Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). This study aimed to determine if SARS-CoV-2 RNA in serum at admission correlated with clinical outcome in COVID-19.
Methods
COVID-19 patients admitted to the infectious diseases department of a tertiary level Swedish hospital and sampled for SARS-CoV-2 RNA in serum at admission during 10 April to 30 June 2020 were included. Primary outcomes were day 28 all-cause mortality and progress to critical disease.
Results
The cohort (N = 167) consisted of 106 SARS-CoV-2 RNA serum-negative and 61 serum-positive patients. Median sampling time for initial SARS-CoV-2 in serum was 1 day (interquartile range [IQR], 1–2 days) after admission, corresponding to day 10 (IQR, 8–12) after symptom onset. Median age was 53 years (IQR, 44–67 years) and 63 years (IQR, 52–74 years) for the serum–negative and -positive patients, respectively. In the serum-negative and -positive groups, 3 of 106 and 15 of 61 patients died, respectively.
The hazard ratios for critical disease and all-cause mortality were 7.2 (95% confidence interval [CI], 3.0–17) and 8.6 (95% CI, 2.4–30), respectively, for patients with serum–positive compared to serum–negative results.
Conclusions
SARS-CoV-2 RNA in serum at hospital admission indicates a high risk of progression to critical disease and death.
Keywords: SARS-CoV-2, COVID-19, viremia, viral load, mortality
Severe acute respiratory syndrome coronavirus 2 RNA in serum at admission was associated with a 7-fold increased risk of critical disease and an 8-fold increased risk of death in a cohort of 167 patients hospitalized for coronavirus disease 2019.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the current coronavirus disease 2019 (COVID-19) pandemic [1]. COVID-19 is characterized by fever, respiratory tract, and gastrointestinal symptoms with multiple organ involvement in severe disease [2]. A proportion of patients develop critical disease with acute respiratory distress syndrome and high mortality rates [2, 3].
A “cytokine storm” has been proposed as the cause of severe COVID-19, based on the detection of high levels of cytokines [4]. Similar findings were described in critically ill patients with severe acute respiratory syndrome (SARS) [5]. In SARS, high serum viral load and detection of virus at multiple sites were predictive of adverse clinical outcome. These findings indicated that adverse outcome was associated with continued, uncontrolled viral replication [6]. Similarly, a high viral load in respiratory samples was associated with more severe disease in Middle East respiratory syndrome (MERS) and has also been described in COVID-19 [7–10]. Oropharyngeal viral load also appears to increase with age in COVID-19 [11]. In line with these findings, SARS-CoV-2 RNA in serum was detected in 5 of 17 critically ill patients, of whom 2 died, and in none of 31 patients with severe or moderate COVID-19 [12]. Moreover, in a very recent study, SARS-CoV-2 RNA was detected in serum in 43 of 58 (74%) of hospitalized COVID-19 patients with an ultrasensitive polymerase chain reaction (PCR) method [13]. The presence and levels of SARS-CoV-2 RNA in serum were correlated to disease severity, and there was a tendency toward a higher risk of poor outcome in the serum-positive group. An inability to control viral replication, and a disseminated hematogenous spread to multiple organs, is thus a possible cause of severe disease or possibly the driving force behind the inflammatory reaction that characterizes severe COVID-19. Detection of viral RNA in serum may thus be an early prognostic marker that is simpler to use compared to more complex risk scoring systems [14, 15]. However, the prognostic value of detecting serum SARS-CoV-2 RNA with a commonly available method has not been evaluated. The primary aim of this study was to determine if SARS-CoV-2 RNA in serum, detected using standard reverse-transcription PCR methods, correlated with clinical outcome in COVID-19.
MATERIALS AND METHODS
Study Design and Setting
This was a retrospective cohort study of patients with confirmed COVID-19, consecutively admitted to the 38-bed Department of Infectious Diseases at Danderyd Hospital, between 10 April and 30 June 2020. Danderyd Hospital is a 500-bed tertiary care hospital in Stockholm, Sweden, that serves a population of approximately 500 000 people.
The study was approved by the Swedish Ethical Review Authority (registration number 2020-01770).
Study Population
The study population consisted of patients with confirmed COVID-19 who were admitted to the Department of Infectious Diseases and had a SARS-CoV-2 RNA serum sample taken within 3 days of admission. All patients admitted to the hospital during the study period with symptoms suggestive of COVID-19 were screened for SARS-CoV-2 in airway (nasopharyngeal, throat, or sputum) samples by PCR assays. Patients with positive results were considered to have a confirmed diagnosis.
At the beginning of the study period, the influx of patients was considerable and patients with confirmed COVID-19 were admitted to medical and surgical wards as well as the Department of Infectious Diseases. During this period there was no particular selection of any patient category to any particular ward.
A phase 1/2 safety and dose-finding study on convalescent plasma was conducted at the Department of Infectious Diseases during the study period. Febrile patients requiring supplemental oxygen therapy were initially eligible for entry into the phase 1 study. Subsequently, patients with SARS-CoV-2 RNA detected in serum were eligible for entry to the phase 2 study. No other interventional studies were ongoing in this population. No antivirals or chloroquine derivatives were in use for the treatment of COVID-19 during the study period. Corticosteroid treatment was introduced for patients with critical disease starting 18 June 2020.
Data Collection
Serum sampling was part of routine and evolving clinical practice. Between 10 April and 10 May 2020, serum samples from patients perceived to be more ill were collected for SARS-CoV-2 PCR analysis. Serum samples for SARS-CoV-2 PCR analysis were systematically collected from patients with confirmed COVID-19 admitted to the Department of Infectious Diseases between 11 May and 30 June 2020.
Department records were accessed to identify all patients with a PCR-confirmed diagnosis of COVID-19 between 10 April and 30 June 2020. The research team accessed the electronic medical records of the patients in the cohort and extracted epidemiological, clinical, laboratory, treatment, and outcome data, retrospectively. Patient characteristics, including routine blood samples and clinical parameters, were noted the same day as the first SARS-CoV-2 PCR analysis from serum was taken. If data were missing, we used the closest known value from ±1 day. No imputation of missing data was done.
All patients were followed for 28 days after SARS-CoV-2 RNA serum sampling. The electronic medical records are linked and synchronized to the Swedish Population Register, which enables follow-up of mortality regardless of cause and place of death.
PCR Analyses
PCR analyses were performed at the Karolinska University Laboratory according to normal routine procedures for SARS-CoV-2 RNA detection. Airway and serum samples were analyzed on the Cobas 6800 instrument, a fully automated test including RNA extraction (Roche Molecular Diagnostics, Pleasanton, California) or an in-house method with RNA extraction (MagNA Pure 96, Roche Diagnostics) followed by a modified version (unpublished data) of the Drosten protocols for PCR analysis [16, 17]. The 2 PCR methods had similar performance in in-house assessments (Supplementary Data). The Cobas targets the envelope (E) and open reading frame 1 (ORF1) genes. The in-house assay targets the E-gene and the RNA-dependent RNA polymerase (RdRp) gene. Amplification of at least 1 gene was considered a positive test. Airway samples were also analyzed using the GeneXpert system (Cepheid, Sunnyvale, California) from 27 April 2020 [18]. Cycle threshold (Ct) values were recorded from serum PCR assays. Ct values were not recorded for airway PCRs as nasopharyngeal, throat swabs, and sputum samples were analyzed and assessed on 3 different systems, preventing meaningful analyses.
Outcome
The primary outcome was all-cause mortality at 28 days after first SARS-CoV-2 serum sampling. The secondary outcome was progression to critical disease within 28 days of the initial SARS-CoV-2 serum sampling. Critical disease was defined as a composite of intensive care unit (ICU) care and mortality. The hospital’s ICU consisted of 1 ward for SARS-CoV-2 patients requiring mechanical ventilation, and 2 wards for patients requiring noninvasive ventilation, high-flow nasal oxygen therapy, or inotropic drugs. During the study period, the ICUs had an admission criterion of peripheral capillary oxygen saturation <90% despite 12 L/minute oxygen on a mask (partial pressure of oxygen/fraction of inspired oxygen ratio approximately 75). At the Department of Infectious Diseases, oxygen was given using a mask but not using noninvasive ventilation or high-flow nasal oxygen.
Statistical Analysis
Patients who were SARS-CoV-2 serum PCR negative at study entry, in effect on admission, were classed as PCR negative. Patients who were SARS-CoV-2 serum PCR positive at entry were classed as PCR positive. Continuous variables are presented as median (interquartile range [IQR]) and were calculated using quantile regression with bootstrap (100 reps). Hazard ratios (HRs) were calculated with 95% confidence intervals (CIs) using univariate and multivariate Cox regression. Variables that have been previously described as predictors for severe outcome (age, sex, body mass index, hypertension, diabetes, C-reactive protein, lymphocyte count, cardiovascular and pulmonary disease) were included in the univariate analyses [19]. Due to missing data and difficulties with standardized interpretations, lactate dehydrogenase and chest radiographs were not included in the statistical analyses. Variables with a P value < .05 in the univariate analysis were included in the multivariate analysis. The proportional hazards assumption was tested with a log-log plot of survival. We constructed Kaplan-Meier survival curves for all-cause mortality and progression to critical disease. The log-rank test was used to test for equality of survivor functions. All tests were 2-sided and a P value < .05 was considered significant. Statistical analyses were done with Stata version 13.1 software (StataCorp, College Station, Texas).
RESULTS
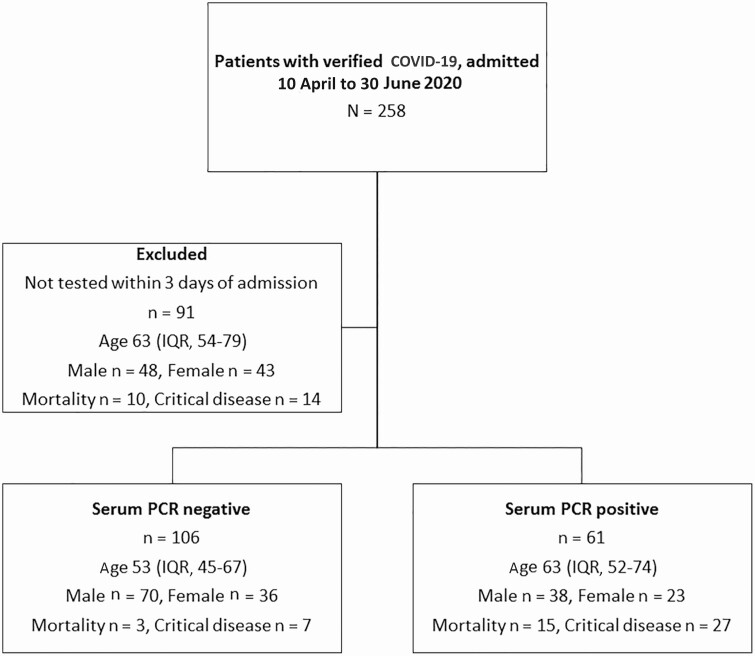
A total of 258 patients with confirmed COVID-19 were admitted to the Department of Infectious Diseases during the study period. Of these, 167 were tested for SARS-CoV-2 RNA in serum within 3 days of admission. SARS-CoV-2 RNA in serum was detected in 61 of 167 (37%) patients and not detected in 106 of 167 (63%) patients. A total of 91 patients were excluded from the study. Of these, 77 were not tested, 10 were tested >3 days after admission, and 4 were tested during/after ICU care. The flow of patients through the study is shown in Figure 1. Baseline characteristics are shown in Table 1. Routine serum sampling of patients admitted to the Department of Infectious Diseases was implemented from 10 May until 30 June. In this subgroup, 69 of 106 (65%) patients were PCR negative and 37 of 106 (35%) were PCR positive.
Figure 1.
Flowchart for study population. Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; PCR, polymerase chain reaction.
Table 1.
Demographic and Clinical Characteristics at Time of First Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Polymerase Chain Reaction (PCR) Assay in Serum, by SARS-CoV-2 Serum PCR Status
| Demographics | PCR Negative (n = 106) |
PCR Positive (n = 61) |
Complete Data, No. (%) |
|---|---|---|---|
| Age, y | 53 (45–67) | 63 (52–74) | 167 (100) |
| Sex, No. (%), female:male | 36:70 (34:66) | 23:38 (38:62) | 167 (100) |
| Any comorbidity, No. (%) | 74 (70) | 48 (79) | 167 (100) |
| Pulmonary disease, No. (%) | 19 (18) | 10 (16) | 167 (100) |
| Any cardiovascular disease, No. (%) | 15 (14) | 18 (30) | 167 (100) |
| Hypertension, No. (%) | 35 (33) | 30 (49) | 167 (100) |
| Diabetes mellitus, No. (%) | 21 (20) | 13 (21) | 167 (100) |
| Liver disease, No. (%) | 3 (3) | 0 (0) | 167 (100) |
| Chronic renal disease, No. (%) | 4 (4) | 8 (13) | 167 (100) |
| Malignancy, No. (%) | 3 (3) | 1 (2) | 167 (100) |
| Immunosuppression, No. (%) | 1 (1) | 5 (8) | 167 (100) |
| Body mass index, kg/m2 | 29 (25–32) | 27 (25–31) | 157 (94) |
| Days after symptom onset for sampling | 10 (8–12) | 9 (8–11) | 165 (99) |
| Days after admission for sampling | 1 (1–2) | 1 (1–2) | 167 (100) |
| Peak temperature, °C | 38.1 (37.4–38.9) | 39 (38.4–39.5) | 167 (100) |
| Peak oxygen demand, L/min | 0 (0–2) | 3 (1–6) | 167 (100) |
| C-reactive protein, mg/L | 93 (49–168) | 115 (74–240) | 165 (99) |
| Procalcitonin, μg/L | 0.17 (0.1–0.4) | 0.32 (0.2–1.2) | 107 (64) |
| Neutrophil, × 109/L | 4.7 (3.4–6.6) | 5.6 (3.6–8.0) | 142 (85) |
| Lymphocyte, × 109/L | 1.1 (0.8–1.4) | 0.8 (0.6–1.3) | 142 (85) |
| Neutrophil/lymphocyte ratio | 4.6 (2.8–7.6) | 6.4 (4.1–10.9) | 142 (85) |
| Troponin T, ng/L | 8 (5–10) | 13 (8–24) | 110 (66) |
| D-dimer, mg/L | 0.8 (0.5–1.3) | 1.0 (0.7–1.8) | 105 (63) |
| Part of phase 1 or 2 convalescent plasma therapy study, No. (%) | 4 (4) | 28 (46) | 167 (100) |
| Corticosteroid treatment, No. (%) | 1 (1) | 3 (5) | 167 (100) |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviation: PCR, polymerase chain reaction.
The median time from admission to initial PCR testing in serum was 1 day (IQR, 1–2 days) in both the PCR-negative and -positive group. The median duration of illness before testing was 10 days (IQR, 8–12 days) and 9 days (IQR, 8–11 days) in the PCR-negative and -positive groups, respectively. Median time to first outcome was 2 days (IQR, 1–2 days) after sampling in the PCR-positive group.
Progression to Critical Disease and Mortality
In total, 18 of 167 (11%) patients died during follow-up, of which 3 of 106 (3%) were in the PCR-negative group and 15 of 61 (25%) in the PCR-positive group. The numbers of patients reaching the composite outcome (ICU care or death) were 34 of 167 (20%) in the whole cohort, 7 of 106 (7%) in the PCR-negative group, and 27 of 61 (44%) in the PCR-positive group. Age, PCR positivity, hypertension, cardiovascular disease, and C-reactive protein were correlated to death or critical diseases in the univariate regression and included in the multivariate Cox regression (Tables 2 and 3). Only PCR positivity and age were significantly associated with mortality and progression to critical disease in the multivariate analysis.
Table 2.
Hazard Ratios for All-cause Mortality at 28 Days (Cox Regression Analysis)
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| PCR positive | 9.9 | (2.8–34) | <.001 | 8.6 | (2.4–30) | .001 |
| Age, y | 1.1 | (1.1–1.2) | <.001 | 1.2 | (1.1–1.2) | <.001 |
| Male sex | 0.8 | (.3–2.1) | .7 | … | … | |
| BMI, kg/m2 | 0.9 | (.8–1.0) | .07 | … | … | |
| Hypertension | 2.6 | (1.0–6.8) | .04 | 1.1 | (.4–2.9) | .9 |
| Diabetes | 2.0 | (.8–5.0) | .17 | … | … | |
| Cardiovascular disease | 3.6 | (1.4–9.1) | .007 | 0.7 | (.2–2) | .5 |
| Pulmonary disease | 1.9 | (.3–3.3) | .9 | … | … | |
| CRP, mg/L | 1.0 | (1.0–1.0) | 1.0 | … | … | |
| Lymphocyte count, × 109/L | 0.4 | (.1–1.3) | .11 | … | … |
Data on age, sex, PCR status, and comorbidities were available for all patients; CRP, BMI, and lymphocyte count were available for 165, 157, and 142 subjects, respectively.
Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; PCR, polymerase chain reaction.
Table 3.
Hazard Ratios for Critical Disease and Death at 28 Days (Cox Regression Analysis)
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| PCR positive | 8.4 | (3.6–19) | <.001 | 7.2 | (3.0–17) | <.001 |
| Age, y | 1.1 | (1.0–1.1) | <.001 | 1.1 | (1.0–1.1) | .001 |
| Male sex | 1.3 | (.6–2.8) | .4 | … | … | |
| BMI, kg/m2 | 1.0 | (.9–1.0) | .2 | … | … | |
| Hypertension | 2.1 | (1.1–4.2) | .03 | 0.9 | (.4–2.0) | .8 |
| Diabetes | 1.0 | (.4–2.3) | 1.0 | … | … | |
| Cardiovascular disease | 2.3 | (1.2–4.7) | .02 | 0.8 | (.3–1.8) | .6 |
| Pulmonary disease | 0.8 | (.3–2.0) | .6 | … | … | |
| CRP, mg/L | 1.0 | (1.0–1.0) | .04 | 1.0 | (1.0–1.0) | .3 |
| Lymphocyte count, × 109/L | 0.5 | (.2–1.1) | .10 | … | … |
Data on age, sex, PCR status, and comorbidities were available for all patients; CRP, BMI, and lymphocyte count were available for 165, 157, and 142 subjects, respectively.
Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; PCR, polymerase chain reaction.
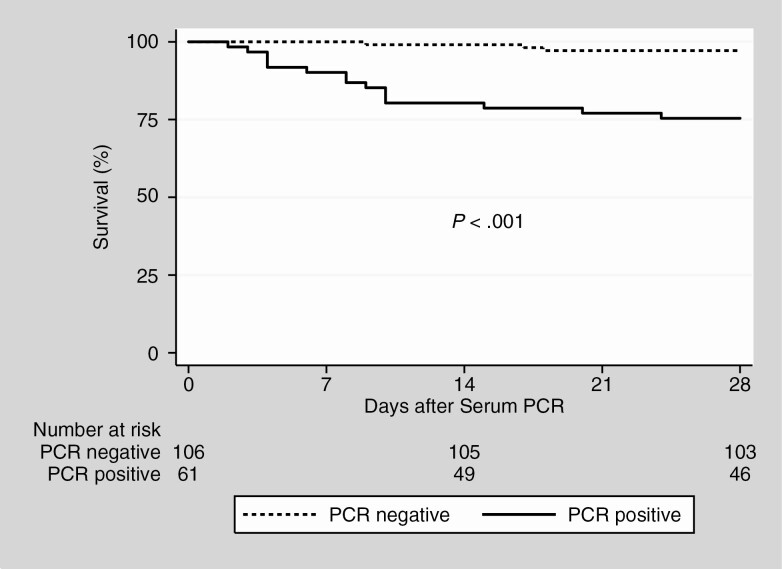
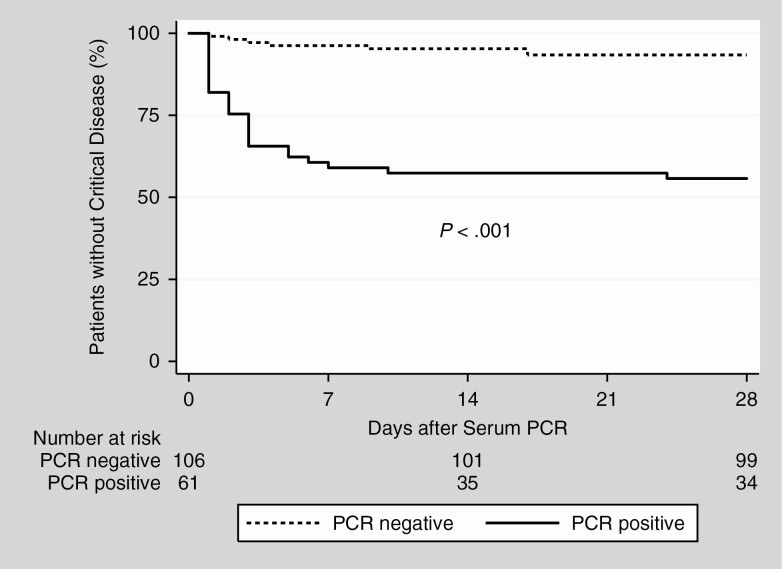
Hazard ratios for serum PCR positivity were 8.6 (95% CI, 2.4–30) for all-cause mortality and 7.2 (95% CI, 3.0–17) for critical disease. HRs for age were 1.2 (95% CI, 1.1–1.2) per year for all-cause mortality and 1.1 (95% CI, 1.0–1.1) for critical disease. There was no significant interaction between age and serum PCR positivity. Kaplan-Meier survival graphs of all-cause mortality and critical disease, comparing serum PCR-positive and -negative patients, are presented in Figures 2 and 3. Performing the analyses after dividing the cohort into the period before and after systematic testing of admitted patients (see Supplementary Materials) did not significantly alter the findings.
Figure 2.
Kaplan-Meier estimates of all-cause mortality. Survival of patients without (dashed line) and with (solid line) severe acute respiratory syndrome coronavirus 2 RNA in serum at admission. Abbreviation: PCR, polymerase chain reaction.
Figure 3.
Kaplan-Meier estimates of progression to critical disease. Estimates of progression to critical disease, defined as a composite of intensive care unit care and all-cause mortality. Patients without (dashed line) and with (solid line) severe acute respiratory syndrome coronavirus 2 RNA in serum at admission. Abbreviation: PCR, polymerase chain reaction.
Correlation Between Age and SARS-CoV-2 Detection in Serum
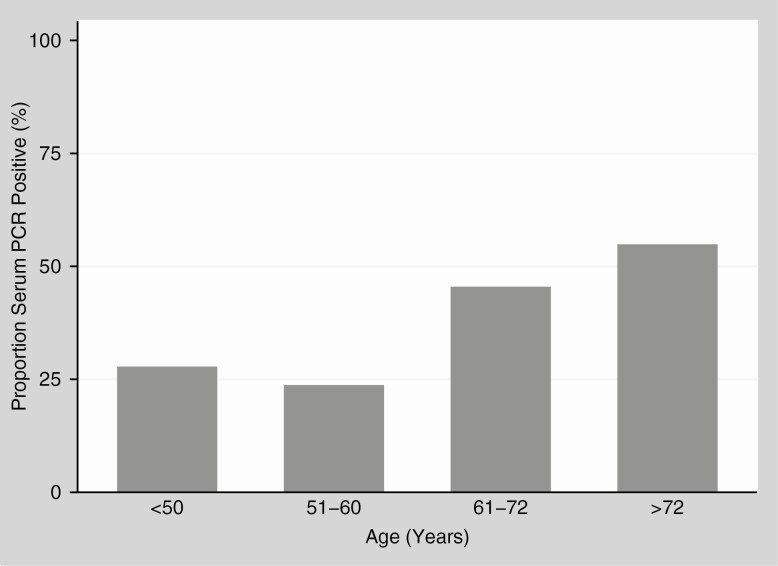
The correlation was assessed in patients admitted between 11 May and 30 June 2020, when serum PCR samples were collected systematically. PCR-negative patients were significantly younger (median, 53 years [IQR, 42–67 years]) compared with PCR-positive patients (median, 62 years [IQR, 50–74] years) (P < .001). The proportion of serum PCR-positive patients increased with age, with a distinct increase in patients older than 60 years, as shown in Figure 4.
Figure 4.
Proportion of severe acute respiratory syndrome coronavirus 2 serum polymerase chain reaction (PCR)–positive patients in different age groups.
Reverse-transcription PCR Results
The in-house PCR method was used in 44 of 61 (72%) of the positive serum samples, and 17 of 61 (28%) were analyzed with the Cobas assay. Median Ct values were 34 (IQR, 32–35) for the E-gene and 33 (IQR, 30–35) for the RdRp gene with the in-house assay, and 37 (IQR, 35–38) for the E-gene with the Cobas assay. Median Ct value for the ORF1 gene from the Cobas assay was not analyzable due to an insufficient number of positive samples.
Correlation Between Convalescent Plasma Therapy and Mortality
Convalescent plasma was given to 28 of 61 serum PCR–positive patients. Plasma from several donors at 7-fold varying doses were used. The proportion of patients who died among serum PCR–positive patients who did and did not receive convalescent plasma was 6 of 28 and 9 of 33, respectively.
Discussion
The principal finding was that detection of SARS-CoV-2 RNA in serum, within 3 days of admission, identified a population with a 7-fold increased risk of progress to critical disease and an 8-fold increased risk of death, compared with patients in whom SARS-CoV-2 RNA was not detected in serum. Equally valuable, not detecting SARS-CoV-2 RNA in serum indicated a high chance of uncomplicated recovery. These data are consistent with a study that reported SARS-CoV-2 RNA in the serum of critically ill patients only (n = 5) and a recent study that identified higher serum viral loads in more severely ill patients [12, 13]. Veyer et al [13] also showed a trend toward worse outcome in the PCR-positive group. The results are also in line with SARS, MERS, and COVID-19 data indicating that detection of virus in serum and viral load in respiratory samples correlated with outcome [6, 7, 9, 10]. However, detection of SARS-CoV-2 in serum was not significantly associated with disease severity in a cohort of 96 patients [8]. A possible explanation is that disease severity was based on clinical characteristics in that cohort, in which there was zero mortality. In the current analysis, the presence of SARS-CoV-2 RNA in serum was assessed as an early predictor of clinical outcome, essentially asking the question the other way around. Thus, these data do not necessarily oppose each other and detection of SARS-CoV-2 in the serum of hospitalized patients seems to be an early indicator of a population at a significantly increased risk of critical disease and death. However, the results need to be verified in other populations.
As shown previously, age was also an independent predictor of critical disease and death [11]. In addition, the proportion of serum PCR–positive patients increased with age, especially in those over the age of 60 years. An age-correlated increased nasopharyngeal viral load has been reported previously, supporting this result [9]. These data suggest that there is an age-correlated, decreased ability to control viral replication that may in turn be causally linked to severe disease. Furthermore, SARS-CoV-2 was found in serum of 78% (28/36) of patients admitted to Danderyd hospital ICU due to an anticipated need of invasive ventilation during a similar time period (unpublished data), which matches the 88% (23/26) among PCR-positive patients with critical disease in a study by Veyer et al [13]. Taken together, this suggests that most patients who developed critical disease had a continued, age-correlated, uncontrolled viral replication as found in SARS [6].
Dysregulation of the inflammatory response resulting in a “cytokine storm” has been proposed as an important factor in the pathogenesis of severe COVID-19 [4]. In line with this, high interleukin 6 (IL-6) levels commonly occur in critically ill patients [3, 12, 20, 21]. Moreover, detection of SARS-CoV-2 in serum correlated with extremely high levels of IL-6 [12]. Detection of SARS-CoV-2 in serum could thus be a result of leakage from tissues damaged by the inflammatory response. However, the presence of SARS-CoV-2 in serum prior to the development of severe disease suggests that the virus is, at least initially, driving the inflammatory response. Detecting SARS-CoV-2 in serum is in line with the multiorgan involvement seen in patients with critical disease and autopsy data reporting viral infection in several organs, indicating hematogenic spread of the virus [2, 22, 23].
The serum PCR Ct values in this study were high with low variance as reported previously, and unlike Ct values from respiratory samples where virus is known to replicate [8]. The low variance and the dichotomous nature of PCR positivity suggests a total viral load reaching a detectable level, predictive of a severe course. In a study using an ultrasensitive PCR method, the viral load in serum differed significantly between patients of different disease severity, and 74% of tested patients were PCR positive [13]. This difference is most likely due to a greater method sensitivity enabling detection of even lower RNA levels in serum, which will be of potential clinical utility in the future.
Early identification of groups with a high or low risk of critical disease, using a commonly available PCR method, helps us make informed treatment decisions. Recent preliminary data indicate that remdesivir given early to moderately ill patients may be an effective COVID-19 treatment [24]. Serum PCR–positive patients are a group in which to primarily assess the efficacy of remdesivir while the supply is limited. Other potential antiviral therapies such as convalescent plasma may also be most effective if given to serum PCR–positive patients before the development of critical disease. The PCR-positive group may also be a target for immunomodulating therapies such as corticosteroids and IL-6 and interleukin 1 inhibitors.
This study has several limitations. First, at the start of the study, only more severely ill patients were tested. This affected the total distribution of negative vs positive samples. However, it did not appear to affect the predictive value of the results as the HRs were not significantly affected by analyzing the data before and after the period of systematic inclusion.
Second, the use of convalescent plasma may have affected the outcome. The use of convalescent plasma from several donors, with differing antibody titers, at 7-fold varying doses prevented a meaningful analysis. However, receiving convalescent plasma did not obviously increase the risk of serum PCR-positive patients dying, at least indicating that convalescent plasma did not cause the deaths seen in the PCR-positive group. Moreover, in a large safety study (n = 20 000) of COVID-19 patients receiving convalescent plasma, the incidence of serious adverse events was low [25].
Third, lactate dehydrogenase and radiological results have been described as predictors for severe outcome. Unfortunately, we were not able to include these variables in our statistical models due to missing data and difficulties with standardized interpretations.
Conclusions
SARS-CoV-2 RNA in serum at admission to hospital is associated with a 7-fold increased risk of progression to critical disease, and an 8-fold increased risk of death. PCR positivity in serum at admission is thus a predictor for risk of progression to critical illness, and identifies a group of patients to whom treatment should be prioritized in a setting with limited drug availability.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. K. H., M. H., P. G.-J., J. D., and J. U. designed the study. K. H., M. H., and J. U. organized and entered the data. K. H., M. H., P. G.-J., J. D., and J. U. contributed to the data analysis. B. H., M. G., and J. D. contributed to the microbiological analyses. K. H. and J. U. drafted the manuscript. All writers contributed to data interpretation, critically revised the drafted manuscript, and approved the submitted manuscript. K. H. and J. U. accept full responsibility for the conduct of the study, had full access to all the data, and controlled the decision to publish. K. H. attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclaimer. No funding source was involved in the planning, implementation, or reporting of the study.
Financial support. This study did not receive any specific grant from funding agencies. J. U. has a position as clinical researcher funded by the Stockholm County Council (award number SLL20160597).
Potential conflicts of interest. P. G. -J. reports personal fees from Pfizer, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vabret N, Britton GJ, Gruber C, et al. Sinai Immunology Review Project . Immunology of COVID-19: current state of the science. Immunity 2020; 52:910–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med 2004; 10:S88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oh MD, Park WB, Choe PG, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med 2016; 375:1303–5. [DOI] [PubMed] [Google Scholar]
- 8. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ 2020; 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magleby R, Westblade LF, Trzebucki A, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020. [Preprint]. June 30, 2020. Available at: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality [manuscript published online ahead of print 6 August 2020]. Lancet Respir Med 2020. doi: 10.1016/S2213-2600(20)30354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020. [Preprint]. April 17, 2020. Available at: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veyer D, Kernéis S, Poulet G, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA shields light on its potential clinical value. Clin Infect Dis 2020. [Preprint]. August 17, 2020. Available at: 10.1093/cid/ciaa1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong J, Ou J, Qiu X, et al. A tool to early predict severe corona virus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis 2020;71:833– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020. [Preprint]. May 12, 2020. doi:10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Cobas SARS-CoV-2. Qualitative assay for use on the Cobas 6800/8800 Systems. Version 1. Available at: https://www.who.int/diagnostics_laboratory/eul_0504-046-00_cobas_sars_cov2_qualitative_assay_ifu.pdf?ua=1. Accessed 9 June 2020.
- 18. Cepheid. Xpert Xpress SARS-CoV-2 Assay CE-IVD ENGLISH [package insert 302–3787 Revision A].2020. Available at: https://www.cepheid.com/Package%20Insert%20Files/Xpress-SARS-CoV-2/Xpert%20Xpress%20SARS-CoV-2%20Assay%20CE-IVD%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20A.pdf. Accessed 9 June 2020.
- 19. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of Covid-19 infection: systematic review and critical appraisal. BMJ 2020; 369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med 2020; 12:e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 2020. [Preprint]. May 22, 2020. doi:10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 25. Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20 000 hospitalized patients. Mayo Clin Proc 2020; 95:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.