Abstract
Present research explored the anti-obesity effect of Moringa olifera seed oil extract and lycopene (LYC). Forty eight male Sprauge Dawely rats were divided equally into 6 groups. Group Ι (C) served as control, group ΙΙ (MC) was given Moringa olifera seed oil extract (800 mg/kg b.wt) for 8 weeks, group ΙΙΙ (LC) was given (20 mg/kg b.wt) LYC for 8 weeks, group ΙV (O) received high fat diet (HFD) for 20 weeks, group Ѵ (MO), was given HFD for 20 weeks and received (800 mg/kg b.wt) Moringa olifera seed oil extract for last 8 weeks and group ѴΙ (LO), received HFD for 20 weeks and was given (20 mg/kg b.wt) LYC for last 8 weeks. Hematology, lipid peroxidation and antioxidants, non-esterified fatty acids (NEFA), glucose, lipid profile, serum liver and kidney biomarkers, inflammatory markers, leptin, resistin and heart fatty acid binding protein (HFABP) were determined. Also histopathology for liver, kidney and aorta were performed besides immunohistochemistry (IHC) for aortic inducible nitric oxide synthase (iNOS). Administration of Moringa olifera seed oil extract and LYC significantly ameliorated the HFD induced hematological and metabolic perturbations as well as reduced leptin and resistin. Both treatments exerted these effects through promotion of antioxidant enzymes and reducing lipid peroxidation as well as inflammatory cytokines along with reduced iNOS protein expression. Administration of Moringa olifera seed oil extract and LYC have anti-obesity potential in HFD induced obesity in male Sprauge Dawely rats.
Keywords: Antioxidants, Inflammatory markers, iNOS, Obesity, Lycopene, Moringa, Fatty infiltrations
1. Introduction
The prevalence of obesity is increasing worldwide, and has already reached alarming levels particularly in the Middle East and North Africa region (Musaiger, 2011). Obesity constitutes a grave health problem and rises the hazard of chronic diseases such as diabetes mellitus and cardiovascular disease (CVD) (Roh and Jung, 2012, Wang and Lobstein, 2006). Obesity is defined as abnormal excessive fat buildup that is accompanied by disproportion in energy intake and expenditure (Kopelman, 2000, Spiegelman and Flier, 2001). In addition, world Health organization defined obesity and overweight as abnormal or excessive fat accumulation that presents a risk to health (WHO, 2000). There are positive correlations between daily lipid intake and body weight as well as fat deposition (Bernardo and Mesquita, 2012). Exposure of animals to high fat diet (HFD) often results in accumulation of visceral fat and obesity (Lutz and Woods, 2012). Visceral fat tissue is considered a dynamic endocrine organ that produces adipokines such as adiponectin, resistin and leptin. The later adipokines have a crucial function in food intake, energy homeostasis, metabolism, insulin sensitivity and production, endothelial function and inflammation (Grundy, 2016).
According to the World Health Organization criteria, obesity is recognized as one of the major causes for the development of chronic and disabling diseases (WHO, 2011). Obesity has been associated with numerous disturbances such as cardiovascular disease (CVD), atherosclerosis (Azimi et al., 2013, Ouimet, 2013), osteoarthritis (Collins et al., 2018), type 2 diabetes (Nath et al., 2006) and hypertension (Dorresteijn et al., 2012), respiratory disorders (Zammit et al., 2010), chronic musculoskeletal problems (Onyemaechi et al., 2016), lumbago (Shiri et al., 2009), skin problems (Yosipovitch et al., 2007), infertility (Bazzano et al., 2015) and certain cancers (Vucenik and Stains, 2012). Also, HFD induces obesity and abnormal lipid metabolism that is subsequently associated with accumulation of fat in the liver (Altunkaynak, 2005, Kameshwaran et al., 2013). Moreover, it is accompanied with low-grade inflammation (Monteiro and Azevedo, 2010) and increased adipose tissue that releases several adipokines and pro-inflammatory factors, such as, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) and interleukin 1 β (IL-1 β) (Henry et al., 2012).
There are many strategies to reduce weight, including use of pharmacological agents, however, some of them has side effect and others withdrawn from the market for safety concerns (Bray, 2009, Van Gaal and Dirinck, 2016).
Other approaches were used such as; rising physical activity, reducing energy intake, and increasing the consumption of food rich in polyphenols as they lead to greater energy expenditure and fat oxidation as well as satiety (Rastmanesh, 2011, Westerterp-Plantenga et al., 2006). Currently, there are no pharmacological treatments that provide a continuous weight loss with minimal adverse effects (Cooke and Bloom, 2006). Thus, several trials have been done to diminish body weight with pharmacological interventions that possess negligible side effects. Herbal supplements are being widely used owing to their efficacy in handling numerous chronic conditions. They are cheap and have minimum or no toxic side effects, when compared to many chemically synthesized drugs (Park et al., 2011). Herbal supplements cover various natural products, including isolated compounds from plants and crude extracts (Hanl et al., 2005, Rayalam et al., 2008).
Moringa olifera is one of the most widely distributed and naturalized species of a monogeneric family which has various medicinal and nutritional values accredited to their bark, roots, flowers, leaves, seeds, and fruits (Vergara-Jimenez et al., 2017). Moringa olifera holds saponins, tannins, flavonoids, glycosides and terpenoids, all of which possess medicinal belongings. The later compounds display effective anti-oxidants, anti-carcinogenic and anti-microbial potential and they can also boost the immune system (Ayoola et al., 2008, Davinelli et al., 2015). These effects are mediated via quenching singlet or triplet oxygens, neutralizing free radicals or decomposing peroxides (Yanishlieva and Heinonen, 2001, Zheng and Wang, 2001). Moreover, Saponins and tannins also have been associated with a reduced energy requirement for protein and lipid biosynthesis, leading to lower body lipid, nutrient utilization, energy retention and growth performance. Therefore, such compounds have been demonstrated to have an efficient anti-obesity potential (Dongmeza et al., 2006).
LYC is a highly unsaturated, non-oxygenated carotenoid that widely used in food and pharmaceutical industries (Stahl et al., 1992). Tomato byproducts, including pizza sauce, tomato juice, and ketchup are affluent sources of LYC (Mangels et al., 1993). The consumption of tomatoes and tomato byproducts containing LYC has been demonstrated to be linked to the diminished risk of chronic diseases, such as cardiovascular diseases and cancer (Mordente et al., 2011). Several studies have accredited the health benefits of LYC to its antioxidant properties (Bahcecioglu et al., 2010, Di Mascio et al., 1989, Viuda-Martos et al., 2014). As, LYC quenches singlet oxygen almost twice as effectively as β-carotene, it can protect tissues and cells against harmful effects of free radicals (Di Mascio et al., 1989). In addition, studies have shown that LYC has a potential neuroprotective effect against neurological disorders (Cao et al., 2019). LYC is mainly stored in adipose tissues and can reduce the production of cytokines that are usually secreted by adipose tissues, therefore leading to a reduction in the risk of pathologies linked with obesity (Gouranton et al., 2011).
The present research aimed to investigate the potential of Moringa olifera oil seed extract and LYC potentials to control obesity and its adverse sequelae. This aim was achieved by determining hematological parameters, lipid profiles, oxidative stress biomarkers, adipokines, liver and kidney functions. As well as detection of aortic inducible nitric oxide synthase (iNOS) protein expression, histopathology of liver, kidney and aorta in male Sprauge Dawely rats.
2. Material and methods
2.1. Animals
Forty eight male Sprauge Dawely rats (110-118 g) were obtained and kept in Laboratory Animal House, Faculty of Veterinary Medicine, Suez Canal University, Egypt. The animals were randomly assigned into six groups, 8 rats per group (each 4 rats kept in a cage). For adaptation and acclimation, rat were maintained under room temperature, natural day light rhythm, and fed ad libitum control diets for 2 weeks. For the 20 weeks experimental period, rats were kept at temperature 24 ± 2 °C and a relative humidity of 60 ± 5%. The experimental protocol was approved by the institutional review board of Faculty of Veterinary Medicine, Suez Canal University (protocol No. 2019030).
2.2. Diet
Control diet was formulated to fulfill the nutritional requirements of rats according to NRC (1995) as shown in Table 1. Induction of obesity in the rats was achieved by feeding HFD, Table 1 for 12 weeks according to Axen and Axen (2006). All diets were given to rats ad libitum.
Table 1.
Composition of experimental diets.
| Ingredients | Control diet (%) | High fat diet (%) |
|---|---|---|
| Yellow corn Corn gluten Soybean* Casein Wheat bran Sucrose Vegetable oil Animal fat Cellulose Methionine Lysine Ground limestone Dicalcium phosphate Common salt Premix** Total Calculated values: CP % ME (Kcal/kg) Ca P |
70.24 5.00 8.80 5.00 4.00 ----- 4.74 ----- ----- 0.34 0.07 0.82 0.56 0.13 0.30 100.00 17.21 3362.80 0.50 0.30 |
29.50 2.1 3.70 13.84 1.68 5.14 1.99 38.20 1.50 0.39 ---- 0.42 1.11 0.13 0.30 100.00 17.20 5001.75 0.50 0.30 |
*Soybeans were autoclaved at 110 0C for 30 min according to (Westfall and Hauge, 1948) to inactivate trypsin inhibitor, tannins, saponins, phytate, protease inhibitors, lectins and goitrogens.
** Each 3 kg contain the following vitamins and minerals: Vit. A 12 mIU, vit. D3 2 mIU, vit. E 1000 mg, vit. k3 1000 mg, vit. B1 1000 mg, vit. B2 5000 mg, vit. B6 1500 mg, vit. B12 10 mg, biotin 50 mg, pantothinic acid 10000 mg, nicotinic acid 30000 mg, folic acid 1000 mg, manganese 60000 mg, zinc 50000 mg, iron 30000 mg, copper 4000 mg, iodine 300 mg, selenium 100 mg, cobalt 100 mg, carrier(CaCO3) to 3 kg. (Golden premix- Selim Pharm Elasher, Egypt.).
2.3. Plant material
Moringa olifera seed oil extract was obtained from Grenera Nutrients Private Limited, India. The dose was selected according to Ajibade et al., 2012, Charles and Hamman, 2015.
Lycopen was obtained as capsules from NOW FOODS Co., USA and prepared in corn oil (5% w/v). The dose of LYC was selected according to Jiang et al. (2016).
2.4. Study design
The rats were splitted into six groups as follows:
Group Ι (C); received control diet during the whole experimental period (20 weeks) and were gavaged corn oil as a vehicle for last 8 weeks.
Group ΙΙ (MC); received control diet for 20 weeks and were given (800 mg/kg b.wt) Moringa olifera seed oil extract daily by oral gavage for the last 8 weeks.
Group ΙΙΙ (LC); received control diet for 20 weeks and were given (20 mg/kg b.wt/ day) LYC in corn oil by intragastric administration for the last 8 weeks.
Group ΙѴ (O); received HFD all over the experimental period (20 weeks) and were gavaged corn oil as a vehicle for last 8 weeks.
Group V (MO); received HFD all over the experimental period (20 weeks) and Moringa olifera seed oil extract (800 mg/kg b.wt/ day) by intragastric administration for last 8 weeks.
Group ѴΙ (LO); received HFD all over the experimental period (20 weeks) and gavaged LYC (20 mg/kg b.wt/ day) for last 8 weeks.
2.5. Feed consumption and body weight
Initial and final body weights were recorded, for each rat, to obtain weight gain. Also feed consumption was recorded.
2.6. Blood and samples collection
Three retro-orbital venous blood samples were drawn from each rats after overnight fasting, under the effect of tetrahydrofurane (THF) inhalation anesthesia. The first blood sample was collected in di-potassium salt of ethylene diamine tetracetate (EDTA) for hematological parameters evaluation. The second blood sample was collected in a plain tube to separate sera for biochemical analysis, fatty acid binding protein, leptin, antioxidant and cytokines analysis. The third blood sample was put in sodium fluoride tube for glucose estimation. Animals were euthanized by over dose of THF. Liver and kidney were weighed and their relative weights in relation to body weight were calculated. Kidneys, liver and aorta were obtained and fixed in 10% formalin for histopathological examination.
2.7. Hematology
Detection of hematological parameters were performed by using standard techniques according to Jain (1986) via improved Neubauer hemocytometer. The percentage for each type of white cells was calculated according to Feldman et al. (2000).
2.8. Lipid peroxidation and antioxidants
Malondialdehyde (MDA) levels in serum was determined according to Armstrong and Browne, 1994, Yagi, 1998 using CELL BIOLABS, INC. , USA kit. Serum catalase (CAT), superoxide dismutase (SOD) and total antioxidative capacity (TAC) were determined according to Wheeler et al., 1990, Koivunen and Krogsrud, 2006, Manoel and Moya, 2015, respectively. The later antioxidants were estimated via commercial kits of (Kamiya Biomedical Co., USA), (Biodiagnostic, Egypt) and (LDN, Germany), repectively.
2.9. Inflammatory markers
The serum levels of IL-6, IL-1 β and TNF-α were assayed using commercial enzyme linked immunoassy (ELISA) kits (IBL Co., Japan). The procedures of the assay were carried out according to Koivunen and Krogsrud (2006).
2.10. Leptin, resistin and heart fatty acid binding protein (HFABP) assay
Serum concentrations of leptin (Kamiya Biomedical Co., USA) and HFABP (GenWay Biotech, Inc., USA) were estimated by ELISA technique. The procedures were followed according to the manufacturers' enclosed pamphlet.
2.11. Biochemical parameters
2.11.1. Lipid profile, non-esterified fatty acids (NEFA) and glucose
Serum level of total cholesterol (TC), triglycerides (TGs) and high density lipoprotein cholesterol (HDL-C) were determined by Spinreact, Spain kits according to the described method of Lopes-Virella et al., 1977, Allain et al., 1974, Fossati and Prencipe, 1982, respectively. Low density lipoprotein cholesterol (LDL-C) was calculated by formula of Friedewald et al. (1972). Serum levels of NEFA were calorimetrically assayed using Elabscience calorimetric kit, USA. Plasma glucose values were determined using Diamond Diagnostic kits, Egypt.
2.11.2. Liver and kidney biomarkers
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were assayed according to the methods of Reitman and Frankel (1957). Serum albumin and total proteins (TP) levels were determined calorimetrically according to Drupt, 1974, Henry, 1964. Serum creatinine and blood urea nitrogen (BUN) levels were determined the methods of according to Henry et al., 1974, Reiss et al., 1965. All these parameters were assayed using Stanbio, Texas, USA commercial kits.
2.12. Histopathology
Formaline fixed liver, kidney and aorta were subjected to routine histopathological procedures according to Bancroft and Stevens (1990). The obtained slides were examined using a light microscope.
2.13. Immunohistochemistry (IHC) and quantification
Protein expression of iNOS, in formalin fixed aorta, was detected via IHC according to Stumm et al. (2002). The iNOS primary polyclonal antibody used in this assay was obtained from Santa Cruz Biotechnology, USA and was used at dilution rate 1:200. Quantification of immuno-reactive parts was performed by ImageJ software, Japan. Six random fields were chosen from each slide of the different experimental groups. Within each field, the integrated density of eight random parts were evaluated in relation to the total area and immune-stained area % (ISA %).
2.14. Statistical analysis
Data of the present study were analyzed using Statistical Package for Social Sciences (SPSS) version 23 (SPSS Inc., Chicago). Results were shown as mean ± SE. One way ANOVA followed by Duncan's test were used for analysis. A P < 0.05 is taken as a level for statistical significance.
3. Results
3.1. Feed consumption, body weight and organs weight
The final body weight and weight gain of rats in group IV significantly (P < 0.05) increased than control (group I). Administration of Moringa olifera seed oil extrect and LYC to rats in group V and VI significantly (P < 0.05) decreased final body weights and weight gain than group IV (O). Feed consumption revealed significant (P < 0.05) reduction in group IV (O) than control group. The administration of Moringa olifera seed oil extrect and LYC to rats in group V and VI significantly (P < 0.05) promoted feed consumption than group IV. A non-significant alteration was observed in relative liver and kidney weights of different groups, Table 2.
Table 2.
Effect of experimental diets, Moringa olifera seed oil extrect and lycopene gavage on body weight, weight gain, feed consumption and relative organs weight.
| Parameters | Group I (C) | Group II (MC) | Group III (LC) | Group IV (O) | Group V (MO) | Group VI (LO) |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 105.83 ± 2.38a | 107.50 ± 2.14 a | 109.17 ± 2.38 a | 105.00 ± 1.82 a | 106.67 ± 1.66 a | 106.67 ± 2.47 a |
| Final body weight (g) | 230.33 ± 10.31b | 226.33 ± 8.53bc | 234.33 ± 6.10 ab | 260.67 ± 6.29 a | 201.17 ± 15.82c | 203.17 ± 11.48c |
| Weight gain (g) | 124.50 ± 8.37b | 118.83 ± 6.46b | 125.17 ± 3.98b | 155.67 ± 4.85 a | 94.50 ± 14.87 bc | 96.50 ± 22.89 bc |
| Feed intake (g) | 29.44 ± 2.86b | 33.26 ± 0.13a | 33.74 ± 0.70a | 25.23 ± 0.43c | 27.77 ± 0.50bc | 28.02 ± 54bc |
| Liver relative weight (%) | 2.59 ± 0.22a | 2.20 ± 0.16a | 2.23 ± 0.04a | 2.41 ± 0.10a | 2.28 ± 0.06a | 2.24 ± 0.08a |
| Kidney relative weight (%) | 0.30 ± 0.01a | 0.30 ± 0.01a | 0.31 ± 0.01a | 0.23 ± 0.04a | 0.25 ± 0.03a | 0.26 ± 0.08a |
| Sub-lumbar Fat relative Weight (%) | 1.11 ± 0.02c | 1.13 ± 0.02c | 1.12 ± 0.02c | 3.23 ± 0.20a | 1.87 ± 0.27b | 1.87 ± 0.18b |
| Epididymal fat relative weight (%) | 0.99 ± 0.10c | 0.97 ± 0.05c | 3.02 ± 0.20a | 1.59 ± 0.16b | 1.58 ± 0.08b | 0.90 ± 0.09c |
Superscripts a,b,c within the same row considered significant at P < 0.05.
3.2. Hematology
Table 3 showed that values of RBCs count, packed cell volume (PCV), hemoglobin (Hb), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were non-significantly changed among all experimental groups. Furthermore, the total WBCs count, monocytes, eosinophils and basophils were also non-significantly changed among all experimental groups. On other side, neutrophils count significantly (P < 0.05) upregulated in group IV (O) than control groups (groups I, II and III), whereas the lymphocytes count significantly (P < 0.05) decreased in obese group compared with control groups (I, II and III). Treatment of rats with Moringa olifera seed oil extract and LYC after HFD significantly (P < 0.05) restored both neutrophils and lymphocytes counts to a level comparable to the control group values.
Table 3.
Effect of experimental diets, Moringa olifera seed oil extract and lycopene (LYC) gavage on hematological parameters.
| Groups Parameters | Group I (C) | Group II (MC) | Group III (LC) | Group IV (O) | Group V (MO) | Group VI (LO) |
|---|---|---|---|---|---|---|
| RBCs (106/μL) | 8.18 ± 0.51a | 8.22 ± 0.10a | 7.47 ± 1.20a | 8.64 ± 0.52a | 8.54 ± 0.56a | 8.21 ± 1.14a |
| Hb (g/dL) | 14.4 ± 0.32a | 14.73 ± 0.19a | 12.93 ± 1.52a | 13.63 ± 0.89a | 14.17 ± 0.93a | 13.00 ± 2.04a |
| PCV (%) | 48.20 ± 1.66a | 48.43 ± 0.55a | 43.73 ± 5.37a | 47.17 ± 3.57a | 46.40 ± 3.78a | 40.10 ± 6.48a |
| MCV (fL) | 56.33 ± 1.16a | 57.63 ± 1.32a | 59.53 ± 2.76a | 54.60 ± 0.80a | 54.37 ± 2.02a | 55.23 ± 1.93a |
| MCH (pg) | 17.70 ± 1.11a | 17.90 ± 0.31a | 17.57 ± 0.97a | 15.73 ± 0.17a | 16.57 ± 0.49a | 15.70 ± 0.36a |
| MCHC (g/dL) | 29.87 ± 0.38a | 30.40 ± 0.57a | 29.60 ± 0.40a | 29.20 ± 0.21a | 30.57 ± 0.48a | 29.43 ± 0.59a |
| WBCs(103/μL) | 13.23 ± 1.76a | 13.80 ± 1.40a | 13.63 ± 1.11a | 17.50 ± 4.42a | 15.60 ± 3.37a | 13.37 ± 3.30a |
| Neutrophils (103/μL) | 30.50 ± 2.96b | 30.21 ± 1.30b | 33.52 ± 1.99b | 70.36 ± 3.54a | 29.28 ± 5.30b | 35.08 ± 5.04b |
| Lymphocytes (103/μL) | 63.60 ± 3.08a | 64.03 ± 1.48a | 60.87 ± 2.41a | 23.97 ± 4.71b | 63.73 ± 5.27a | 58.8 ± 4.93a |
| Monocytes (103/μL) | 3.78 ± 0.40a | 3.69 ± 0.35a | 3.48 ± 0.27a | 3.57 ± 0.79a | 3.78 ± 0.39a | 3.40 ± 0.39a |
| Eosinophils (103/μL) | 1.86 ± 0.10a | 1.78 ± 0.14a | 1.86 ± 0.10a | 1.80 ± 0.40a | 1.95 ± 0.36a | 2.46 ± 0.22a |
| Basophils (103/μL) | 0.26 ± 0.09a | 0.29 ± 0.07a | 0.28 ± 0.08a | 0.30 ± 0.08a | 0.27 ± 0.08a | 0.26 ± 0.09a |
Superscripts a,b within the same row considered significant at P < 0.05.
3.3. Lipid peroxidation and antioxidants
The induction of obesity in male Sprauge Dawely rats using a HFD induced a significant (P < 0.05) increment in MDA (group IV) than control, Table 4. Rats of group IV showed depleted CAT, TAC and SOD levels when compared to control groups (I, II and III). Treatment of HFD fed rats with Moringa olifera seed oil extract and LYC for 8 weeks produced a significant (P < 0.05) reduction in MDA level, and upregulated TAC, SOD and CAT activities than group IV (O).
Table 4.
Effect of experimental diets, Moringa olifera seed oil extract and lycopene (LYC) gavage on lipid peroxidation, antioxidant enzymes, inflammatory cytokines, leptin, resistin and heart fatty acid binding protein (HFABP).
| Groups Parameters | Group I (C) | Group II (MC) | Group III (LC) | Group IV (O) | Group V (MO) | Group VI (LO) |
|---|---|---|---|---|---|---|
| MDA (μM) | 0.76 ± 0.02d | 0.76 ± 0.02d | 0.76 ± 0.02d | 1.65 ± 0.03a | 0.97 ± 0.01c | 1.16 ± 0.03b |
| CAT (n mol/ml) | 6.52 ± 0.05a | 6.66 ± 0.05a | 6.67 ± 0.06a | 4.12 ± 0.06d | 5.54 ± 0.08b | 4.83 ± 0.09c |
| TAC (n mol/ml) | 2.39 ± 0.06a | 2.36 ± 0.04a | 2.32 ± 0.10a | 0.87 ± 0.00d | 1.86 ± 0.03b | 1.35 ± 0.03c |
| SOD (µg/mL) | 4.57 ± 0.12a | 4.62 ± 0.06a | 4.64 ± 0.06a | 2.81 ± 0.06d | 3.82 ± 0.08b | 3.32 ± 0.08c |
| TNF-α (pg/ml) | 5.13 ± 0.14d | 5.16 ± 0.04d | 5.08 ± 0.08d | 19.47 ± 0.20a | 11.92 ± 0.22c | 15.03 ± 0.19b |
| IL-1β (pg/ml) | 3.89 ± 0.04d | 3.84 ± 0.03d | 3.85 ± 0.02d | 7.45 ± 0.07a | 4.92 ± 0.11c | 6.16 ± 0.21b |
| IL-6 (pg/ml) | 10.51 ± 0.07d | 10.52 ± 0.04d | 10.46 ± 0.04d | 23.74 ± 0.11a | 15.31 ± 0.05c | 18.84 ± 0.28b |
| Leptin (pg/mL) | 2.64 ± 0.06d | 2.60 ± 0.03d | 2.61 ± 0.06d | 4.42 ± 0.07a | 3.26 ± 0.06c | 3.68 ± 0.13b |
| Resistin (pg/mL) | 3.44 ± 0.03d | 3.22 ± 0.04d | 3.25 ± 0.06d | 6.46 ± 0.11a | 4.56 ± 0.08c | 5.39 ± 0.15b |
| HFABP (ng/mL) | 15.51 ± 0.32d | 14.94 ± 0.26d | 14.99 ± 0.34d | 43.22 ± 0.75a | 23.72 ± 0.36c | 32.61 ± 1.05b |
Superscripts a,b,c,d within the same row considered significant at P < 0.05.
3.4. Inflammatory markers
The cytokines’ levels (IL-6, IL-1β and TNF-ɑ) significantly (P < 0.05) amplified in group IV than control groups. The administration of Moringa olifera seed oil extract or LYC (groups VI, V) to HFD fed rats resulted in a significant (P < 0.05) decrease in the levels of these cytokines when compared to group IV. The Moringa olifera seed oil extract and LYC administration to HFD fed rats produced a significant (P < 0.05) decrease in IL-6, IL-1β and TNF-ɑ levels than LO group (group VI) as shown in Table 4.
3.5. Leptin, resistin and HFABP assay
Serum leptin, resistin and HFABP significantly (P < 0.05) upregulated in group IV (O) than control groups (groups I, II and III). The administration of Moringa olifera seed oil extract or LYC to HFD fed rats for 8 weeks caused a significant (P < 0.05) reduction in the former parameters in groups V and VI than group IV, Table 4.
3.6. Lipid profile, Non-esterified fatty acids (NEFA) and glucose
Table 5 revealed that induction of obesity with HFD induced a significant (P < 0.05) elevation in the levels of TC, TGs, LDL-C, VLDL-C, NEFA and glucose in group IV (O) than control groups (groups I, II and III). The treatment of rats with Moringa olifera seed oil extract and LYC after HFD administration significantly (P < 0.05) reduced the former parameters than group IV, and sometimes their levels were comparable to control. LDL-C levels in the control group that was given LYC (group III) significantly (P < 0.05) diminished than control group. Whereas, levels of HDL-C were significantly (P < 0.05) dropped in group IV than control groups (groups I, II and III). Treatment of rats with Moringa olifera seed oil extract and LYC after inducing obesity via HFD administration significantly (P < 0.05) raised HDL-C levels compared to group IV (O). Also, HDL-C levels in group III that administrated LYC were significantly (P < 0.05) promoted than control group (C).
Table 5.
Effect of experimental diets, Moringa olifera seed oil extract and lycopene (LYC) gavage on lipid profile, non-esterified fatty acids (NEFA), liver and kidney biomarkers.
| Groups Parameters | Group I (C) | Group II (MC) | Group III (LC) | Group IV (O) | Group V (MO) | Group VI (LO) |
|---|---|---|---|---|---|---|
| TC (mg/dl) | 210.67 ± 1.20bc | 199.67 ± 2.03de | 190.33 ± 2.60e | 245.67 ± 2.96a | 217.67 ± 2.33b | 203.67 ± 6.12 cd |
| TGs (mg/dl) | 129.03 ± 0.90c | 118.07 ± 1.53c | 125.7 ± 3.73c | 194.75 ± 2.80a | 145.66 ± 8.21b | 126.40 ± 3.01c |
| HDL-C (mg/dl) | 40.40 ± 0.61ab | 42.67 ± 1.45a | 42.00 ± 0.58a | 36.93 ± 1.24c | 39.83 ± 0.62abc | 38.10 ± 0.67bc |
| LDL-C (mg/dl) | 144.46 ± 0.83bc | 133.39 ± 3.25 cd | 123.19 ± 3.17d | 169.78 ± 4.46a | 148.70 ± 1.32b | 140.29 ± 7.38bc |
| VLDL-C (mg/dl) | 25.81 ± 0.18c | 23.61 ± 0.31c | 25.14 ± 0.75c | 38.95 ± 0.56a | 29.13 ± 1.64b | 25.28 ± 0.60c |
| NEFA (mmol/L) | 0.19 ± 0.01c | 0.15 ± 0.01c | 0.14 ± 0.01c | 0.77 ± 0.04a | 0.40 ± 0.02b | 0.46 ± 0.03b |
| Glucose (mg/dl) | 110.00 ± 2.08c | 106.67 ± 4.63c | 95.33 ± 3.84c | 172.33 ± 8.95a | 134.33 ± 3.18b | 128.00 ± 3.61b |
| ALT (IU/l) | 39.92 ± 1.45c | 39.68 ± 0.72c | 38.90 ± 0.72c | 60.66 ± 1.47a | 47.08 ± 0.97b | 41.13 ± 1.01c |
| AST (IU/l) | 56.33 ± 6.43bc | 49.21 ± 0.94bc | 42.15 ± 0.83c | 84.88 ± 8.49a | 61.83 ± 5.78b | 48.83 ± 1.02bc |
| Albumin (g/dl) | 4.47 ± 0.18a | 4.03 ± 0.07a | 4.13 ± 0.27a | 3.33 ± 0.09b | 4.00 ± 0.23a | 4.07 ± 0.18a |
| TP (g/dl) | 7.57 ± 0.09a | 7.47 ± 0.42ab | 7.30 ± 0.10ab | 6.63 ± 0.33b | 7.17 ± 0.22ab | 6.93 ± 0.26ab |
| Creatinine (mg/dl) | 0.84 ± 0.02b | 0.78 ± 0.05bcd | 0.71 ± 0.02d | 1.14 ± 0.04a | 0.81 ± 0.02bc | 0.73 ± 0.02 cd |
| BUN (mg/dl) | 36.77 ± 2.80bc | 39 ± 2.32bc | 32.4 ± 2.36c | 54.83 ± 1.78a | 44.67 ± 3.20b | 43.38 ± 1.62b |
| Uric Acid (mg/dl) | 2.41 ± 0.18b | 1.87 ± 0.54b | 2.01 ± 0.02b | 3.75 ± 0.53a | 1.81 ± 0.42b | 1.94 ± 0.08b |
Superscripts a,b,c,d within the same row considered significant at P < 0.05.
3.7. Serum liver and kidney biomarkers
Induction of obesity via HFD in male Sprauge Dawely rats produced significant (P < 0.05) elevations in ALT, AST, creatinine, uric acid, and BUN than control groups (groups I, II and III). Treatment of HFD fed rats with Moringa olifera seed oil extract and LYC significantly (P < 0.05) declined ALT, AST, creatinine, BUN and uric acid levels than group IV (O). Total protein and albumin values were significantly (P < 0.05) decreased in group IV than control groups. Treatments of HFD fed groups with Moringa olifera seed oil extract and LYC for 8 weeks significantly (P < 0.05) increased total protein and albumin values than group IV, Table 5.
3.8. Histopathology
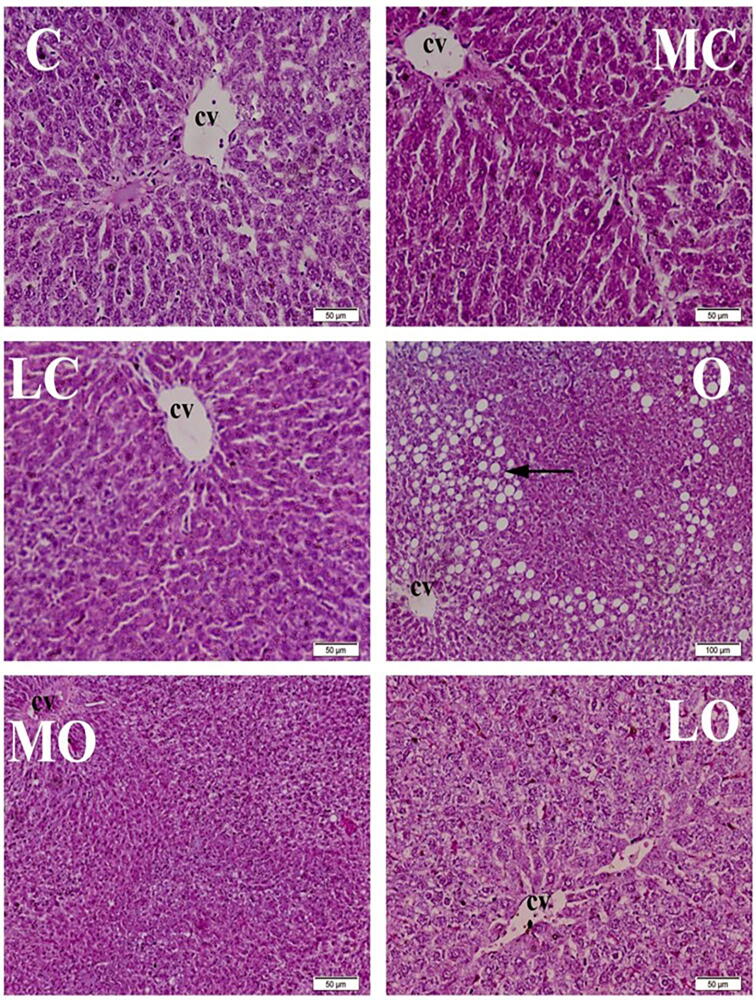
Histopathological examination of liver showed that the control groups (C, MC and LC) showed normal histological architecture. Hepatic lobules were of the same size and shape with normal hepatic areas, centrally located central veins and normal radiating columns of hepatic cells. Hepatic cells were hexagonal in shape with abundant eosinophilic cytoplasm and centrally located basophilic nuclei. Hepatic sinusoids were normal. All of the livers of the rats from obese group (O) showed multifocal to extensive fat deposition inside the hepatocytes which was represented by severe vacuolar degeneration; the vacuoles coalesce to form large vacuoles pushing the nuclei to the periphery of cells forming the characteristic signet ring appearance. The liver of MO group showed mild to moderate fatty infiltrations in hepatocytes and focal lymphocytes’ intrusions with presence of few macrophages around hepatic areas. The LO rats revealed mild to moderate infiltrations with fat as well as focal leukocytic infiltrations around hepatic areas, Fig. 1.
Fig. 1.
Histopathological examination of liver, 8 weeks after treatment showing normal histological structure of normal groups [control (C), Moringa olifera seed oil extract (MC) and lycopene (LC)]. Marked to severe fatty infiltration of hepatic cells were observed in high fat diet fed (HFD) induced obese group (O). Marked improvement of obese Moringa olifera seed oil extract treated group (MO). Mild vacuolar degeneration was noticed in high fat diet (HFD) induced obese group treated with lycopene (LO). H&E. Bar 50 μm.*(cv) central vein, (arrow) fat infiltration.
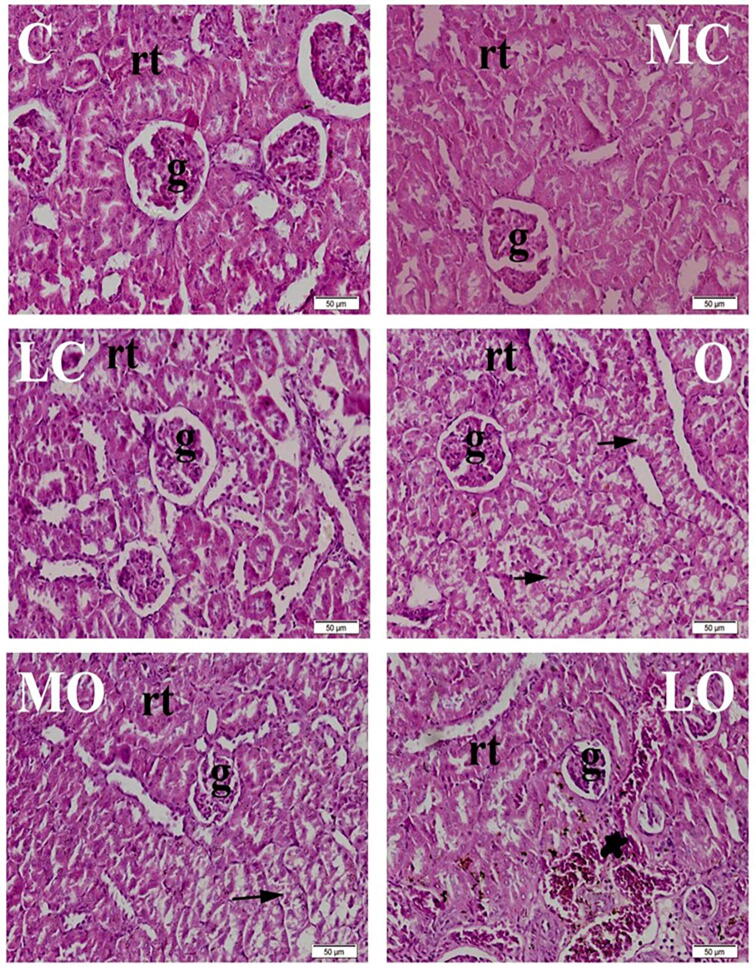
Histopathological examination of the kidney showed that the control groups (C, MC and LC) demonstrated normal histological architecture. Normal glomeruli with normal capillary tufts and normal renal tubules in both cortex and medulla. All kidneys of the O group showed multifocal to diffuse degeneration of renal tubular epithelium. The kidneys of the MO group revealed mild to moderate focal degenerative changes of renal tubules, especially in cortex. LO group rats showed mild degeneration of renal tubules along with multifocal congestion of inter-tubular capillaries, Fig. 2.
Fig. 2.
Histopathological examination of kidney, 8 weeks after treatment showing normal histological structure of normal groups [control (C), Moringa olifera seed oil extract (MC) and lycopene (LC)]. Diffuse degeneration of renal tubular epithelium was observed in high fat diet (HFD) fed group (O). Focal, mild degeneration of renal tubular epithelium was observed in high fat diet (HFD) induced obese group treated with Moringa olifera seed oil extract (MO). Mild to moderate congestion of intertubular capillaries was noticed in high fat diet (HFD) induced obese group treated with lycopene (LO). H&E. Bar 50 μm.*(rt) renal tubules. (g) glomeruli. (arrow) renal tubular degeneration. (star) congestion.
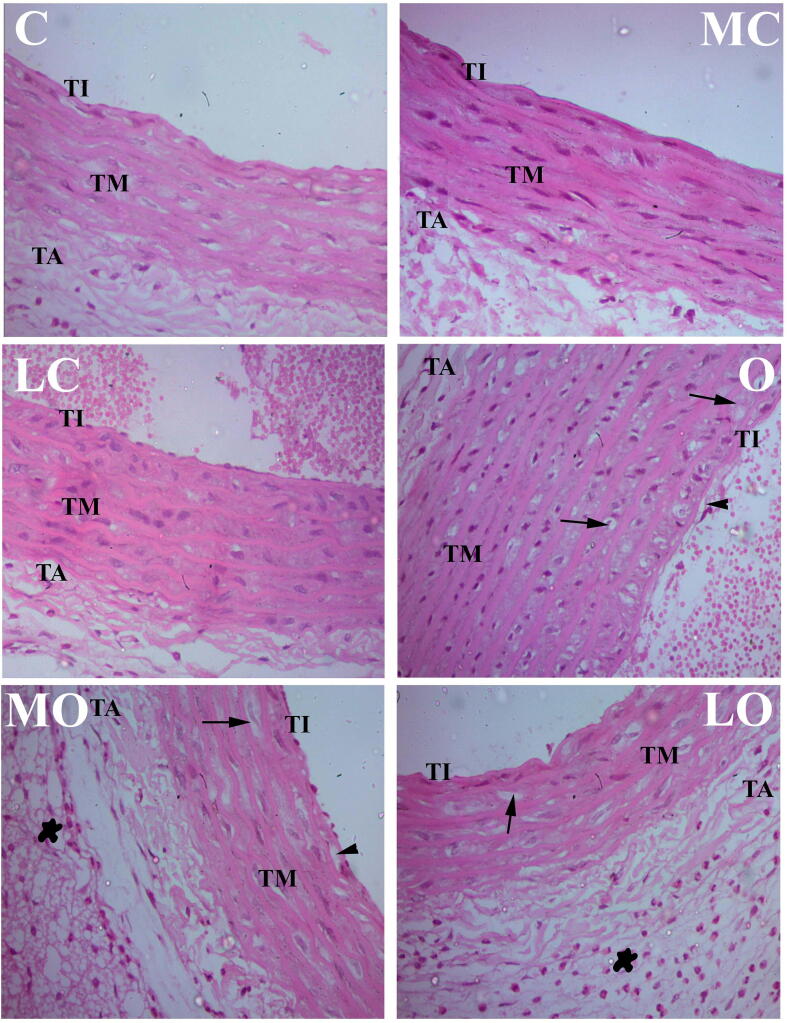
Histopathological examination of H & E stained sections of rats’ aorta in the control groups (C, MC and LC) revealed normal histological building of the three tunics of the aorta; smooth regular tunica intima, tunica media and tunica adventitia. The aorta of HFD fed group (O) group revealed structural changes in the three tunics accompanied with marked increase in the perivascular deposition of adipose cells admixed with large number of eosinophils. Tunica intima showed swelling, vacuolation and irregularity of the endothelial surface. Focal shedding of the endothelial cells accompanied with adhesion of mononuclear leukocytes, in-addition to sub-intimal proliferation of mononuclear cells and macrophages infiltration with foam cells formation. The Tunica media showed marked proliferation of smooth muscle cells with pronounced increase in thickness along with an increased number of foam cells. Examination of HFD fed groups treated with Moringa olifera seed oil extract and LYC showed noticeable amelioration of the histopathological changes of the aorta when compared to group IV (O), signposted by a reduced foam cells formation, intimal damage and smooth muscle cells proliferation, Fig. 3.
Fig. 3.
Aortic histophatological image of normal group [control (C), Moringa olifera seed oil extract (MC) and lycopene (LC)] showed normal tunica intimae (TI), tunica media (TM), and tunica adventitia (TA). High fat diet (HFD) fed group showed irregular tunica intimae (TI) with foam cells (arrow head), irregular tunica media (TM) with fat deposits (arrows) and dense irregular nuclei. Administration of Moringa olifera seed oil extract (MO) and lycopene (LO) to HFD induced obese groups reduced retrogressive changes produced by obesity. X 400, scale bar: 50 μm.
3.9. Immunohistochemistry (IHC) and quantification
The expression of iNOS protein was represented as brownish coloration among the layers of aorta. The ISA% significantly (P < 0.05) increased in in group IV than control groups (C, M and L). The administration of Moringa olifera seed oil extrect and LYC to HFD fed rats (groups V and VI) resulted in a significant (P < 0.05) reduction in iNOS ISA% than non-treated HFD rats (group IV) as shown in Fig. 4.
Fig. 4.
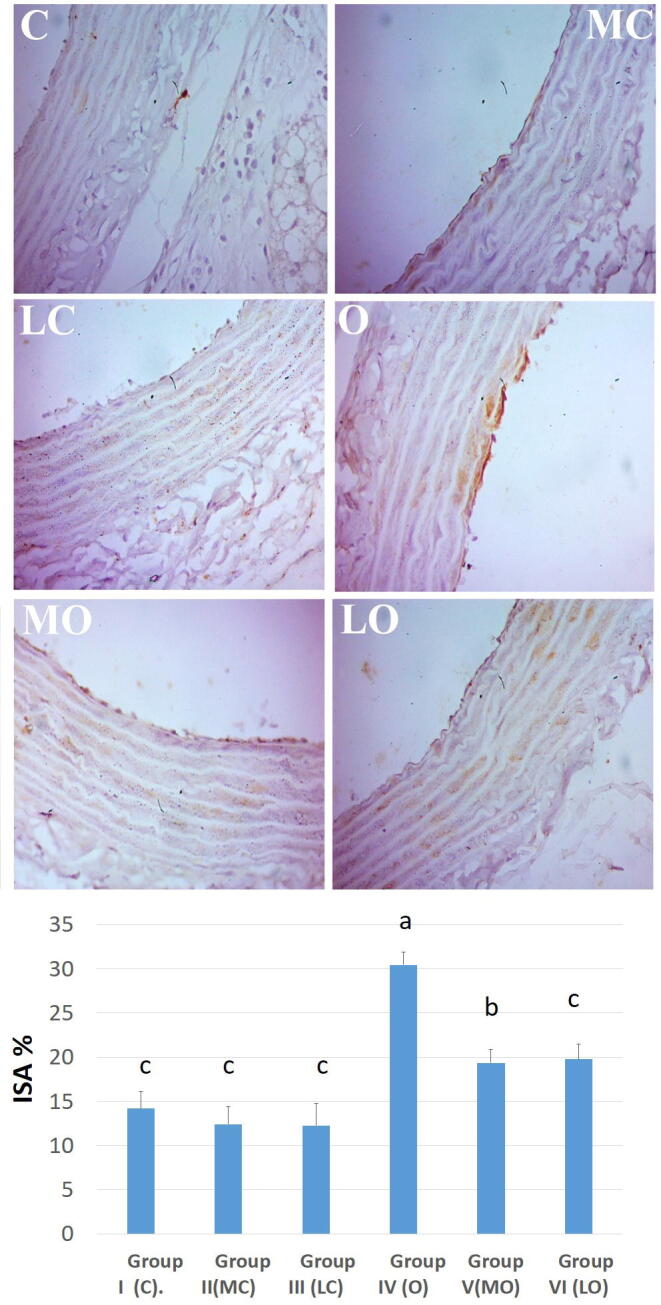
Aortic inmmunohistochemical reaction of inducible nitric oxide synthase (iNOS) of normal groups [control (C), Moringa olifera seed oil extract (MC) and lycopene (LC)] showed lower expression of iNOS protein. The down chart expressed semi-quantitative analysis of immune-stained area % (ISA%) of iNOS. The high fat diet fed (HFD) induced obese group ( O) showed higher expression of iNOS protein. Administration of Moringa olifera seed oil extract (MO) and lycopene (LO) to HFD induced obese groups ameliorated the increased iNOS protein expression than HFD fed induced obese group. X 400, scale bar: 50 μm.
4. Discussion
Obesity is excessive or abnormal fat accumulation generated by disproportion in energy expenditure and intake (Kopelman, 2000, Spiegelman and Flier, 2001). It is associated with the development of several chronic complications, such as hyperglycemia, impaired glucose tolerance, dyslipidemia and hormonal imbalance (Gregor and Hotamisligil, 2011, Marinou et al., 2010, Saito, 2012). Moreover, obesity is a chief risk issue for increased morbidity and mortality and is linked to several medical ailments (Wang and Lobstein, 2006). There is no pharmacological management delivers a suitable weight loss with minimal adverse effects. Therefore, the current study was designed to explore the impact of Moringa olifera seed oil and LYC in the management of obesity induced metabolic disorders. In the current study, obesity was induced to rats by a HFD for examining the metabolic effect of Moringa olifera seed oil and LYC on different parameters.
In present study, the feed intake in HFD group was significantly reduced than control, however, weight gain increased as well as relative fat mass due to high dietary fat content. These results were in harmony with the records of Santos et al., 2016, Santos et al., 2019, Kostrycki et al., 2019. The possible attribution is that higher energy intake in HFD group caused promotion of weight gain consequent to the increased fat mass. Treatment of HFD fed rats with Moringa olifera seed oil extract and LYC produced a significant decline in body weight than HFD fed group that was in harmony with results of Bais et al., 2014, Jiang et al., 2016, respectively. These results demonstrated the ability of Moringa olifera seed oil and LYC to reduce body weight as well as fat mass.
For the hematological parameters, the current results showed that RBCs count, Hb value, PCV, MCV, MCH, MCHC, WBCs count, monocytes, eosinophils and basophiles were non-significantly altered among all experimental groups. These results coincided with Shawky, 2015, Ajibade et al., 2012, Periago et al., 2016. The absence of differences produced by Moringa olifera and LYC on most of hematological findings suggested that these two plant extracts are so safe and therefore do not produce a deleterious effect on the hematological parameters (Ajibade et al., 2012, Phachonpai et al., 2013). The neutrophil count was significantly promoted in HFD fed rats than control ones while lymphocytes significantly decreased which are similar to the results of Auwal et al. (2013). Neutrophilia in obese rats may be due to the fact that obesity can induce glucocorticoids production as well as IL-6 that play role in bone marrow granulopoiesis. They enhance the mobilization of neutrophils from the bone marrow and also causes prolongation of their intravascular half-life (Rusten et al., 1994, Suwa et al., 2000, Ulich et al., 1989). Lymphocytopenia in obese rats is a common finding during the systemic inflammatory response due to reduction of T-cells in peripheral blood, spleen, and thymus (Nunez et al., 2011, Tanaka et al., 1998). Moreover, stress of obesity that may lead to increase of corticosteroids production (Baudrand and Vaidya, 2015) that has been shown to produce lymphocytopenia (Claman, 1975, Hurdle et al., 1966). Amelioration of HFD induced neutrophilia and lymphopenia could be attributed for their anti-obesity and antioxidant potentials that reduced oxidative stress promotion by corticosteroids (Paliwal et al., 2011) and cytokines as IL-6 (Fard et al., 2015, Göncü et al., 2016) that were observed in the present results.
For the lipid peroxidation and antioxidants levels, the current study showed a significant elevation of MDA with a significant reduction of CAT, TAC and SOD as a result of obesity induction. This data is in accordance to Emami et al., 2016, Piña-Zentella et al., 2016, Agrawal and Singh, 2017, Guo et al., 2018. Increasing MDA during obesity is an indication for extensive lipid peroxidation, which plays a crucial part in oxidative stress development and may disturb the soundness of cellular membranes (Mozos et al., 2017, Yefsah-Idres et al., 2016). Administration of Moringa olifera seed oil extract and LYC perfectly ameliorated the obesity induced oxidative stress and promoted antioxidant reserve. The antioxidant effects of Moringa olifera may be due to its richness with various types of natural antioxidant compounds such as β-carotene, tannins, saponins, flavonoids, terpenoids, glycosides, phenolic compounds, vitamin A, β-sitosterol and ascorbic acid (Anwar et al., 2007, Dillard and German, 2000, Lopez-Teros et al., 2017, Murillo and Fernandez, 2017, Siddhuraju and Becker, 2003). These combination of antioxidants found in Moringa olifera has been established to have more efficacy than a single antioxidant, due to increased antioxidant cascade mechanisms as well as their synergistic mechanisms (Ferreira et al., 2008, Mishra et al., 2011, Tejas et al., 2012). Phenolic compounds act as primary antioxidants (Murillo and Fernandez, 2017) due to their capability for prevention of free radicals formation coming out the decomposition of hydroperoxides or inactivation of lipid free radicals. The later properties constitute a principal corner stone in quenching singlet or triplet oxygen, neutralizing free radicals and decomposition of peroxides (Pokorny et al., 2001, Zheng and Wang, 2001). Also, LYC has a potent antioxidant potential that directly scavenges free radicals (Bahcecioglu et al., 2010, Di Mascio et al., 1989, Viuda-Martos et al., 2014) and modulates the assembly of antioxidant enzymes, such as SOD and CAT (Pereira et al., 2017).
The present study has demonstrated a significantly altered lipid profile of HFD fed rats than control ones causing dyslipidemia along with elevate NEFA and glucose levels. These changes were also found by Guo et al., 2018, Udomkasemsab et al., 2019, Xia et al., 2019. The hallmarks of dyslipidemia in obesity are elevated TGs in combination with elevated levels of the majority of small dense LDL-C as well as low HDL-C that was observed in HFD group. This is mainly attributed to the elevated NEFA resulted from their excessive spillover due to HFD (Karpe et al., 2011). Increased levels of NEFA can adversely affect insulin signaling, decrease muscle uptake of glucose, amplify TGs synthesis and induce gluconeogenesis in the liver (Mlinar et al., 2007). All these factors could contribute to the observed hyperglycemia in HFD fed group that is considered a common sequelae of obesity (Martin et al., 2008). Moreover, hyperglycemia may be attributed to oxidative stress resulted from obesity. Oxidative stress increases free radical levels that can impede insulin function as well as peripheral tissues glucose utilization (Ceriello and Motz, 2004). Moringa olifera seed oil extract lowered NEFA levels, reduced dyslipidemia as well as hyperglycemia observed in HFD fed rats. The antidyslipidemic and hypoglycemic effects of Moringa olifera against obesity may be attributed to the phytochemicals such as phenolic, flavonoids, β-sitosterol and saponins contents of this plant extract as they play important roles in lipid regulation. These phytochemicals significantly reduce and delay cholesterol absorption. Also, they can increase the binding to bile acids forming insoluble complexes and increasing their fecal excretion which can lead to decreased plasma cholesterol concentrations (Adisakwattana and Chanathong, 2011, Siasos et al., 2013, Toma et al., 2012). Moreover, Saponins in Moringa olifera can prevent cholesterol absorption via binding to bile acids, resulting in reduction bile acids’ enterohepatic circulation and elevated the levels of cholesterol fecal excretion, resulting in lower plasma cholesterol (Oyedepo et al., 2013), as well as NEFA spillover thus reducing insulin resistance and hyperglycemia. LYC has lipid lowering properties which work by reducing TC, TGs and LDL-C oxidation as well as the synthesis of dysfunctional HDL-C (Mozos et al., 2018). Also, LYC could inhibit cholesterol synthesis (Palozza et al., 2011) via inhibition of HMG-CoA reductase that enhances LDL-C degradation (Agarwal and Rao, 1998, Bobek, 1999, Fuhrman et al., 1997). In addition, other studies have suggested LYC could inhibit the nitric oxide (NO) production pathway (Li et al., 2017), which could result in further stimulation of adipose cells lipolysis and NEFA production (Hong et al., 2015, Jobgen et al., 2006).
Our results declared that induction of obesity via HFD significantly elevated AST and ALT while reduced albumin and TP. Moreover, serum creatinine, urea and uric acid that were measured to evaluated functional kidney damage were significantly increased in HFD fed group than control. These results concurred with Faran et al. (2019). Elevated liver and kidney enzymes were an expected result of increased MDA during obesity, which is an indicator for extensive lipid peroxidation. MDA plays a substantial role in oxidative stress and may disrupt the integrity of hepatic and renal cell membranes (Mozos et al., 2017, Yefsah-Idres et al., 2016), which causes leakage of their enzymes into blood. These changes were clear in histopathological sections and could be seen as hepatic vacuolar degeneration and multifocal to diffuse degeneration of renal tubular epithelium. TP and albumin are mainly produced via hepatic synthesis (Rothschild et al., 1977, Schreiber et al., 1976), therefore their reduction in the serum is indicative of hepatic diseases (Dooley et al., 2018). Reduction of serum albumin may attributed to albuminuria, which is associated with renal damage induced by obesity (Thoenes et al., 2009). Also, albumin is considered a negative acute phase protein, which declines during obesity induced inflammatory conditions (Hübner and Voss, 1978, Schreiber et al., 1986). The results of albumin were in harmony with the promoted oxidative stress in obese rats, as albumin was thought to have antioxidant criteria (Taverna et al., 2013). Administration of Moringa olifera seed oil and LYC significantly ameliorated the perturbed hepatic and renal markers as well as histopathological lesions. These hepatoprotective and nephroprotective effects of Moringa olifera and LYC may be attributed to their antioxidant, singlet-oxygen and free radical hunting capacity (Agarwal and Rao, 2000, Espíndola-Antunes and Kater, 2007, Pokorny et al., 2001, Velmurugan et al., 2004, Zheng and Wang, 2001). The antioxidant power of both Moringa olifera seed oil extrect and LYC helped to maintain the integrity of liver and kidney and also their functions, with reductions in ALT, AST, creatinine, BUN and uric acid with promotion of hepatic synthesis of TP and albumin.
Increasing fat mass in HFD fed rats led to increased serum leptin and resistin levels, and this coincided with the results of Aborehab et al., 2016, Santos et al., 2019. Increasing fat mass in the current study led to promotion of inflammatory cytokines as well as adipokines production such as leptin (Saucillo et al., 2014) and resistin (Ribot et al., 2008). TNF-α and IL-6 are considered adipokines that produced and promoted in adiposity (Fain et al., 2004, Hotamisligil et al., 1993). The observed hyperleptinemia is suggestive for leptin resistance, a state that resulted in altered energy expenditure, as well the observed decline in food intake (Enriori et al., 2007). Resistin, is directly related to the development of insulin resistance. Moreover, NEFA can act not only as a source of energy but it can also signal for regulation of intracellular protein kinases, including PKC and JNK, resulting in impairments in insulin signaling (Guo, 2014). The present study also denoted increased IL-6, IL-1β and TNF-ɑ levels which can function as inflammatory cytokines. Our results were parallel to those obtained by Wang and He, 2018, Campbell et al., 2019, Santos et al., 2019. Induction of obesity and accumulation of fat usually accompany dysfunctional lipid metabolism, including extensive lipolysis, which in turn increases secretion and production of free fatty acids (FFAs). Elevated FFAs levels can promote an abnormal pro-inflammatory response, and subsequently the progression of insulin resistance (Sobczak et al., 2019). In addition, depletion of intracellular antioxidants in the fat tissue, which is principally attributed to increased generation of reactive oxygen species (ROS), could result in oxidative stress. The later can lead to further increased inflammatory cytokines production (Naik and Dixit, 2011) and insulin resistance (Dludla et al., 2019). This was supported by the results showing elevated levels of glucose, increased lipid peroxidation and depleted antioxidant enzymes in obese group. Administration of Moringa olifera seed oil and LYC to HFD fed rats significantly ameliorated the elevated leptin and resistin levels, and caused a reduction of inflammatory cytokines levels. These effects are attributed to their fat mass reducing effect that is the main source of adipokines, as well as their potential in adjusting lipid metabolism (Aborehab et al., 2016, Xie et al., 2018). Furthermore, both Moringa olifera seed oil extrect and LYC possess an antioxidant potential that restores the antioxidant reserve and decreased MDA, thus reducing inflammatory cytokines production and restoring glucose level toward normal values.
The induction of obesity is a predisposing factor for the dysfunction of blood vessel endothelial as well as cardiac injury (Hadi et al., 2005). Present study revealed there was a significant elevation in HFABP, biomarker for myocardial injury, in the HFD fed rats than control ones. This result can be considered early prediction for obesity induced myocardial injury, especially in groups where HFABP directly correlated (Hasić et al., 2011). Increasing NEFA in obese subjects potentially increased oxidative stress and promoted inflammatory cytokines output (IL-6, IL-1β and TNF-ɑ). These cytokines could cause electrophysiological remodeling in cardiac muscle (Ali et al., 2018, Pilz and März, 2008). Administration of Moringa olifera seed oil and LYC significantly ameliorated obesity related increase in HFABP which may be due to their antioxidant potentials and lipid lowering effects observed in this study.
The present study showed that there was significant promotion of iNOS, a key enzyme in NO production, in the wall of aorta along with focal shedding of endothelium and adhesion of mononuclear leukocytes in HFD fed group. In addition to sub-intimal mononuclear cells infiltration with formation of foam cells. High dietary fat can increase NEFA, which can promote nuclear factor kappa B (NF-κB) induced inflammatory cytokines production, oxidative stress and iNOS mediated NO production (Ghosh et al., 2017). The increased level of NO could further stimulate lipolysis and fatty acid oxidation in fat cells, thus triggering the inflammatory portfolio responsible for endothelial dysfunction and insulin resistance (Hong et al., 2015, Jobgen et al., 2006, Noronha et al., 2005). Increased pro-inflammatory cytokines are responsible for mononuclear cells and macrophages infiltration in to the sub intima of aorta. Upregulation of endothelial iNOS in obese subjects may be associated with O2− production due to deficiency of NO substrate that reduces NO bioavailability via activation of NADPH oxidase activity (Lobato et al., 2012). These events promote nitrosative stress, where NO reacts with O2−, leading to peroxynitrite generation, with consequent nitration of protein tyrosine, and cell toxicity (Ballinger et al., 2000) all of which causes endothelial dysfunction as well as hypertention (McIntyre et al., 1999, Spieker et al., 2000). Elevated uric acid is another causal factor that results in increased inflammatory cytokines by activation of NF-κB that mediates the observed endothelial dysfunction (Cai et al., 2017). Administration of Moringa olifera seed oil and LYC significantly ameliorated the over expression of iNOS induced inflammatory portfolio to induce endothelial dysfunction. Both Moringa olifera seed oil extrect and LYC increased the levels of CAT, SOD and TAC all of which can scavenge NADPH oxidase produced O2–, which is responsible for HFD nitrosative stress seen in the aortas. Subsequently, NF-κB induced inflammatory cytokines TNF-α and IL-6, that produce mononuclear cells adhesion and endothelial dysfunction, were reduced (Li et al., 2017, Randriamboavonjy et al., 2017, Xu et al., 2012).
5. Conclusion
The induction of obesity by HFD is associated with metabolic perturbations that can be resulted from oxidative stress and inflammation which adversely influenced liver, kidney and endothelial blood vessels function through induction of iNOS protein expression. Administration of Moringa olifera seed oil extrect and LYC to HFD induced obese rats produced significant amelioration of obesity induced metabolic perturbations, liver, kidney and endothelial dysfunction. These effects were achieved via their antioxidant potential, as this could reduce obesity induced inflammatory cytokines as well as the elevated iNOS protein expression.
Declaration of Competing Interest
None.
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aborehab N.M., El Bishbishy M.H., Waly N.E. Resistin mediates tomato and broccoli extract effects on glucose homeostasis in high fat diet-induced obesity in rats. BMC Complement. Altern. Med. 2016;16:225. doi: 10.1186/s12906-016-1203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisakwattana S., Chanathong B. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011;15:803–808. [PubMed] [Google Scholar]
- Agarwal S., Rao A.V. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998;33:981–984. doi: 10.1007/s11745-998-0295-6. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Rao A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000;163:739–744. [PMC free article] [PubMed] [Google Scholar]
- Agrawal N., Singh S.J. Obesity: An independent risk factor for oxidative stress. Int. J. Adv. Med. 2017;4:718–721. http://dx.doi.org/10.18203/2349-3933.ijam20172260. [Google Scholar]
- Ajibade T., Olayemi F., Arowolo R. The haematological and biochemical effects of methanol extract of the seeds of Moringa oleifera in rats. J. Med. Plant. Res. 2012;6:615–621. doi: 10.5897/JMPR10.544. [DOI] [Google Scholar]
- Ali A., Boutjdir M., Aromolaran A.S. Cardiolipotoxicity, inflammation, and arrhythmias: Role for interleukin-6 molecular mechanisms. Front. Physiol. 2018;9:1866. doi: 10.3389/fphys.2018.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. doi: 10.1093/clinchem/20.4.470. [DOI] [PubMed] [Google Scholar]
- Altunkaynak Z. Effects of high fat diet induced obesity on female rat livers (a histochemical study) Eur. J. Gen. Med. 2005;2:100–109. https://doi.org/10.29333/ejgm/82319. [Google Scholar]
- Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phytother. Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Armstrong, D., Browne, R., 1994. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory, In: Free radicals in diagnostic medicine. Springer, pp. 43-58. [DOI] [PubMed]
- Auwal M., Tijjani A., Sadiq M., Saka S., Mairiga I., Shuaibu A., Adawaren E., Gulani I. Antibacterial and haematological activity of Moringa oleifera aqueous seed extract in Wistar albino rats. SJVS. 2013;11:28–37. doi: 10.4314/sokjvs.v11i1.5. [DOI] [Google Scholar]
- Axen K.V., Axen K. Very low-carbohydrate versus isocaloric high-carbohydrate diet in dietary obese rats. Obesity. 2006;14:1344–1352. doi: 10.1038/oby.2006.152. [DOI] [PubMed] [Google Scholar]
- Ayoola G., Coker H., Adesegun S., Adepoju-Bello A., Obaweya K., Ezennia E., Atangbayila T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008;7:1019–1024. doi: 10.4314/tjpr.v7i3.14686. [DOI] [Google Scholar]
- Azimi A., Charlot M.G., Torp-Pedersen C., Gislason G.H., Køber L., Jensen L.O., Thayssen P., Ravkilde J., Tilsted H.-H., Lassen J.F. Moderate overweight is beneficial and severe obesity detrimental for patients with documented atherosclerotic heart disease. Heart. 2013;99:655–660. doi: 10.1136/heartjnl-2012-303066. [DOI] [PubMed] [Google Scholar]
- Bahcecioglu, I.H., Kuzu, N., Metin, K., Ozercan, I.H., Ustündag, B., Sahin, K., Kucuk, O., 2010. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010, Article ID 262179. https://doi.org/10.4061/2010/262179. [DOI] [PMC free article] [PubMed]
- Bais, S., Singh, G.S., Sharma, R., 2014. Antiobesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. J. adv. biol. 2014, Article ID 162914. https://doi.org/10.1155/2014/162914.
- Ballinger S.W., Patterson C., Yan C.-N., Doan R., Burow D.L., Young C.G., Yakes F.M., Van Houten B., Ballinger C.A., Freeman B.A. Hydrogen peroxide–and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ. Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- Bancroft J.D., Stevens A. Churchill Livingstone, Edinburgh; New York: 1990. Theory and Practice of Histological Techniques. [Google Scholar]
- Baudrand R., Vaidya A. Cortisol dysregulation in obesity-related metabolic disorders. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22:143–149. doi: 10.1097/MED.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano M., Torelli C., Pustovrh M., Paz D., Elia E. Obesity induced by cafeteria diet disrupts fertility in the rat by affecting multiple ovarian targets. Reprod. Biomed. Online. 2015;31:655–667. doi: 10.1016/j.rbmo.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Bernardo M.A., Mesquita M.F. Food intake, body mass index and body fat mass in elderly. Asian J. Clin. Nutr. 2012;4:107–115. doi: 10.3923/ajcn.2012.107.115. [DOI] [Google Scholar]
- Bobek P. Dietary tomato and grape pomace in rats: Effect on lipids in serum and liver, and on antioxidant status. Br. J. Biomed. Sci. 1999;56:109–113. [PubMed] [Google Scholar]
- Bray G.A. Lifestyle and pharmacological approaches to weight loss: Efficacy and safety. J. Clin. Endocrinol. Metab. 2009;94 doi: 10.1210/jc.2008-1294. 324 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, W., Duan, X.-M., Liu, Y., Yu, J., Tang, Y.-L., Liu, Z.-L., Jiang, S., Zhang, C.-P., Liu, J.-Y., Xu, J.-X., 2017. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res. Int. 2017, ID 4391920. https://doi.org/10.1155/2017/4391920. [DOI] [PMC free article] [PubMed]
- Campbell C.L., Yu R., Li F., Zhou Q., Chen D., Qi C., Yin Y., Sun J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. 2019;12:97–107. doi: 10.2147/DMSO.S192228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Wang P., Gao X., Shao B., Zhao S., Li Y. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J. Inorg. Biochem. 2019;193:143–151. doi: 10.1016/j.jinorgbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- Charles D.E., Hamman L.L. Effect of aqueous extract of Moringa oleifera seed on haematological parameters and the spleen in male albino rats. IOSR-JDMS. 2015;14:35–41. doi: 10.9790/0853-14493541. [DOI] [Google Scholar]
- Claman H. How corticosteroids work. J. Allergy Clin. Immunol. 1975;55:145–151. doi: 10.1016/0091-6749(75)90010-x. [DOI] [PubMed] [Google Scholar]
- Collins K.H., Hart D.A., Seerattan R.A., Reimer R.A., Herzog W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res. 2018;7:274–281. doi: 10.1302/2046-3758.74.BJR-2017-0201.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke D., Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat. Rev. Drug Discov. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- Davinelli S., Bertoglio J.C., Zarrelli A., Pina R., Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015;34:28–33. doi: 10.1080/07315724.2015.1080108. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Dillard C.J., German J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. 10.1002/1097-0010(20000915)80:12 1744::AID-JSFA725 3.0.CO;2-W. [Google Scholar]
- Dludla, P., Nkambule, B., Jack, B., Mkandla, Z., Mutize, T., Silvestri, S., Orlando, P., Tiano, L., Louw, J., Mazibuko-Mbeje, S., 2019. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 11, pii: E23. https://doi.org/10.3390/nu11010023. [DOI] [PMC free article] [PubMed]
- Dongmeza E., Siddhuraju P., Francis G., Becker K. Effects of dehydrated methanol extracts of moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus (L.)) Aquac. 2006;261:407–422. doi: 10.1016/j.aquaculture.2006.08.006. [DOI] [Google Scholar]
- Dooley J.S., Lok A.S., Garcia-Tsao G., Pinzani M. John Wiley & Sons; 2018. Sherlock's diseases of the liver and biliary system. [Google Scholar]
- Dorresteijn J., Visseren F., Spiering W. Mechanisms linking obesity to hypertension. Obes. Rev. 2012;13:17–26. doi: 10.1111/j.1467-789X.2011.00914.x. [DOI] [PubMed] [Google Scholar]
- Drupt F. Colorimetric method for determination of albumin. Pharm. Biol. 1974;9:777–779. [Google Scholar]
- Emami S.R., Jafari M., Haghshenas R., Ravasi A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J. Exerc. Nutrition Biochem. 2016;20:29–35. doi: 10.20463/jenb.2016.03.20.1.5. https://doi.org/10.20463/jenb.2016.03.20.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori P.J., Evans A.E., Sinnayah P., Jobst E.E., Tonelli-Lemos L., Billes S.K., Glavas M.M., Grayson B.E., Perello M., Nillni E.A. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Espíndola-Antunes D., Kater C. Adipose tissue expression of 11beta-hydroxysteroid dehydrogenase type 1 in Cushing's syndrome and in obesity. Arq. Bras. Endocrinol. Metabol. 2007;51:1397–1403. doi: 10.1590/S0004-27302007000800027. [DOI] [PubMed] [Google Scholar]
- Fain J.N., Madan A.K., Hiler M.L., Cheema P., Bahouth S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- Faran S.A., Asghar S., Khalid S.H., Khan I.U., Asif M., Khalid I., Farooq Gohar U., Hussain T. Hepatoprotective and renoprotective properties of lovastatin-loaded ginger and garlic oil nanoemulsomes: Insights into serum biological parameters. Medicina. 2019;55:579. doi: 10.3390/medicina55090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard M.T., Arulselvan P., Karthivashan G., Adam S.K., Fakurazi S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn. Mag. 2015;11:S556–S563. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B.F., Zinkl J.G., Jain N.C., Schalm O.W. 5th ed. Lippincot Williams and Wilkins; Philadelphia: 2000. Schalms veterinary hematology. [Google Scholar]
- Ferreira, Pinheiro P.M., Farias, Felipe D., Oliveira J.T.D.A., Carvalho A.D.F.U. Moringa oleifera: bioactive compounds and nutritional potential. Rev. Nutr. 2008;21:431–437. doi: 10.1590/S1415-52732008000400007. [DOI] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. doi: 10.1093/clinchem/28.10.2077. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Fuhrman B., Elis A., Aviram M. Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 1997;233:658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Gao L., Thakur A., Siu P.M., Lai C.W. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017;24:50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göncü T., Oğuz E., Sezen H., Koçarslan S., Oğuz H., Akal A., Adıbelli F.M., Çakmak S., Aksoy N. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq. Bras. Oftalmol. 2016;79:357–362. doi: 10.5935/0004-2749.20160102. [DOI] [PubMed] [Google Scholar]
- Gouranton E., Thabuis C., Riollet C., Malezet-Desmoulins C., El Yazidi C., Amiot M., Borel P., Landrier J. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J. Nutr. Biochem. 2011;22:642–648. doi: 10.1016/j.jnutbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Gregor F.M., Hotamisligil S.G. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Grundy S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016;64:1082–1086. doi: 10.1136/jim-2016-000155. [DOI] [PubMed] [Google Scholar]
- Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models to disease mechanisms. J. Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.-X., Wang Y., Wang K., Ji B.-P., Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B. 2018;19:559–569. doi: 10.1631/jzus.B1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi H.A., Carr C.S., Suwaidi J.A. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- Hanl, K., Kimura, Y., Okuda, H., 2005. Anti-obesity effects of natural products, In: Studies in natural products chemistry. Elsevier, pp. 79-110.
- Hasić S., Jadrić R., Ćosović E., Kiseljaković E., Mornjaković Z., Winterhalter-Jadrić M. Heart-type fatty acid-binding protein and its relation with morphological changes in rat myocardial damage model induced by isoproterenol. Bosn. J. Basic Med. Sci. 2011;11:240–244. doi: 10.17305/bjbms.2011.2557. https://doi.org/10.17305/bjbms.2011.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.J. Harper & Row; New York: 1964. Clinical chemistry, principles and technics; p. 152. [Google Scholar]
- Henry R.J., Cannon D.C., Winkelman J.W. second ed. Harper and Row, Hagerstown; London: 1974. Clinical chemistry: principles and techniques. [Google Scholar]
- Henry S.L., Bensley J.G., Wood-Bradley R.J., Cullen-McEwen L.A., Bertram J.F., Armitage J.A. White adipocytes: more than just fat depots. Int. J. Biochem. Cell Biol. 2012;44:435–440. doi: 10.1016/j.biocel.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hong M.Y., Hartig N., Kaufman K., Hooshmand S., Figueroa A., Kern M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr. Res. 2015;35:251–258. doi: 10.1016/j.nutres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hübner G., Voss C. Protein fractions and total proteins in serum of rats on high fat diets. Nahrung. 1978;22:85–88. doi: 10.1002/food.19780220113. [DOI] [PubMed] [Google Scholar]
- Hurdle A., Gyde O., Willoughby J. Occurrence of lymphopenia in heart failure. J. Clin. Pathol. 1966;19:60–64. doi: 10.1136/jcp.19.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.C. fourth ed. Lea & Febiger; Philadelphia, USA: 1986. Schalm's veterinary hematology. [Google Scholar]
- Jiang W., Guo M.-H., Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016;22:10180–10188. doi: 10.3748/wjg.v22.i46.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobgen W.S., Fried S.K., Fu W.J., Meininger C.J., Wu G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kameshwaran S., Jothimanivannan C., Senthilkumar R., Kothai A. Anti-obesity and hypolipidemic activity of methanol extract of tecoma stans flowers on atherogenic diet induced obesity in rats. Pharmacologia. 2013;4:77–81. doi: 10.3923/pharmacologia.2013.77.81. [DOI] [Google Scholar]
- Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen M.E., Krogsrud R.L. Principles of immunochemical techniques used in clinical laboratories. Lab. Med. 2006;37:490–497. doi: 10.1309/MV9RM1FDLWAUWQ3F. [DOI] [Google Scholar]
- Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kostrycki I.M., Wildner G., Donato Y.H., Dos Santos A.B., Beber L.C.C., Frizzo M.N., Ludwig M.S., Keane K.N., Cruzat V., Rhoden C.R., Heck T. Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate matter. J. Diabetes Res. 2019;2019:4858740. doi: 10.1155/2019/4858740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-N., Lin J., Xia J., Qin L., Zhu S.-Y., Li J.-L. Lycopene mitigates atrazine-induced cardiac inflammation via blocking the NF-κB pathway and NO production. J. Funct. Foods. 2017;29:208–216. doi: 10.1016/j.jff.2016.12.029. [DOI] [Google Scholar]
- Lobato N.D.S., Filgueira F.P., Akamine E.H., Tostes R., Carvalho M.H.C.D., Fortes Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz. J. Med. Biol. Res. 2012;45:392–400. doi: 10.1590/S0100-879X2012007500058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Virella M.F., Stone P., Ellis S., Colwell J.A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977;23:882–884. doi: 10.1093/clinchem/23.5.882. [DOI] [PubMed] [Google Scholar]
- Lopez-Teros V., Ford J.L., Green M.H., Tang G., Grusak M.A., Quihui-Cota L., Muzhingi T., Paz-Cassini M., Astiazaran-Garcia H. Use of a “super-child” approach to assess the vitamin A equivalence of Moringa oleifera leaves, develop a compartmental model for vitamin A kinetics, and estimate vitamin A total body stores in young Mexican children. J. Nutr. 2017;147:2356–2363. doi: 10.3945/jn.117.256974. [DOI] [PubMed] [Google Scholar]
- Lutz T.A., Woods S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012;58:5–61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels A.R., Holden J.M., Beecher G.R., Forman M.R., Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J. Am. Diet. Assoc. 1993;93:284–296. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- Manoel H.R., Moya H.D. A comprehensive study of the use of Cu(I)/4,4’-dicarboxy-2,2’-biquinoline complexes to measure the total reducing capacity: application in herbal extracts. Molecules. 2015;20:22411–22421. doi: 10.3390/molecules201219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinou K., Tousoulis D., Antonopoulos A.S., Stefanadi E., Stefanadis C. Obesity and cardiovascular disease: from pathophysiology to risk stratification. Int. J. Cardiol. 2010;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- Martin S.S., Qasim A.N., Reilly M.P. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre M., Bohr D.F., Dominiczak A.F. Endothelial function in hypertension: the role of superoxide anion. Hypertension. 1999;34:539–545. doi: 10.1161/01.HYP.34.4.539. [DOI] [PubMed] [Google Scholar]
- Mishra G., Singh P., Verma R., Kumar S., Srivastav S., Jha K., Khosa R.J.D.P.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der Pharm. Lett. 2011;3:141–164. [Google Scholar]
- Mlinar B., Marc J., Janež A., Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin. Chim. Acta. 2007;375:20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Monteiro, R., Azevedo, I., 2010. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, pii: 289645. https://doi.org/10.1155/2010/289645. [DOI] [PMC free article] [PubMed]
- Mordente A., Guantario B., Meucci E., Silvestrini A., Lombardi E., Martorana E.G., Giardina B., Bohm V. Lycopene and cardiovascular diseases: an update. Curr. Med. Chem. 2011;18:1146–1163. doi: 10.2174/092986711795029717. [DOI] [PubMed] [Google Scholar]
- Mozos I., Borzak G., Caraba A., Mihaescu R.J.O., therapy Arterial stiffness in hematologic malignancies. Onco. Targets Ther. 2017;10:1381–1388. doi: 10.2147/OTT.S126852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozos I., Stoian D., Caraba A., Malainer C., Horbańczuk J.O., Atanasov A.G. Lycopene and vascular health. Front. Pharmacol. 2018;9:521. doi: 10.3389/fphar.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo A., Fernandez M. The relevance of dietary polyphenols in cardiovascular protection. Curr. Pharm. Des. 2017;23:2444–2452. doi: 10.2174/1381612823666170329144307. [DOI] [PubMed] [Google Scholar]
- Musaiger, A.O., 2011. Overweight and obesity in eastern mediterranean region: prevalence and possible causes. J. Obes. 2011, Article ID 407237. https://doi.org/10.1155/2011/407237. [DOI] [PMC free article] [PubMed]
- Naik E., Dixit V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath D., Heemels M.-T., Anson L. Obesity and diabetes. Nature. 2006;444 doi: 10.1038/444839a. 839 839. [DOI] [Google Scholar]
- Noronha B.T., Li J.-M., Wheatcroft S.B., Shah A.M., Kearney M.T. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54:1082–1089. doi: 10.2337/diabetes.54.4.1082. [DOI] [PubMed] [Google Scholar]
- NRC, 1995. Nutrient requirements of laboratory animals. National Research Council, National Academic Press, Washington, DC, USA.
- Nunez J., Minana G., Bodi V., Nunez E., Sanchis J., Husser O., Llàcer A. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 2011;18:3226–3233. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- Onyemaechi N.O., Anyanwu G.E., Obikili E.N., Onwuasoigwe O., Nwankwo O.E. Impact of overweight and obesity on the musculoskeletal system using lumbosacral angles. Patient Prefer Adherence. 2016;10:291–296. doi: 10.2147/PPA.S90967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M. Autophagy in obesity and atherosclerosis: Interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2013;1831:1124–1133. doi: 10.1016/j.bbalip.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Oyedepo T., Babarinde S., Ajayeoba T. Evaluation of anti-hyperlipidemic effect of aqueous leaves extract of Moringa oleifera in alloxan induced diabetic rats. Int. J. Biochem. Res. Rev. 2013;3:162–170. doi: 10.9734/IJBCRR/2013/3639. [DOI] [Google Scholar]
- Paliwal R., Sharma V., Pracheta J. A review on horse radish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance. Asian J. Biotechnol. 2011;3:317–328. doi: 10.3923/ajbkr.2011.317.328. [DOI] [Google Scholar]
- Palozza P., Simone R., Catalano A., Parrone N., Monego G., Ranelletti F.O. Lycopene regulation of cholesterol synthesis and efflux in human macrophages. J. Nutr. Biochem. 2011;22:971–978. doi: 10.1016/j.jnutbio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Park J.P., Kim J.H., Park M.K., Yun J.W. Potential agents for cancer and obesity treatment with herbal medicines from the green garden. Biotechnol. Bioproc. E. 2011;16:1065–1076. doi: 10.1007/s12257-011-0215-3. [DOI] [Google Scholar]
- Pereira B.L., Reis P.P., Severino F.E., Felix T.F., Braz M.G., Nogueira F.R., Silva R.A., Cardoso A.C., Lourenço M.A., Figueiredo A.M., Chiuso-Minicucci F., Azevedo P., Polegato B., Okoshi K., Fernandes A., Paiva S., Zornoff L., Minicucci M. Tomato (Lycopersicon esculentum) or lycopene supplementation attenuates ventricular remodeling after myocardial infarction through different mechanistic pathways. J. Nutr. Biochem. 2017;46:117–124. doi: 10.1016/j.jnutbio.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Periago M.J., Martín-Pozuelo G., González-Barrio R., Santaella M., Gómez V., Vázquez N., Navarro-González I., García-Alonso J. Effect of tomato juice consumption on the plasmatic lipid profile, hepatic HMGCR activity, and fecal short chain fatty acid content of rats. Food Funct. 2016;7:4460–4467. doi: 10.1039/c6fo00344c. [DOI] [PubMed] [Google Scholar]
- Phachonpai W., Muchimapura S., Tong-un T., Wattanathorn J., Thukhammee W., Thipkaew C., Sripanidkulchai B., Wannanon P. Acute toxicity study of tomato pomace extract in rodent. Online J. Biol. Sci. 2013;132834:28–34. doi: 10.3844/ojbssp.2013.28.34. [DOI] [Google Scholar]
- Pilz S., März W. Free fatty acids as a cardiovascular risk factor. Clin. Chem. Lab. Med. 2008;46:429–434. doi: 10.1515/CCLM.2008.118. [DOI] [PubMed] [Google Scholar]
- Piña-Zentella R.M., Rosado J.L., Gallegos-Corona M.A., Madrigal-Pérez L.A., García O.P., Ramos-Gomez M. Lycopene improves diet-mediated recuperation in rat model of nonalcoholic fatty liver disease. J. Med. Food. 2016;19:607–614. doi: 10.1089/jmf.2015.0123. [DOI] [PubMed] [Google Scholar]
- Pokorny J., Yanishlieva N., Gordon M.H. CRC Press; USA: 2001. Antioxidants in food: practical applications. [Google Scholar]
- Randriamboavonjy, J.I., Rio, M., Pacaud, P., Loirand, G., Tesse, A., 2017. Moringa oleifera seeds attenuate vascular oxidative and nitrosative stresses in spontaneously hypertensive rats. Oxid. Med. Cell Longev. 2017, Article ID 4129459. https://doi.org/10.1155/2017/4129459. [DOI] [PMC free article] [PubMed]
- Rastmanesh R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem. Biol. Interact. 2011;189:1–8. doi: 10.1016/j.cbi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Rayalam S., Della-Fera M.A., Baile C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008;19:717–726. doi: 10.1016/j.jnutbio.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Reiss D., Muraine J., Dainciart A. Transaminases in Serum. Am. J. Clin. Pathology Bull. Soc. Pharm. Bordeaux. 1965:104–173. [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Ribot J., Rodríguez A.M., Rodríguez E., Palou A. Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity. 2008;16:723–730. doi: 10.1038/oby.2008.113. [DOI] [PubMed] [Google Scholar]
- Roh C., Jung U. Screening of crude plant extracts with anti-obesity activity. Int. J. Mol. Sci. 2012;13:1710–1719. doi: 10.3390/ijms13021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild, M.A., Oratz, M., Schreiber, S.S., 1977. Albumin synthesis, In: Albumin: Structure, Function and Uses. Elsevier, pp. 227-253.
- Rusten L.S., Jacobsen F.W., Lesslauer W., Loetscher H., Smeland E., Jacobsen S. Bifunctional effects of tumor necrosis factor alpha (TNF alpha) on the growth of mature and primitive human hematopoietic progenitor cells: involvement of p55 and p75 TNF receptors. Blood. 1994;83:3152–3159. doi: 10.1182/blood.V83.11.3152.bloodjournal83113152. [DOI] [PubMed] [Google Scholar]
- Saito I. Epidemiological evidence of type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease in Japan. Circulation. 2012;76:1066–1073. doi: 10.1253/circj.CJ-11-1519. [DOI] [PubMed] [Google Scholar]
- Santos E.W., de Oliveira D.C., Hastreiter A., de Oliveira Beltran J.S., Rogero M.M., Fock R.A., Borelli P. High-fat diet or low-protein diet changes peritoneal macrophages function in mice. Nutrire. 2016;41:6. doi: 10.1186/s41110-016-0006-x. [DOI] [Google Scholar]
- Santos E.W., Oliveira D.C., Hastreiter A., Silva G.B., Beltran J.S.D.O., Rogero M.M., Fock R.A., Borelli P. Short-term high-fat diet affects macrophages inflammatory response, early signs of a long-term problem. Braz. J. Pharm. Sci. 2019;55 doi: 10.1590/s2175-97902019000117561. [DOI] [Google Scholar]
- Saucillo D.C., Gerriets V.A., Sheng J., Rathmell J.C., MacIver N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Aldred A.R., Thomas T., Birch H.E., Dickson P.W., Guo-Fen T., Heinrich P.C., Northemann W., Howlett G.J., De Jong F.A. Levels of messenger ribonucleic acids for plasma proteins in rat liver during acute experimental inflammation. Inflammation. 1986;10:59–66. doi: 10.1007/BF00916041. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Urban J., Edwards K., Dryburgh H., Inglis A.S. Mechanism and regulation of albumin synthesis in liver and hepatomas. Adv. Enzyme Regul. 1976;14:163–184. doi: 10.1016/0065-2571(76)90012-1. [DOI] [PubMed] [Google Scholar]
- Shawky S.M. Effect of Short-Term High Fat Diet Inducing Obesity on Hematological, Some Biochemical Parameters and Testicular Oxidative Stress in Male Rats. J. Adv. Vet. Res. 2015;5:151–156. [Google Scholar]
- Shiri R., Karppinen J., Leino-Arjas P., Solovieva S., Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am. J. Epidemiol. 2009;171:135–154. doi: 10.1093/aje/kwp356. [DOI] [PubMed] [Google Scholar]
- Siasos G., Tousoulis D., Tsigkou V., Kokkou E., Oikonomou E., Vavuranakis M., Basdra E., Papavassiliou A., Stefanadis C. Flavonoids in atherosclerosis: an overview of their mechanisms of action. Curr. Med. Chem. 2013;20:2641–2660. doi: 10.2174/0929867311320210003. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Sobczak, A.I.S., Blindauer, C.A., Stewart, A.J., 2019. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 11, pii: E2022. https://doi.org/10.3390/nu11092022. [DOI] [PMC free article] [PubMed]
- Spiegelman B.M., Flier J.S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Spieker L., Noll G., Ruschitzka F., Maier W., Lüscher T. Working under pressure: the vascular endothelium in arterial hypertension. J. Hum. Hypertens. 2000;14:617–630. doi: 10.1038/sj.jhh.1001012. [DOI] [PubMed] [Google Scholar]
- Stahl W., Schwarz W., Sundquist A.R., Sies H. Cis-trans isomers of lycopene and β-carotene in human serum and tissues. Arch. Biochem. Biophys. 1992;294:173–177. doi: 10.1016/0003-9861(92)90153-N. [DOI] [PubMed] [Google Scholar]
- Stumm M.M., D'Orazio D., Sumanovski L.T., Martin P.Y., Reichen J., Sieber C.C. Endothelial, but not the inducible, nitric oxide synthase is detectable in normal and portal hypertensive rats. Liver. 2002;22:441–450. doi: 10.1034/j.1600-0676.2002.01653.x. [DOI] [PubMed] [Google Scholar]
- Suwa T., Hogg J.C., English D., Van Eeden S.F. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2954–H2960. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- Tanaka S.-I., Isoda F., Yamakawa T., Ishihara M., Sekihara H. T lymphopenia in genetically obese rats. Clin. Immunol. Immunopathol. 1998;86:219–225. doi: 10.1006/clin.1997.4467. [DOI] [PubMed] [Google Scholar]
- Taverna M., Marie A.-L., Mira J.-P., Guidet B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care. 2013;3:4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejas G., Umang J., Payal B., Tusharbinu D., Pravin T. A panoramic view on pharmacognostic, pharmacological, nutritional, therapeutic and prophylactic values of Moringa oleifera Lam. Int. Res. J. Pharm. 2012;3:1–7. [Google Scholar]
- Thoenes M., Reil J.-C., Khan B.V., Bramlage P., Volpe M., Kirch W., Böhm M. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc. Health Risk Manag. 2009;5:577–585. doi: 10.2147/VHRM.S5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma A., Makonnen E., Debella A., Tesfaye B. Antihyperglycemic effect on chronic administration of butanol fraction of ethanol extract of Moringa Stenopetala leaves in alloxan induced diabetic mice. Asian Pac. J. Trop. Biomed. 2012;2:S1606–S1610. doi: 10.1016/S2221-1691(12)60461-4. [DOI] [Google Scholar]
- Udomkasemsab A., Ngamlerst C., Kwanbunjun K., Krasae T., Amnuaysookkasem K., Chunthanom P., Prangthip P. Maoberry (Antidesma bunius) improves glucose metabolism, triglyceride levels, and splenic lesions in high-fat diet-induced hypercholesterolemic rats. J. Med. Food. 2019;22:29–37. doi: 10.1089/jmf.2018.4203. [DOI] [PubMed] [Google Scholar]
- Ulich T.R., del Castillo J., Guo K. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989;73:108–110. [PubMed] [Google Scholar]
- Van Gaal L., Dirinck E. Pharmacological approaches in the treatment and maintenance of weight loss. Diabetes Care. 2016;39:S260–S267. doi: 10.2337/dcS15-3016. [DOI] [PubMed] [Google Scholar]
- Velmurugan B., Santhiya S.T., Nagini S. Protective effect of S-allylcysteine and lycopene in combination against N-methyl-N'-nitro-N-nitrosoguanidine-induced genotoxicity. Pol. J. Pharmacol. 2004;56:241–246. [PubMed] [Google Scholar]
- Vergara-Jimenez M., Almatrafi M., Fernandez M. Bioactive components in Moringa Oleifera leaves protect against chronic disease. Antioxidants. 2017;6:91. doi: 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viuda-Martos M., Sanchez-Zapata E., Sayas-Barberá E., Sendra E., Pérez-Álvarez J., Fernández-López J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: a review. Crit. Rev. Food Sci. Nutr. 2014;54:1032–1049. doi: 10.1080/10408398.2011.623799. [DOI] [PubMed] [Google Scholar]
- Vucenik I., Stains J.P. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M., Diepvens K., Joosen A.M., Bérubé-Parent S., Tremblay A. Metabolic effects of spices, teas, and caffeine. Physiol. Behav. 2006;89:85–91. doi: 10.1016/j.physbeh.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Westfall R.J., Hauge S.M. The nutritive quality and the trypsin inhibitor content of soybean flour heated at various temperatures. J. Nutr. 1948;35:379–389. doi: 10.1093/jn/35.3.379. [DOI] [PubMed] [Google Scholar]
- Wheeler C.R., Salzman J.A., Elsayed N.M., Omaye S.T., Korte D.W., Jr Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990;184:193–199. doi: 10.1016/0003-2697(90)90668-y. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Health Communications: 2000. The Asia-Pacific perspective: redefining obesity and its treatment, Sydney. [Google Scholar]
- WHO, 2011. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. World Health Organization.
- Xia H.M., Wang J., Xie X.J., Xu L.J., Tang S.Q. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. Int. J. Molec. Med. 2019;44:1523–1530. doi: 10.3892/ijmm.2019.4285. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang Y., Jiang W.-W., Luo X.-F., Dai T.-Y., Peng L., Song S., Tao L., Shi C.-Y., Hao R.-S., Xiao R., Tian Y., Sheng J. Moringa oleifera leaf petroleum ether extract inhibits lipogenesis by activating the AMPK signaling pathway. Front. Pharmacol. 2018;9:1447. doi: 10.3389/fphar.2018.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-R., Zou Z.-Y., Huang Y.-M., Xiao X., Ma L., Lin X.-M. Serum carotenoids in relation to risk factors for development of atherosclerosis. Clin. Biochem. 2012;45:1357–1361. doi: 10.1016/j.clinbiochem.2012.07.101. [DOI] [PubMed] [Google Scholar]
- Yagi, K., 1998. Free Radicals and Antioxidant Protocols. 108: 101-106. activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism. Cell Death Dis. 8, e2545. [DOI] [PMC free article] [PubMed]
- Yanishlieva N., Heinonen I. Sources of natural antioxidants: vegetables, fruits, herbs, spices and teas. Antioxidant in Food: Pract. Appl. Elsevier. 2001:210–249. [Google Scholar]
- Yefsah-Idres A., Benazzoug Y., Otman A., Latour A., Middendorp S., Janel N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016;7:2862–2869. doi: 10.1039/c6fo00095a. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G., DeVore A., Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J. Am. Acad. Dermatol. 2007;56:901–916. doi: 10.1016/j.jaad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zammit C., Liddicoat H., Moonsie I., Makker H. Obesity and respiratory diseases. Int. J. Gen. Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]