Abstract
Extracellular vesicles (EVs) are lipid bilayered compartments released by virtually all living cells, including fungi. Among the diverse molecules carried by fungal EVs, a number of immunogens, virulence factors and regulators have been characterized. Within EVs, these components could potentially impact disease outcomes by interacting with the host. From this perspective, we previously demonstrated that EVs from C. albicans could be taken up by and activate macrophages and dendritic cells to produce cytokines and express costimulatory molecules. Moreover, pre-treatment of Galleria mellonella larvae with fungal EVs protected the insects against a subsequent lethal infection with C. albicans yeasts. These data indicate that C. albicans EVs are multi-antigenic compartments that activate the innate immune system and could be exploited as vaccine formulations. Here we investigated whether immunization with C. albicans EVs induces a protective effect against murine candidiasis in immunosuppressed mice. Total and fungal antigen-specific serum IgG antibodies increased by 21 days after immunization, confirming the efficacy of the protocol. Vaccination decreased fungal burden in the liver, spleen and kidney of mice challenged with C. albicans. Splenic levels of cytokines indicated a lower inflammatory response in mice immunized with EVs when compared with EVs+Freund’s adjuvant (ADJ). Higher levels of IL-12p70, TNFα and IFNγ were detected in mice vaccinated with EVs+ADJ, while IL-12p70, TGFβ, IL-4 and IL-10 were increased when no adjuvants were added. Full protection of lethally challenged mice was observed when EVs were administered, regardless the presence of adjuvant. Physical properties of the EVs were also investigated and EVs produced by C. albicans were relatively stable after storage at 4, −20 or −80 °C, keeping their ability to activate dendritic cells and to protect G. mellonella against a lethal candidiasis. Our data suggest that fungal EVs could be a safe source of antigens to be exploited in vaccine formulations.
Keywords: Extracellular vesicles, Candida albicans, vaccines, fungal pathogenesis
INTRODUCTION
Candida albicans is regularly found as part of the human microbiota, colonizing the oral cavity, skin, and the gastrointestinal and genitourinary tracts (Iliev and Leonardi, 2017). Nonetheless, it is also one of the major opportunistic fungal pathogens, causing superficial cutaneous-mucosa (oral and vaginal) and life threatening disseminated infections (Kim and Sudbery, 2011; Gow et al., 2012). This species is the most frequent pathogen causing recurrent vulvovaginal candidiasis (RVVC), affecting over 370 million woman during their lifetime (Denning et al., 2018). In addition, C. albicans causes a systemic disease in immunocompromised patients, the so-called invasive candidiasis (Pappas et al., 2018a). Oral and vulvovaginal candidiasis (VVC) in patients that are not critically ill are usually treated with azole derivatives, such as fluconazole (Sobel, 2016). However, echinocandins recently became the initial choice for treatment of RVVC and systemic candidiasis (Cornely et al., 2012b; Pappas et al., 2016b; Bassetti et al., 2018b). Amphotericin B is also recommended to combat systemic candidiasis, but only when azoles and echinocandins are limited by intolerance, resistance or unavailability (Cornely et al., 2012a; Pappas et al., 2016a). Despite the recent changes and advances in treatment, systemic candidiasis remains associated with high mortality rates (Pappas et al., 2018b; Bassetti et al., 2018a). Moreover, there are reports of increasing numbers of C. albicans strains resistant to current antifungal drugs (Fisher et al., 2018; Pappas et al., 2018b). Thus, the development of alternative therapeutic strategies and prophylactic tools is imperative.
Considering that systemic candidiasis is usually associated with altered immune status, such as neutrophil or CD4+ T cells immunodeficiencies, adjunctive therapies that stimulate effector functions in the immune response are potential alternatives (Cassone and Rappuoli, 2010; Cassone, 2013; Kullberg BJ, van de Veerdonk F, 2014; Scriven et al., 2017). This stimulation should be specially effective when the pre-existing immunity is modified or strongly reduced by immunosuppressing conditions (Cassone, 2013). In this context, live attenuated strains, cytoplasm and cell wall extracts as well as purified proteins and conjugated polysaccharides have been explored in vaccine formulations to control disseminated candidiasis in murine models (Vilanova et al., 2004a; Thomas et al., 2006b; Wu et al., 2007; Raska et al., 2008b; Saville et al., 2009; Li et al., 2011a; Ahmad et al., 2012; De Bernardis et al., 2012; Sui et al., 2017a). Although attenuated strains have provided full protection (Saville et al., 2009) they are particularly risky under conditions of limited immune response. Immunization with cell wall (Thomas et al., 2006a) and cytoplasmic (Ahmad et al., 2012) fungal extracts are protective against murine candidiasis, but the methods of extraction and the batch-to-batch differences are problems that have yet to be circumvented. In addition, it is likely that the methods used for antigen extraction from the cell wall promote changes the native structure of the immunogens. For instance, native cryptococcal glucuronoxylomannan is biologically different from the detergent-extracted polysaccharide (Frases et al., 2008). A number of univalent vaccines, formulations carrying a single antigen, have been investigated in mice models and two of them tested in human clinical trials (Vilanova et al., 2004b; Raska et al., 2008a; Li et al., 2011b; De Bernardis et al., 2012; Sui et al., 2017b). However, according to Cassone (Cassone and Rappuoli, 2010; Cassone, 2013), the use of univalent formulations would be able to induce protection limited to specific body niches sites. Indeed, the use of univalent vaccine formulations seems to protect mice and humans in VVC and RVVC, but not against disseminated candidiasis (Cassone, 2013). Thus, the development of a safe and well-designed multivalent vaccine formulation could promote protection to different targets and against disseminated candidiasis. The major limitations include the elevated cost of development and the ability to determine the perfect combination of antigens.
Recent studies suggest that fungal extracellular vesicles (EVs) may represent a new alternative for the development of multivalent vaccine formulations (Rodrigues et al., 2014; Joffe et al., 2016; Nimrichter et al., 2016; Rizzo et al., 2017; Colombo et al., 2019). Fungal EVs were isolated in 2007 from the culture supernatant of Cryptococcus neoformans (Rodrigues et al., 2007a). Since then, fungal EVs have been characterized in several fungal species including C. albicans, Candida glabrata, Paracoccidioides brasiliensis, Sporothrix brasiliensis, Cryptococcus gattii, Histoplasma capsulatum, Saccharomyces cerevisiae, Pichia fermentans, Malassezia sympodialis, Alternaria infectoria and Aspergillus fumigatus (Albuquerque, 2004; Oliveira et al., 2010b; Vallejo et al., 2011; Silva et al., 2014; Vargas et al., 2015; Rayner et al., 2017; Bielska et al., 2018; Ikeda et al., 2018; Leone et al., 2018; Souza et al., 2019b). As multi-antigenic compartments, fungal EVs carry a number of native structures such as proteins, pigments, polysaccharides, lipids and nucleic acids (Rodrigues et al., 2007a; Rodrigues et al., 2008; Eisenman et al., 2009; Vallejo et al., 2011; Vargas et al., 2015; Da Silva et al., 2015; Nimrichter et al., 2016). Some of these components are conserved among the different species, but others are species-specific (Nimrichter et al., 2016). EVs isolated from pathogens contain a diverse array of virulence factors and regulators as well as highly immunogenic components that could directly contribute to disease development. In vitro, these EVs are able to stimulate macrophages and dendritic cells (Vargas et al., 2015; Da Silva et al., 2016; Zamith-Miranda et al., 2018). They regulate cytokine production in phagocytes, macrophage polarization and the expression of co-stimulatory molecules in dendritic cells (DCs) (Vargas et al., 2015). In addition, pre-treatment of Galleria mellonella larvae with fungal EVs stimulated a protective response against a lethal challenge with C. albicans or C. neoformans (Vargas et al., 2015; Colombo et al., 2019). In other models, the exposure of host cells to fungal EVs have been also associated with disease development, as demonstrated for C. neoformans and S. brasiliensis (Huang et al., 2012; Ikeda et al., 2018). In addition, fungal EVs seem to participate in the development of antifungal resistance and as a messenger compartment for virulence transference (Bielska et al., 2018; Mitchell et al., 2018).
Based on the composition and biological activities of C. albicans EVs we investigated whether these compartments could be used as a multivalent antigenic vaccine formulation in a lethal murine candidiasis model. We initially developed an intraperitoneal prime-boost immunization murine model using C. albicans EVs and then evaluated total and specific serum immunoglobulin levels, fungal burden in different tissues, cytokine production in the spleen and mice survival during a lethal challenge with yeasts of C. albicans. Then we tested the stability and morphological properties of fungal EVs stored at low temperatures using dynamic light scattering and transmission electron microscopy. Stored EVs were tested according to their ability to activate murine bone-marrow derived DCs and protect G. mellonella larvae challenged with a lethal inoculum of C. albicans yeasts. Our results demonstrated that fungal EVs are stable formulations with the potential to combat candidiasis.
MATERIAL AND METHODS
Culture of fungal cells.
C. albicans strain 11 is a clinical bloodstream isolated from a 46-year-old male patient, kindly provided by Dr. Marcos Dornelas (Laboratory of Microbiology and Mycology, State Institute of Hematology Arthur de Siqueira Cavalcanti, HemoRio). The strain was stored in solid Sabouraud/glycerol agar medium and maintained at −80 °C. Yeast cells were cultured in liquid Sabouraud for 48 hours at 30 °C under agitation (150 rpm).
Preparation of fungal EVs (Vargas et al., 2015).
Fungal EVs were isolated from C. albicans culture supernatants. Yeasts were inoculated in a 100 mL Erlenmeyer flask containing 20 mL of liquid Sabouraud medium and cultured under agitation (150 rpm) at room temperature for 48 hours. The pre-inoculum was then transferred to a 1000 ml Erlenmeyer flask containing 400 ml of liquid Sabouraud medium and cultivated under the same conditions. The yeasts were separated from the culture supernatant according to the protocol previously described in our laboratory (Vargas et al., 2015). The whole preparation was developed at 4 °C. Briefly, the culture was centrifuged at 4,000 x g for 15 minutes. The supernatants were collected and further centrifuged at 15,000 x g for 15 minutes to remove cell debris. Residual cells and debris were removed after a step of filtration using a 0.8 μm membrane filter (Merck Millipore). The cell-free supernatant was concentrated about 25-fold using an Amicon ultrafiltration system (100 kDa membrane). The concentrated supernatant was then centrifuged at 100,000 x g for 1 hour. The pellet was washed twice with 0.1 M phosphate-buffered saline (PBS) pH 7.4. Fungal EVs were suspended in PBS and aliquots were plated onto Sabouraud agar plates to confirm the absence of any contaminant, confirmed by no colony observation. Quantification of EVs was developed using the quantitative Amplex Red Sterol Assay Kit (Invitrogen) and the BCA Protein Assay Kit (ThermoFisher). Quality control of EVs preparation was performed by dynamic light scattering (DLS) as described below (Vargas et al., 2015).
Detection of antibody classes.
Serum pool of five animals was used for antibody detection. For IgM and global IgG analysis, goat anti-mouse IgM or IgG (SouthernBiotech, AL), 1 μg/mL diluted in PBS, were pre-immobilized to half-area-high binding 96 well ELISA plates, following incubation at 4 °C overnight. Afterwards, the plates were subjected to four washes with PBS and blocked with PBS containing 1% bovine serum albumin (BSA) for 1 hour and 30 minutes at room temperature. BSA was removed and the plates were washed with PBS, serum samples were diluted 1:100 in PBS-1% BSA and serially three-fold and five-fold diluted to determine the concentration of IgM and IgG, respectively. IgM and IgG (1 μg/mL) diluted 1: 3 and 1: 5, respectively, were used as the standard curve. The samples were incubated at 4 °C overnight. Plates were washed four times with PBS and incubated with 50 μL of goat anti-mouse IgM (1:4000) and IgG (1:8000) (SouthernBiotech, AL) conjugated to peroxidase (HRP) diluted in PBS 1% BSA and incubated at room temperature for 1 h 30 min. At the end, the plates were again subjected to four washes with PBS and then developed with TMB (3,3′, 5,5;-tetramethylbenzidine) (Thermo Fisher); the reaction was stopped with 50 μL of 3 N HC1 and the reading performed on a 450 and 650 nm filter microplate reader (iMark, BioRad, US).
Protein extraction.
To extract proteins from C. albicans an adaptation of the method used by Dojnov and colleagues was used (Dojnov et al., 2007). Briefly, C. albicans yeasts (108 cells) were suspended in 1 mL of lysis buffer (0.06 M Tris HC1, pH 6, 10% glycerol, 5% 2-mercaptoethanol) and the suspension was submitted to 6 cycles of 1 min in ultrasonic bath (22 KHZ) alternating with 30 sec pauses. Suspensions were centrifuged at 5,000xg for 15 min and the supernatants collected. The cells were suspended in the same lysis buffer and transferred to 2 mL tubes containing glass beads (0.5-0.75 mm) (1:3 v/v). The cells were disrupted using a Thermo Savant Fastprep FP120 device using 10 cycles of 2 min, with 2 min alternating pauses at 4 °C. The samples were then centrifuged at 5,000 xg-for 15 min. The supernatants (cytosolic proteins) were saved and the pellets, containing the cell wall antigens, were suspended in lysis buffer supplemented with 2% SDS and boiled for 2 min at 100 °C for protein extraction. After centrifugation under the same conditions described above all the supernatants were combined and the pellets discarded. Total protein was quantified using the BCA protein quantification kit (Pierce, US).
Detection of antibody subclasses.
Serum pooled from five animals was used for antibody detection. For IgG subclass determination, 50 μl/well of antigens (EVs, proteins from C. albicans yeasts and BCG) were added to half-area-high binding 96 well ELISA plates in a final concentration of 10 μg/ml and the samples were incubated overnight incubation at 4 °C. The wells were subjected to four washes with PBS and blocked with PBS containing 1% BSA for 90 minutes at room temperature. Serum samples were diluted 1:40; 1:120; 1:360 and 1:1080 in PBS-1% BSA and then added to the wells. The plates were incubated at 4 °C overnight, followed by four more washing steps with PBS. A final volume of 50 μL of the subclasses reporter antibodies (anti-IgG1, IgG2a, IgG2b and IgG3, diluted 1:1000 in PBS 1% BSA) conjugated to HRP were added to the wells and incubated for 90 min at room temperature. The wells were again subjected to four washes with PBS and then developed with TMB; the reaction was stopped with 50 μL of 3 N HC1 and read on a 450 and 650 nm filter microplate reader (iMark, BioRad, US).
Mouse model.
Female BALB/c mice (6-8 weeks, 11 animals per group) were immunized intraperitoneally four times, one week apart, with 200 μL of an EV suspension at a final sterol concentration of 10 μM in PBS, formulated or not with Freund’s adjuvant (ADJ) (Sigma-Aldrich) diluted 1:1 (v/v) in the EV suspension (fresh preparations kept at 4 °C up to 36 hours). PBS and PBS-Adjuvant (ADJ) (Sigma-Aldrich) were included as controls. Four days after the third boost the animals were immunosuppressed with cyclophosphamide (200 mg/kg) intraperitoneally. Under these conditions, cyclophosphamide induces mice neutropenia with maximum effect between days 3 and 4 (Segal E, 1981). Neutropenia is usually restored 10 post-administration. Three days later, the animals were inoculated intraperitoneally with a lethal inoculum of C. albicans (3x107 yeasts per mouse). Blood was collected at days 0, 7, 14, 21, 25 and 28 days. The blood was centrifuged at 10,000 x g for 10 min, and the serum (upper phase) was collected and stored at −80 °C for further analysis. All mice were treated according to the ethical guidelines for animal experimentation (Protocol 01200.001568/2013-17, approved in 2019 by CEUA, UFRJ). The number of deaths was evaluated daily. Survival curves were plotted, and statistical analyses were performed using the log—rank (Mantel—Cox) survival test.
Fungal burden and cytokine measurements.
Following the immunizations and immunosupression, three and five days after fungal infection mice were euthanized and the organs (kidney, liver and spleen) excised, weighted and homogenized in 5 ml of PBS for analysis of the fungal load by the enumeration of colony forming units (CFU) and determination of the cytokines profile. The CFU were determined after plating successive dilutions of organs homogenate onto Sabouraud agar and incubation at 30 °C for 48h. Homogenates were also used for the measurement of cytokines. The cytokines TNF-α, IL-12, IL-10, TGF-β, IL-4, IL-6 and INF-γ were quantified using commercial kits from eBioscience (US) according to the manufacturer’s protocols. Statistical analysis was performed using one-way analysis of variance (ANOVA), and the difference between groups were analyzed by Tukey post test.
EVs stability at low temperatures.
To investigate the stability of the fungal EVs, 200 μL aliquots (final concentration of 10 μM based on sterol content) were stored at distinct temperatures; 4 °C, −20 °C and −80 °C. After 7 days, the aliquots were thawed at room temperature and then their dimensions were analyzed by two independent approaches. First, EVs were analyzed using dynamic light scattering (DLS) in a 90Plus / BIMAS Multi-Angle Particle Size Analyzer (Brookhaven Instruments) as described (Vargas et al., 2015). In addition, the EVs were submitted to NTA on an LM10 nanoparticle analysis system, coupled with a 488-nm laser and equipped with an SCMOS camera and a syringe pump (Malvern Panalytical, Malvern, United Kingdom) as detailed by Reis and colleagues (Reis et al., 2019). All samples were 700-fold diluted in filtered PBS. Samples were injected using a syringe pump speed of 100, and three videos of 60 s duration captured per sample, with the camera level set to 15, gain set to 3, and viscosity set to that of water (0.954 to 0.955 cP). For data analysis, the gain was set to 10 to 15 and the detection threshold was set to 2 to 3 for all samples. Levels of blur and maximum jump distance were automatically set. The data were acquired and analyzed using the NTA 3.0 software (Malvern Panalytical). Finally, EVs were visualized by transmission electron microscopy (TEM) using a negative staining technique. Briefly, the EVs suspension was adhered to 200-mesh copper grids previously treated with Formvar 0.3% and discharged at PELCO easiGlow™ Glow Discharge Cleaning System. The EVs were then negatively contrasted with 1% (m/v) uranyl acetate. The micrographs were performed in an JEOL 1200EX 80 kV electronic microscope. A fresh preparation of EVs was used as control for DLS, NTA and EM analyses.
Cytokine production by dendritic cells treated with fungal EVs.
To investigate whether storage would impact fungal EVs ability to activate host cells, bone marrow-derived DCs were treated with pre-stored EVs and the levels of IL-6 and IL-10 evaluated. Bone marrow-derived DCs were obtained from femur and tibia of BALB/c female mice (4 to 12 weeks old) following the protocol established by Lutz et al (Lutz et al., 1999). Briefly, femur and tibia of BALB/c mice (female), aged 4 to 12 weeks, were removed. After removal of the musculature and tendons, the intact bones were left in 70% ethanol for 2 to 5 minutes for disinfection followed by washing with sterile PBS. Both ends were sheared with scissors and the bone-marrow washed with Hanks’ medium containing 2% FBS using a syringe with a 0.45 mm diameter needle. The supernatant was then centrifuged for 5 minutes at 1500 rpm and suspended in 2 mL of RPMI and 10% FBS. Cells were counted in Neubauer’s chamber and plated at the final suspensions of 106 cells in 10 ml of RPMI 1640 (Roswell Park Memorial Institute) medium supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM MEM (“Minimal Essential Medium”) nonessential amino acids, 50 μM 2-β-mercaptoethanol and 20 ng/mL rGM-CSF (recombinant granulocyte macrophage - colony stimulating factor) at 37 °C and 5% CO2. Cells were then incubated for 3 days at 37 °C and 5% CO2. On day 2, 10 mL of RPMI supplemented with 10% FBS and 20 ng/mL rGM-CSF was added. On day 10 the cells were washed in RPMI medium supplemented with 10% FBS, counted and plated at 105 cells per well in 96-well plate. Bone-marrow derived DCs were then incubated with C. albicans EVs (at final concentration of 10 μM sterol per well) and incubated overnight under the same conditions. The supernatants were collected and the cytokines IL-6 and IL-10 were determined by enzyme-linked immunosorbent assay as recommended by the manufacturer eBioscience (US). Lipopolysaccharide (LPS) (Sigma) (1 μg/mL) and PBS were used as a positive and negative control respectively. Statistical analysis was performed using one-way analysis of variance (ANOVA), and the difference between groups were analyzed by Tukey post test.
Galleria mellonella infection.
G. mellonella larvae in the final instar larval stage were selected according to similarity in weight (0.10—0.15 g). Larvae (20 per group) were inoculated with 10 μL of EV suspensions (10 μM per insect, based on sterol quantification) using an 30G insulin syringe into the haemocoel through the last proleg as described by Brennan et al. (Brennan et al., 2002). The same volume of PBS was used as negative control. The larvae were then placed in sterile Petri dishes and kept in the dark at 37 °C for two days. Subsequently, all larvae were inoculated with 10 μL of a suspension containing 2 × 105 yeasts of C. albicans (strain 11). A control group received only the EVs to determine whether these compartments are toxic to the larvae. Larvae were kept under the same conditions above and their mortality was monitored by checking twice daily. Death was assessed by the lack of movement in response to stimulation. Survival curves were plotted, and statistical analyses were performed using the log-rank (Mantel–Cox) survival test and displayed results represent the mean percentage survival of larvae from all assays.
Statistics.
Statistical analyses were performed with the GraphPad 5 software (La Jolla, CA). Group comparisons were submitted to one-way analysis of variance (ANOVA) with Bonferroni or Tukey post test, according to each experiment. Survival analysis were performed by Log-rank (Mantel-Cox) test.
RESULTS
Fungal EVs from C. albicans activate the humoral immune response.
To investigate the ability of C. albicans EVs to stimulate the humoral response we carried out a prime-and-boost immunization protocol using EVs stored at 4°C, the same conditions used for all the studies previously performed in our laboratory (Oliveira et al., 2010a; Vargas et al., 2015). Figure 1 shows our vaccination strategy. We first aimed to determine the number of immunizations required to induce antibody production in mice. Prior to the first immunization step, serum from all mice was collected. Then EVs, EVs + ADJ, ADJ or PBS were inoculated intraperitoneally, and the serum collected before each of the three additional boosts to follow up the production of IgM and IgG. We detected an increase of total IgM one week after the first immunization only with EVs + ADJ (day 7) (Figure 2A). However, total IgG increased after the third immunization (day 21), for all groups except for the PBS treated mice (control) (Figure 2A). Based on these results we decided to immunize the mice four times before infection.
Figure 1 – Experimental design.
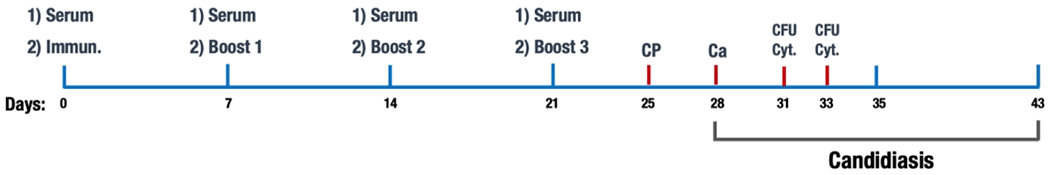
The strategy used to immunize and infect the mice was represented as a function of time. All treatments were intraperitoneal. First immunization was given on day 1 right after serum collection, followed by three boosts and cyclophosphamide treatment (CP). Three days after CP treatment the mice were infected with C. albicans. Colony forming units (CFU) and cytokines (Cyt) were measured three and five days after infection.
Figure 2-. Total and fungal antigen-specific IgM and IgG production in mice immunized with EVs from C. albicans.
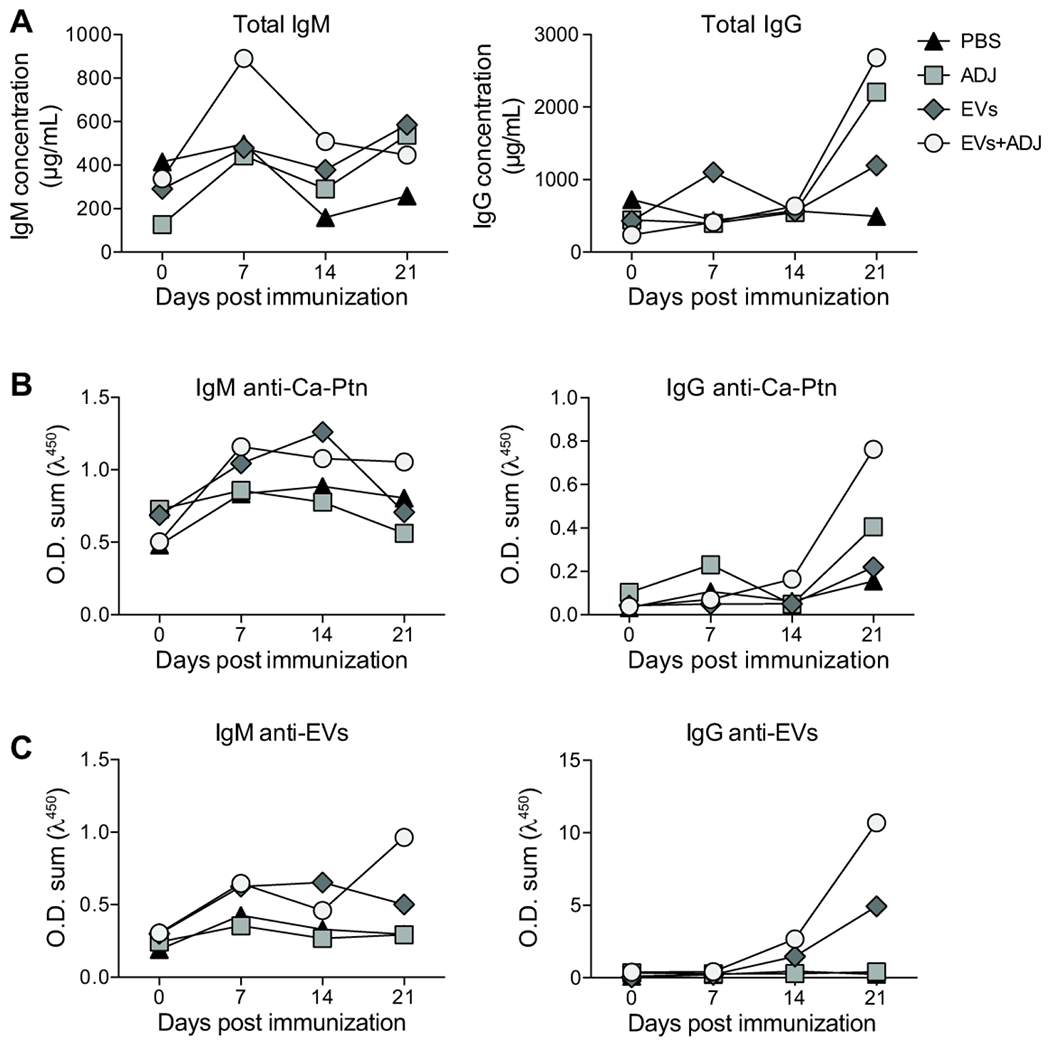
Mice were vaccinated intraperitoneally with EVs from C. albicans with (EVs+ADJ) or without (EVs) complete Freund’s adjuvant (ADJ) four times as described in Figure 1. ADJ and PBS were used as controls. Total IgM and IgG (A) were determined. C. albicans protein extract (Ca-Ptn) and EVs were used to detect specific IgM (B) and IgG (C). These data represent one of two consistent experiments.
We then investigated the specificity of the IgM and IgG produced after immunization against C. albicans protein extract (Ca-Ptn, Figure 2B) and EVs (and Figure 2C). In general, we observed a slight increase in IgM production against both Ca-Ptn and EVs at day 7, with low variation during later periods. Similar to serum IgG concentration, a significant increase of IgG anti-Ca-Ptn and EVs at day 21 was observed in mice immunized with EVs + ADJ and EVs. The predominant IgG classes reactive to Ca-Ptn were IgG1 from mice immunized with EVs + ADJ. IgG1 was also the major class recognizing EVs antigens when mice were immunized with EVs + ADJ or EVs (Figure 1). In addition, the increased reactivity of IgG antibodies from mice immunized with EVs + ADJ was highly associated with BCG antigens present in the ADJ (Figure S1).
Vaccination with EVs decreased fungal burden in mice tissues.
To determine whether treatment with C. albicans EVs can control C. albicans dissemination in immunosuppressed mice, the immunization protocol was followed by a single dose of cyclophosphamide (CP) (Figure 1) and two days later the mice were infected intraperitoneally with a lethal inoculum of C. albicans yeast cells. Three- and five-days post-infection, fungal burden in the kidney, spleen and liver were determined (Figure 3). Immunization with fungal EVs and EVs + ADJ significantly decreased the CFU in all organs, suggesting a protective effect. When the CFU was compared between the formulations containing EVs the presence of adjuvants decreased the CFU at day 3 in spleen and liver, and at day 5 in all organs.
Figure 3-. Vaccination with C. albicans EVs reduces fungal burden.
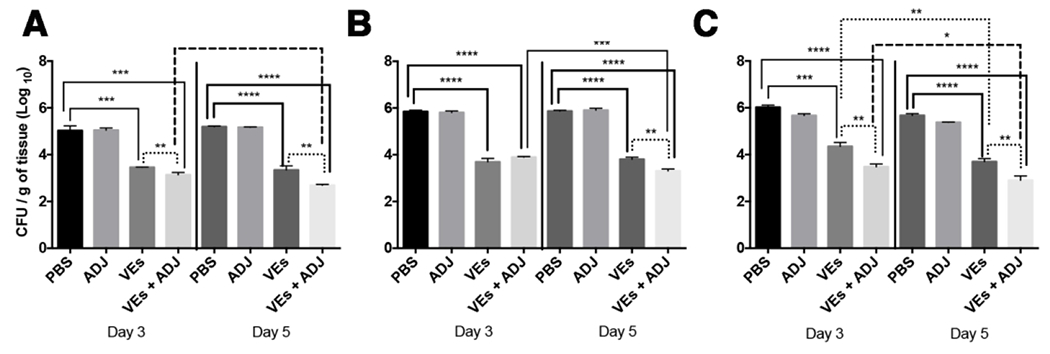
Mice were immunized with C. albicans EVs, immunosuppressed with cyclophosphamide (CP) and then challenged with a lethal inoculum of C. albicans yeasts. Figure shows colony forming units (CFU) in spleen (B), kidney (C) and liver (D) are showed. Results are representative of two independent experiments. Statistical analysis was performed using one-way analysis of variance, and the difference between groups were analyzed by Tukey post test. * p<0.05; ** p<0.001; *** p<0.002 and **** p<0.0001.
Immunization with C. albicans EVs induces different profiles of cytokines.
In order to better understand the impact of immunization with fungal EVs during the immune response we also measured the cytokines produced in the spleens of mice at different time-points after infection (3 and 5 days). Mice vaccinated with fungal EVs produced higher of IL-12p70 at days 3 and 5, while treatment with EVs + ADJ displayed substantially high levels for this cytokine at day 3, returning to levels similar to EVs stimulation at day 5 (Figure 4A). Also, day 3 demonstrated an increase of TNF-α production when mice were immunized with EVs and EVs + ADJ (Figure 4B). After 5 days all conditions showed an increased production of TNFα, but only EVs + ADJ displayed a statistically significant raise (Figure 4B, p<0,05). Remarkably, only spleens from mice immunized with EVs showed a higher level of IL-10 (day 3), TGF-β (days 3 and 5) and IL-4 (day 3) (Figure 4C, D and E). The cytokines IFN-γ and IL-6 were not detected after 3 days of infection; however, both increased for all mice after 5 days. A higher and significant level was observed for both cytokines only when fungal EVs + ADJ were used as a vaccine formulation (Figure 4F and G).
Figure 4 -. Vaccination with C. albicans EVs modified the cytokine production in response to C. albicans.
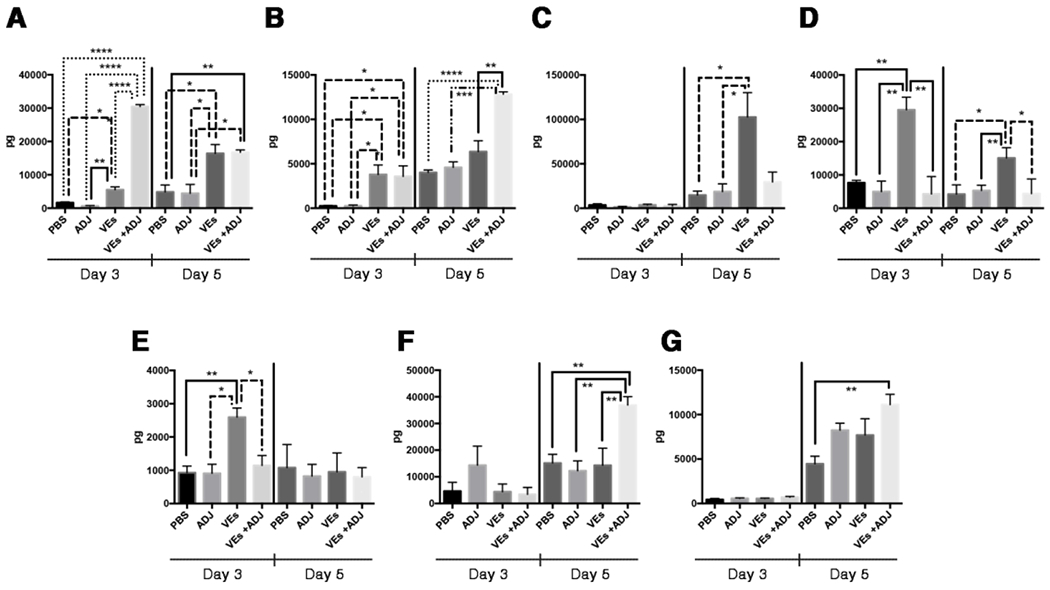
Mice were immunized, immunosuppressed and infected with C. albicans yeasts as described in Figure 5. After three and five days of infection mice were euthanized, and the spleen was excised for cytokine determination. (A) IL12p70, (B) TNFα, (C) IL-10, (D) TGFβ, (E) IL-4, (F) IFNγ and (G) IL-6 were measured. Results are representative of two independent experiments. Statistical analysis was performed using one-way analysis of variance, and the difference between groups were analyzed by Tukey post test. * p<0.05; ** p<0.001; *** p<0.002 and **** p<0.0001.
Immunization with EVs protected mice against a lethal infection with C. albicans
After a lethal infection with C. albicans yeast cells, all non-immunized or ADJ-immunized mice died by 15 days of infection (Figure 5). However, immunization with EVs alone or EVs-ADJ formulations induced full protection, with all mice surviving until the end of the experiments (p<0.05).
Figure 5-. Vaccination with C. albicans EVs induced protection against disseminated candidiasis in mice.
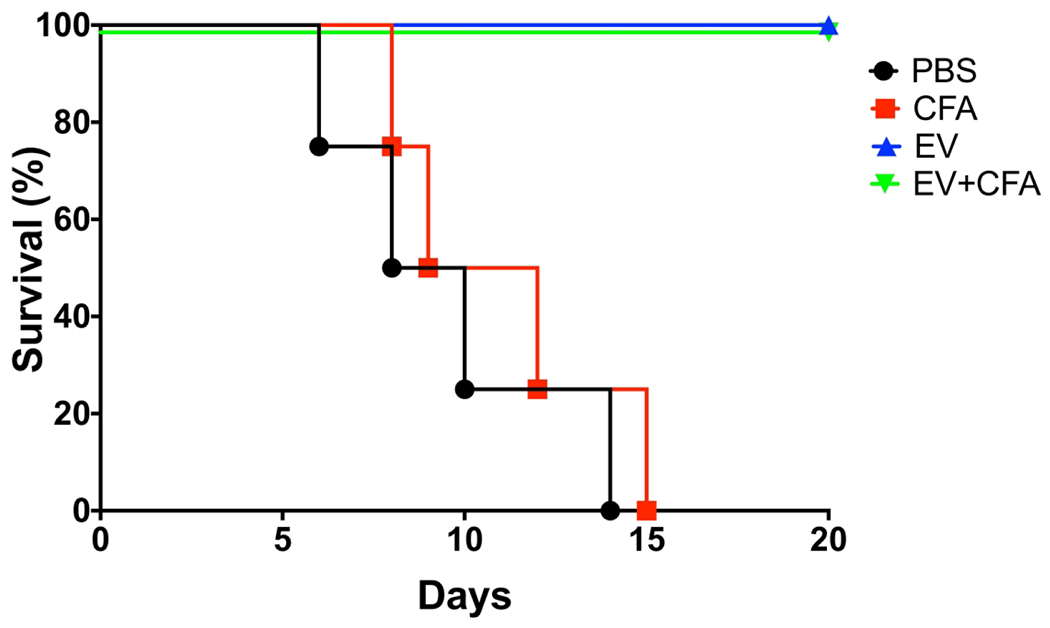
Mice were immunized with C. albicans EVs, immunosuppressed with cyclophosphamide (CP) and then challenged with a lethal inoculum of C. albicans yeasts. The survival curves evidenced the protective effect of EVs and EVs-ADJ immunization. Statistical analysis was performed using one-way analysis of variance, and the difference between groups were analyzed by Log-rank (Mantel-Cox) test, p<0.0001. Results are representative of two independent experiments.
Fungal EVs from C. albicans were preserved after storage at low temperatures.
Considering the protective effects of fungal EVs, we evaluated their stability as a potential parameter for determining their future applicability as vaccine candidates. Maintenance of EV dimensions have been proposed as a parameter of vesicular stability (Wolf et al., 2012; Almeida et al., 2017). We therefore adopted EV dimensions in association with microscopic observation to evaluate the effects of storage on EV stability. Initial experiments were performed to investigate the stability of fungal EVs after storage at 4, −20 and −80 °C. The average diameter size was evaluated using DLS and distribution of EVs after storage showed a very modest increase in diameter by DLS analysis (Figure 6). For all samples, a bimodal distribution was visualized. Two populations of fungal EVs were detected, ranging between 30-75 and 140-230 nm. TEM of fresh and stored EVs demonstrated the presence of round shaped bilayered membranous structures within the size rates observed by DLS analysis. In order to confirm the size of the EVs we also evaluated the samples using nanoparticle tracking analysis (NTA). Minimal changes in size were observed when the EVs were stored at 4, −20 or −80 °C, in comparison to fresh samples (Figure 6). In all tested conditions the population of EVs ranged between 60 and 350 nm. However, EVs kept at 4 °C showed a less homogeneous distribution in size. Finally, no agglutination or membrane fragments were observed in TEM micrographies or DLS analysis, since DLS is also able to detect micelles or smaller particles (Moniruzzaman et al., 2010; Stetefeld et al., 2016).
Figure 6-. Effect of low temperature storage on fungal EVs size and stability.
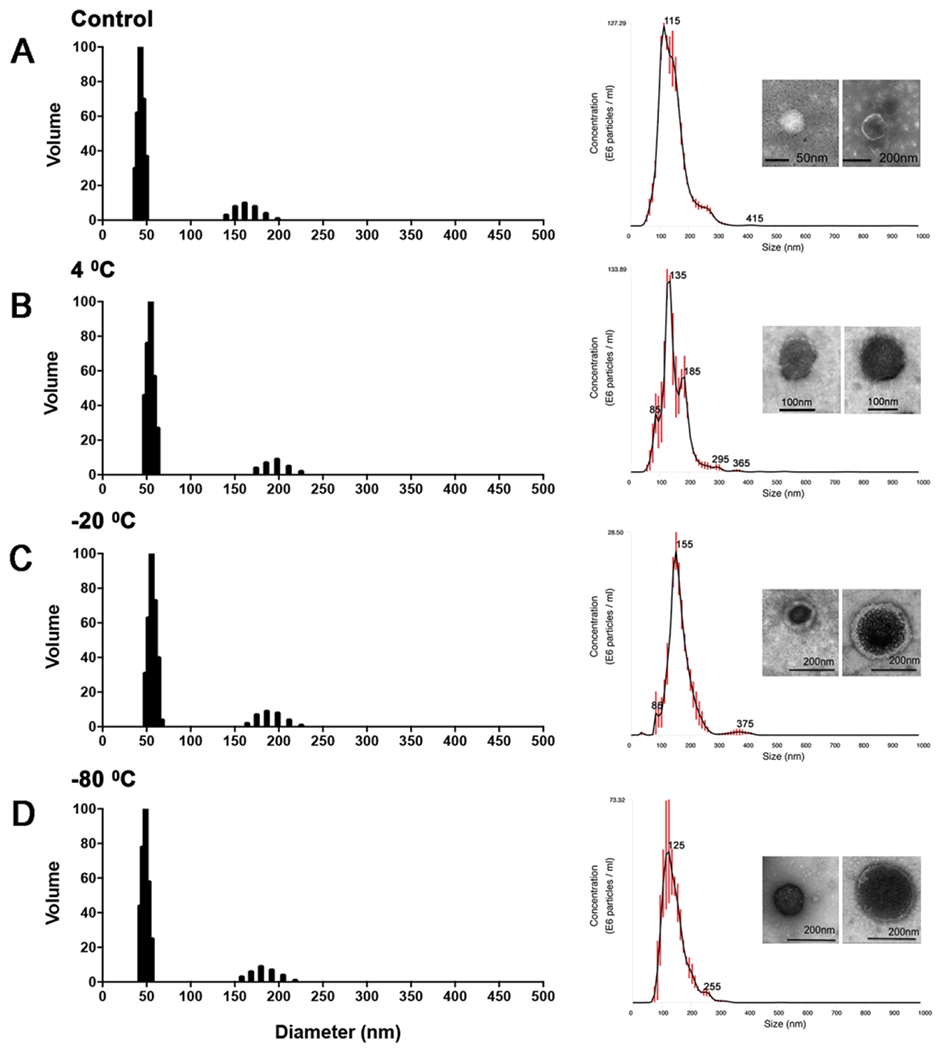
DLS, NTA and TEM analysis were performed in fresh (A) and stored fungal EVs at 4 °C (B), −20 °C (C) and −80 °C (D). The data depicted here is representative of two consistent, independent experiments.
Fungal EVs induced production of IL-6 by dendritic cells.
We recently demonstrated that C. albicans EVs modulate the activity of bone marrow-derived DCs, suggesting that these compartments could activate immune cells involved directly with the innate response (Vargas et al., 2015). To investigate whether storage would modify the ability of C. albicans EVs to activate DCs we examined IL-6 and IL-10 production after overnight stimulation with these compartments. Independent of the storage conditions all fungal EVs induced IL-6. However, stimulation with EVs kept at −80 °C was significatively reduced when compared with fresh and that storage at −20 °C. On the other hand, IL-10 production was minimal for all EV samples (Figure 7).
Figure 7– Effects of storage conditions on the induction of IL-6 and IL-10 in dendritic cells (DCs).
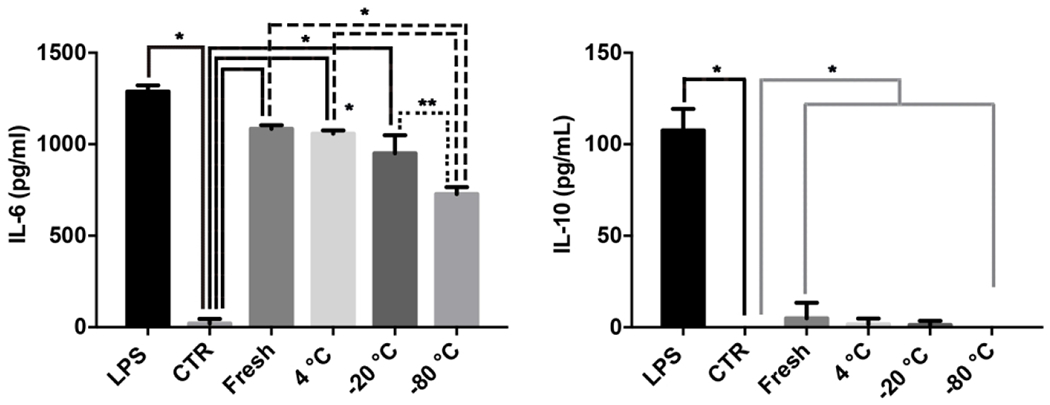
DCs were pretreated with fungal EVs and the levels of IL-6 and IL-10 were determined. Results are representative of two independent experiments. Statistical analysis was performed using one-way ANOVA analysis of variance, and the difference between groups were analyzed by Bonferroni post test, * p<0.0001; ** p=0.0015.
Pretreatment with fungal EVs decreased the lethality of C. albicans in a G. mellonella model of infection.
We used G. mellonella larvae to study whether fungal EVs storage could impact their ability to protect the insect against a lethal challenge with C. albicans. Fungal EVs were administered in concentrations previously described by our group as capable of significantly reduce larvae mortality and fungal burden (Vargas et al., 2015). The G. mellonella survival data indicated that all fungal EVs tested were able to protect the larvae from C. albicans infection, independent of their storage conditions (Figure 8). However, fresh fungal EVs resulted in the highest survival rates (62%).
Figure 8 – Pretreatment with fungal EVs reduced the mortality of G. mellonella larvae infected with C. albicans.
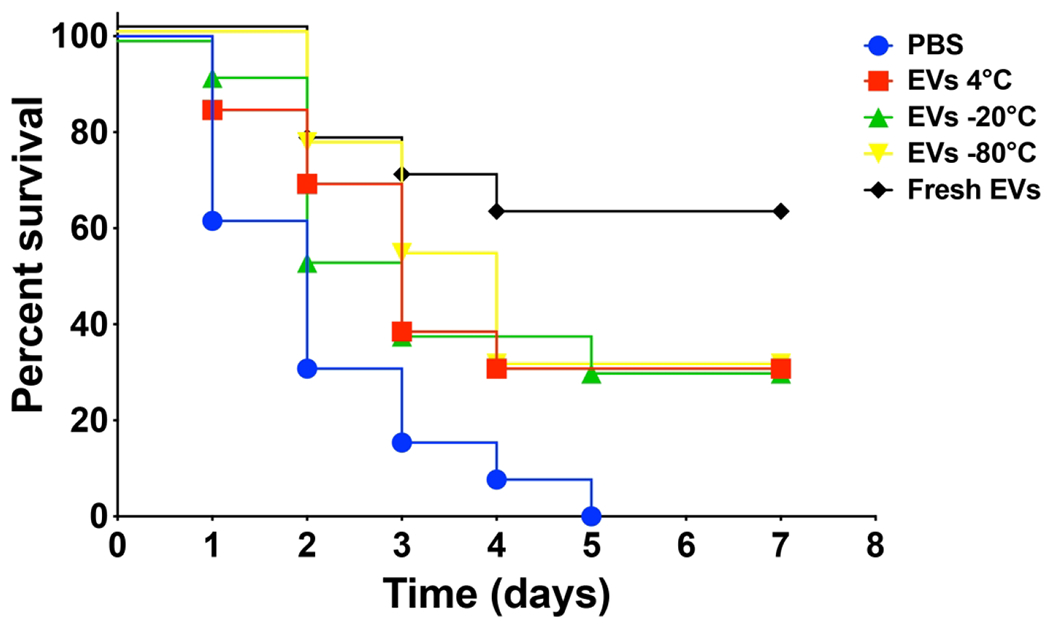
Survival curves of larvae pretreated with fungal EVs stored under different conditions (10 μl of a 10 μM suspension based on the sterol content) and then infected two days later with a lethal inoculum of C. albicans yeasts are shown. Statistical analysis was performed using oneway analysis of variance, and the survival differences between each EV groups and PBS were analyzed by individual paired Log-rank (Mantel-Cox) (Supplemental Table 1). Results are representative of two independent experiments.
DISCUSSION
Fungal EVs can directly modulate the innate immune system with a promising potential to trigger the development of adaptive responses (Vargas et al., 2015). Since fungal EVs carry a combination of native immunogens in a cell-free system, they may be a safe source of immunogens for the development of vaccine formulations. In fact, the immunomodulatory activity of fungal EVs has been clearly demonstrated in several in vitro models (Oliveira et al., 2010a; Vargas et al., 2015; Joffe et al., 2016; Da Silva et al., 2016; Baltazar et al., 2018; Bielska et al., 2018; Souza et al., 2019a), as well as their ability to prolong the survival of invertebrate models of infection with C. albicans or C. neoformans (Vargas et al., 2015; Colombo et al., 2019). However, the protective effects of fungal EVs in mammalian models of fungal infections have not yet been demonstrated, which hampers the design of fungal EV-based vaccine formulations. In this study, we demonstrated that fungal EVs efficiently protect immunosuppressed mice against a lethal C. albicans infection.
As demonstrated previously by our group, DCs treated with C. albicans EVs exhibited an increase in MHCII and CD86 expression, suggesting that these compartments could mediate an efficient communication between the innate and the adaptive immune response (Vargas et al., 2015). To investigate this possibility, we developed a protocol for mice immunization using fungal EVs in the presence or absence of classic vaccine adjuvants. Our results demonstrated that one initial vaccination and three additional boosts are required to stimulate a significant increase in antibody production. We found that most of the serum antibodies that recognize fungal antigens, both C. albicans crude extract and EVs, belong to the IgGl subclass. Although our data indicate that the addition of adjuvants promotes a polyclonal B cell response, further studies are required to determine whether the antibodies produced after immunization with C. albicans EVs are specific and/or effector components of the immune response.
The reduced fungal burden in the spleen, liver and kidney of mice immunized with EV formulations clearly shows that vaccination decreases C. albicans dissemination. It is noteworthy that during these time intervals, mice are still neutropenic and susceptible to fungal dissemination, supporting the efficiency of the vaccination protocol in immunosuppressed animals. These data corroborate with the full protection observed in the survival experiment using both EVs or EVs+ADJ as vaccine formulations. However, the cytokine profile in the spleen of mice was distinct when immunization with EVs and EVs+ADJ were compared suggesting a different protective immunological regulation for both formulations. Although the pro-inflammatory cytokines IL12p70 and TNFα increased at day 3 for both conditions, IL-4 and TGFP were significantly higher in the absence of adjuvants. In general, IL-4 is associated with TH1 inhibition and has a pivotal role inducing a TH2 response and susceptibility to candidiasis (Puccetti et al., 1994; Tonnetti et al., 1995). However, IL-4 can also play a protective role in candidiasis. Presence of IL-4 was required for protection through a mechanism involving the induction of CD4+ TH1 response (Romani et al., 1994; Spaccapelo et al., 1995). An increased production of IL-4 was detected in memory CD4+ T cells after a secondary challenge with C. albicans yeasts (Romani et al., 1996). Corroborating with that, TGFP seems to be involved with acquired resistance and its presence is required for optimal TH1 development and long-term anticandidal activities (Spaccapelo et al., 1995). Of note, the participation of TGFP as an inducer of memory CD4 cells in mice has been reported (Swain et al., 1991). Interestingly, TGF-β was able to enhance both memory phenotype of antigen-specific murine TH1 cells and their effector function in experimental autoimmune encephalomyelitis (Weinberg et al., 1992).
A higher inflammatory activity mediated by EVs+ADJ was confirmed at day 5, when increased levels of TNFα, IFNγ and IL-6 were detected. In mice immunized with EVs the IL-6 increase in combination with the presence of TGFP could trigger the development of a TH17 response (Mangan et al., 2006), also supported by the potential TH1 inhibitory activity of IL-4. Excepting for the increase in IL-10 and TGFP at day 5, all the cytokines detected after EV immunization were similar to PBS and ADJ. Under these conditions, the cytokines associated with anti-inflammatory responses would be important for restoring normal conditions. Based on our data we could not rule out that the infection was resolved faster when the EVs were used alone. However, the fungal burden was similar during days 3 and 5 after immunization with EVs or EVs+ADJ, which argues against this hypothesis. The addition of adjuvant apparently favored an inflammatory response.
Our results confirm that fungal EVs are promising multi-antigenic compartments to be exploited as vaccine formulations. However, the mechanisms by which EVs mediate this protection remains to be determined. A number of components carried by C. albicans EVs could be individually and / or collectively involved in the protective effects. Indeed, proteomic analysis of C. albicans EVs was previously performed and a list of proteins successfully used in murine vaccination models were characterized, including (i) enolase-1 (Enol), (ii) MP65, (iii) Bgl-2, (iv) the agglutinin-like sequence protein 3 (Alsp3) and (v) the secreted aspartyl protease 2 (Sap2) (Vargas et al., 2015; Gil-Bona et al., 2015b). Thus, the mechanism by which the fungal EVs induce protection is most probably dependent on a combination of antigens that stimulates: (i) phagocyte activation with the upregulation of cytokines (Oliveira et al., 2010a; Vargas et al., 2015), (ii) activation of antigen presenting cells (Vargas et al., 2015), with potential induction of TH17 response and (iii) antibody production. The presence of mannosylated proteins and the mechanism of internalization of C albicans EVs suggests the participation of cell surface receptors (Vargas etal., 2015; Gil-Bona et al., 2015a). This activation could be at least in part be mediated by MP65, a mannoprotein enriched in EVs that modulates the expression of CD86 and MHCII by dendritic cells (Gomez etal., 1996; Pietrella et al., 2006) and induces the production of cytokines, similarly to what has been observed when DCs were incubated with C. albicans EVs (Vargas etal., 2015). Two specific proteins carried by C. albicans EVs, Als-3 and Sap2, have been extensively investigated in vaccine studies (De Bernardis et al., 2012; Sui et al., 2017b). The N-terminal domain of Als-3 was formulated with aluminum hydroxide as adjuvant (NDV-3 vaccine). In humans, this formulation was safe, tolerable and stimulated an immunogenic response (Schmidt et al., 2012) and is currently in a phase 2 trial. In addition, recombinant Sap2 was incorporated into influenza virosomes (PEV7) and has completed phase 1 clinical trial (De Bernardis et al., 2018). Protection in mice by these antigens is mediated through mechanisms that include shifts in TH1 and TH17 cytokines as well as antibody production (De Bernardis et al., 2012; De Bernardis et al., 2015).
The combination of all these immunogens in their native structure could potentiate the efficacy of C. albicans EVs as a vaccine. In fact, the use of combined antigens has been previously investigated before. Torosantucci and colleagues (Torosantucci et al., 2005) conjugated /Z-glucan to CRM197, a non-toxic mutant of diphtheria toxin that functions as a carrier protein, and this formulation protected mice against vulvovaginal and systemic candidiasis. Additional optimized formulations were recently tested by Bundle and colleagues (Bundle et al., 2018) who conjugated β-mannans to β-glucans to obtain more efficient immunogens. Other highly conserved EVs components, such as metabolic enzymes (e.g. from the glycolytic pathway), heat shock proteins and the glycosphingolipid glucosylceramide (GlcCer), potentially stimulate the immune system against a variety of fungal species (Nimrichter et al., 2004; Rodrigues et al., 2007b; Raska et al., 2008a). In combination these antigens could induce crossed protection against other fungal pathogens, which still has to be experimentally addressed for fungal EVs. In other models, immunization with C. albicans aldolase, a metabolic enzyme that is abundant in EVs, conferred protection against C. glabrata (Medrano-Diaz et al., 2018). In addition, immunization of mice with GlcCer protected mice against a lethal inoculum of C. neoformans (Mor et al., 2016). We previously demonstrated that passive immunization with anti-GlcCer antibodies prolongs the survival of mice challenged with C. neoformans (Rodrigues et al., 2007b).
The crossed protection activity of C. albicans EVs is currently under investigation in our laboratory, as well as of the potential EV-associated cell surface pattern recognition receptors (PRRs) and pathogen associated molecular patterns (PAMPs). EVs produced by C. albicans and other fungal species are the vehicles of RNA export (Da Silva et al., 2015). Therefore, it is reasonable to expected that, if released within the host cells, vesicular RNA could potentially activate TLR9. Alternatively, since TLR9 can be recruited to the phagosomes when dectin-1 is activated (Khan et al., 2016), trafficking of EVs to phagosomes could also trigger TLR9 activation. Finally, we cannot rule out the participation of polysaccharides in the activation of innate responses. Recently, Silva and colleagues demonstrated that EVs from Paraccoidioides brasiliensis are recognized by DC-SIGN in vitro (Peres da Silva et al., 2015). However, such components have not been reported in C. albicans EVs.
The use of fungal EVs in vaccine preparations depends on the reproducibility of their protective effects, which is directly related to their stability. Indeed, intact fungal EVs could function as a delivery system similarly to liposomes, preventing antigen degradation, which would increase their relative concentration and facilitate the delivery of fungal components to antigen presenting cells (Giddam et al., 2012; Peres da Silva et al., 2015). Independent of their mechanism for promoting protection, EVs must be carefully isolated and stored to keep their functional epitopes structurally stable. Although fungal EV isolation protocols have been significantly improved to speed up and optimize the preparation of vesicular fractions (Reis et al., 2019), storage is a key issue to be considered and thoroughly investigated, since it directly impacts the applicability of fungal EVs as vaccine candidates. In EVs released by mammalian cells, the impact of storage at different temperatures has been investigated, but the results seem to diverge according to the cell line tested, suggesting that each model must be individually investigated (Sokolova et al., 2011; Gamonet et al., 2017; Maroto et al., 2017; Park et al., 2018; Frank et al., 2018). The effects of storage on fungal EVs are poorly known. In C. neoformans, vesicle stability appears to be distinct according to the EV population (Wolf et al., 2012). Larger EVs, varying from 100 to 200 nm, were stable for at least 72 hours at room temperature in conditioned medium. In contrast, smaller vesicles, ranging from 40–60 nm, decreased in size after 24 hours of storage. In our experiments C. albicans EVs were kept at lower temperatures for 7 days and their size compared with fresh preparations. Using DLS as a tool to measure size we showed that EVs from C. albicans consist of two populations, comparable to what was previously described by our group (Vargas et al., 2015). The particle range measured by DLS after storage was relatively homogeneous and similar to the fresh prepared EVs. A small increase in size was observed in the smaller population of EVs after storage at 4 and −20 °C. The larger vesicles were also slightly increased when storage was carried out at 4 °C. These modest changes could be explained by EV fusion or aggregation. Increasing in EV size has been reported after freezing, caused by multilamellar formation due to expansion of ice nano- or micro-crystals in the lipid bilayer(Lee et al., 2016). This effect was not evident in our TEM images after storage at −20 and −80 °C. Despite our multiple approaches suggesting that the EVs were stable and intact, the data does not absolutely rule out that there was no disruption and reassembly of the vesicles. However, the absence of membrane fragments or debris, which would be detected both by TEM and DLS, suggests that most of the EVs were intact. The preservation of their dimensions was confirmed by NTA, which revealed EV populations very similar in dimensions independently of storage conditions. However, it is noteworthy that, so far, there are no authoritative methods to determine the size of EVs (Thery et al., 2018). A combination of fluorescent labeling EVs and particle counting technologies, such as NTA, is recommended. However, antibodies and lipid dyes can form particles that could interfere with data analysis. Thus, although there remains a theoretical methodological limitation in our current investigation, our data indicate that although fungal EVs were relatively stable and that the −80 °C temperature was apparently the best storage condition, at least for C. albicans fungal EVs.
Since EVs integrity was maintained under different storage conditions, we subsequently investigated whether the immunogens also conserved their antigenic activity. Most importantly, storage conditions did not abrogate the ability of EVs to activate DCs. Treatment with EVs increased the production of the pro-inflammatory cytokine IL-6, consistent with previous data from our lab confirming the activation of DCs through EVs recognition (Vargas et al., 2015). In addition, lower levels of IL-10 were detected. Fungal EV storage at lower temperatures did not affect protection against C. albicans in G. mellonella, a reliable model to investigate the activation of innate immune responses (Fuchs and Mylonakis, 2006). Our results not only confirmed previous experiments published by our lab, showing that fungal EVs stimulated the innate immune response and protected the insect (Vargas et al., 2015), but also demonstrated that C. albicans EVs could be stored at lower temperatures without losing their ability to activate DCs and the innate immune system.
Our present study shows that immunization with C. albicans EVs stimulates a protective immune response in a murine model of candidiasis. These results, in association to the stability of fungal EVs preparations, open a new venue for the development of novel and efficient vaccines to prevent candidiasis and potentially other fungal infections. The use of mutant strains lacking virulence factors can also be explored as well as the development of platforms to generate EVs tailored with heterologous antigens originally from pathogenic fungi and expressed in non-pathogenic fungal species. Furthermore, antigens from bacteria, viruses and parasites could also be combined with fungal components for immunological assays of prophylaxis or treatment by boosting the immune system.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the Brazilian agency Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, grants 405520/2018-2, 440015/2018-9, and 301304/2017-3 to M.L.R.; 311179/2017-7 and 408711/2017-7 to L.N.), FAPERJ (E-26/202.809/2018 to L.N and E-26/202.696/2018 to A.J.G) and Fiocruz (grants VPPCB-007-FIO-18 and VPPIS-001-FIO18) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001). JDN was supported in part by R21AI124797.
Footnotes
CONFLICTS OF INTEREST
The authors state that there are no conflicts of interests.
REFERENCES:
- Ahmad E, Fatima MT, Saleemuddin M, and Owais M (2012) Plasma beads loaded with Candida albicans cytosolic proteins impart protection against the fungal infection in BALB/c mice. Vaccine 30: 6851–6858. [DOI] [PubMed] [Google Scholar]
- Albuquerque PC (2004) Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Science (80-) 10: 1695–1710 http://www.sciencemag.org/content/306/5703/1895.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F, Wolf JM, Silva T.A. da , DeLeon-Rodriguez CM, Rezende CP, Pessoni AM, et al. (2017) Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun 8: 1968 https://pubmed.ncbi.nlm.nih.gov/29213074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar LM, Zamith-Miranda D, Burnet MC, Choi H, Nimrichter L, Nakayasu ES, and Nosanchuk JD (2018) Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci Rep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Righi E, Montravers P, and Cornely OA (2018a) What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother 73: i14–i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Righi E, Montravers P, and Cornely OA (2018b) What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother 73: i14–i25 https://www.ncbi.nlm.nih.gov/pubmed/29304208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardis F. De , Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, et al. (2012) A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 30: 4490–4498 http://www.sciencedirect.com/science/article/pii/S0264410X12006159. [DOI] [PubMed] [Google Scholar]
- Bernardis F. De , Arancia S, Sandini S, Graziani S, and Norelli S (2015) Studies of Immune Responses in Candida vaginitis. Pathog (Basel, Switzerland) 4: 697–707 https://www.ncbi.nlm.nih.gov/pubmed/26473934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardis F.De , Graziani S, Tirelli F, and Antonopoulou S (2018) Candida vaginitis: virulence, host response and vaccine prospects. MedMycol 56: S26–S31 10.1093/mmy/myx139. [DOI] [PubMed] [Google Scholar]
- Bielska E, Sisquella MA, Aldeieg M, Birch C, O’Donoghue EJ, and May RC (2018) Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat Commun 9 10.1038/s41467-018-03991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M, Thomas DY, Whiteway M, and Kavanagh K (2002) Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 34: 153–157 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Bundle DR, Paszkiewicz E, Elsaidi HRH, Mandal SS, and Sarkar S (2018) A Three Component Synthetic Vaccine Containing a β-Mannan T-Cell Peptide Epitope and a β-Glucan Dendritic Cell Ligand. Molecules 23: 1961 https://pubmed.ncbi.nlm.nih.gov/30082627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A (2013) Development of vaccines for Candida albicans: fighting a skilled transformer. Nat Rev Microbiol 11: 884 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]
- Cassone A, and Rappuoli R (2010) Universal Vaccines: Shifting to One for Many. MBio 1: e00042–10 http://mbio.asm.org/content/1/1/e00042-10.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AC, Rella A, Normile T, Joffe LS, Tavares PM, S Araujo G.R. de, et al. (2019) Cryptococcus neoformans Glucuronoxylomannan and Sterylglucoside Are Required for Host Protection in an Animal Vaccination Model. MBio 10: e02909–18 https://www.ncbi.nlm.nih.gov/pubmed/30940711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely OA, Bassetti M, Calandra T, Garbino I, Kullberg BJ, Lortholary O, et al. (2012a) ESCMID* guideline for the diagnosis and management of <em>Candida</em> diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18: 19–37. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Bassetti M, Calandra T, Garbino I, Kullberg BJ, Lortholary O, et al. (2012b) ESCMID* guideline for the diagnosis and management of <em>Candida</em> diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18: 19–37 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- Denning DW, Kneale M, Sobel JD, and Rautemaa-Richardson R (2018) Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18: e339–e347 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- Dojnov B, Bozic N, Bulajic N, and Vujcic Z (2007) Preparation of combined extract of cell wall and cytosol antigens of Candida albicans for immunoblot analysis. J Clin Lab Anal 21: 406–412 https://www.ncbi.nlm.nih.gov/pubmed/18022925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Frases S, Nicola AM, Rodrigues ML, and Casadevall A (2009) Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155: 3860–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Hawkins NJ, Sanglard D, and Gurr SJ (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science (80-) 360: 739–742 https://science.sciencemag.org/content/360/6390/739. [DOI] [PubMed] [Google Scholar]
- Frank J, Richter M, Rossi C. de, Lehr C-M, Fuhrmann K, and Fuhrmann G (2018) Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci Rep 8: 12377 https://www.ncbi.nlm.nih.gov/pubmed/30120298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S, Nimrichter L, Viana NB, Nakouzi A, and Casadevall A (2008) Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BB, and Mylonakis E (2006) Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 9: 346–351 http://www.sciencedirect.com/science/article/pii/S1369527406000877. [DOI] [PubMed] [Google Scholar]
- Gamonet C, Mourey G, Aupet S, Biichle S, Petitjean R, Vidal C, et al. (2017) How to quantify microparticles in RBCs? A validated flow cytometry method allows the detection of an increase in microparticles during storage. Transfusion 57: 504–516 10.1111/trf.13989. [DOI] [PubMed] [Google Scholar]
- Giddam AK, Zaman M, Skwarczynski M, and Toth I (2012) Liposome-based delivery system for vaccine candidates: constructing an effective formulation. Nanomedicine 7: 18771893 10.2217/nnm.12.157. [DOI] [PubMed] [Google Scholar]
- Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, and Gil C (2015b) Proteomics Unravels Extracellular Vesicles as Carriers of Classical Cytoplasmic Proteins in Candida albicans. JProteome Res 14: 142–153. [DOI] [PubMed] [Google Scholar]
- Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, and Gil C (2015b) Proteomics Unravels Extracellular Vesicles as Carriers of Classical Cytoplasmic Proteins in Candida albicans. J Proteome Res 14: 142–153 10.1021/pr5007944. [DOI] [PubMed] [Google Scholar]
- Gomez MJ, Torosantucci A, Arancia S, Maras B, Parisi L, and Cassone A (1996) Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect Immun 64: 2577 LP–2584 http://iai.asm.org/content/64/7/2577.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NAR, Veerdonk F.L. Van De , Brown AJP, and Netea MG (2012) Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat Rev Microbiol 10: 112–122 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Wu CH, Chang YC, Kwon-Chung KJ, Brown RJ, and Jong A (2012) Cryptococcus neoformans-Derived Microvesicles Enhance the Pathogenesis of Fungal Brain Infection. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda MAK, Almeida J.R.F. de, Jannuzzi GP, Cronemberger-Andrade A, Torrecilhas ACT, Moretti NS, et al. (2018) Extracellular Vesicles From Sporothrix brasiliensis Are an Important Virulence Factor That Induce an Increase in Fungal Burden in Experimental Sporotrichosis. Front Microbiol 9: 2286 https://www.frontiersin.org/article/10.3389/fmicb.2018.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, and Leonardi I (2017) Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol 17: 635 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe LS, Nimrichter L, Rodrigues ML, and Poeta M. Del (2016) Potential roles of fungal extracellular vesicles during infection. mSphere 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NS, Kasperkovitz PV, Timmons AK, Mansour MK, Tam JM, Seward MW, et al. (2016) Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan. J Immunol 196: 2249–2261 https://pubmed.ncbi.nlm.nih.gov/26829985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, and Sudbery P (2011) Candida albicans, a major human fungal pathogen. J Microbiol 49: 171 10.1007/sl2275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- Kullberg BJ, van de Veerdonk F, N.M. (2014) Immunotherapy: a potential adjunctive treatment for fungal infection. Curr Opin Infect Dis 27: 511–516. [DOI] [PubMed] [Google Scholar]
- Lee M, Ban J-J, Im W, and Kim M (2016) Influence of storage condition on exosome recovery. Biotechnol Bioprocess Eng 21: 299–304 10.1007/s12257-015-0781-x. [DOI] [Google Scholar]
- Leone F, Bellani L, Muccifora S, Giorgetti L, Bongioanni P, Simili M, et al. (2018) Analysis of extracellular vesicles produced in the biofilm by the dimorphic yeast Pichia fermentans. J Cell Physiol 233: 2759–2767 10.1002/jcp.25885. [DOI] [PubMed] [Google Scholar]
- Li W. qing, Hu X. chu, Zhang X, Ge Y, Zhao S, Hu Y, and Ashman RB (2011a) Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine 29: 5526–5533 http://www.sciencedirect.com/science/article/pii/S0264410X11007407. [DOI] [PubMed] [Google Scholar]
- Li W. qing, Hu X. chu, Zhang X, Ge Y, Zhao S, Hu Y, and Ashman RB (2011b) Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine 29: 5526–5533. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie ALJ, RoBner S, Koch F, Romani N, and Schuler G (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92 http://www.sciencedirect.com/science/article/pii/S002217599800204X. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. (2006) Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231–234. [DOI] [PubMed] [Google Scholar]
- Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. (2017) Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. JExtracell vesicles 6: 1359478 https://www.ncbi.nlm.nih.gov/pubmed/28819550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano-Diaz CL, Vega-Gonzalez A, Ruiz-Baca E, Moreno A, and Cuellar-Cruz M (2018) Moonlighting proteins induce protection in a mouse model against Candida species. Microb Pathog 124: 21–29 http://www.sciencedirect.com/science/article/pii/S0882401018308519. [DOI] [PubMed] [Google Scholar]
- Mitchell KF, Azadi P, Jaromin A, Sanchez H, Dominguez E, Andes DR, et al. (2018) Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLOS Biol 16: e2006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M, Kamiya N, and Goto M (2010) Ionic liquid based microemulsion with pharmaceutically accepted components: Formulation and potential applications. J Colloid Interface Sci 352: 136–142 http://www.sciencedirect.com/science/article/pii/S0021979710009306. [DOI] [PubMed] [Google Scholar]
- Mor V, Farnoud AM, Singh A, Rella A, Tanno H, Ishii K, et al. (2016) Glucosylceramide Administration as a Vaccination Strategy in Mouse Models of Cryptococcosis. PLoS One 11: e0153853–e0153853 https://www.ncbi.nlm.nih.gov/pubmed/27082428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrichter L, Barreto-Bergter E, Mendonqa-Filho RR, Kneipp LF, Mazzi MT, Salve P, et al. (2004) A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microbes Infect 6. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Souza M.M. De, Poeta M. Del, Nosanchuk JD, Joffe L, Tavares PDM, and Rodrigues ML (2016) Extracellular vesicle-associated transitory cell wall components and their impact on the interaction of fungi with host cells. Front Microbiol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, and Nimrichter L (2010a) Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJP, Nosanchuk JD, et al. (2010b) Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. (2016a) Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62: 409–417. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. (2016b) Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62: 409–417 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, and Kullberg BJ (2018a) Invasive candidiasis. Nat Rev Dis Prim 4: 18026 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, and Kullberg BJ (2018b) Invasive candidiasis. Nat Rev Dis Prim 4: 18026. [DOI] [PubMed] [Google Scholar]
- Park SJ, Jeon H, Yoo S-M, and Lee M-S (2018) The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. Vitr Cell Dev Biol - Anim 54: 423–429 10.1007/s11626-018-0261-7. [DOI] [PubMed] [Google Scholar]
- Peres da Silva R, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, et al. (2015) Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep 5: 14213 https://www.ncbi.nlm.nih.gov/pubmed/26387503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Bistoni G, Corbucci C, Perito S, and Vecchiarelli A (2006) Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol 8: 602–612 10.1111/j.1462-5822.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, et al. (1994) Cure of Murine Candidiasis by Recombinant Soluble Interleukin-4 Receptor. J Infect Dis 169: 1325–1331. [DOI] [PubMed] [Google Scholar]
- Raska M, Belakova J, Raska M, Belakova J, Horynova M, Krupka M, et al. (2008a) Systemic and mucosal immunization with Candida albicans hsp90 elicits hsp90-specific humoral response in vaginal mucosa which is further enhanced during experimental vaginal candidiasis. Med Mycol 46: 411–420. [DOI] [PubMed] [Google Scholar]
- Raska M, Belakova J, Raska M, Belakova J, Horynova M, Krupka M, et al. (2008b) Systemic and mucosal immunization with Candida albicans hsp90 elicits hsp90-specific humoral response in vaginal mucosa which is further enhanced during experimental vaginal candidiasis. Med Mycol 46: 411–420 10.1080/13693780701883508. [DOI] [PubMed] [Google Scholar]
- Rayner S, Bruhn S, Vallhov H, Andersson A, Billmyre RB, and Scheynius A (2017) Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci Rep 7: 1–9 10.1038/srep39742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis FCG, Borges BS, Jozefowicz LJ, Sena BAG, Garcia AWA, Medeiros LC, et al. (2019) A Novel Protocol for the Isolation of Fungal Extracellular Vesicles Reveals the Participation of a Putative Scramblase in Polysaccharide Export and Capsule Construction in Cryptococcus gattii. mSphere 4: e00080–19 http://msphere.asm.org/content/4/2/e00080-19.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo J, Nimrichter L, and Rodrigues ML (2017) What Is New? Recent Knowledge on Fungal Extracellular Vesicles. Curr Fungal Infect Rep 11. [Google Scholar]
- Rodrigues ML, Nakayasu ES, Almeida IC, and Nimrichter L (2014) The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteomics 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, and Casadevall A (2008) Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. (2007a) Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Shi L, Barreto-Bergter E, Nimrichter L, Farias SE, Rodrigues EG, et al. (2007b) Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, et al. (1996) Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med 183: 1345–1355 https://www.ncbi.nlm.nih.gov/pubmed/8666893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Puccetti P, Mencacci A, Spaccapelo R, Cenci E, Tonnetti L, and Bistoni F (1994) Tolerance to staphylococcal enterotoxin B initiated Th1 cell differentiation in mice infected with Candida albicans. InfectImmun 62: 4047–4053 https://www.ncbi.nlm.nih.gov/pubmed/7914883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, and Lopez-Ribot JL (2009) Efficacy of a genetically engineered Candida albicans tet-NRG1 strain as an experimental live attenuated vaccine against hematogenously disseminated candidiasis. Clin Vaccine Immunol 16: 430–432 https://www.ncbi.nlm.nih.gov/pubmed/19144791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, et al. (2012) NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 30: 7594–7600 https://www.ncbi.nlm.nih.gov/pubmed/23099329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven JE, Tenforde MW, Levitz SM, and Jarvis JN (2017) Modulating host immune responses to fight invasive fungal infections. Curr Opin Microbiol 40: 95–103 https://www.ncbi.nlm.nih.gov/pubmed/29154044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E S-LH (1981) Experimental vaccination with Candida albicans ribosomes in cyclophosphamide-treated animals. Sabouraudia 19: 267–273. [PubMed] [Google Scholar]
- Silva BMA, Prados-Rosales R, Espadas-Moreno J, Wolf JM, Luque-Garcia JL, Gonçalves T, and Casadevall A (2014) Characterization of Alternaria infectoria extracellular vesicles. Med Mycol 52: 202–210 https://www.ncbi.nlm.nih.gov/pubmed/24576997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R.P. Da, Puccia R, Rodrigues MLML, Oliveira DLDL, Joffe LSLS, César GVGV, et al. (2015) Extracellular vesicle-mediated export of fungal RNA. Sci Rep 5: 7763 https://www.ncbi.nlm.nih.gov/pubmed/25586039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva T.A. Da, Roque-Barreira MC, Casadevall A, and Almeida F (2016) Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci Rep 6: 1–10 10.1038/srep35867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JD (2016) Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 214: 15–21 10.1016/j.ajog.2015.06.067. [DOI] [PubMed] [Google Scholar]
- Sokolova V, Ludwig A-K, Hornung S, Rotan O, Horn PA, Epple M, and Giebel B (2011) Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surfaces B Biointerfaces 87: 146–150 http://www.sciencedirect.com/science/article/pii/S0927776511002724. [DOI] [PubMed] [Google Scholar]
- Souza JAM, Baltazar L. de M, Carregal VM, Gouveia-Eufrasio L, Oliveira A.G. de, Dias WG, et al. (2019a) Characterization of Aspergillus fumigatus Extracellular Vesicles and Their Effects on Macrophages and Neutrophils Functions. Front Microbiol 10: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JAM, Baltazar L de M, Carregal VM, Gouveia-Eufrasio L, Oliveira AG de, Dias WG, et al. (2019b) Characterization of Aspergillus fumigatus Extracellular Vesicles and Their Effects on Macrophages and Neutrophils Functions. Front Microbiol 10: 2008 https://www.ncbi.nlm.nih.gov/pubmed/31551957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccapelo R, Romani L, Tonnetti L, Cenci E, Mencacci A, Sero G.Del , et al. (1995) TGF-beta is important in determining the in vivo patterns of susceptibility or resistance in mice infected with Candida albicans. J Immunol 155: 1349 LP–1360 http://www.jimmunol.org/content/155/3/1349.abstract. [PubMed] [Google Scholar]
- Stetefeld J, McKenna SA, and Patel TR (2016) Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev 8: 409–427 https://pubmed.ncbi.nlm.nih.gov/28510011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Yan L, and Jiang Y (2017a) The vaccines and antibodies associated with Als3p for treatment of Candida albicans infections. Vaccine 35: 5786–5793 http://www.sciencedirect.com/science/article/pii/S0264410X17311829. [DOI] [PubMed] [Google Scholar]
- Sui X, Yan L, and Jiang Y (2017b) The vaccines and antibodies associated with Als3p for treatment of Candida albicans infections. Vaccine 35: 5786–5793. [DOI] [PubMed] [Google Scholar]
- Swain SL, Huston G, Tonkonogy S, and Weinberg A (1991) Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol 147: 2991 LP–3000 http://www.jimmunol.org/content/147/9/2991.abstract. [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DP, Viudes A, Monteagudo C, Lazzell AL, Saville SP, and López-Ribot JL (2006a) A proteomic-based approach for the identification of Candida albicans protein components present in a subunit vaccine that protects against disseminated candidiasis. Proteomics 6: 6033–6041. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Viudes A, Monteagudo C, Lazzell AL, Saville SP, and López-Ribot JL (2006b) A proteomic-based approach for the identification of Candida albicans protein components present in a subunit vaccine that protects against disseminated candidiasis. Proteomics 6: 6033–6041 10.1002/pmic.200600321. [DOI] [PubMed] [Google Scholar]
- Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman RL, et al. (1995) Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol 25: 1559–1565. [DOI] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P, Bernardis F De, Berti F, Galli C, et al. (2005) A novel gly co-conjugate vaccine against fungal pathogens. J Exp Med 202: 597–606 https://pubmed.ncbi.nlm.nih.gov/16147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Matsuo AL, Ganiko L, Medeiros LCS, Miranda K, Silva LS, et al. (2011) The Pathogenic Fungus Paracoccidioides brasiliensis Exports Extracellular Vesicles Containing Highly Immunogenic α-Galactosyl Epitopes. Eukaryot Cell 10: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Rocha JDB, Oliveira DL, Albuquerque PC, Frases S, Santos SS, et al. (2015) Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol 17. [DOI] [PubMed] [Google Scholar]
- Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, et al. (2004a) Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology 111: 334–342 https://www.ncbi.nlm.nih.gov/pubmed/15009435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, et al. (2004b) Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology 111: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg AD, Whitham R, Swain SL, Morrison WJ, Wyrick G, Hoy C, et al. (1992) Transforming growth factor-beta enhances the in vivo effector function and memory phenotype of antigen-specific T helper cells in experimental autoimmune encephalomyelitis. J Immunol 148: 2109 LP–2117 http://www.jimmunol.org/content/148/7/2109.abstract. [PubMed] [Google Scholar]
- Wolf JM, Rivera J, and Casadevall A (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol 14: 762–773 10.1111/j.1462-5822.2012.01757.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Lipinski T, Carrel FR, Bailey JJ, and Bundle DR (2007) Synthesis and immunochemical studies on a Candida albicans cluster glycoconjugate vaccine. Org Biomol Chem 5: 3477–3485 10.1039/B709912F. [DOI] [PubMed] [Google Scholar]
- Zamith-Miranda D, Nimrichter L, Rodrigues ML, and Nosanchuk JD (2018) Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


