found to be effective against some Candida species (Mardani et al., 2018). Controversially, another study showed that the petroleum ether, chloroform, methanol, and aqueous extracts of the fruits, leaves, seeds, and stems of Ziziphus sp. collected from Khartoum, Sudan showed no antifungal activity against Aspergillus niger and Candida albicans (Ali et al., 2015). Previous studies on Rumex sp. had some antimicrobial effects, mainly antibacterial (Al Aboody, 2015, Laouini and Ouahrani, 2017), despite a previous study reported the antifungal effect of ether extract of Rumex sp. leaves against the Penicillium funiculosum and Fusarium oxysporum (Shah et al., 2015).
3.2. Identification of selected plant pathogenic fungal genera
The sequences of Helminthosporium sp. and Alternaria spp. identified at the species level were analyzed by using 16S rRNA analysis procedure. The results revealed 99% identity with Alternaria brassicae and 100% with Helminthosporium rostratum (or Exserohilum rostratum) for the accession numbers JN108910.1 and GU966507, respectively. Exserohilum rostratum or the killing fungus which is a graminicolous species excluded from the genus Helminthosporium (also known as Drechslera) while the taxonomy of this species is complicated with many changes and refinements since its discovery (Sharma et al., 2014). This fungus is a member of Ascomycota which are characterized by the asexual (anamorphic) reproduction by mito-spores or conidia while Exserohilum rostratum represents an anamorph/teleomorph (sexual reproduction) connection which was previously recognized with a teleomorph phase Setosphaeria rostrate in 1974 by K.J. Leonard.
3.3. Assessment of the in vitro antifungal activity of aqueous plant extracts
In this study, the antifungal activity of the aqueous extracts of Rumex sp. and Ziziphus sp. was evaluated against Helminthosporium rostratum, Alternaria brassicae, Fusarium oxysporum and Rhizoctonia solani, in a dose-dependent manner. The results revealed that Rumex concentration at 10 mg/ml, significantly, reduced the growth of Helminthosporium rostratum and Fusarium oxysporum, however, there was no significant growth reduction of both Alternaria brassicae and Rhizoctonia solani. By concentrations increment to 25 and 50 mg/ml of R. vesicarius, it was found that Helminthosporium rostratum and Fusarium oxysporum growth decreased significantly and their colonies diameter shrunk to 4.2 and 4.02 mm, respectively, despite there was no significant difference between the 25 mg/ml and 50 mg/ml doses [Table 2], while, in the case of and Ziziphus sp. aqueous extract, it was found that the lowest used concentration (10 mg/ml) significantly reduced only Helminthosporium rostratum growth, while the growth inhibition of Fusarium oxysporum appeared at the concentration of 50 mg/ml [Table 2]. The inhibition (IC50) of Fusarium oxysporum and Helminthosporium rostratum was at the Rumex sp. extract concentration 25 and 50 mg/ml, respectively while the inhibition (IC50) of Helminthosporium rostratum occurred at the Ziziphus sp. extract concentration of 25 mg/ml.
Table 2.
Effect of different concentrations of aqueous extracts of R. vesicarius and Z. spina-christi on mycelial growth diameter (mm) of selected phytopathogenic fungi on Czapak Dox agar medium after 7 days of incubation at 25 °C.
| Extract Conc. (mg/ml) |
Helminthosporium rostratum |
Alternaria brassicae |
Fusarium oxysporum |
Rhizoctonia solani |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Growth (mm) | Inhibition % | Growth (mm) | Inhibition % | Growth (mm) | Inhibition % | Growth (mm) | Inhibition % | ||
| R. vesicarius | 0 | 8.50a ± 0.00 | 0 | 7.78 a ± 0.07 | 0 | 8.50 a ± 0.00 | 0 | 8.50 a ± 0.00 | 0 |
| 10 | 6.68b ± 0.88 | 21.41 | 7.42 a ± 0.10 | 4.63 | 5.45b ± 0.23 | 35.88 | 8.37 a ± 0.07 | 1.53 | |
| 25 | 5.10c ± 0.61 | 40.00 | 7.40 a ± 0.21 | 4.88 | 4.17c ± 0.24 | 50.94 | 8.35 a ± 0.08 | 1.76 | |
| 50 | 4.20c ± 0.03 | 50.59 | 7.37 a ± 0.13 | 5.27 | 4.02c ± 0.02 | 52.71 | 8.35 a ± 0.08 | 1.76 | |
| LSD 0.05 | 1.007 | --- | 0.452 | --- | 0.538 | --- | 0.207 | --- | |
| Z. spina-christi | 0 | 8.50 a ± 0.00 | 0 | 7.45 a ± 0.03 | 0 | 8.50 a ± 0.00 | 0 | 8.50 a ± 0.00 | 0 |
| 10 | 6.43b ± 0.41 | 24.35 | 7.45 a ± 0.03 | 0.00 | 8.50 a ± 0.00 | 0.00 | 8.50 a ± 0.00 | 0.00 | |
| 25 | 3.95c ± 0.08 | 53.53 | 6.78b ± 0.14 | 8.99 | 7.97 ab ± 0.28 | 6.24 | 8.12b ± 0.07 | 4.47 | |
| 50 | 3.17 d ± 0.18 | 62.71 | 4.73c ± 0.13 | 36.51 | 7.65b ± 0.18 | 10.00 | 4.70c ± 0.10 | 44.71 | |
| LSD 0.05 | 0.739 | --- | 0.322 | --- | 0.548 | --- | 0.201 | --- | |
Data represented as mean ± standard error. Means in the same column followed by the same letter are not significantly different based on LSD at p = 0.05 according to Duncan’s multiple range test.
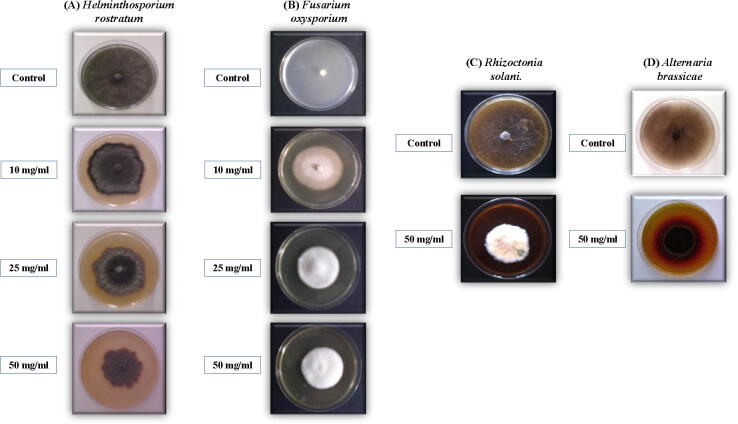
The higher concentrations of Rumex sp. reduces the growth of Alternaria brassicae and Rhizoctonia solani, as well, despite it was statistically non-significant. The aqueous extract of Rumex sp. induced an obvious change in the colony characterization (or colony morphotype), as well. In the case of Helminthosporium rostratum, the formed fungal hyphae were very weak with partial faint color [Fig. 1A]. Rumex sp., significantly, inhibited the growth of Fusarium oxysporum (as a radical growth diameter) but the mycelium appeared to grow in the vertical expansion [Fig. 1B]. The colonies of Rhizoctonia solani [Fig. 1C] and Alternaria spp. [Fig. 1D] exhibited darker colors with a remarkable heavier growth.
Fig. 1.
Effect of different concentrations of Rumex aqueous extract on the growth of different phyto-pathogenic fungi cultured on Czapak Dox agar medium. (A) Helminthosporium rostratum, (B) Fusarium oxysporium, (C) Rhizoctonia solani, and (D) Alternaria brassicae.
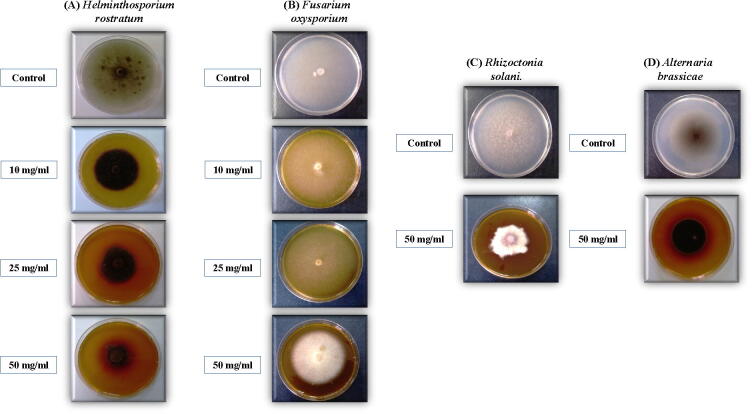
The growth of Helminthosporium rostratum, Alternaria brassicae, and Rhizoctonia solani were significantly inhibited at 50 mg/ml dose of the aqueous extract of Ziziphus sp., as well. Similar to Rumex sp., Ziziphus sp. caused a notable reduction in colony diameter (3.17 mm) of Helminthosporium rostratum, besides, the formed fungal hyphae were very weak with a short vertical expansion of the mycelia [Fig. 2A]. The mycelial growth of Fusarium oxysporum [Fig. 2B] and Rhizoctonia solani [Fig. 2C] in the treated plate looked a bright white color with a vertical heavy growth, similar to the shape of coral reefs. In the case of Alternaria brassicae, the colonies’ color became darker compared to the control [Fig. 2D]. In a comparison between the two plant extracts, it appeared that Ziziphus sp. demonstrated a significant degree of activity against the growth of overall tested fungal genera which might clarify the differential antifungal activity of these plants extracts with different fungal genera.
Fig. 2.
Effect different concentrations of Ziziphus Aqueous extract on the growth of different phyto-pathogenic fungi cultured on Czapak Dox agar medium. (A) Helminthosporium rostratum, (B) Fusarium oxysporium, (C) Rhizoctonia solani, and (D) Alternaria brassicae.
In agreement with our findings, a previous study showed that the aqueous extract of Ziziphus sp. had an inhibitory effect against the Fusarium solani growth and the rate of inhibition increased gradually by increasing the concentration (5, 10, 15, 20, and 25%) (Haikal, 2007) while, in another study, the methanolic extract of the leaves had an inhibitory effect at 4 mg/ml dose on mycelial growth of Botrytis fabae by 95.56%, Alternaria solani by 91.11%, and by 65–70.% at Fusarium oxysporum and Fusarium solani (El-Khateeb et al., 2013). For the best of our knowledge, there weren’t any previous studies for the antifungal effect of aqueous extract of Rumex vesicarius, despite the usage of ether extract of the leaves had an inhibitory effect against the Fusarium oxysporum, as mentioned before (Shah et al., 2015). Another recent study demonstrated that the methanolic extract of Ziziphus sp. leaves had antifungal effect on Fusarium moniliforme despite it didn’t affect Aspergillus niger or Aspergillus flavus (Ibraheem, 2017).
3.4. Effect of aqueous plant extracts on sporogenesis (formation of vegetative reproduction spores)
Sporogenesis or conidiogenesis is a form of asexual reproduction of fungi, and the term is also used to refer to the process of reproduction via spores. Reproductive spores are formed in many eukaryotic organisms, such as fungi, during their normal reproductive life cycle (Riquelme et al., 2018). Fungal genera, Helminthosporium, Alternaria and Fusarium are common plant-infesting fungi that reproduce asexually by forming mitotically derived spores called conidia. Although this study aimed to evaluate the antifungal activity of the aqueous extracts of Rumex sp. and Ziziphus sp., the sporulation or formation of vegetative reproduction spore was assessed by the two plants extracts to investigate the characteristic behavior of the selected fungi. Rhizoctonia solani was excluded because its vegetative reproduction occurs by mycelial fragmentation; it does not form conidia, only sterile mycelium [Table 3].
Table 3.
The Effect of different concentrations of plant aqueous extracts of R. vesicarius and Z. spina-christi on sporogenesis of selected phytopathogenic fungi.
| Plants | Extract Conc. (mg/ml) | Helminthosporium rostratum | Alternaria brassicae | Fusarium oxysporum |
|---|---|---|---|---|
| R. vesicarius | 0 | 48 a ± 11.314 | 22.4c ± 3.92 | 41.6 d ± 6.4 |
| 10 | 12.8b ± 3.2 | 41.6b ± 3.92 | 89.6c ± 9.6 | |
| 25 | 0b | 48b ± 10.12 | 128b ± 8.76 | |
| 50 | 0b | 73.6 a ± 3.92 | 230.4 a ± 19.98 | |
| Z. spina-christi | 0 | 16 a ± 7.16 | 22.4 d ± 3.92 | 38.4c ± 3.92 |
| 10 | 22.4 a ± 3.92 | 57.6c ± 3.92 | 60.8c ± 5.98 | |
| 25 | 16 a ± 0.00 | 76.8 a ± 5.99 | 115.2b ± 11.76 | |
| 50 | 12.8 a ± 3.20 | 67.2 ab ± 9.33 | 182.4 a ± 11.97 |
Means in the same column followed by the same letter are not significantly different based on LSD at p = 0.05 according to Duncan’s multiple range test.
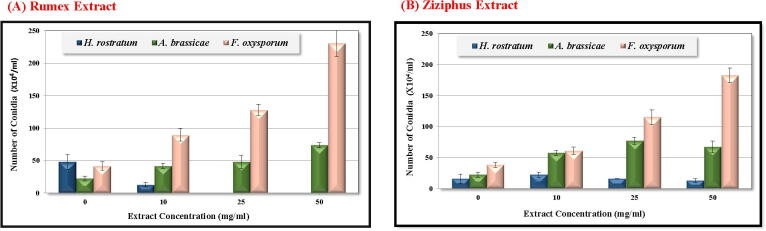
Our results indicated that Rumex sp. aqueous extract significantly suppressed vegetative spores’ formation of Fusarium oxysporum and Helminthosporium rostratum at the lowest tested concentration (10 mg/ml), while sporogenesis completely inhibited at higher concentrations. In the case of Alternaria brassicae, there was a significant increase in the number of spores at the 50 mg/ml concentration [Fig. 3A]. Similarly, the treatment with the aqueous extract of Ziziphus sp. increased, significantly, the number of spores formed in the cases of Alternaria brassicae and Fusarium oxysporum, in a dose dependent-manner while with Helminthosporium rostratum the sporogenesis wasn’t significantly affected by all of the used concentrations [Fig. 3B]. Our results indicated that the sporulation of these fungi might be controlled by different doses of these plant extracts. Both extracts activated the sporogenesis of either Alternaria brassicae or Fusarium oxysporum, however, only Rumex sp. could significantly reduce the sporogenesis of Helminthosporium rostratum without any noticeable effect of Ziziphus sp.
Fig. 3.
Influence of plants extracts at various concentrations on sporogenesis of selected phytopathogenic fungi. Data represented as mean ± standard error. (A) Rumex extract, (B) Ziziphus extract.
In an agreement with our results, a previous study on the extracts from nine wild edible herbaceous species showed a significant reduction of conidial germination on the black mold fungi (Aspergillus carbonarius and Aspergillus niger), in a dose-dependent manner while in some cases, the antifungal activity increased as the phenolic concentration increased because of higher concentrations (Gatto et al., 2016). On contrary, the spore production of Fusarium solani was affected by plant extracts and failed to produce spores when treated with aqueous extracts of Rumex sp. and Ziziphus sp. at the concentration of 20% which elevated an evident that plant extracts could provide a potential source of antifungal compounds (Abu-Taleb et al., 2011). Evaluation of plant extracts effectiveness that triggers morphological differentiation would be useful for designing strategies to control selected fungal genera colonization.
3.5. Effect of aqueous plant extracts on fungal spore germination
Spore germination, is the events induces the loss of the spore-specific properties, is an essentially biophysical process, and requires many spore-specific proteins which are, mainly, associated with the inner spore membrane (Setlow, 2014). According to the previous results, the antifungal activity of the aqueous extract of Rumex sp. was elevated against Helminthosporium rostratum and Fusarium oxysporum while the aqueous extract of Ziziphus sp. inhibited the growth of Helminthosporium rostratum and Alternaria brassicae, a dose-dependent manner. The current results illustrated the effect of these extracts on the spore germination of these species as shown in [Table 4].
Table 4.
Effect of different concentrations of plant aqueous extracts of R. vesicarius and Z. spina-christi on spore germination percent of selected phytopathogenic fungi.
| Plants | Extract Conc. (mg/ml) | Helminthosporium rostratum | Fusarium oxysporum | Alternaria brassicae |
|---|---|---|---|---|
| R. vesicarius | 0 | 61.91a ± 3.07 | 54.02 a ± 4.89 | ــــــــــــــــــــ |
| 10 | 61.69 a ± 4.60 | 14.25b ± 3.12 | ــــــــــــــــــــ | |
| 25 | 2.04b ± 2.04 | 14.89b ± 4.38 | ــــــــــــــــــــ | |
| 50 | 2.86b ± 2.86 | 5.66b ± 3.02 | ــــــــــــــــــــ | |
| LSD 0.05 | 9.56 | 11.48 | ــــــــــــــــــــ | |
| Z. spina-christi | 0 | 64.21 a ± 5.26 | ــــــــــــــــــــ | 60.24 a ± 3.99 |
| 10 | 46.77b ± 5.37 | ــــــــــــــــــــ | 61.97 a ± 5.11 | |
| 25 | 23.18c ± 0.90 | ــــــــــــــــــــ | 42.12b ± 2.72 | |
| 50 | 6.64 d ± 2.40 | ــــــــــــــــــــ | 4.65c ± 3.20 | |
| LSD 0.05 | 11.59 | ــــــــــــــــــــ | 11.28 |
Data were represented as mean ± standard error. Means in the same column followed by the same letter are not significantly different based on the Least Significant Difference (LSD) at p = 0.05 according to Duncan’s multiple range test.
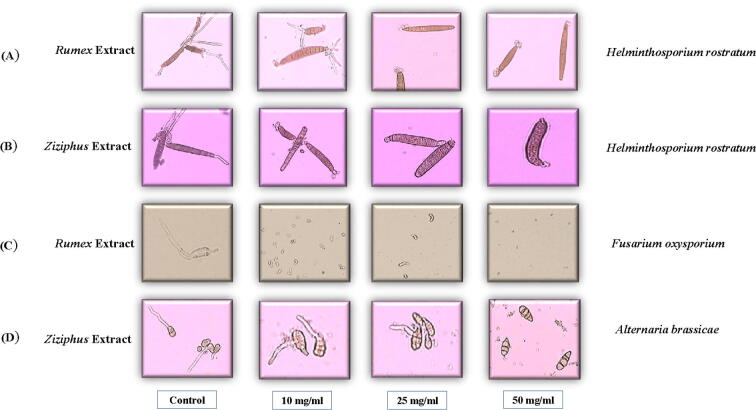
It appeared that the spore’s germination percent of Helminthosporium rostratum were reduced, significantly, at all concentrations of both plants compared to the control. It was noticeable that the reduction induced by Rumex sp. was stronger than that of Ziziphus sp. [Fig. 4A and 4B]. Moreover, the Rumex sp. aqueous extract, significantly, reduced spores’ germination of Fusarium oxysporum to 5.66 ± 3.02% at the concentration of 50 mg/ml [Fig. 4C] Ziziphus sp., significantly, reduced spores’ germination of Alternaria brassicae to 4.65 ± 3.20% at the concentration of 50 mg/ml, as well, compared to the control [Fig. 4D]. Moreover, aqueous extracts from both species were effective in reducing the germ tube elongation also when a slight inhibition of conidial germination was observed.
Fig. 4.
Effect of different concentrations of aqueous plant extracts on the spore’s germination (A) effect of Rumex extract on Helminthosporium rostratum, (B) effect of Ziziphus extract on Helminthosporium rostratum, (C) effect of Rumex extract on Fusarium oxysporium, (D) effect of Ziziphus extract on Alternaria brassicae.
In agreement with our results, the study of Abu-Taleb et al. (2011) reported that conidia germination of Drechslera biseptata were inhibited (18.7%) when treated with aqueous extract of Ziziphus sp. at the concentration of 20% and it was evident that this plant extract could provide a potential source of antifungal compounds (Abu-Taleb et al., 2011). Another study showed that the extract of Ziziphus sp. leaves showed stronger inhibition on spore germination of Alternaria alternata (Lang et al., 2018). Generally, extracts showed a remarkable inhibition of the germ tube elongation rather than the spore germination which might be a result of some “toxic” compounds in the tested extracts. The effects of the antifungal compounds on spore germination may be due to the effect of these compounds on the permeability of the cell wall which might suppress the early stages of mycelial growth leading to inhibition of fungal growth (Sadhasivam et al., 2019).
3.6. Morphological and ultrastructural characterizations of fungal growth by SEM and TEM
SEM and TEM were used to clarify the morphological changes of viable fungal mycelium as a response of exposure to the effective concentration of tested aqueous plant extracts (Rumex sp. and Ziziphus sp.) and their effect on the integrity of the cell wall and internal organelles.
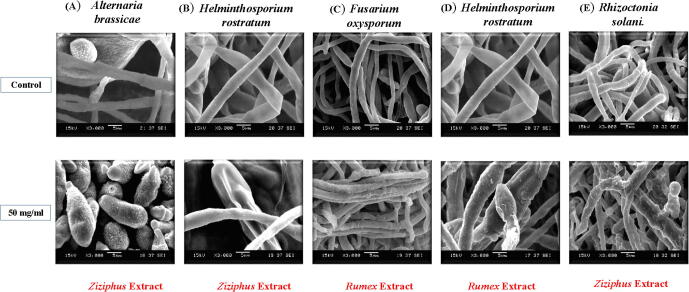
The results of SEM showed that the characteristic morphology of Alternaria brassicae (untreated) is naturally with a lengthened hypha of constant diameter, sub-parallel and with rounded or lightly tapering apex, smooth external surface, and granulated elliptical or oval conidia. After the treatment with 50 mg/ml of the Ziziphus sp. extract, the morphology reflects that of the control except for the presence of undulations and deformations along the hyphal border. Otherwise, most of the formed fungal mycelia appeared curly and shrunken. Besides, SEM micrographs revealed differences in the enumeration, size, and shape of the formed conidia. In the treated samples, conidia were larger in the size and count with beaks as well as granulated and wrinkled surfaces. In spite of the increment of conidia formation, conidia have deformation that is could be an explanation for decreasing and inhibition of conidia germination [Fig. 5A].
Fig. 5.
The effect of plant extracts on the morphology of tested fungi as shown in conidia size, hyphae structure and attached hilum, by SEM at X3.000. magnification, (A) effect of Ziziphus extract on Alternaria brassicae (B) effect of Ziziphus extract on Helminthosporium rostratum, (C) effect of Rumex extract on Fusarium oxysporium (D) effect of Rumex extract on Helminthosporium rostratum.
The SEM results of the Fusarium oxysporum (untreated) showed that it’s characterized with lengthened hyphae, of constant diameter, sub-parallel and with rounded or lightly tapering apex, smooth external surface and rarely conidia. Meanwhile, most of the formed fungal mycelia appeared to grow parallel and adherent. The SEM micrographs of the sample treated with 50 mg/ml of Rumex sp. extract, clearly, showed curly hyphae, in varied diameter, deformed and wrinkled external surfaces, besides, the formed conidia were embedded and overlapping [Fig. 5C].
The SEM results of Helminthosporium rostratum (untreated) revealed that it had lengthened hyphae, of mostly constant diameter and smooth external surface. Besides, microscopic examination described straight-cylindrical conidia as “most distinctive peculiarity ”. The conidium bears a conspicuous hilum. The hilum is the tip of the spore that originally attached to the conidiophores. When the samples were treated with 50 mg/ml of either extract of Rumex sp. [Fig. 5B] or Ziziphus sp. [Fig. 5D], the morphology reflects that of the control except for the presence of undulations and deformations along the hyphal surface. Otherwise, most of the formed fungal mycelia appeared curly, granulated and shrunken. Moreover, SEM micrographs of the treated samples revealed severe differences and deformation in the formed conidia which appeared shrunken, wrinkled, and seemed like dried or empty conidia that might be the possible explanation for the inhibition of conidia germination.
In the case of Rhizoctonia solani, the SEM results of the fungus (untreated) showed that it had a lengthened hypha, of constant diameter and with rounded or lightly tapering apex and smooth external surface. After, the treatment with an aqueous extract of Ziziphus sp. the mycelia formed reflected many morphological alterations such as the presence of undulations and deformations along the hyphal border. Otherwise, most of the formed fungal mycelia, appeared shrunken, granular, and curly as well as the irregular swelling and excessive branching of the hyphae [Fig. 5E].
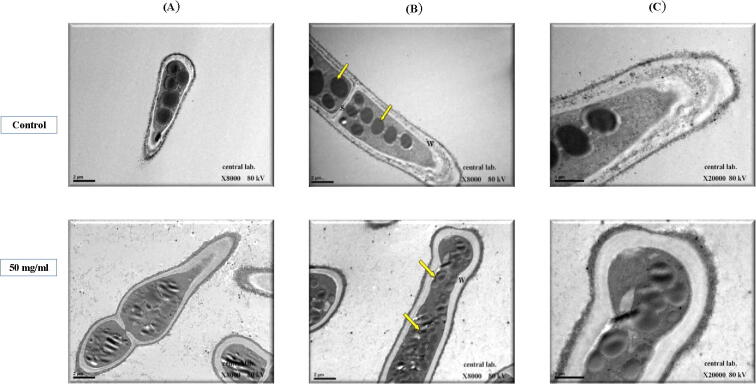
In TEM micrographs, Alternaria brassicae hyphae treated with Ziziphus sp. extract showed ultrastructural changes including the presence of largely fragmented conidia, the external sheath, and the cell wall had irregular shape on the outside, the organelles and cytoplasm were not visible for dispersed many electrons dense material along the hyphae. The most significant change observed was the coating of thread-like material on a swollen and de-shaped hyphal tip. Moreover, we noticed the presence of some unknown substances on the hyphal surface which were completely hollow and deformed [Fig. 6].
Fig. 6.
Transmission electron micrographs showing a cross-sectional view of Alternaria brassicae. Untreated (control) and treated with 50 mg/ml Ziziphus extract. Changes were observed in the conidia morphology including the sheath, cell wall, organelles, and cytoplasm of the hyphae. A. Longitudinal section of a mature conidia (X8000); B. The hyphal tip, in which showing a cluster of electron-dense vacuoles (yellow arrow), cell wall (W) and septa (S) are visible (X8000) and C. Cell wall and sheath of hyphal tip (X20000).
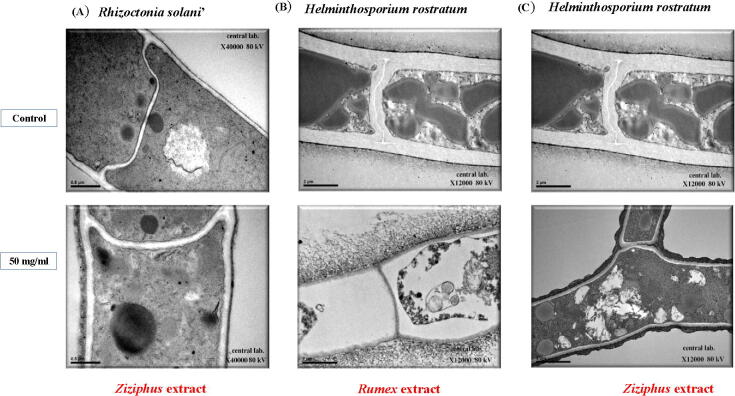
In TEM micrographs, Rhizoctonia solani hyphae treated with Ziziphus sp. extract showed certain ultrastructural changes. An increase in the thickness of the cell wall accompanied by the disappearance of the organelles and cytoplasm were observed in the dispersed electron-dense material along the hyphae. The most significant change observed was the presence of some unknown material coating the surface of hyphae and the hyphal tip [Fig. 7A]. TEM micrographs showed of Helminthosporium rostratum the ultrastructural changes in hyphae treated with Rumex sp. The most important observations were the cytoplasm degeneration and deterioration of organelles. Hyphal ultrastructural alterations consisted of cell distortion, shrinkage of internal components, cavity, or electron-dense material in hyphal cells [Fig. 7B]. TEM micrographs of Helminthosporium rostratum treated with Ziziphus sp. extract indicated ultrastructural changes, as well. It caused morphological and structural alterations, like irregular swelling and excessive branching of hyphae, considerable thickening of the hyphal cell walls, very frequent septation and presence of some unknown material coating the surface of hyphae in addition to the presence of much electron-dense material [Fig. 7C]. Similarly, the TEM micrographs of Fusarium oxysporum hyphae treated with Rumex sp. extract revealed ultrastructural changes such as the deformation and damage in the cell wall, besides, the presence of much electron-dense material along the hyphae [Fig. 8]. So far, the prominent changes in the mycelial morphology indicated that the cell wall might be the expected target of Ziziphus sp. or Rumex sp. extracts action.
Fig. 7.
Transmission electron micrographs showing the effect 50 mg/ml of plant extracts on the tested fungi the micrographs show a cross-sectional view the hyphae of the untreated (control) and treated at X40000. Changes were observed in the conidia morphology including the sheath, cell wall, organelles, and cytoplasm of the hyphae. (A) Rhizoctonia solani treated with Ziziphus extract, (B) Helminthosporium rostratum treated with Rumex extract, and (C) Helminthosporium rostratum treated with Ziziphus extract.
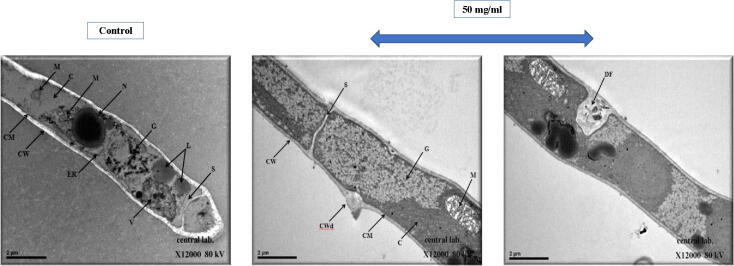
Fig. 8.
Transmission electron micrographs showing a cross-sectional view of Fusarium oxysporum’s hyphae. Changes were observed in the conidia morphology including the sheath, cell wall, organelles, and cytoplasm of the hyphae. Untreated (left panel) and treated with 50 mg/ml of Rumex extract (right panel). C. cytoplasm; CW. cell wall; CM. Cytoplamic membrane; ER. Endoplasmic reticulum; G. glycogen; L. lipids; M. mitochondrion; N. nucleus; S. septum; V. vacuole; CWd. cell wall damage; DF. Deformation (X12000).
All the above-mentioned observations emphasized the previous results of the inhibitory effect induced by the experimented plant extracts against the tested fungi as the morphological alterations correlate with extract concentration. The changes in the cell wall permeability which might lead to leakage of the cytoplasm, besides the metal chelation which interrupting the fungal enzymatic process and might contribute to the inhibitory mechanisms induced by these plants against the tested fungi.
SEM and TEM were used in some previous studies to show that the plant extracts were able to delay the fungal germination; the observations by SEM and TEM showed severe changes in the morphology and ultrastructure of Alternaria solani when treated with the ethanol extract of the Calotropis procera leaves at a concentration of 20% (Baka and Rashad, 2016). In another study, SEM surveys showed that hypha of the treated Trichophyton rubrum with allicin and garlic extract had rough and granular like surface, abnormal and irregularly-shaped with distinguishable disintegration of cytoplasm caused by the cracking of the cell membrane, collapsing of hypha, and dissolution of the cytoplasm component (Aala et al., 2014).
In another study, SEM showed the hydroethanolic extracts from the leaves Sapindus saponaria L. caused morphological changes to the Colletotrichum gloeosporioides by reducing the size of hyphae at the concentration of 50 mg/ml, while at 100 mg/ml damages in the hyphal structure were more severe and induced mycelial rupture (Marinho et al., 2018). In a recent study, SEM of Candida albicans shows the effect of the 150 µl/ml of Ziziphus sp. extract that caused cellular cavities and shrinkage of the cell wall with reduction of the cells number to only a few abnormal cells (Al-Ali and Al-Judaibi, 2019). More studies showed that the Scutellaria baicalensis Georgi root extract induced shrinkage or cracking of fungal filaments with deformation of the fungal surface structure with a cotton-like degenerated cytosol surroundings the bodies of Trichophyton rubrum, Trichophyton mentagrophytes, Aspergillus fumigatus, and Candida albicans (Da et al., 2019).
All over, at the ultrastructural level, all untreated understudying fungal species exhibit formally with normal hyphae covered with the cell wall and have the organelles that usually present in the fungal cells, like a nucleus, vacuoles, mitochondria, lipid bodies, endoplasmic reticulum, etc. However, the treatment with either Rumex sp. or Ziziphus sp. aqueous extracts, caused the damage of hyphae at the cellular level, the organelles were partially and/or entirely disappeared or destroyed, the cytoplasm degenerated and the electron-dense material appeared in the hyphal cells. The external sheath was slightly modified and the cell wall had an irregular shape on the outer surfaces. The loss of hyphae’s viability might be due to the sedimentation of the cytoplasm and the destruction of the organelles and nucleus.
4. Conclusion
The observed efficacy of the aqueous extracts of Rumex sp. and Ziziphus sp. explores the possibilities of controlling the fungal pathogenesis by using plant extracts and encouraging the possible applications in agriculture after in vivo field investigations.
Funding
This research project was supported by a grant from the Researchers Supporting Project number (RSP2019/114), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Research Supporting Project number: RSP-2019/114, King Saud University, Riyadh, Saudi Arabia.
Author contributions
Both of FOA and EHA contributed to the conception and study design. FOA contributed to the editing and reviewing of the intellectual contents, experimental applications, data acquisition and analysis, statistical analysis, manuscript preparation, editing and reviewing besides, she acts as a guarantor and corresponding author. EHA and GA were responsible for the literature search besides the editing and reviewing of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aala F., Yusuf U.K., Nulit R., Rezaie S. Inhibitory effect of allicin and garlic extracts on growth of cultured hyphae. Iran J. Basic Med. Sci. 2014;17:150–154. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4016684/ [PMC free article] [PubMed] [Google Scholar]
- Abouziena H.F., Haggag W.M. Weed control in clean agriculture: A Review. Planta Daninha [online]. 2016;2016(34):377–392. doi: 10.1590/S0100-83582016340200019. [DOI] [Google Scholar]
- Abu-Taleb A.M., El-Deeb K., El-Otibi F.O. Assessment of antifungal activity of Rumex vesicarius L. and Ziziphus spina-christi (L) wild extract against two phytopathogenic fungi. Afr. J. Microbiol. Res. 2011;5:1001–1011. doi: 10.5897/AJMR10.826. [DOI] [Google Scholar]
- Ads E.N., Rajendrasozhan S., Hassan S.I., Sharawy S.M.S., Humaidi J.R. Phytochemical, antimicrobial and cytotoxic evaluation of Ziziphus spina-christi (L.) stem bark. Biomed. Res. 2017;28:6646–6653. doi: 10.4066/biomedicalresearch.29-17-1668. [DOI] [Google Scholar]
- Al Aboody M.S. In vitro screening of phytochemical, antibacterial and antioxidant activities of Rumex vesicarius L. Int. J. Curr. Microbiol. App. Sci. 2015;4:884–893. [Google Scholar]
- Al-Ali S., Al-Judaibi A. Biochemical and molecular effects of Phoenix dactylifera and Ziziphus spina-christi extracts on Candida albicans. J. Biosci. Med. 2019;7:29–43. doi: 10.4236/jbm.2019.73004. [DOI] [Google Scholar]
- Ali, A.B., Almagboul, A.Z., Mohammed, O.M., 2015. Antimicrobial activity of fruits, leaves, seeds and stems extracts of Ziziphus spina -christi. Arabian Journal of Medicinal and Aromatic Plants (AJMAP). 1, 94-107. Retrieved from: https://revues.imist.ma/index.php?journal=AJMAP& page=article&op=view&path%5B%5D=4325&path%5B%5D=3113.
- Baka Z., Rashad Y. Alternative control of early blight of tomato using plant extracts from Acacia nilotica, Achillea fragrantissima and Calotropis procera. Phytopathologia Mediterranea. 2016;55:121–129. doi: 10.14601/phytopathol_mediterr-17161. [DOI] [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddou F., Bekhechi C., Ksouri R., Chabane Sari D., Atik Bekkara F. Potential assessment of Rumex vesicarius L. as a source of natural antioxidants and bioactive compounds. J. Food Sci. Technol. 2015;52:3549–3560. doi: 10.1007/s13197-014-1420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Cheng Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant. Sci. 2015;6:1020. doi: 10.3389/fpls.2015.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da X., Nishiyama Y., Tie D., Hein K.Z., Yamamoto O., Morita E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-38916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khateeb A.Y., Elsherbiny E.A., Tadros L.K., Ali S.M., Hamed H.B. Phytochemical analysis and antifungal activity of fruit leaves extracts on the mycelial growth of fungal plant pathogens. J. Plant Pathol. Microb. 2013;4:199. doi: 10.4172/2157-7471.1000199. [DOI] [Google Scholar]
- Gatto M.A., Ippolito A., Sergio L., Di Venere D. Extracts from wild edible herbs for controlling postharvest rots of fruit and vegetables. Acta Hortic. 2016;1144:349–354. doi: 10.17660/ActaHortic.2016.1144.51. [DOI] [Google Scholar]
- Haikal N.Z. Improving biological control of Fusarium root-rot in cucumber (Cucumis sativus L.) by allelopathic plant extracts. Int. J. Agric. Biol. 2007;9:459–461. Retrieved from: [Google Scholar]
- Ibraheem A.A. Using natural polyphenol extracts of Sider (Ziziphus spina-christi L.) leaves and seeds as anti-microbial. Middle East J. Appl. Sci. 2017;7:262–271. http://www.curresweb.com/mejas/mejas/2017/262-271.pdf Retrieved from: [Google Scholar]
- Johnson J.S., Spakowicz D.J., Hong B.Y., Petersen L.M., Demkowicz P., Chen L. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019;10:5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane J.R., Dürr C., Schwanck A.A., Robin M.H., Sarthou J.P., Cellier V. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017;37:1–25. doi: 10.1007/s13593-017-0417-y. [DOI] [Google Scholar]
- Lang J.F., Tian X.L., Shi M.W., Ran L.X. Identification of endophytes with biocontrol potential from Ziziphus jujuba and its inhibition effects on Alternaria alternata, the pathogen of jujube shrunken-fruit disease. PLoS ONE. 2018;13:e0199466. doi: 10.1371/journal.pone.0199466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouini S.E., Ouahrani M.R. Phytochemical screening, In vitro antioxidant and antibacterial activity of Rumex vesicarius L. extract. Sci. Study & Res. – Chem. & Chem. Eng., Biotechnol., Food Industry. 2017;18:367–376. http://pubs.ub.ro/?pg=revues&rev=cscc6&num=201704&vol=4&aid=4633 Retrieved from: [Google Scholar]
- Lushchak V.I., Matviishyn T.M., Husak V.V., Storey J.M., Storey K.B. Pesticide toxicity: a mechanistic approach. EXCLI. J. 2018;17:1101–1136. doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlo S.M., Chauke H.R., McGaw L., Eloff J. Antioxidant and antifungal activity of selected medicinal plant extracts against phytopathogenic fungi. Afr. J. Tradit. Complement. Altern. Med. 2016;13:216–222. doi: 10.21010/ajtcam.v13i4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani M., Badiee P., Gharibnavaz M., Jassebi A., Jafarian H., Ghassemi F. Comparison of anti-Candida activities of the ancient plants Lawsonia inermis and Ziziphus spina christi with antifungal drugs in Candida species isolated from oral cavity. J. Conserv. Dent. 2018;21:359–362. doi: 10.1371/journal.pone.0199466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho G.J.P., Klein D.E., Luis Junior C.S. Evaluation of soapberry (Sapindus saponaria L.) leaf extract against papaya anthracnose. Summa Phytopathologica. 2018;44:127–131. doi: 10.1590/0100-5405/175605. [DOI] [Google Scholar]
- McDonald B.A., Stukenbrock E.H. Rapid emergence of pathogens in agro-ecosystems: global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016;371:20160026. doi: 10.1098/rstb.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D.G., Pauli G., Mascarin G.M., Delalibera I. A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J. Microbiol. Methods. 2015;119:44–52. doi: 10.1016/j.mimet.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Riquelme M., Aguirre J., Bartnicki-García S., Braus G.H., Feldbrügge M., Fleig U., Hansberg W. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018;82:e00068–e117. doi: 10.1128/MMBR.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, C., Akter, N., Sarkar, M.K., PK, M.M., Begum, N., Zenat, E., et al., 2019. Control of early blight of tomato caused by Alternaria solani and screening of tomato varieties against the pathogen. Open Microbiol. J.13,41-50. https://doi.org/10.2174/1874285801913010041.
- Sadhasivam S., Shapiro O.H., Ziv C., Barda O., Zakin V., Sionov E. Synergistic inhibition of mycotoxigenic fungi and mycotoxin production by combination of pomegranate peel extract and azole fungicide. Front. Microbiol. 2019;10:1919. doi: 10.3389/fmicb.2019.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales M.D.C., Costa H.B., Fernandes P.M.B., Ventura J.A., Meira D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016;6:26–31. doi: 10.1016/j.apjtb.2015.09.026. [DOI] [Google Scholar]
- Schütte G., Eckerstorfer M., Rastelli V., Reichenbecher W., Restrepo-Vassalli S., Ruohonen-Lehto M., Saucy A.W., Mertens M. Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017;29:5. doi: 10.1186/s12302-016-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y., Kim Y.H. Potential reasons for prevalence of Fusarium wilt in oriental melon in Korea. Plant Pathol. J. 2017;33:249–263. doi: 10.5423/PPJ.OA.02.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Germination of spores of Bacillus species: what we know and do not know. J. Bacteriol. 2014;196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Sharma P., Vijayvergia R. Antimicrobial properties of different solvents extract of Rumex Vesicarius Linn. on some selected bacterial and fungal isolates. Int. J. Pharm. Sci. Rev. Res. 2015;6:1107–1114. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- Sharma K., Goss E.M., Dickstein E.R., Smith M.E., Johnson J.A., Southwick F.S., van Bruggen A.H. Exserohilum rostratum: characterization of a cross-kingdom pathogen of plants and humans. PLoS ONE. 2014;9:e108691. doi: 10.1371/journal.pone.0108691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuping D.S.S., Eloff J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017;14:120–127. doi: 10.21010/ajtcam.v14i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurbaar J., Mosobil R., Donkor A.M. Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. BMC Res. Notes. 2017;10:660. doi: 10.1186/s13104-017-3001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whish J.P.M., Herrmann N.I., White N.A., Moore A.D., Kriticos D.J. Integrating pest population models with biophysical crop models to better represent the farming system. Environ. Modell. Softw. 2015;2015(72):418–425. doi: 10.1016/j.envsoft.2014.10.010. [DOI] [Google Scholar]
Further Reading
- Marin, Y., Hernández-Restrepo, M., Iturrieta González, I., García, D., Gené, J., Groenewald, J.Z., et al., 2019. Genera of phytopathogenic fungi: GOPHY 3. Stud. Mycol. 94, 1–124. 10.1016/j.simyco.2019.05.001. [DOI] [PMC free article] [PubMed]