Abstract
Background
Population-based literature suggests severe acute respiratory syndrome coronavirus 2 infection may disproportionately affect racial/ethnic minorities; however, patient-level observations of hospitalization outcomes by race/ethnicity are limited. Our aim in this study was to characterize coronavirus disease 2019 (COVID-19)–associated morbidity and in-hospital mortality by race/ethnicity.
Methods
This was a retrospective analysis of 9 Massachusetts hospitals including all consecutive adult patients hospitalized with laboratory-confirmed COVID-19. Measured outcomes were assessed and compared by patient-reported race/ethnicity, classified as white, black, Latinx, Asian, or other. Student t test, Fischer exact test, and multivariable regression analyses were performed.
Results
A total of 379 patients (aged 62.9 ± 16.5 years; 55.7% men) with confirmed COVID-19 were included (49.9% white, 13.7% black, 29.8% Latinx, 3.7% Asian), of which 376 (99.2%) were insured (34.3% private, 41.2% public, 23.8% public with supplement). Latinx patients were younger, had fewer cardiopulmonary disorders, were more likely to be obese, more frequently reported fever and myalgia, and had lower D-dimer levels compared with white patients (P < .05). On multivariable analysis controlling for age, gender, obesity, cardiopulmonary comorbidities, hypertension, and diabetes, no significant differences in in-hospital mortality, intensive care unit admission, or mechanical ventilation by race/ethnicity were found. Diabetes was a significant predictor for mechanical ventilation (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.11–3.23), while older age was a predictor of in-hospital mortality (OR, 4.18; 95% CI, 1.94–9.04).
Conclusions
In this multicenter cohort of hospitalized COVID-19 patients in the largest health system in Massachusetts, there was no association between race/ethnicity and clinically relevant hospitalization outcomes, including in-hospital mortality, after controlling for key demographic/clinical characteristics. These findings serve to refute suggestions that certain races/ethnicities may be biologically predisposed to poorer COVID-19 outcomes.
Keywords: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), healthcare disparities, race/ethnicity
While early observations suggest coronavirus disease 2019 (COVID-19) disproportionately affects racial/ethnic minorities, patient-level examination of hospitalization outcomes remained limited. Among hospitalized COVID-19 patients, we found no association between race/ethnicity and clinical outcomes including in-hospital mortality, after controlling for comorbidities.
(See the Editorial Commentary by Linas and Cunningham on pages e4139–40.)
Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first detected in Wuhan, China, in 2019, the single-stranded RNA virus has led to life-threatening disease that has reached pandemic proportions [1, 2]. This novel virus, which causes coronavirus disease 2019 (COVID-19), has impacted more than 200 countries; however, the United States has surpassed all other countries in the number of laboratory-confirmed cases of COVID-19 and associated deaths. As the United States has become the epicenter of the SARS-CoV-2 crisis, large-scale studies to examine manifestations of the disease and health-related outcomes are beginning to shed light on the impact of this pandemic nationwide [1, 3].
Alongside this sharp rise in US COVID-19 cases, population-level concerns have emerged regarding the virus disproportionately impacting racial/ethnic minorities. Recently released data by the Centers for Disease Control and Prevention (CDC) shows that blacks have a higher rate of hospitalization than their white counterparts, suggesting this group may be disproportionately affected by COVID-19 with higher infection rates, higher disease severity, or both [4]. Despite these early observations, patient-level studies with granular clinical data examining how presenting symptoms, comorbid conditions, baseline patient characteristics, and clinical outcomes differ by race/ethnicity among hospitalized adults with COVID-19 are lacking. Our primary aim in this study was to assess presenting symptoms and clinically relevant hospitalization outcomes of COVID-19 and how they differ by race/ethnicity in a large hospital system in Massachusetts.
METHODS
This was a multicenter, retrospective analysis that included all consecutive adult patients age ≥18 years hospitalized from 22 March 2020 through 2 April 2020 with a laboratory-confirmed diagnosis of COVID-19. SARS-CoV-2 infection was confirmed in all cases via polymerase chain reaction nasopharyngeal swab testing. All patients who had completed hospitalization (ie, discharge or death) were included. Data were collected from the 2 tertiary medical centers and the 7 community hospitals that comprise Mass General Brigham (formerly Partners Healthcare), all of which are located in Massachusetts. Study approval was obtained from the Partners Healthcare Institutional Review Board.
Patient characteristics, including gender, race/ethnicity, age, health insurance status, body mass index (BMI; kg/m2), preexisting comorbid medical conditions, COVID-19–associated symptoms at time of initial presentation, initial laboratory data, clinical course, and clinical outcomes, including use of mechanical ventilation and in-hospital mortality, were abstracted manually from electronic medical records using a structured abstraction tool. Race/ethnicity information was self-reported and classified as white, black, Latinx, Asian, or other by patients at the time of initial telephone hospital registration, first emergency department presentation, or hospital admission. All patients in our hospital system are asked standard questions with validated explanations and examples to guide them in accurately reporting their race and ethnicity.
Continuous variables were expressed as means and standard deviations, with categorical data reported using numbers and frequencies. Each patient-level characteristic was then compared by race/ethnicity. Univariate analyses were performed using the Student t test or Fisher exact test. Multivariable analyses were performed using logistic regression. All data were analyzed using Statistical Analysis Software 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Of 386 patients hospitalized for COVID-19 infection during the study period, 379 (98.2%) had completed hospitalization (discharge or death) and met inclusion criteria at the time of analysis. The mean age of the included cohort was 62.9 ± 16.5 years and a majority (55.7%) were men. Within this cohort, 189 (49.9%) patients identified as white, 52 (13.7%) as black, 113 (29.8%) as Latinx, 14 (3.7%) as Asian, and 11 (2.9%) as other. Nearly all patients (99.2%) in the cohort had health insurance, with 34.3% private, 41.2% public, and 23.8% public supplemented with private coverage. White patients were significantly less likely to only have public insurance coverage compared with Latinx (18.0% vs 62.8%, P < .001) and black (18.0% vs 40.4%, P < .001) patients.
Compared with white patients, Latinx patients were significantly younger (53.7 ± 14.5 vs 68.2 ± 15.2 years, P < .001) and less likely to have a history of pulmonary disorders (10.6% vs 24.3%, P = .003), cardiac arrhythmias (7.1% vs 21.2%, P = .02), or hypertension (38.9% vs 62.4%, P = .01). However, Latinx patients were more likely to be obese (BMI ≥30 kg/m2; 56.6% vs 40.7%; P = .03) with higher mean BMI compared with white patients (31.8 ± 6.7 vs 29.8 ± 6.5; P = .03; Table 1). Black patients were more likely to have a history of diabetes mellitus than white patients (47.1% vs 26.5%, P = .005). No other significant differences were noted between hospitalized black patients and white patients with regard to age or comorbid conditions. Of note, Asian patients were the most likely racial/ethnic group to report a diagnosis of diabetes mellitus (57%), although the size of this group was small.
Table 1.
Baseline Clinical Characteristics of Hospitalized Coronavirus Disease 2019 Patient Cohort
| Characteristic | All Coronavirus Disease 2019 (N = 379) | White (n = 189) | Black (n = 52) | Latinx (n = 113) | Asian (n = 14) | Others (n = 11) |
|---|---|---|---|---|---|---|
| Age, ±SD, y | 62.9 ± 16.5 | 68.2 ± 15.2 | 62.9 ± 15.4 | 53 .7 ± 14.5a | 69.5 ± 12.9 | 58.5 ± 23.9 |
| Male, n (%) | 211 (55.7) | 105 (55.6) | 25 (48.1) | 67 (59.3) | 7 (50.0) | 7 (63.6) |
| BMI, ±SD, kg/m2 | 30.3 ± 6.6 | 29.8 ± 6.5 | 30.1 ± 6.9 | 31.8 ± 6.7a | 26.1 ± 4.4 | 28.4 ± 3.9 |
| Obesity (BMI >30 kg/m2), n (%) | 167 (44.1) | 77 (40.7) | 19 (36.5) | 64 (56.6) a | 3 (21.4) | 4 (36.4) |
| Comorbidities, n (%) | ||||||
| Hypertension | 214 (56.5) | 118 (62.4) | 35 (67.3) | 44 (38.9) a | 10 (71.4) | 7 (63.6) |
| Hyperlipidemia | 172 (45.4) | 99 (52.4) | 20 (38.5) | 40 (35.4) | 9 (64.3) | 4 (36.4) |
| Diabetes | 123 (32.5) | 50 (26.5) | 24 (47.1) a | 39 (34.5) | 8 (57.1) | 2 (18.2) |
| Pulmonary disorders | 76 (20.1) | 46 (24.3) | 12 (23.1) | 12 (10.6) a | 2 (14.3) | 4 (36.4) |
| Cardiac arrhythmia | 57 (15.0) | 40 (21.2) | 6 (11.5) | 8 (7.1) a | 2 (14.3) | 1 (9.1) |
| Coronary artery disease | 54 (14.3) | 31 (16.4) | 6 (11.5) | 11 (9.7) | 3 (21.4) | 3 (27.3) |
| Thyroid disorders | 52 (13.7) | 33 (17.5) | 4 (7.7) | 12 (10.6) a | 1 (7.1) | 2 (18.2) |
| Chronic renal insufficiency | 44 (12.6) | 27 (15.3) | 7 (14.3) | 8 (8.0) | 1 (7.7) | 1 (9.1) |
| Tobacco use | 42 (11.1) | 26 (13.8) | 6 (11.5) | 8 (7.1) | 0 (0) | 2 (18.2) |
| Congestive heart failure | 38 (10.1) | 21 (11.2) | 6 (11.5) | 10 (8.9) | 1 (7.1) | 0 (0) |
| Cerebrovascular accidents | 12 (3.2) | 6 (3.2) | 2 (3.9) | 4 (3.5) | 0 (0) | 0 (0) |
| Insurance | ||||||
| Public | 156 (41.2) | 34 (18.0) | 21 (40.4) a | 71 (62.8) a | 2 (14.3) | 2 (18.2) |
| Private | 130 (34.3) | 82 (43.4) | 23 (44.2) | 37 (32.7) | 8 (57.1) | 6 (54.6) |
| Combination private/public | 90 (23.8) | 73 (38.6) | 7 (13.5) | 3 (2.7) | 4 (28.6) | 3 (27.3) |
| No insurance | 3 (0.79) | 0 (0) | 1 (1.9) | 2 (1.8) | 0 (0) | 0 (0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
a P < .05 vs white.
Overall, these COVID-19 patients presented to the hospital with a wide array of symptoms, including systemic, pulmonary, cardiac, and gastrointestinal symptoms, as detailed in Table 2. On presentation, Latinx patients more frequently reported fevers (91.2% vs 64.6%, P = .009) and myalgias (62.0% vs 33.3%, P = .002). Examination of initial laboratory values revealed higher alanine aminotransferase levels for Latinx patients compared with white patients (48.3 ± 42.9 vs 34.0 ± 31.5, P = .002). Black, Latinx, and Asian patients all had lower white blood cell (WBC) counts on average than did white patients. Otherwise, there were no significant differences in presenting symptoms or laboratory results by race/ethnicity (Table 2).
Table 2.
Clinical Symptoms and Laboratory Values of Hospitalized Coronavirus Disease 2019 Patients at Time of Presentation
| Clinical Characteristics | All Coronavirus Disease 2019 (N = 379) | White (n = 189) | Black (n = 52) | Latinx (n = 113) | Asian (n = 14) | Others (n = 11) |
|---|---|---|---|---|---|---|
| General symptoms, n (%) | ||||||
| Fever | 313 (82.6) | 149 (78.8) | 42 (80.8) | 103 (91.2) a | 10 (71.4) | 9 (81.8) |
| Fatigue | 223 (58.8) | 122 (64.6) | 23 (44.2) a | 60 (53.1) | 10 (71.4) | 8 (72.7) |
| Myalgia | 157 (41.4) | 63 (33.3) | 12 (23.1) | 70 (62.0) a | 6 (42.9) | 6 (54.6) |
| Chills | 94 (24.8) | 43 (22.8) | 11 (21.2) | 35 (31.0) | 2 (14.3) | 3 (27.3) |
| Diaphoresis | 23 (6.1) | 16 (8.5) | 1 (1.9) | 5 (4.4) | 0 (0) | 1 (9.1) |
| Arthralgia | 14 (3.7) | 8 (4.2) | 1 (1.9) | 4 (3.5) | 0 (0) | 1 (9.1) |
| Airway symptoms, n (%) | ||||||
| Cough | 302 (79.7) | 153 (81.0) | 38 (73.1) | 93 (82.3) | 9 (64.3) | 9 (81.8) |
| Dyspnea | 242 (63.9) | 116 (61.4) | 30 (57.7) | 80 (70.8) | 6 (42.9) | 10 (90.1) |
| Sore throat | 71 (18.8) | 37 (19.6) | 3 (5.9) | 29 (25.7) | 1 (7.1) | 1 (9.1) |
| Sputum production | 60 (15.8) | 32 (16.9) | 13 (25.0) | 13 (11.5) | 1 (7.1) | 1 (9.1) |
| Rhinorrhea | 56 (14.8) | 28 (14.8) | 4 (7.8) | 22 (19.5) | 1 (7.1) | 1 (9.1) |
| Loss of smell or taste, n (%) | ||||||
| Anosmia | 36 (9.5) | 18 (9.5) | 5 (9.6) | 10 (8.9) | 0 (0) | 3 (27.3) |
| Ageusia | 27 (7.2) | 11 (5.9) | 5 (9.8) | 9 (8.0) | 1 (7.1) | 1 (9.1) |
| Gastrointestinal symptoms, n (%) | ||||||
| Anorexia | 136 (36.0) | 70 (37.0) | 21 (41.2) | 36 (31.9) | 4 (28.6) | 5 (45.5) |
| Diarrhea | 124 (32.7) | 69 (36.5) | 13 (25.0) | 36 (31.9) | 3 (21.4) | 3 (27.3) |
| Nausea | 98 (25.9) | 49 (25.9) | 12 (23.1) | 27 (23.9) | 7 (50.0) | 3 (27.3) |
| Vomiting | 58 (15.3) | 31 (16.4) | 6 (11.5) | 16 (14.2) | 4 (28.6) | 1 (9.1) |
| Abdominal pain | 54 (14.3) | 24 (12.8) | 7 (13.5) | 21 (38.9) | 1 (7.1) | 1 (9.1) |
| Weight loss | 41 (10.9) | 28 (14.9) | 5 (9.6) | 6 (5.3) | 2 (14.3) | 0 (0) |
| Laboratory results | ||||||
| White blood cell count, ×109/L | 7.7 ± 9.5 | 8.6 ± 13.2 | 6.6 ± 2.7 | 6.8 ± 2.9 | 6.7 ± 2.7 | 8.2 ± 2.9 |
| Lymphocytes, ×109/L | 1.7 ± 8.2 | 2.3 ± 11.6 | 1.2 ± 0.9 | 1.2 ± 1.0 | 0.78 ± 0.35 | 1.0 ± 0.6 |
| Hemoglobin, g/L | 18.2 ± 12.8 | 18.3 ± 11.5 | 17.0 ± 12.0 | 17.2 ± 9.8 | 26.4 ± 36.1 | 17.3 ± 9.8 |
| Platelets, ×109/L | 196.5 ± 83.2 | 190.5 ± 82.7 | 209.8 ± 101.2 | 198.7 ± 79.0 | 180.8 ± 53.3 | 218.5 ± 67.3 |
| International normalized ratio | 1.22 ± 0.47 | 1.29 ± 0.56 | 1.22 ± 0.53 | 1.12 ± 0.20 | 1.11 ± 0.14 | 1.18 ± 0.15 |
| Creatinine | 2.16 ± 13.3 | 2.31 ± 15.7 | 1.14 ± 0.45 | 2.59 ± 13.6 | 1.38 ± 1.34 | 1.10 ± 0.30 |
| Aspartate aminotransferase, U/L | 53.9 ± 67.5 | 48.7 ± 54.9 | 69.6 ± 130.9 | 56.5 ± 43.8 | 50.9 ± 49.7 | 48.6 ± 45.3 |
| Alanine aminotransferase, U/L | 38.1 ± 35.3 | 34.0 ± 31.5 | 31.5 ± 22.3 | 48.3 ± 42.9a | 39.9 ± 49.0 | 34.9 ± 28.6 |
| Alkaline phosphate, mmol/L | 81.7 ± 43.4 | 84.0 ± 47.2 | 74.5 ± 34.2 | 84.4 ± 41.7 | 73.1 ± 39.9 | 54.3 ± 12.8 |
| Total bilirubin, mmol/L | 0.62 ± 1.01 | 0.60 ± 0.59 | 0.84 ± 2.29 | 0.58 ± 0.69 | 0.48 ± 0.25 | 0.60 ± 0.35 |
| C-reactive protein | 97.4 ± 83.7 | 95.7 ± 90.5 | 99.5 ± 81.2 | 97.4 ± 74.8 | 76.6 ± 59.4 | 118.4 ± 78.7 |
| D-dimer, nmol | 1501.7 ± 2031 | 1557.4 ± 2265 | 1750.5 ± 1780 | 1265.1 ± 1796 | 1399.9 ± 1010 | 2224.1 ± 2545 |
| Ferritin | 835.2 ± 922.4 | 807.4 ± 871.3 | 882.0 ± 1429 | 865.9 ± 813.6 | 811.8 ± 585.4 | 819.6 ± 829.9 |
| Lactate | 1.64 ± 1.02 | 1.60 ± 0.68 | 1.54 ± 0.85 | 1.70 ± 0.48 | 2.48 ± 0.82 | 1.46 ± 0.94 |
a P < .05 vs white.
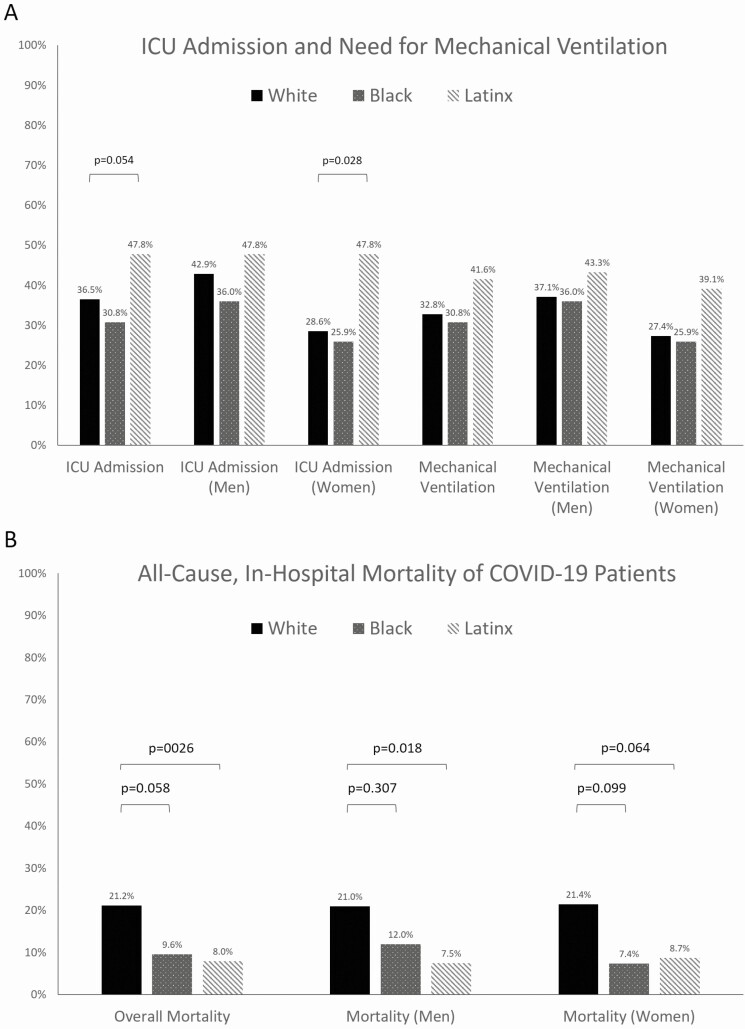
Next, we examined the clinical outcomes of intensive care unit (ICU) admission, need for mechanical ventilation, and all-cause, in-hospital mortality. Overall, there were 150 (39.6%) ICU admissions (69/189 [36.5%] whites, 16/52 [30.8%] blacks, and 54/113 [47.8%] Latinx), most notably with a significantly higher rate of ICU admission among Latinx patients. A total of 135 (35.6%) patients required mechanical ventilator support, with 96 (71.9%) successfully extubated and 38 (28.1%) deaths (ie, in-hospital mortality). The need for mechanical ventilator support did not differ significantly by race/ethnicity (62/189 [32.8%] whites, 16/52 [30.8%] blacks, and 47/113 [41.6%] Latinx; Figure 1A). When these outcomes were further examined by both gender and race/ethnicity, the difference in rate of ICU admission between Latinx and white patients was mainly driven by the lower ICU admission rate among white women compared with white men and Latinx women. The other relationships between race/ethnicity and the outcomes of ICU admission, need for mechanical ventilator support, and in-hospital mortality observed on univariate analyses were similar across genders. On multivariable analysis, using logistic regression controlling for age, obesity, gender, and cardiopulmonary comorbidities, race/ethnicity was not significantly associated with ICU admission or with a need for mechanical ventilation (Table 3). There were no significant predictors of ICU admission found in this multivariable analysis; however, male gender and history of diabetes were strong predictors of ICU admission. A history of diabetes mellitus was the only significant predictor of needing mechanical ventilation, while male gender and a history of hypertension or cardiac disease were strong but nonsignificant predictors of needing mechanical ventilation.
Figure 1.
Hospitalization outcomes of patients with COVID-19 infection by race/ethnicity. A, ICU admission rate and need for mechanical ventilation. B, All-cause, in-hospital mortality rate. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Table 3.
Multivariable Models for Predictors of Need for Mechanical Ventilation and In-Hospital Mortality on Presentation Among Hospitalized Coronavirus Disease 2019 Patients
| Multivariable Regression Model for Need for Mechanical Ventilation | |||
|---|---|---|---|
| Covariate | OR | 95% CI | P Value |
| Race | |||
| Black vs White | 0.89 | .45–1.79 | .45 |
| Latinx vs White | 1.34 | .79–2.28 | .21 |
| Age | 1.51 | .89–2.54 | .13 |
| Sex (Male) | 1.53 | .95–2.45 | .08 |
| Obesity | 1.07 | .67–1.71 | .78 |
| History of cardiac diseasea | 0.54 | .28–1.04 | .06 |
| History of pulmonary disordersb | 1.10 | .61–1.99 | .76 |
| Hypertension | 0.59 | .35–1.01 | .05 |
| Diabetes | 1.89 | 1.11–3.23 | .02 |
| Multivariable Regression Model for Intensive Care Unit | |||
| Covariate | OR | 95% CI | P Value |
| Race | |||
| Black vs White | 0.77 | .39–1.53 | .17 |
| Latinx vs White | 1.50 | .89–2.52 | .15 |
| Age | 1.35 | .81–2.25 | .25 |
| Sex (Male) | 1.50 | .95–2.37 | .08 |
| Obesity | 1.04 | .66–1.65 | .86 |
| History of cardiac diseasea | 0.70 | .38–1.30 | .26 |
| History of pulmonary disordersb | 0.90 | .50–1.62 | .73 |
| Hypertension | 0.73 | .44–1.22 | .23 |
| Diabetes | 1.55 | .93–2.61 | .10 |
| Multivariable Regression Model for In-Hospital Mortality | |||
| Covariate | OR | 95% CI | P Value |
| Race | |||
| Black vs White | 0.39 | .13–1.12 | .23 |
| Latinx vs White | 0.55 | .23–1.29 | .79 |
| Age | 4.18 | 1.94–9.04 | .0003 |
| Sex (Male) | 1.07 | .55–2.08 | .85 |
| Obesity | 0.69 | .35–1.37 | .29 |
| History of cardiac diseasea | 1.22 | .58–2.53 | .60 |
| History of pulmonary disordersb | 1.52 | .72–3.20 | .27 |
| Hypertension | 2.15 | .94–4.91 | .07 |
| Diabetes | 1.97 | .97–4.02 | .06 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aIncludes a history of coronary artery disease, congestive heart failure, or arrhythmia.
bIncludes a history of asthma, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary hypertension, or pulmonary embolism.
Overall, 57 (15.0%) patients in this cohort died during their hospitalization with COVID-19. All-cause, in-hospital mortality was higher among white patients than black patients (21.1% vs 9.6%, P = .058) and Latinx patients (21.2% vs 8.0%, P = .003), as can be seen in Figure 1B. When mortality was examined by both gender and race/ethnicity, we found that these relationships were similar between male and female patients. On multivariable analysis using logistic regression controlling for age, gender, obesity, and cardiopulmonary comorbidities, no significant association was found between race/ethnicity and in-hospital mortality (Table 3). Older age was the only significant predictor of in-hospital mortality (odds ratio, 4.18; 95% confidence interval, 1.94–9.04) in this multivariable analysis, while a history of diabetes mellitus or hypertension was a strong but nonsignificant predictor of in-hospital mortality.
DISCUSSION
In the midst and aftermath of pandemics or natural disasters, racial/ethnic minority populations have been shown to have decreased access to healthcare and higher rates of adverse health outcomes [5–7]. Additionally, preexisting societal inequities, many of which are a result of long-standing structural racism, place black and Latinx communities and individuals at greater risk of being adversely affected by such disasters. Densely populated communities and homes, along with commutes dependent upon public transportation to “essential jobs” and high comorbid rates of cardiopulmonary diseases and diabetes mellitus, generally lead to increased rates of transmission and mortality from communicable diseases. Unfortunately, this further exacerbates existing healthcare disparities [8, 9]. Consistent with this dire historic trend, a disproportionate impact of the COVID-19 epidemic on black and Latinx communities has already been well documented, including substantially higher infection rates, need for hospitalization, and COVID-19–related death [4, 10, 11].
Our study aimed to examine whether hospitalized patients with COVID-19 have similar presenting symptoms and comorbid conditions by race/ethnicity and whether these patients ultimately have similar outcomes. The state of Massachusetts has developed an excellent dashboard for tracking COVID-19 cases, hospitalizations, and deaths, yet few conclusions can be drawn regarding how COVID-19 varies in terms of incidence or outcomes by race/ethnicity throughout the state [12] due to the sizable proportion of patients with missing race and ethnicity identity data. It is quite apparent from data available from the city of Boston [13], however, that there is a substantially higher COVID-19 infection rate among black residents, especially among those from lower-income sections of the city. Furthermore, in the neighboring city of Chelsea, which has the highest COVID-19 incidence in Massachusetts, an alarmingly disproportionate infection rate exists among Latinx patients [13–15]. Our findings reported here do not refute those data but rather demonstrate that despite this disproportionate infection rate among black and Latinx individuals, when their disease is severe enough to require hospitalization, these patients do just as well in terms of important outcomes, including mortality.
Thus, this study provides one of the first insights into the outcomes of patients hospitalized with COVID-19 by race/ethnicity within Boston and in Massachusetts more broadly. These findings are similar to those from another recent retrospective, patient-level study that used coded data from electronic medical records of ambulatory and hospitalized patients in Louisiana [16]. In that study of more than 3000 patients, black race was not associated with higher in-hospital mortality when compared with white race, after adjustment for potential confounders. Another study from California demonstrated that several sociodemographic and clinical characteristics, including race/ethnicity, were likely to increase the risk of hospitalization, with black patients having a rate of hospitalization more than double that of white patients, even after controlling for confounders [17]. While black patients in Massachusetts may also have a higher likelihood of hospitalization for COVID-19, we did not have the ability to assess this point. We specifically performed extensive chart review to examine outcomes and clinically relevant measures that occurred during the hospital stay, finding no significant association between race/ethnicity and key outcomes of hospitalization, including ICU admission, need for mechanical ventilation, and in-hospital mortality.
We found few differences in presenting symptoms or laboratory studies by race/ethnicity. We did, however, find that black, Latinx, and Asian patients all had lower WBC counts on presentation when compared with white patients. This finding of heterogeneity of hematologic parameters among racial/ethnic groups has been described previously, particularly among black individuals [18, 19]. Importantly, however, these lower initial WBC counts were not associated with poorer health outcomes among these non-white patients. In our cohort, hospitalized Latinx patients were younger, with fewer underlying cardiopulmonary comorbidities compared with white patients, and they were significantly more likely to present with fever and myalgias. This may suggest that Latinx patients presented later or with a more severe disease phenotype among younger, healthier patients; although, interestingly, markers of disease severity and risk of deterioration on admission, such as mean D-dimer and ferritin levels, were not statistically different between the groups. Of note, Latinx patients had a higher mean BMI, with a majority of them (56.6%) classified as obese. Obesity may be an early marker of impaired metabolic health and has emerged as an important risk factor for severe illness among those with COVID-19 [20]. Despite the disproportionate hospitalization rate of black patients noted by others and the different clinical characteristics of our Latinx cohort, we found no statistically significant difference in hospitalization outcomes based upon race/ethnicity, even after adjusting for age, gender, BMI, and cardiopulmonary comorbidities [4, 17].
Our findings may be explained in part by the characteristics of our included population. While multicenter in design, this was a retrospective analysis limited to the largest health system in Massachusetts, a state where the vast majority of residents have health insurance due to healthcare reform in 2006 [21]. In fact, nearly every patient (99.2%) in our cohort had some form of health insurance coverage. As demonstrated by prior studies, disparities, while still present, are less prevalent in Massachusetts post healthcare reform, with improved coverage likely mitigating delays in presentation due to lack of insurance or under-insurance and its subsequent financial burden [22, 23]. However, our cohort contained a relatively small percentage of black patients, and the care experience of this group of black patients may differ from that of black patients admitted to hospitals that serve mostly black patients and other patients of color. Therefore, given this healthcare system’s location, as well as the substantial resources available and utilized to treat all patients, our findings may not be generalizable to patients treated at safety net hospitals or in settings where more patients are uninsured or underinsured. It is possible that safety net hospitals and other hospitals that primarily serve black and/or Latinx patients may not be able to achieve outcomes similar to those from our system; these hospitals are often underresourced and understaffed, especially in times of crisis such as the current pandemic [16, 17, 24]. Furthermore, in addition to the delays in evaluation that result from safety net hospitals being overwhelmed amidst the crisis, patient awareness of resource limitations and hospital constraints may cause patients to delay seeking care, ultimately worsening their outcome [24]. While we endeavored to evaluate whether outcomes differed by race or ethnicity within the largest healthcare system within the state of Massachusetts, for the reasons detailed above, our results may not be generalizable to the general US population. Nonetheless, these results provide insight into outcomes by race/ethnicity when there is equal access to care in terms of health insurance coverage and geography and when the quality of inpatient care is similar, with few resource limitations, including equal access to clinical trials.
There are several important strengths to this study, including the strict enrollment criteria of laboratory-confirmed SARS-CoV-2 infection, patient-level examination of hospitalized cases, inclusion of both tertiary and community hospitals, and the detailed manual review of data, including patient self-identified race/ethnicities. Importantly, our hospital system has a system-wide consistent and validated methodology for collecting patients’ self-reported race/ethnicity. These highly accurate data on racial/ethnic self-identity are found in nearly all patients’ medical records, with few missing data, which enhances the validity of our analysis and findings. Furthermore, despite this study’s modest size, to our knowledge, this is the largest cohort to date of hospitalized US adults to specifically examine the impact of race/ethnicity on COVID-19 symptoms and health-related outcomes. As of 2 April 2020, there were 8966 confirmed cases of COVID-19 within the state of Massachusetts, of which 813 were hospitalized [25]. Thus, 46.6% of all COVID-19 hospitalizations to date in Massachusetts were included in this study. While there is evidence of black and Latinx individuals being affected and potentially infected at higher rates compared with white individuals (at least in Boston and nearby cities), their outcomes, once hospitalized, did not appear to differ by their race/ethnicity in our cohort.
Our study also has several important limitations. One limitation, mentioned previously, is the high rate of health insurance among residents of the state of Massachusetts, which differs from many other states, thus limiting this study’s generalizability to locations in which a higher proportion of the population, especially black and Latinx individuals, are uninsured or underinsured. A second and related limitation, as detailed above, is that our hospitals may not be representative of the type of setting where most black and Latinx patients receive care in the United States. A third limitation is that our study included only patients hospitalized in the early weeks of the COVID-19 pandemic in Massachusetts. While we believe that these weeks are representative of the entire pandemic in the state, it is unknown whether this may have in some way biased our findings. Finally, we only examined outcomes among hospitalized patients. It may be that pre-hospital or post-hospitalization deaths due to COVD-19 may differ by race/ethnicity and thus remain unaccounted for in this study. This is important as there is evidence of population-level disproportionate death rates among black and Latinx individuals in Massachusetts at the time of the COVID-19 pandemic, which are not explained by our study’s findings [26].
In summary, we found no association between race/ethnicity and clinically relevant outcomes of hospitalization, including mortality, among patients hospitalized with COVID-19 in the largest hospital system in Massachusetts, after controlling for key demographic and clinical characteristics. These findings serve to refute potential hypotheses that certain races or ethnicities may be biologically predisposed to poorer outcomes of COVID-19. Furthermore, while societal inequities, including structural racism, have led to higher COVID-19 infection rates and higher hospitalization rates among black and Latinx individuals in the United States, our data, along with those from Louisiana, demonstrate that these disparities do not necessarily lead to higher in-hospital mortality rates [10, 16, 27]. Further large-scale, population-based studies are needed, particularly in areas with less equitable health insurance coverage and geographic access to care, to further assess both the immediate and long-term differential impact of COVID-19 outcomes by race/ethnicity.
Notes
Author contributions. Study concept and design: T. R. M., K. E. H., W. D. R., L. S., and W. W. C. Data acquisition: W. D. R., J. C. Z., K. E. H., T. R. M., N. J. R., A. N. B., C. N., D. W., and L. S. Paper preparation and statistical analyses: T. R. M., K. E. H., W. D. R., Q. D. T., J. C. Z., A. N. B., V. E. S., and W. W. C. Critical revisions: W. D. R., J. C. Z., K. E. H., T. R. M., A. N. B., C. N., D. W., Q. D. T., L. S., V. E. S., and W. W. C. Administrative support and overall study supervision: W. W. C.
Financial support. This work was funded, in part, by a National Institutes of Health grant (T32 DK007533–35 to K. E. H.). Q. D. T. is supported by a Health Services Research pilot test grant from the Defense Health Agency and an unrestricted educational grant from the Vattikuti Urology Institute.
Potential conflicts of interest. Q. D. T. reports personal fees from Astellas, Bayer, Janssen, Insightec, and Intuitive Surgical. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johns Hopkins University and Medicine. Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/map.html. Accessed 1 May 2020.
- 2.World Health Organization. Coronarvirus Disease (COVID-19) Pandemic. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 1 May 2020.
- 3. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Cases in U.S. Updated April 11, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 12 April 2020.
- 4. Garg S, Kim L, Whitaker M, O’Hallran A, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. United States Centers for Disease Control and Prevention. Morb Mortal Wkly Rep 2020; 69:458–64. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6915e3-H.pdf. Accessed 12 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrulis DP, Siddiqui NJ, Gantner JL. Preparing racially and ethnically diverse communities for public health emergencies. Health Aff 2007; 26:1269–79. [DOI] [PubMed] [Google Scholar]
- 6. Fothergill A, Maestas EG, Darlington JD. Race, ethnicity and disasters in the United States: a review of the literature. Disasters 1999; 23:156–73. [DOI] [PubMed] [Google Scholar]
- 7. Hutchins SS, Fiscella K, Levine RS, Ompad DC, McDonald M. Protection of racial/ethnic minority populations during an influenza pandemic. Am J Public Health 2009; 99(Suppl 2):S261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blumenshine P, Reingold A, Egerter S, Mockenhaupt R, Braveman P, Marks J. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis 2008; 14:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinn SC, Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur Bioterror 2014; 12:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yancy CW. COVID-19 and African Americans. JAMA 2020; 323:1891–2. [DOI] [PubMed] [Google Scholar]
- 11. New York State Department of Health. COVID-19 Fatalities. Updated April 22, 2020. Available at: https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n. Accessed 12 April 2020.
- 12. Massachusetts Department of Public Health COVID-19 Dashboard, April 22, 2020. Available at: https://www.mass.gov/doc/covid-19-dashboard-april-22–2020/download. Accessed 22 April 2020.
- 13. COVID-19 Health Inequities Task Force–Boston Mayor’s Office. Racial Data On Boston Resident COVID-19 Cases. Available at: https://www.boston.gov/departments/mayors-office/racial-data-boston-resident-covid-19-cases. Accessed 22 April 2020.
- 14. City of Chelsea Massachusetts. A Message to Chelsea Residents. Posted April 9, 2020. Available at: https://www.chelseama.gov/home/news/message-chelsea-residents. Accessed 22 April 2020.
- 15.Massachusetts Department of Public Health. Coronavirus Disease 2019 (COVID-19) Cases in Massachusetts as of May 6, 2020: Count and Rate (per 100,000) of Confirmed COVID-19 Cases in MA by City/Town, January 1, 2020–May 6, 2020. Available at: https://www.mass.gov/doc/confirmed-covid-19-cases-in-ma-by-citytown-january-1-2020-may-6-2020-0/download. Accessed 8 May 2020.
- 16. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020; 382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff 2020. doi:101377hlthaff202000598. [DOI] [PubMed] [Google Scholar]
- 18. Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. Int J Lab Hematol 2010; 32:590–7. [DOI] [PubMed] [Google Scholar]
- 19. Saxena S, Wong ET. Heterogeneity of common hematologic parameters among racial, ethnic, and gender subgroups. Arch Pathol Lab Med 1990; 114:715–9. [PubMed] [Google Scholar]
- 20. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol 2020; 16:341–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. 187th General Court of the Commonwealth of Massachusetts. Massachusetts General Laws, chap. 6A, sec. 16L, Health Care Quality and Cost Council. Available at: https://malegislature.gov/Laws/SessionLaws/Acts/2006/Chapter58. Accessed 14 April 2020.
- 22. Kolstad JT, Kowalski AE. The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ 2012; 96:909–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loehrer AP, Song Z, Auchincloss HG, Hutter MM. Massachusetts health care reform and reduced racial disparities in minimally invasive surgery. JAMA Surg 2013; 148:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khatana SAM, Groeneveld PW. Health disparities and the coronavirus disease 2019 (COVID-19) pandemic in the USA. J Gen Intern Med 2020; 35:2431–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massachusetts Department of Public Health. Coronavirus Disease 2019 (COVID-19) Cases in MA: as of April 2, 2020. Available at: https://www.mass.gov/doc/covid-19-cases-in-massachusetts-as-of-april-2-2020/download. Accessed 14 April 2020.
- 26. Chen JT, Waterman PD, Krieger N. COVID-19 and the unequal surge in mortality rates in Massachusetts, by city/town and ZIP Code measures of poverty, household crowding, race/ethnicity, and racialized economic segregation. Harvard Center for Population and Development Studies Working Paper Series, Volume 19, Number 2. May 9, 2020. Available at: https://www.hsph.harvard.edu/population-development/research/working-papers/harvard-pop-center-working-paper-series/. Accessed 10 June 2020. [Google Scholar]
- 27. Williams DR, Cooper LA. COVID-19 and health equity— a new kind of “herd immunity.” JAMA 2020. doi: 10.1001/jama.2020.8051. [DOI] [PubMed] [Google Scholar]