Abstract
Background
Zoonotic coronaviruses have emerged as a global threat by causing fatal respiratory infections. Given the lack of specific antiviral therapies, application of human convalescent plasma retaining neutralizing activity could be a viable therapeutic option that can bridges this gap.
Methods
We traced antibody responses and memory B cells in peripheral blood collected from 70 recovered Middle East respiratory syndrome coronavirus (MERS-CoV) patients for 3 years after the 2015 outbreak in South Korea. We also used a mouse infection model to examine whether the neutralizing activity of collected sera could provide therapeutic benefit in vivo upon lethal MERS-CoV challenge.
Results
Anti-spike-specific IgG responses, including neutralizing activity and antibody-secreting memory B cells, persisted for up to 3 years, especially in MERS patients who suffered from severe pneumonia. Mean antibody titers gradually decreased annually by less than 2-fold. Levels of antibody responses were significantly correlated with fever duration, viral shedding periods, and maximum viral loads observed during infection periods. In a transgenic mice model challenged with lethal doses of MERS-CoV, a significant reduction in viral loads and enhanced survival was observed when therapeutically treated with human plasma retaining a high neutralizing titer (> 1/5000). However, this failed to reduce pulmonary pathogenesis, as revealed by pathological changes in lungs and initial weight loss.
Conclusions
High titers of neutralizing activity are required for suppressive effect on the viral replication but may not be sufficient to reduce inflammatory lesions upon fatal infection. Therefore, immune sera with high neutralizing activity must be carefully selected for plasma therapy of zoonotic coronavirus infection.
Keywords: MERS-CoV, neutralizing antibody, plasma therapy
Anti-spike-specific immunoglobulin G responses persisted for up to 3 years, especially in sera of Middle East respiratory syndrome (MERS) patients recovered from severe pneumonia. Additionally, sera with high neutralizing titers (> 1:5000) could provide therapeutic benefit in vivo mouse model upon lethal MERS-CoV challenge.
Zoonotic coronaviruses have been continuously emerging as global threats on public health by causing fatal respiratory diseases [1]. Severe acute respiratory syndrome coronavirus (SARS-CoV) arose in China and infected more than 8000 victims, resulting in 774 deaths in 27 countries during 2002 to 2003 [1]. Middle East respiratory syndrome coronavirus (MERS-CoV), originating from camels in the Middle East area, continues to cause outbreaks with high mortality (~ 35%) in 27 countries since 2012 [1, 2]. In December 2019, another novel coronavirus (SARS-CoV-2) emerged in Wuhan, China, and has currently caused more than 20 million human infections with a global 3.6% mortality (https://covid19.who.int/). Despite the disastrous impact of the continuous emergence and spread of zoonotic coronaviruses on the human population, there is currently no specific antiviral therapy [3]. In addition, preventative vaccines against coronaviruses have not yet been approved for human application, although ongoing studies have demonstrated the potential of various candidate vaccines and monoclonal antibodies, especially those targeting viral spike antigens [4].
Application of human sera or plasma collected from recovered patients that retain neutralizing activity has been clinically investigated for therapeutic use because of the ready availability of sera and plasma compared to other therapeutic options [5–7]. Although most of the studies were low quality, lacked control groups, and at moderate or high risk of bias, they consistently showed a reduction in mortality, especially when convalescent plasma is administered early after symptom onset [7]. However, the minimum level of neutralizing activity of immune plasma for effective therapeutic application has been poorly defined [5, 6]. Therefore, evidence for this therapy would be strengthened by a well-designed clinical trial or other formal evaluation [7–9].
Here, we traced antibody levels against spike antigen of MERS-CoV in recovered Korean patients who had confirmed MERS-CoV infection during the 2015 Korean outbreak. Spike-specific antibody levels and neutralizing activity were extensively assessed by various techniques. In addition, we used a mouse infection model to examine whether the neutralizing activity of collected sera could provide therapeutic benefit in vivo upon lethal MERS-CoV challenge.
METHODS
Study Design and Participants
We recruited 73 recovered MERS patients; their baseline characteristics are summarized in Table 1. Sera and peripheral blood mononuclear cells (PBMCs) were collected from the participants at 3-to-6-month intervals from 6 months after symptom onset. Sera from 9 patients assessed in a previous study [10] were also included in this study. Clinical data and specimens obtained from the MERS patients were used in this study after ethnical approval granted by the institutional review boards of Chungnam National University Hospital (CNUH, 2017–12–004), National Medical Center (H-1510–059–007), Seoul National University Hospital (1509–103–705 and 1511–117–723), Seoul National University Boramae Medical Center (26–2016–8), Seoul Medical Center (Seoul, 2015–12–102), and Dankook University Hospital (DKUH,2016–02–014). This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions. All the participants provided written and informed consent to participate.
Table 1.
Baseline Characteristics of the Enrolled Patients
| Clinical severity groups | |||
|---|---|---|---|
| Group I (n = 18) | Group II (n = 37) | Group III (n = 18) | |
| Age (years) | |||
| Mean (± SD) | 52 (± 15.9) | 50 (± 11.5) | 48 (± 11.9) |
| Sex | |||
| Female, n (%) | 9 (50%) | 18 (49%) | 3 (17%) |
| Male, n (%) | 9 (50%) | 19 (51%) | 15 (83%) |
| Fever duration (d) | |||
| Mean (± SD) | 5 (± 4.7) | 11 (± 8.9) | 20 (± 11.5) |
| Person with underlying diseasesa, n (%) | 7 (39%) | 14 (38%) | 7 (39%) |
| Smokers, n (%) | - | 8 (22%) | 6 (33%) |
aDiabetes, chronic (heart, kidney, lung, or liver) diseases, cancer, hypertension.
All the other experimental methods are available in Supplementary Method.
RESULTS
The recovered patients were classified into 3 groups based on disease severity during the Korean MERS outbreak in 2015 [11]. Group I (G-I) included 18 persons who were asymptomatic or had mild fever without developing pneumonia. Group II (G-II) included 37 participants who developed mild pneumonia without hypoxemia. Eighteen people recovered after more prolonged and severe pneumonia and were classified as group III (G-III). G-III participants experienced hypoxemia and were treated with oxygen during hospitalization. Baseline characteristics of the severity groups are summarized in Table 1.
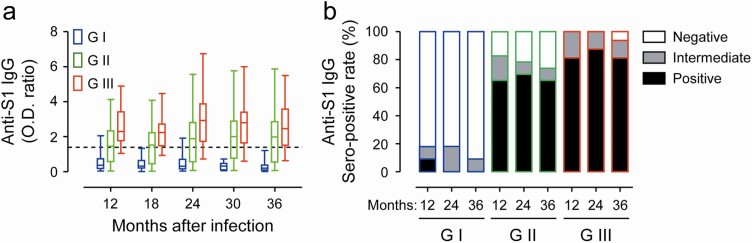
First, we traced antibody responses against MERS-CoV spike antigen in 70 participants whose sera were available from 12 to 36 months after symptom onset (Figure 1A). The average OD ratios against S1 spike antigen in all the samples were generally sustained for up to 36 months (mean ± SD: 1.56 ± 1.22, 1.90 ± 1.69, and 1.83 ± 1.55 at 12, 24, and 36 months, respectively) and were positively correlated with disease severity. In particular, the average antibody levels in patients of G-II (mean ± SD: 1.52 ± 1.06, 1.96 ± 1.58, and 1.92 ± 1.36 at 12, 24, and 36 months, respectively), and G-III (mean ± SD: 2.59 ± 1.16, 3.06 ± 1.70, and 2.74 ± 1.58 at 12, 24, and 36 months, respectively) did not significantly change, whereas those of G-I (mean ± SD: 0.56 ± 0.57, 0.48 ± 0.53, and 0.30 ± 0.34 at 12, 24, and 36 months, respectively) gradually declined. There were 50 patients whose sera were available at all the 3-year points and across all 3 groups. Only 1 person (9.1%, 1/11) in G-I was sero-positive at 12 months after infection, while the rest remained negative (90.9%, 10/11) throughout the follow-up period (Figure 1B). Among the 23 individuals in G-II, 16 (69.6%) were persistently positive with antibody levels for up to 36 months. In addition, 81.3% (13/16) of G-III participants showed seroconversion and persistent antibody responses throughout the study period. Only 2 patients in G-III failed to elicit positive responses, and 1 positive person turned intermediate at 36 months after infection. Therefore, MERS patients who recovered from more severe pneumonia developed significantly higher levels of anti-spike IgG antibody responses and these antibody responses persisted for at least 3 years after symptom onset in most of the recovered patients.
Figure 1.
Kinetic changes of IgG antibody responses against S1 antigen of MERS-CoV in 70 participants from 12 to 36 months after symptom onset. A, Collected sera were tested by a commercial ELISA kit. The assay was semi-quantitatively evaluated by calculating a ratio of the extinction value of the patient sample over the extinction value of the calibrator. Optical density (OD) ratios < 0.7 were considered negative, ratios > 1.4 (dashed line) were considered positive, and ratios ≥ 0.7 and ≤ 1.4 were considered as intermediate. B, Relative proportion of sera with negative, positive, and intermediate OD ratio values is presented in clinical severity groups (GI ~ GIII) at the indicated time points (G-I: n = 9 ~ 18, G-II: n = 20 ~ 33, and G-III: n = 12 ~ 18). Abbreviations: IgG, immunoglobulin G; MERS-CoV, Middle East respiratory syndrome coronavirus.
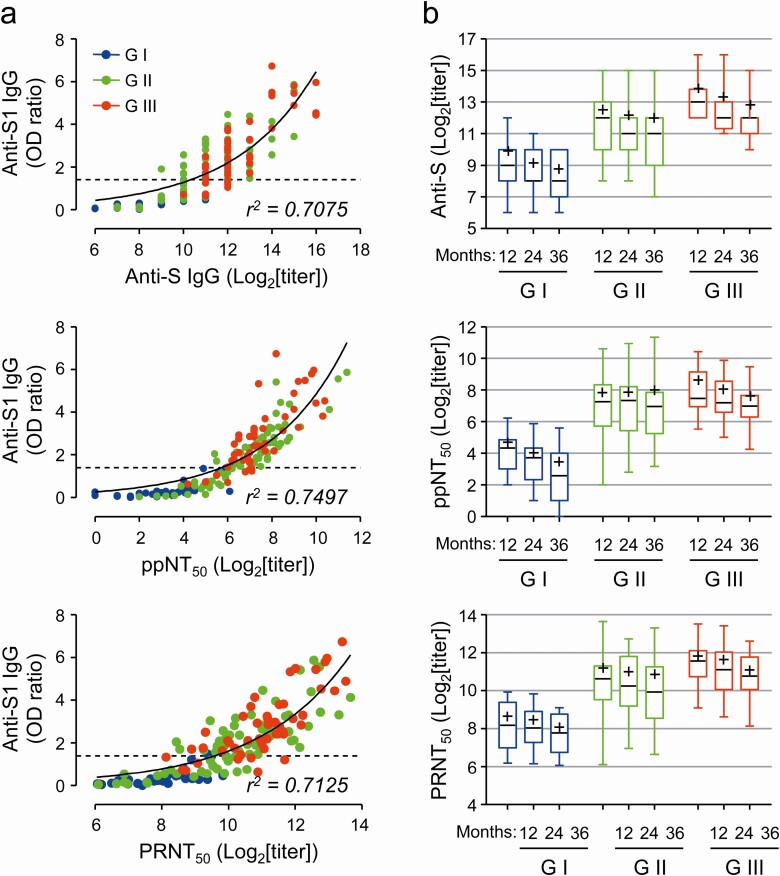
We also measured antibody titers against spike antigen and neutralizing activity against spike pseudotyped lentivirus (ppNT50) and MERS-CoV (PRNT50) using sera from the 50 patients (Figure 2). The antibody titers against spike antigen and the neutralizing titers against the pseudotyped lentivirus and MERS-CoV correlated well with OD ratio values against S1 antigen (Figure 2, left panels). In addition, the titers were generally higher in the patients who suffered from more severe MERS than those with milder symptoms, as indicated by OD ratio values (Figure 2, right panels). Antibody titers against spike antigen and the neutralizing titers generally declined, but by less than 2-fold every year. Average titers declined more rapidly in G-I and G-III groups, when compared to those of G-II. For example, average PRNT50 titers of G-II patients were reduced by 20.5% at the third year (mean ± SD: 1840 ± 2350) when compared to those of the first year (2322 ± 2774), whereas those of G-I and G-III groups were reduced by 35.3% and 40.8%, respectively, at the third year (G-I: 268 ± 166, G-III: 2220 ± 1962) when compared to those of the first year (G-I: 415 ± 330, G-III: 3751 ± 3105).
Figure 2.
Correlation of anti-S1 OD ratio with anti-spike IgG titer and neutralizing activity (ppNT50 and PRNT50) in sera from 50 patients. A, Correlation of OD ratio values against S1 antigen with the antibody titers against spike antigen and the neutralizing titers against the pseudotyped lentivirus (ppNT50) or MERS-CoV (PRNT50) were assessed (left panels). Nonlinear regression curves (exponential growth) and goodness of curve fit (r2 value) are presented. B, Kinetic changes of anti-S IgG titers and neutralizing activity (ppNT50 and PRNT50) in sera samples are presented in clinical severity groups (GI ~ GIII). Box and whiskers (min to max) plots including median (black line) and mean (+) values of each plot are presented at the indicated time points (G-I: n = 11, G-II: n = 23, and G-III: n = 16). Abbreviations: IgG, immunoglobulin G; MERS-CoV, Middle East respiratory syndrome coronavirus; OD, optical density.
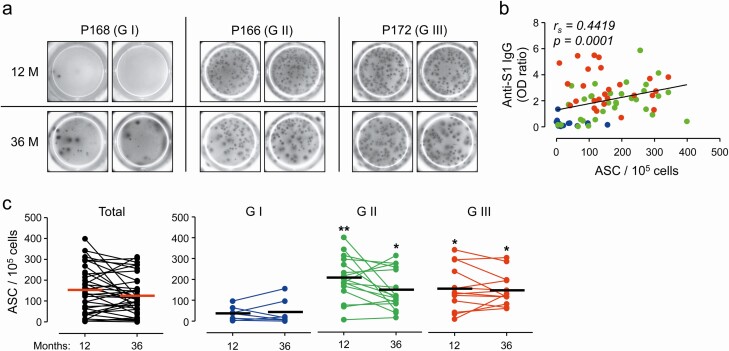
In order to further confirm the spike-specific antibody responses, we measured spike-specific memory B cell responses on a cellular level by the immunoassay ELISPOT. PBMCs were taken at 12 and 36 months after infection from 36 subjects (G-I: n = 7, G-II: n = 16, and G-III: n = 13) and applied for analysis of spike antigen-specific IgG secreting memory B cells (Figure 3). The number of spots in the 72 samples was overall positively correlated with OD ratio against S1 antigen (P = .0001). A positive correlation was observed in specimens from G-II subjects (P = .0150), whereas there were no significant correlations of the OD ratio with the spot counts in G-I and G-III samples (Figure 3B). Average counts of spike-specific IgG-secreting B cells in the 36 persons at 36 months (mean ± SD: 127.6 ± 91.0 cells/105 PBMCs) were slightly, but not significantly, decreased when compared to those at 12 months (mean ± SD: 154.6 ± 112.5 cells/105 PBMCs). Consistent with the levels of antibody responses, the counts of B cells secreting spike-specific IgG were significantly higher in G-II (mean ± SD: 203.2 ± 104.7 cells/105 PBMCs) and G-III (mean ± SD: 158.4 ± 106.3 cells/105 PBMCs) subjects than those of G-I (mean ± SD: 36.6 ± 34.4 cells/105 PBMCs) at 12 months after infection and similar trends of B cells counts were also observed at 36 months after infection (Figure 3C). These results clearly indicate that antibody responses and the levels of memory B cells against the spike antigen of MERS-CoV are significantly higher in MERS patients who recovered from pneumonia than those who had mild symptoms or were asymptomatic. In addition, these responses barely waned and persisted for at least 3 years after the initial infection.
Figure 3.
Quantification of spike-specific memory B cells in peripheral blood mononuclear cells (PBMCs) taken at 12 and 36 months after infection in 36 subjects. A, Representative images of B cell ELISPOT results. B, Correlation of OD ratio values against S1 antigen with anti-S IgG-secreting B cell counts were assessed by linear regression (black line) and Spearman’s rank test (rs and P value). PBMCs were taken at 12 and 36 months after infection from 36 subjects (G-I: n = 7, G-II: n = 16, and G-III: n = 13) and applied for analysis of spike antigen-specific IgG secreting memory B cells. C, Kinetic changes of anti-S IgG-secreting B cells in PBMCs are presented. Statistical analysis was performed using one-way ANOVA, followed by the Newman–Keuls t-test for comparisons of values among the severity groups at the indicated time points. Abbreviation: ASC, antibody-secreting cells; IgG, immunoglobulin G; OD, optical density. *, P < .05; **, P < .01.
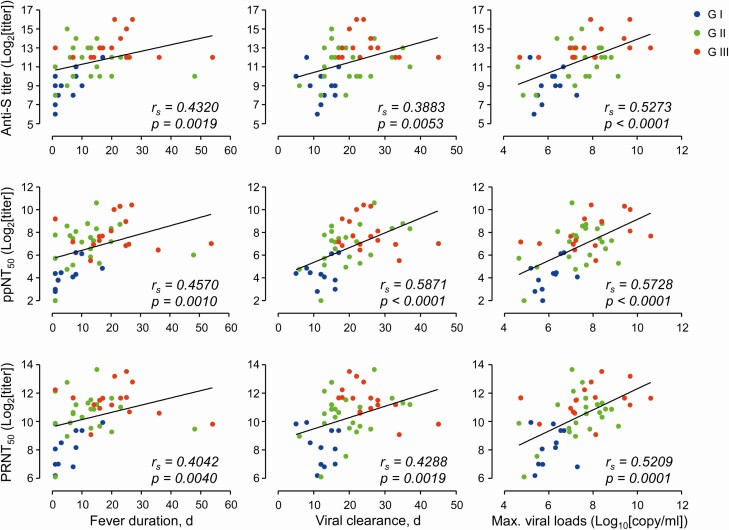
Next, we investigated the potential correlation of the levels of antibody responses at 12 months with fever duration, viremic periods, or maximum viral loads in respiratory secretions during the initial infection phase (Figure 4). The levels of antibodies, including anti-spike IgG titer, ppNT50, and PRNT50, in the 50 recovered patients were significantly and positively correlated with fever duration, viremic periods, and maximum viral loads. It is notable that the maximum viral loads in the respiratory samples during the acute phase of infection showed the best correlation with the antibody levels regardless of severity group. However, the antibody and neutralizing titers were not positively correlated with fever duration and viremic periods in G-III recovered patients. Mean fever duration and viremic period of 6 patients (2 in G-II and 4 in G-III) with the highest neutralization titers (ppNT50 > 1/1000 or PRNT50 > 1/5000) were 19.3 days (SD: ± 8.1) and 22.3 days (SD: ± 4.4), respectively.
Figure 4.
Correlation of antibody levels with fever duration, viremic period, and maximum viral loads during infection period. Correlations of antibody levels (anti-S IgG titer, ppNT50, and PRNT50 in sera collected at 1 year after infection) with the indicated parameters observed during infection periods in 50 subjects (G-I: n = 11, G-II: n = 23, and G-III: n = 16) were assessed by linear regression (black line) and Spearman’s rank test (rs and P value). Abbreviations: IgG, immunoglobulin G; Max., maximum.
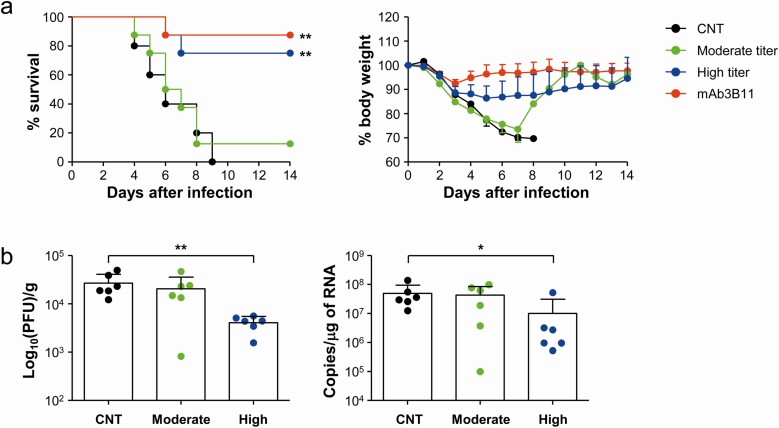
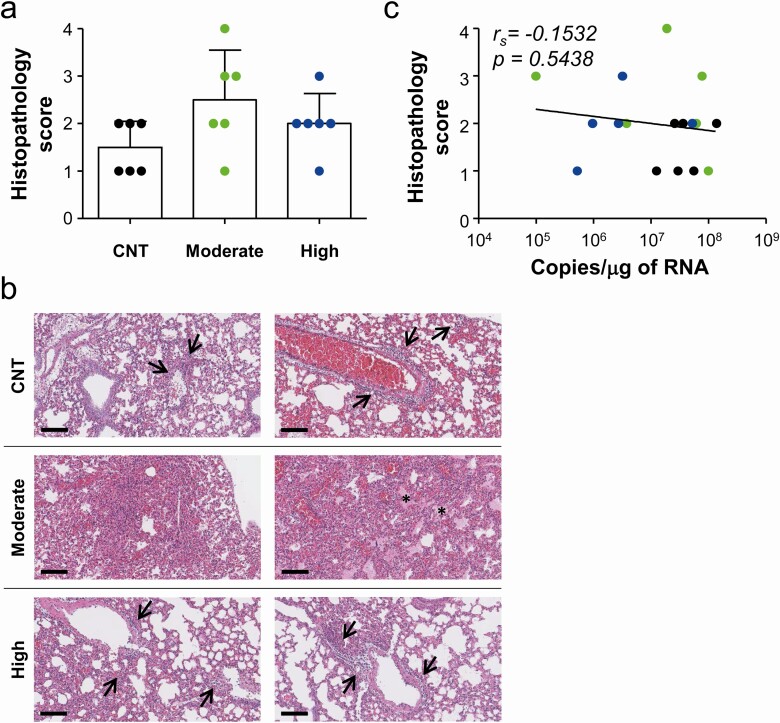
Finally, we evaluated the therapeutic efficacy of sera from the recovered patients. We selected sera from 3 patients with intermediate PRNT50 titers (~ 1/1000) and 3 additional sera with high PRNT50 titers (> 1/5000) to generate pooled sera. A therapeutic human monoclonal antibody (3B11) [12, 13] against spike antigen was used as a positive control, and pooled sera from healthy volunteers who had never contacted MERS-CoV were used as a negative control. The antibody levels of each pooled sera were assessed by measuring OD ratio, anti-spike IgG titer, and PRNT50 titer (Table 2). hDPP4-Tg mice were challenged intranasally with MERS-CoV at 2500 plaque forming units (PFU)/mouse (5 × LD50) and then treated with pooled sera (100 µL/mouse) or therapeutic mAb (20 µg in 100 µL of PBS/mouse) 4 times (1 hour and 1, 2, and 3 days postinfection). Mice were monitored for their change in weight and survival for 2 weeks after infection (Figure 5). Results showed that administration of therapeutic mAb or pooled sera with high PRNT50 titer significantly enhanced survival rate (87.5% [7/8] and 75.0% [6/8], respectively). Body weights of mice that ultimately expired continuously decreased, but some (3/8 in high titer group and 1/8 in therapeutic mAb group) of the surviving mice gradually lost 25% ~ 30% of the initial body weight until 8 days postinfection before gradually recovering. In contrast, all the mice that received control sera and 87.5% (7/8) of mice treated with moderate titer sera died within 8 days after infection. It is also notable that weight loss in mice treated with moderate titer sera progressed more rapidly, but not significantly, during the early phase of infection than that of mice administered control sera or immune-sera with high neutralizing activity. To investigate the inhibitory effect of the sera on virus replication in the lungs during the acute phase of lethal infection, viral loads in lungs were assessed at 4 days after intranasal infection (Figure 5B). Consistent with the morbidity and mortality results, adoptive transfer of immune-sera with high neutralizing antibody titer significantly suppressed productive viral infection and replication (mean ± SD: 4.1 × 103 ± 1.4 × 103 PFU/g of lung tissue and 1.0 × 107 ± 2.0 × 107 copies/µg of RNA) in the lungs of challenged mice when compared to those of mice administered non-immune sera (2.7 × 104 ± 1.4 × 104 PFU/g of lung tissue and 5.0 × 107 ± 4.6 × 107 copies/µg of RNA). In contrast, mice treated with moderate levels of neutralizing antibody failed to efficiently control viral replication in the lungs (2.0 × 104 ± 1.5 × 104 PFU/g of lung tissue and 4.3 × 107 ± 4.1 × 107 copies/µg of RNA), with much larger individual variations. Interestingly, lung histology studies at 4 days after infection revealed various degrees of lung inflammation, as indicated by infiltration of inflammatory cells into perivascular and pulmonary parenchyma, and the presence of interstitial and alveolar edema [14], in all the mice groups regardless of plasma therapy. Most of the infiltrating inflammatory cells were lymphocytes, monocytes/macrophages, plasma cells, and a few neutrophils. In addition, there was no significant difference in pulmonary pathology among the experimental groups, although the group treated with immune-sera with moderate neutralizing activity showed more variation in pathological grade (Figure 6A and B). Moreover, there was no significant correlation between the degree of lung pathology and viral copies (Figure 6C). These results indicate that systemic administration of immune-sera with high neutralizing antibody titer may not be sufficient to reduce pulmonary inflammation during the acute phase of lethal infection, but could provide protective effect by suppressing viral replication and spread of MERS-CoV. However, immune-sera with moderate levels of neutralizing activity failed to efficiently control viral replication, and resulted in more variable degree of lung inflammation and damage during the acute phase of infection with lethal dose of MERS-CoV when compared to those treated with non-immune sera.
Table 2.
Summary of Anti-spike Antibody Titers and Neutralizing Titers in Pooled Sera for Therapy
| Groups | OD ratio | Anti-S titer | PRNT50 |
|---|---|---|---|
| Negative control | 0.029 | - | - |
| Moderate titer sera | 1.946 | 4096 | 1081 |
| High titer sera | 4.997 | 32 786 | 7046 |
Abbreviation: OD, optical density.
Figure 5.
Evaluation of therapeutic efficacy of pooled sera from recovered patients in hDPP4-Tg mice. A, hDPP4-Tg mice were challenged intranasally with MERS-CoV at 2500 PFU/mouse (5 × LD50) and then treated with pooled sera (100 µL/mouse) or therapeutic mAb/3B11 (20 µg in 100 µL of PBS/mouse) four times (1 hour and 1, 2, and 3 days postinfection). The antibody titers and neutralizing activity (PRNT50) of pooled sera (negative, moderate, and high titer) are summarized in Table 2. Virus-challenged mice were monitored for 14 days to evaluate survival rate (left) and body weight changes (right). The body weight data are presented as means + SD of mice in each group (CNT: n = 5, moderate, high titer, and mAb/3B11: n = 8). Significant differences between the experimental group and control group (CNT) treated with non-immune sera are indicated (**, P < .01). B, MERS-CoV viral loads were assessed by measuring PFU (left) and copy numbers of viral RNA (right) in lung tissues collected at 4days after infection. Statistical significance between the experiment group and control group was tested by using a two-tailed Student’s t-test. Abbreviation: MERS-CoV, Middle East respiratory syndrome coronavirus. *, P < .05; **, P < .01.
Figure 6.
Pathological changes in lungs of hDD4-Tg mice infected with lethal dose of MERS-CoV. A and B, Lung tissue sections collected from mice at 4 days after infection were stained with hematoxylin and eosin. Pathological scores of infected lungs (n = 6/group) (bar graphs: mean + SD, A) and representative scanned images are presented (B). Bar, 100 μm. C, Correlation of histopathological scores with viral loads (copy numbers of viral RNAs) was assessed by linear regression (black line) and Spearman’s rank test (rs and P value). Abbreviations: CNT, control group; MERS-CoV, Middle East respiratory syndrome coronavirus.
DISCUSSION
We performed a large-scale follow-up study on the quality and longevity of antibody responses specific to spike antigen of MERS-CoV in 70 recovered patients who had confirmed infection and complete clinical and virological datasets during the 2015 Korean outbreak. Specific IgG responses, including neutralizing antibodies, persisted for up to 3 years after the outbreak, especially in MERS patients who suffered from viral pneumonia, even though antibody titers gradually declined by less than 2-fold every year (Figures 1 and 2). Similar trends of antibody kinetics have been reported in other longitudinal studies of smaller sample sizes within less than 3 years after initial MERS-CoV infection [10, 15, 16]. They showed that levels of specific antibody responses were significantly dependent on clinical severity and the duration of viral persistence [11, 16–19]. We also found that levels of antibody response are significantly correlated with fever duration, viral shedding periods, and maximum viral loads (Figure 4). Of note, maximum viral loads in respiratory samples during the acute phase of infection showed the best correlation with antibody levels regardless of severity group in our study. These were further confirmed by the presence and persistence of memory B cells secreting specific antibodies (Figure 3). Since the specific antibody responses and memory B cells gradually decreased to undetectable levels in SARS patients at 6 years after infection [20], the longevity and persistence of the MERS-specific humoral immunity needs to be determined in future studies.
Persistence of specific antibodies, neutralizing activity, and B cell memory in the recovered patients could be protective against reinfection [21]. Experimentally infected animals were protected or partially protected against reinfection of MERS-CoV [22]. However, evidence of natural reinfection of camels that were previously seropositive suggests that prior infection does not provide complete immunity from reinfection [23]. In addition, reinfection of MERS-CoV enhanced pulmonary inflammation in New Zealand white rabbits in the absence of neutralizing antibody [24]. Nevertheless, there is some evidence that prior infection eliciting neutralizing activity or passive transfer of neutralizing antibody can protect animals from subsequent reinfection [24, 25]. Since there have been limited studies on reinfection in humans, careful monitoring of potential reinfection cases needs to be continued.
Passive antibody therapy using plasma from convalescent patients has been emergently applied in epidemics, such as the current COVID-19 pandemic, where there is insufficient time or resources to generate immunoglobulin therapy [26]. Indeed, several previous studies reported clinical benefits of plasma therapy by reducing viral loads and improving clinical symptoms in patients infected with emerging coronaviruses such as SARS-CoV-1, MERS-CoV, and SARS-CoV-2 [5, 8, 27–29]. Even though most of them have limitations such as small size samples, study design, and concomitant treatment modalities (ie, simultaneous use of antiviral drugs and/or anti-inflammatory treatments), administration of convalescent plasma against the emerging CoVs is generally safe and provides clinical benefits, leading to calls for the wider adoption of plasma therapy for current viral pandemics [9, 26].
The antibodies present in immune sera can bind to a given pathogen, thereby directly neutralizing its infectivity, while other antibody-mediated effector functions including complement activation, antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP), may also contribute to its therapeutic effect [26]. Here, we observed significant reduction in viral loads and enhanced survival of a mouse model challenged with lethal MERS-CoV only when treated with plasma containing high titer (1/7046) of neutralizing activity. However, this failed to reduce pulmonary pathogenesis upon lethal viral infection, and plasma with moderate neutralizing titer (1/1081) did not provide any clinical benefit. These results indicate that only high titers of neutralizing activity can provide suppressive effect on viral replication and subsequent spread, but is still not sufficient to reduce inflammatory lesions upon fatal MERS-CoV infection, as revealed by pathological changes in lungs and by initial weight loss regardless of plasma therapy. Similar results were also reported in in vivo studies using common marmosets treated with hyperimmune plasma or monoclonal antibody against MERS-CoV [30], and mouse model administered immune sera from camels [31]. Extra-neutralizing functions of antibodies, including complement-dependent cytotoxicity, ADCP, and ADCC, may have both protective and pathological consequences [32]. Antibody-dependent enhancement (ADE) of coronavirus entry into host cells has been continuously reported [33–35], suggesting that ADE may occur under specific conditions in vivo, depending on antibody dose, binding affinity of the antibodies, and expression of viral and Fcµ receptors. In addition, a recent report showed that vaccine-induced antibodies may directly promote enhanced disease via macrophage-induced inflammatory chemokines and cytokines, resulting in lung injury during acute SARS-CoV infection [36]. Moreover, enhanced activation of complement [37] and/or elevated ADCC [38], driven by antigen–antibody complexes, may also contribute to pulmonary pathogenesis during acute respiratory viral infection. Dissecting these properties of antibody response against emerging coronavirus infections is necessary for defining precise metrics of immunity, required for effective vaccines and therapeutics [32]. Therefore, feasibility, safety, and clinical effects of convalescent plasma therapy have to be properly assessed in ongoing clinical trials by determining appropriate neutralizing antibody titer, dose, or dosing range [39].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. S. K., H. S. S., and N. H. C. conceptualized the study. Y. S. K., A. A., U. P., Y. K., and N. H. C. designed the methodology. Y. S. K., A. A., U. P., Y. K., H. P., J. Y. C., Y. K. J., and N. H. C. conducted the investigation. Y. S. K., J. Y. R., J. P. C., W. B. P., S. W. P., Y. K, K. S. I., D. G. L., and H. S. S. provided resources. Y. S. K., H. S. S., and N. H. C. provided funding. Y. S. K., A. A., U. P., H. S. S., and N. H. C. wrote the article.
Financial support. This work was supported by a grant (HI15C3227) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), and a grant (2017N-ER5307/4834–300–210–13) from Korea Center for Disease Control and Prevention, funded by the Ministry of Health & Welfare, and by a grant (2017M3A9E4061998) of the National Research Foundation of Korea (NRF). A. A., U. P., and Y. K. received a scholarship from the BK21-plus education program provided by the National Research Foundation of Korea.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 2018; 18:e217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pruijssers AJ, Denison MR. Nucleoside analogues for the treatment of coronavirus infections. Curr Opin Virol 2019; 35:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu J, Jia W, Wang P, et al. Antibodies and vaccines against Middle East respiratory syndrome coronavirus. Emerg Microbes Infect 2019; 8:841–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 2016; 22:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther 2018; 23:617–22. [DOI] [PubMed] [Google Scholar]
- 7. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. ; Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 2020; 323:1561–2. [DOI] [PubMed] [Google Scholar]
- 10. Choe PG, Perera R, Park WB, et al. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis 2017; 23:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang XC, Agnihothram SS, Jiao Y, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A 2014; 111:E2018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cockrell AS, Yount BL, Scobey T, et al. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol 2016; 2:16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li K, Wohlford-Lenane CL, Channappanavar R, et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A 2017; 114:E3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Payne DC, Iblan I, Rha B, et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis 2016; 22:1824–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alshukairi AN, Khalid I, Ahmed WA, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis 2016; 22:1113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis 2015; 21:2186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko JH, Müller MA, Seok H, et al. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis 2017; 89:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang F, Quan Y, Xin ZT, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol 2011; 186:7264–8. [DOI] [PubMed] [Google Scholar]
- 21. Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity 2007; 27:384–92. [DOI] [PubMed] [Google Scholar]
- 22. Adney DR, Bielefeldt-Ohmann H, Hartwig AE, Bowen RA. Infection, replication, and transmission of Middle East respiratory syndrome coronavirus in alpacas. Emerg Infect Dis 2016; 22:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hemida MG, Alnaeem A, Chu DK, et al. Longitudinal study of Middle East respiratory syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014–2015. Emerg Microbes Infect 2017; 6:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houser KV, Broadbent AJ, Gretebeck L, et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog 2017; 13:e1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subbarao K, McAuliffe J, Vogel L, et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol 2004; 78:3572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020; 130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005; 24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 2005; 56:919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Doremalen N, Falzarano D, Ying T, et al. Efficacy of antibody-based therapies against Middle East respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res 2017; 143:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao J, Perera RA, Kayali G, Meyerholz D, Perlman S, Peiris M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J Virol 2015; 89:6117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zohar T, Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol 2020; 20:392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol 2020; 94:e02015–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang SF, Tseng SP, Yen CH, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun 2014; 451:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaume M, Yip MS, Cheung CY, et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J Virol 2011; 85:10582–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019; 4:e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carvelli J, Demaria O, Vely F, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ye ZW, Yuan S, Poon KM, et al. Antibody-dependent cell-mediated cytotoxicity epitopes on the hemagglutinin head region of pandemic H1N1 influenza virus play detrimental roles in H1N1-infected mice. Front Immunol 2017; 8:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus 2015; 4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.