Abstract
Background
Antibacterials may be initiated out of concern for bacterial coinfection in coronavirus disease 2019 (COVID-19). We determined prevalence and predictors of empiric antibacterial therapy and community-onset bacterial coinfections in hospitalized patients with COVID-19.
Methods
A randomly sampled cohort of 1705 patients hospitalized with COVID-19 in 38 Michigan hospitals between 3/13/2020 and 6/18/2020. Data were collected on early (within 2 days of hospitalization) empiric antibacterial therapy and community-onset bacterial coinfections (positive microbiologic test ≤3 days). Poisson generalized estimating equation models were used to assess predictors.
Results
Of 1705 patients with COVID-19, 56.6% were prescribed early empiric antibacterial therapy; 3.5% (59/1705) had a confirmed community-onset bacterial infection. Across hospitals, early empiric antibacterial use varied from 27% to 84%. Patients were more likely to receive early empiric antibacterial therapy if they were older (adjusted rate ratio [ARR]: 1.04 [1.00–1.08] per 10 years); had a lower body mass index (ARR: 0.99 [0.99–1.00] per kg/m2), more severe illness (eg, severe sepsis; ARR: 1.16 [1.07–1.27]), a lobar infiltrate (ARR: 1.21 [1.04–1.42]); or were admitted to a for-profit hospital (ARR: 1.30 [1.15–1.47]). Over time, COVID-19 test turnaround time (returned ≤1 day in March [54.2%, 461/850] vs April [85.2%, 628/737], P < .001) and empiric antibacterial use (ARR: 0.71 [0.63–0.81] April vs March) decreased.
Conclusions
The prevalence of confirmed community-onset bacterial coinfections was low. Despite this, half of patients received early empiric antibacterial therapy. Antibacterial use varied widely by hospital. Reducing COVID-19 test turnaround time and supporting stewardship could improve antibacterial use.
Keywords: SARS-CoV, COVID-19, antibiotic stewardship, pneumonia, coinfection
In 38 Michigan hospitals, early empiric antibacterials were prescribed to 56.6% (965/1705) of patients hospitalized with COVID-19, while 3.5% (59/1705) had a confirmed community-onset bacterial coinfection. Among hospitals, empiric antibacterial use varied from 27% to 84%.
(See the Editorial Commentary by Furukawa and Graber on pages e542–4.)
Coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), frequently presents as a febrile respiratory illness that may progress to pneumonia and respiratory failure [1–3]. In the absence of bacterial coinfection, antibacterial therapy has no known benefit in patients with COVID-19. However, patients with COVID-19 may be at risk of concomitant bacterial infections that would require antibacterial treatment [4]. Data on bacterial coinfections are sparse and variable, with reports of coinfections occurring in 3–30% of patients with COVID-19 [5–10]. Specifically, patients with COVID-19 are often started empirically on antibacterials when first hospitalized [2, 11]. However, it is unclear if bacterial coinfections are present early during hospitalization or develop later, after additional hospital exposures. To guide efforts to improve antibiotic therapy, more data are needed on the prevalence of community-onset bacterial coinfections.
In a multicenter cohort study of hospitalized patients with COVID-19 at 38 Michigan hospitals, we aimed to determine patterns and predictors of early empiric antibacterial therapy and community-onset bacterial coinfection.
METHODS
MI-COVID19
MI-COVID19 is a statewide multi-institutional collaborative quality initiative sponsored by Blue Cross Blue Shield of Michigan and Blue Care Network [12] to improve care for hospitalized patients with COVID-19. Institutional participation in MI-COVID19 is voluntary. The data abstraction and collection templates were adapted from the Michigan Hospital Medicine Safety Consortium (HMS) [13]. Of the 92 noncritical access, nonfederal hospitals in Michigan, 38 (41.3%) elected to participate in MI-COVID19. Hospitals participating in MI-COVID19 were diverse in terms of size, teaching status, and ownership structure (Table 1). Trained abstractors collected data via medical record review; data collection and quality-assurance procedures have been previously described [14].
Table 1.
Patient Characteristics and Bivariable Predictors of Early Empiric Antibacterial Therapy in Patients With Coronavirus Disease 2019 (COVID-19)
| Total (N = 1705) | Received Early Empiric Antibacterialsa (n = 965) | Did Not Receive Early Empiric Antibacterials (n = 740) | Rate Ratio (95% CI) | P | |
|---|---|---|---|---|---|
| Hospital characteristics | |||||
| Teaching hospital | 1560 (91.5) | 891 (92.3) | 669 (90.4) | 1.06 (.85–1.33) | .59 |
| Ownership | |||||
| Nonprofit | 1470 (86.2) | 779 (80.7) | 691 (93.4) | Ref | |
| For profit | 235 (13.8) | 186 (19.3) | 49 (6.6) | 1.46 (1.32–1.62) | <.001 |
| Bed size median (IQR) (rate ratio reported per 100-bed increase), beds | 391 (250–537) | 404 (250–537) | 391 (250–632) | .98 (.96–1.01) | .24 |
| Admission month | |||||
| March | 834 (48.9) | 556 (57.6) | 278 (37.6) | Ref | |
| April | 745 (43.7) | 348 (36.1) | 397 (53.6) | .73 (.65–.81) | <.001 |
| May | 111 (6.5) | 52 (5.4) | 59 (8.0) | .75 (.57–.98) | .04 |
| June | 15 (0.9) | 9 (0.9) | 6 (0.8) | .86 (.65–1.16) | .32 |
| Demographics | |||||
| Age, median (IQR) (rate ratio reported per 10-year increase), y | 64.7 (53.0–76.7) | 66.3 (54.5–78.1) | 62.8 (51.3–74.1) | 1.05 (1.02–1.09) | .001 |
| Women | 820 (48.1) | 471 (48.8) | 349 (47.2) | 1.02 (.94–1.10) | .65 |
| Race | |||||
| White | 732 (42.9) | 394 (40.8) | 338 (45.7) | Ref | |
| Black | 802 (47.0) | 473 (49.0) | 329 (44.5) | 1.05 (.90–1.21) | .56 |
| Other | 171 (10.0) | 98 (10.2) | 73 (9.9) | 1.06 (.92–1.22) | .40 |
| Comorbidities | |||||
| Body mass index, kg/m2 | 29.8 (25.5–35.9) | 29.4 (25.4–35.6) | 30.4 (25.7–36.7) | .99 (.98–1.00) | .06 |
| Charlson comorbidity score, median (IQR) | 1 (0–3) | 2 (0–3) | 1 (0–3) | 1.03 (1.01–1.05) | .003 |
| COPD | 200 (11.7) | 121 (12.5) | 79 (10.7) | 1.06 (.94–1.20) | .33 |
| Asthma | 215 (12.6) | 112 (11.6) | 103 (13.9) | .91 (.77–1.08) | .27 |
| Moderate or severe chronic kidney disease | 449 (26.3) | 275 (28.5) | 174 (23.5) | 1.12 (1.02–1.22) | .02 |
| On dialysis | 57 (3.3) | 30 (3.1) | 27 (3.6) | .94 (.77–1.15) | .57 |
| On immune-suppressive medications | 166 (9.7) | 98 (10.2) | 68 (9.2) | 1.08 (.97–1.20) | .16 |
| Admission from skilled nursing or subacute rehabilitation facility | 236 (13.8) | 155 (16.1) | 81 (10.9) | 1.18 (1.05–1.32) | .006 |
| Severity of illness | |||||
| Initial admission to intensive care unit | 185 (10.9) | 132 (13.7) | 53 (7.2) | 1.31 (1.20–1.44) | <.001 |
| Highest mode of respiratory support on day 1 or 2 of hospitalization | |||||
| No supplemental oxygen | 595 (34.9) | 278 (28.8) | 317 (42.8) | Ref | |
| Low-flow oxygen | 937 (55.0) | 554 (57.4) | 383 (51.8) | 1.24 (1.12–1.36) | <.001 |
| Heated high-flow nasal cannula | 44 (2.6) | 33 (3.4) | 11 (1.5) | 1.58 (1.35–1.85) | <.001 |
| Noninvasive positive-pressure ventilation | 13 (0.8) | 9 (0.9) | 4 (0.5) | 1.50 (1.16–1.95) | .002 |
| Mechanical ventilation | 116 (6.8) | 91 (9.4) | 25 (3.4) | 1.61 (1.35–1.92) | <.001 |
| Sepsis on day 1 or 2 of hospitalizationb | 1260 (73.9) | 748 (77.5) | 512 (69.2) | 1.23 (1.11–1.36) | <.001 |
| Severe sepsis on day 1 or 2 of hospitalizationb | 481 (28.2) | 319 (33.1) | 162 (21.9) | 1.25 (1.15–1.36) | <.001 |
| Septic shock on day 1 or 2 of hospitalizationb | 138 (8.1) | 101 (10.5) | 37 (5.0) | 1.35 (1.19–1.53) | <.001 |
| Signs/symptoms potentially indicating bacterial infection | |||||
| Highest white blood cell count on day 1 or 2 of hospitalization, median (IQR), 103/μL | 6.8 (5.2–9.2) | 7.0 (5.3–9.8) | 6.6 (4.9–8.6) | 1.01 (1.00–1.01) | .11 |
| Initial chest X-ray or chest CT was normal | 196 (11.5) | 74 (7.7) | 122 (16.5) | .66 (.57–.76) | <.001 |
| Initial chest X-ray or chest CT showed lobar infiltrate | 87 (5.1) | 63 (6.5) | 24 (3.2) | 1.29 (1.14–1.47) | <.001 |
| Initial procalcitonin value (N = 910c) | |||||
| 0–0.1 ng/mL | 288 (31.6) | 133 (26.7) | 155 (37.7) | Ref | |
| 0.1–0.25 ng/mL | 278 (30.5) | 144 (28.9) | 134 (32.6) | 1.15 (.97–1.35) | .11 |
| 0.25–0.5 ng/mL | 139 (15.3) | 76 (15.2) | 63 (15.3) | 1.22 (1.02–1.46) | .03 |
| >0.5 ng/mL | 205 (22.5) | 146 (29.3) | 59 (14.4) | 1.57 (1.39–1.77) | <.001 |
| Initial CRP (N = 999c), mg/dL | 18.8 (7.2–92.3) | 22.8 (8.9–107.7) | 15.2 (5.4–67.5) | 1.001 (1.000–1.002) | .001 |
| Sputum production | 223 (13.1) | 131 (13.6) | 92 (12.4) | 1.04 (.93–1.16) | .51 |
Data are presented as n (%) unless otherwise indicated; N = 1705. Missing data: 86 patients were missing body mass index, 23 patients were missing white blood cell count.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; IQR, interquartile range; Ref, reference.
aEmpiric antibacterial therapy was defined as any intravenous or oral antibacterial therapy prescribed on day 1 or 2 of hospitalization. Does not include patients who received azithromycin only.
bSepsis was defined as ≥2 of the following: temperature >38°C or <36°C, heart rate >90 beats/minute, respiratory rate >20 breaths/minute, and leukocyte count >12 × 109 cells/L or <4 × 109 cells/L or >10% immature bands. Severe sepsis was defined as sepsis plus evidence of organ dysfunction, defined as any of the following: systolic blood pressure <90 mm Hg (or initiation of vasopressors), lactate level >2 mmol/L, platelet count <100 × 109 cells/L, bilirubin level >2 mg/dL (without documentation of moderate or severe liver disease), creatinine level >2 mg/dL (without documentation of moderate or severe chronic kidney disease), or ventilatory support (ie, noninvasive positive-pressure ventilation or mechanical ventilation). Septic shock included any vasopressor requirement (vasopressors include angiotensin II, dobutamine, epinephrine, norepinephrine, phenylephrine, or vasopressin).
cNot missing at random; 910 patients had a procalcitonin test result and 999 had a CRP test result.
Inclusion/Exclusion Criteria
Our primary cohort of interest was hospitalized adults with positive COVID-19 polymerase chain reaction (PCR) testing during the 2020 COVID-19 surge in the state of Michigan. Patients were excluded if they were pregnant, less than 18 years old, left against medical advice, were in comfort care within 3 hours of hospitalization, or were transferred from another hospital. For patients with multiple hospitalizations, only the first was included.
Sampling
A pseudo-random sample of COVID-19–positive cases discharged between 13 March 2020 and 18 June 2020 from each hospital was included. When hospitals had abstractor capacity to include all eligible patients, they did. Hospitals unable to abstract all cases (eg, due to high COVID-19 volumes) followed a pseudo-randomization procedure in which daily eligible cases were sorted by time stamp of discharge and included in order of smallest minute value until abstraction capacity was reached.
Data Collection
Similar to prior studies [15, 16], data on included patients were collected from 90 days prior to admission until death or hospital discharge. Data collected included demographics, comorbidities, antibacterial use (daily inpatient utilization), daily signs and symptoms (eg, laboratory results, vital signs, organ support), radiographic results, and microbiologic data. Data were collected from medical records using a standardized data dictionary and operations manual and entered into the MI-COVID19 registry using a structured data collection template.
Outcomes
The primary outcome was the percentage of patients prescribed early empiric antibacterial therapy (any intravenous or oral antibacterial on day 1 or 2 of hospitalization). Antibacterial therapy started after day 2 was not considered empiric as it may have been aimed toward hospital-onset infection. Empiric antibacterials were categorized as targeting (1) community-acquired organisms only (defined using 2019 American Thoracic Society and Infectious Diseases Society of America guidelines to include ampicillin/sulbactam, cefotaxime, ceftriaxone, moxifloxacin, levofloxacin, ceftaroline; see Supplementary Appendix for details) [17], (2) methicillin-resistant Staphylococcus aureus (MRSA), or (3) Pseudomonas aeruginosa. Azithromycin in the absence of other antibacterial treatment was not included as many MI-COVID19 hospitals recommended its use as COVID-19–specific therapy [18]. We also calculated antibiotic duration based on daily administration and discharge prescriptions.
The secondary outcome of interest was community-onset bacterial coinfection. Coinfections were identified by (1) blood or respiratory culture positive for a typically pathogenic bacterium (for a list of excluded contaminants, see the Supplementary Appendix), (2) positive Legionella pneumophila or Streptococcus pneumonia antigen, or (3) positive Mycoplasma pneumonia or Chlamydophila pneumonia PCR test. We also report how many patients had a community-onset viral infection based on respiratory virus PCR testing. Coinfections were considered community-onset if the positive culture or test was collected in the first 3 days of hospitalization.
Predictor Variables
Variables of interest included the following: (1) patient demographics, (2) comorbidities, (3) symptoms (eg, cough), (4) disease severity on admission (admission to intensive care, highest mode of respiratory support on day 1 or 2 of hospitalization, sepsis, severe sepsis, septic shock), and (5) features potentially indicating bacterial infection (chest imaging showing lobar infiltrate, sputum production, elevated procalcitonin, elevated white blood cell count, elevated C-reactive protein [CRP]). Hospital-level variables included bed size, profit type, and self-reported teaching status.
Statistical Analysis
Descriptive statistics (percentages and medians with interquartile range [IQR]) were used to characterize the cohort. To evaluate individual predictors associated with empiric antibacterial use or community-onset bacterial coinfection, we performed bivariable analyses using generalized estimating equation (GEE) Poisson models with robust standard errors and compound symmetry correlation structure, accounting for hospital clustering. We used Poisson models rather than logistic regression because the odds ratio only approximates the rate ratio when the outcome is rare [19]. For multivariable analysis, we used Poisson GEE models with backwards elimination starting with all variables that had a P value of less than .10 in bivariable analyses and eliminating variables until all had a P value less than .050. C-reactive protein and procalcitonin were not missing at random (both variables were linked to hospital test availability and practice) and thus could not be imputed and were not included in the multivariable model. When describing hospital variation in empiric antibacterial use, we included hospitals with at least 10 included patients positive for COVID-19 (N = 32 hospitals). All statistical tests were 2-sided; P-values less than .050 were significant. SAS version 9.4 (SAS Institute) was used for analyses. We followed EQUATOR (Enhancing the QUAlity and Transparency Of health Research) reporting guidelines (STROBE [Strengthening the Reporting of Observational Studies in Epidemiology] in the Supplementary Appendix).
Institutional Review Board Approval
This project received nonregulated status prior to data collection by the University of Michigan Institutional Review Board.
RESULTS
A total of 3412 patients with COVID-19 were eligible for inclusion. After pseudo-randomization, a total of 1705 patients from 38 hospitals were included. Patient characteristics are shown in Table 1.
Early Empiric Antibacterial Therapy
The majority (56.6% [965/1705]) of hospitalized patients with COVID-19 were prescribed early empiric antibacterial therapy (Table 2). The most commonly prescribed empiric antibacterials were ceftriaxone (38.9% [663/1705]), vancomycin (13.8% [235/1705]), doxycycline (10.9% [185/1705]), and cefepime (10.4% [177/1705]). Of patients who received empiric antibiotic therapy (N = 965), the majority (63.4%, 612) were only prescribed antibacterials targeting community-acquired pathogens; however, 25.8% (249) received antibacterials targeting MRSA, and 26.3% (254) received antibacterials targeting Pseudomonas aeruginosa. In those who received empiric antibacterial therapy, the median inpatient duration was 3 days (IQR, 2–6 days). Only 11.4% (110/965) of those prescribed antibiotics were prescribed antibiotics at discharge (median, 4 days; IQR, 3–5 days’ duration after discharge). Total days of inpatient, postdischarge, and total antibacterial therapy were 4158 days/1000 patients, 484 days/1000 patients, and 4628 days/1000 patients, respectively.
Table 2.
Diagnostic Testing, Coinfection, and Antibacterial Use in Hospitalized Patients With Coronavirus Disease 2019 (COVID-19)
| Values | |
|---|---|
| Cultures obtained within first 3 days of hospitalization | |
| Blood or respiratory culture obtained | 1095 (64.2) |
| Blood culture | 1063 (62.3) |
| Respiratory culture | 131 (7.7) |
| Nonculture testing performed | 934 (54.8) |
| Respiratory PCR test | 783 (45.9) |
| Urine legionella antigen | 413 (24.2) |
| Urine pneumococcal antigen | 304 (17.8) |
| Had a community-onset bacterial coinfectiona | 59 (3.5) |
| Positive blood or respiratory culture | 55 (3.2) |
| Positive blood culture | 31 (1.8) |
| Positive respiratory culture | 25 (1.5) |
| Had a community-onset viral coinfectionb | 9 (0.5) |
| Influenza A or B | 1 (0.1) |
| Other viral pathogen | 8 (0.5) |
| Empiric antibacterial therapyc | 965 (56.6) |
| Community-acquired empiric coverage onlyd | 612 (35.9) |
| Ampicillin/sulbactam | 41 (2.4) |
| Cefotaxime | 5 (0.3) |
| Ceftriaxone | 663 (38.9) |
| Moxifloxacin | 4 (0.2) |
| Levofloxacin | 20 (1.2) |
| Ceftaroline | 1 (0.1) |
| Empiric anti-MRSA therapye | 249 (14.6) |
| Vancomycin | 235 (13.8) |
| Empiric anti-pseudomonal therapyf | 254 (14.9) |
| Cefepime | 177 (10.4) |
| Piperacillin/ tazobactam | 72 (4.2) |
| Empiric anti-MRSA and anti-pseudomonal therapye,f | 184 (10.8) |
| Turnaround time for COVID-19 PCR test, median (IQR), d | 1 (0–2) |
| Length of hospital stay, median (IQR), d | 5 (3–9) |
Data are presented as n (%) unless otherwise indicated; N = 1705.
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction.
aCommunity-onset bacterial coinfection included any positive blood or respiratory culture or microbiological test obtained in the first 3 days of hospitalization (contaminants excluded).
bCommunity-onset viral coinfection included any viruses identified on a respiratory PCR obtained in the first 3 days of hospitalization.
cEmpiric antibacterial therapy was defined as any intravenous or oral antibacterial therapy prescribed on day 1 or 2 of hospitalization. Does not include patients who received azithromycin monotherapy. May add up to more than 100% as patients may be in multiple rows.
dIncludes patients who received antibacterials recommended for empiric community-acquired pneumonia (CAP) treatment in 2019 CAP guidelines [17] (ie, ampicillin/sulbactam, cefotaxime, ceftriaxone, moxifloxacin, levofloxacin, ceftaroline) and did not receive empiric anti-MRSA or anti-pseudomonal coverage.
eAnti-MRSA antibacterials include vancomycin, linezolid, sulfamethoxazole/trimethoprim, or clindamycin.
fAnti-pseudomonal antibacterials include piperacillin/tazobactam, aminoglycosides, ceftazidime, aztreonam, meropenem, imipenem, ceftolozane/tazobactam, polymixin B, colistin, ciprofloxacin, cefepime, ceftazadime-avibactam, or meropenem-vaborbactam.
Bacterial Coinfections in Patients Positive for COVID-19
Community-onset bacterial coinfections were confirmed in 3.5% (59/1705) of all patients, including 1.8% (31/1705) who had a positive blood culture and 1.7% (29/1705) who had a bacterial respiratory pathogen identified (from respiratory culture or nonculture diagnostic test). Community-onset bacterial infections occurred in 4.9% (47/965) of patients who received early empiric antibacterial therapy versus 1.6% (12/740) of those who did not (P < .001), of whom 33.3% (4/12) were subsequently started on antibiotics. Patients were more likely to have a community-onset bacterial infection if they were older, had a lower body mass index, had kidney disease, were admitted from a skilled nursing facility, were more severely ill (eg, admitted to intensive care), or had more signs of a bacterial infection (eg, higher white blood cell count; see Table 3 for details). Although 55.9% (19/34) of patients with a community-onset bacterial coinfection had a procalcitonin level greater than 0.5 ng/mL, so did 21.2% (186/876) of those without a community-onset bacterial coinfection. Thus, the positive-predictive value of a procalcitonin level greater than 0.5 ng/mL was 9.3% for community-onset bacterial coinfection. In contrast, the negative-predictive value of a procalcitonin level of 0.1 ng/mL or less was 98.3%. Compared with patients without a confirmed community-onset bacterial infection, those with a confirmed infection had a longer length of stay (median, 7 [IQR, 4–10] vs 5 [3–8] days; P = .003) and had higher in-hospital mortality (47.5% [28/71] vs 18.0% [297/1634]; P < .001).
Table 3.
Patient Characteristics and Bivariable Predictors of Community-Onset Bacterial Coinfection in Patients With Coronavirus Disease 2019 (COVID-19)
| Confirmed Community-Onset Bacterial Coinfectiona (n = 59) | No Confirmed Bacterial Coinfection (n = 1646) | Rate Ratio (95% CI) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) (rate ratio reported per 10-year increase), y | 72.6 (61.9–85.4) | 64.5 (52.7–76.5) | 1.30 (1.08–1.57) | .006 |
| Women | 32 (54.2) | 788 (47.9) | 1.30 (.70–2.41) | .41 |
| Race | ||||
| White | 33 (55.9) | 699 (42.5) | Ref | |
| Black | 22 (37.3) | 780 (47.4) | .63 (.33–1.17) | .14 |
| Other | 4 (6.8) | 167 (10.1) | .50 (.17–1.46) | .21 |
| Comorbidities | ||||
| Body mass index; kg/m2 | 26.6 (22.7–31.1) | 30.0 (25.6–36.1) | .94 (.90–.99) | .009 |
| Charlson comorbidity score, median (IQR) | 2 (1–5) | 1 (0–3) | 1.20 (1.10–1.31) | <.001 |
| COPD | 10 (16.9) | 190 (11.5) | 1.52 (.67–3.46) | .31 |
| Asthma | 5 (8.5) | 210 (12.8) | .66 (.31–1.39) | .27 |
| Moderate or severe chronic kidney disease | 26 (44.1) | 423 (25.7) | 2.19 (1.35–3.57) | .002 |
| On immune suppressive medications | 8 (13.6) | 158 (9.6) | 1.44 (.83–2.51) | .20 |
| Admission from skilled nursing or subacute rehabilitation facility | 23 (39.0) | 213 (12.9) | 3.96 (2.44–6.43) | <.001 |
| Severity of illness | ||||
| Initial admission to intensive care unit | 21 (35.6) | 164 (10.0) | 4.45 (2.87–6.88) | <.001 |
| Highest mode of respiratory support on day 1 or 2 of hospitalization | ||||
| No supplemental oxygen | 12 (20.3) | 583 (35.4) | Ref | |
| Low-flow oxygen | 29 (49.2) | 908 (55.2) | 1.52 (.75–3.09) | .24 |
| Heated high-flow nasal cannula | 1 (1.7) | 43 (2.6) | 1.13 (.15–8.33) | .91 |
| Noninvasive positive-pressure ventilation | 1 (1.7) | 12 (0.7) | 3.65 (.57–23.52) | .17 |
| Mechanical ventilation | 16 (27.1) | 100 (6.1) | 6.74 (3.43–13.25) | <.001 |
| Sepsis on day 1 or 2 of hospitalizationb | 52 (88.1) | 1208 (73.4) | 2.55 (1.28–5.08) | .008 |
| Severe sepsis on day 1 or 2 of hospitalizationb | 35 (59.3) | 446 (27.1) | 3.62 (2.16–6.07) | <.001 |
| Septic shock on day 1 or 2 of hospitalizationb | 17 (28.8) | 121 (7.4) | 4.60 (2.65–8.00) | <.001 |
| Signs/symptoms potentially indicating bacterial infection | ||||
| Highest white blood cell count on day 1 or 2 of hospitalization, median (IQR), 103/μL | 10 (5.9–15.8) | 6.8 (5.1–9.0) | 1.03 (1.01–1.04) | <.001 |
| Initial chest X-ray or chest CT was normal | 7 (11.9) | 189 (11.5) | 1.04 (.42–2.58) | .93 |
| Initial chest X-ray or chest CT showed lobar infiltrate | 4 (6.8) | 83 (5.0) | 1.30 (.44–3.86) | .64 |
| Initial procalcitonin value (N = 910c) | ||||
| 0–0.1 ng/mL | 5 (14.7) | 283 (32.3) | Ref | |
| 0.1–0.25 ng/mL | 9 (26.5) | 269 (30.7) | 1.88 (.70–4.69) | .22 |
| 0.25–0.5 ng/mL | 1 (2.9) | 138 (15.8) | .44 (.07–2.68) | .37 |
| >0.5 ng/mL | 19 (55.9) | 186 (21.2) | 4.99 (1.87–13.33) | .001 |
| Initial CRP (N = 999c), mg/dL | 26.2 (14.9–123.0) | 18.5 (7.1–90.0) | 1.00 (1.00–1.00) | .15 |
| Sputum production | 12 (20.3) | 211 (12.8) | 1.69 (.93–3.08) | .09 |
Data are presented as n (%) unless otherwise indicated; N = 1705. Missing data: 86 patients were missing body mass index, 23 patients were missing white blood cell count.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; IQR, interquartile range; Ref, reference.
aCommunity-onset bacterial coinfections include any positive blood or respiratory culture or microbiological test obtained in the first 3 days of hospitalization (contaminants excluded).
bSepsis was defined as ≥2 of the following: temperature >38°C or <36°C, heart rate >90 beats/minute, respiratory rate >20 breaths/minute, and leukocyte count >12 × 109 cells/L or <4 × 109 cells/L or >10% immature bands. Severe sepsis was defined as sepsis plus evidence of organ dysfunction, defined as any of the following: systolic blood pressure <90 mm Hg (or initiation of vasopressors), lactate level >2 mmol/L, platelet count <100 × 109 cells/L, bilirubin level >2 mg/dL (without documentation of moderate or severe liver disease), creatinine level >2 mg/dL (without documentation of moderate or severe chronic kidney disease), or ventilatory support (ie, noninvasive positive pressure ventilation or mechanical ventilation). Septic shock included any vasopressor requirement (vasopressors include angiotensin II, dobutamine, epinephrine, norepinephrine, phenylephrine, or vasopressin).
cNot missing at random; 910 patients had a procalcitonin test result and 999 had a CRP test result.
Nearly half (45.9%, 783/1705) of patients had respiratory PCR testing while only 0.5% (9/1705) had an identified community-onset viral coinfection. There was no difference in early empiric antibiotic use in those with an identified community-onset viral coinfection compared with those without (66.7% vs 56.5%; P = .74).
Variation in Empiric Antibacterial Therapy in Patients With COVID-19
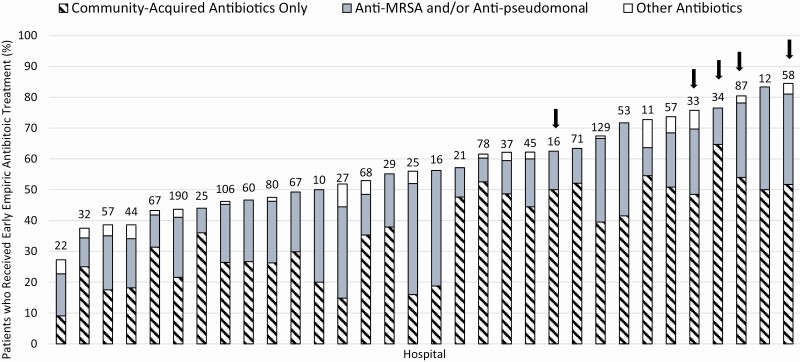
Thirty-two hospitals had at least 10 patients with COVID-19 included in MI-COVID19. Within these sites, the percentage of patients with COVID-19 who were prescribed empiric antibacterials varied from 27% to 84%. Similarly, the percentage of patients receiving antibacterial therapy targeting community-acquired versus anti-MRSA and/or anti-pseudomonal coverage also varied widely by hospital (Figure 1).
Figure 1.
Early empiric antibiotic treatment in hospitalized patients with COVID-19, by hospital (N = 32 hospitals; 1667 patients). Each bar represents 1 hospital. The number of COVID-19–positive cases included per hospital is shown at the top of each bar. Arrows indicate for-profit hospitals. Hospitals with less than 10 included COVID-19–positive cases are not shown (n = 6). Abbreviations: COVID-19, coronavirus disease 2019; MRSA, methicillin-resistant Staphylococcus aureus.
Predictors of Empiric Antibacterial Therapy in Patients With COVID-19
Bivariable predictors of empiric antibacterial therapy are shown in Table 1. On multivariable analysis, patients were more likely to receive early empiric antibacterial therapy if they were older, had a lower body mass index, had more severe disease (eg, respiratory support, severe sepsis), had a lobar infiltrate, or were admitted to a for-profit hospital; patients admitted at a later date in the surge were less likely to receive empiric antibacterials (see Table 4).
Table 4.
Multivariable Predictors of Early Empiric Antibiotic Therapy in Patients With Coronavirus Disease 2019 (COVID-19)
| Adjusted Rate Ratio (95% CI) | P | |
|---|---|---|
| Age (for each 10 additional years) | 1.04 (1.01–1.08) | .02 |
| Body mass index (per additional point) | .99 (.99–1.00) | .03 |
| Highest level of respiratory support on day 1 or 2 of hospitalization | ||
| None | Ref | |
| Low-flow oxygen (nasal cannula, oxygen mask) | 1.18 (1.06–1.31) | .002 |
| Heated high-flow nasal cannula | 1.50 (1.28–1.76) | <.001 |
| Noninvasive positive-pressure ventilation | 1.35 (.98–1.85) | .07 |
| Invasive mechanical ventilation | 1.29 (1.07–1.54) | .007 |
| Severe sepsis on admissiona | 1.16 (1.07–1.27) | <.001 |
| Initial chest X-ray or chest CT was normal | .72 (.62–.84) | <.001 |
| Initial chest X-ray or chest CT showed lobar infiltrate | 1.21 (1.04–1.42) | .02 |
| Admission month | ||
| March | Ref | |
| April | .71 (.63–.81) | <.001 |
| May | .76 (.58–1.01) | .06 |
| June | .79 (.59–1.06) | .11 |
| Hospital ownership | ||
| Nonprofit | Ref | |
| For profit | 1.30 (1.15–1.47) | <.001 |
N = 1705. Empiric antibacterial therapy was defined as any intravenous or oral antibacterial therapy prescribed on day 1 or 2 of hospitalization. Does not include patients who received azithromycin only. Multivariable analysis used Poisson GEE (generalized estimating equation) models with backwards elimination starting with all variables that had P-value <.10 in bivariable analyses and eliminating variables until all remaining had P-value <.050.
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; CT, computed tomography.
aSevere sepsis was defined as sepsis plus evidence of organ dysfunction, defined as any of the following: systolic blood pressure <90 mm Hg (or initiation of vasopressors), lactate level >2 mmol/L, platelet count <100 × 109 cells/L, bilirubin level >2 mg/dL (without documentation of moderate or severe liver disease), creatinine level >2 mg/dL (without documentation of moderate or severe chronic kidney disease), or ventilatory support (ie, noninvasive positive-pressure ventilation or mechanical ventilation).
Empiric Antibacterial Therapy and COVID-19 PCR Tests
Of patients with COVID-19 who were prescribed empiric antibacterials and had their COVID-19 test return before the end of their hospitalization, 453 of 832 (54.4%) had their antibacterials stopped within 1 day after COVID-19 tests returned positive. Of the 379 who had antibacterials continued, only 28 (7.4%) had a confirmed community-onset bacterial coinfection. Of those who had antibacterials continued and did not have a confirmed community-onset bacterial coinfection, 35.9% (126/351) had fewer than 5 days’ total inpatient antibacterial duration; 39.6% (139/351) had 5 to 7 days and 24.5% (86/351) had more than 7 days.
Excluding 4 patients with missing COVID-19 test dates, the percentage of patients who had a COVID-19 PCR test turnaround time of 1 day or less was 64.9% (624/962) in patients who received early empiric antibacterial therapy compared with 76.4% (565/739) in patients without early empiric therapy (P < .001). Antibacterial therapy was often given prior to clinicians knowing the results of COVID-19 tests. For example, 13.6% (131/962) of patients who received early empiric therapy did not have a COVID-19 test return until after discharge. Furthermore, about half (52.5%) of antibacterial treatment duration occurred prior to COVID-19 PCR tests showing a positive result. Turnaround times decreased over time (54.2% [461/850] returned ≤1 day in March; April: 85.2% [628/737]; May: 89.2% [91/102]; June: 75.0% [9/12]; P < .001 for monthly trend). Similarly, the percentage of patients with COVID-19 who were treated empirically with antibacterials decreased over time: March, 66.7% (95% confidence interval [CI]: 63.4–69.9; April, 46.7% (95% CI: 43.1–50.4); May, 46.9% (95% CI: 37.3–56.6); June, 60.0% (95% CI: 32.3–83.7) (P < .001 for monthly trend).
DISCUSSION
In this large, multicenter cohort of patients hospitalized with COVID-19, we found that 56.6% were treated with early empiric antibacterial therapy. Despite concerns that patients with COVID-19 might be at high risk of bacterial coinfections, we found only 3.5% of patients positive for COVID-19 had a confirmed community-onset bacterial coinfection. Early empiric antibacterial use varied from 27% to 84% across hospitals and decreased over time.
Similar to other studies [4, 9, 11, 20], we found that patients hospitalized with COVID-19 were often treated with early empiric antibacterials. Notably, we saw wide variability between hospitals in early empiric antibacterial use, suggesting a need for a standardized approach to antibiotic use, diagnostic testing, and antibiotic stewardship in patients with COVID-19. Although the reasons for variation in empiric antibiotic use are unclear, variability could relate to existing antibiotic stewardship infrastructure or hospital culture. Supportively, we found for-profit hospitals had more empiric antibacterial use even after adjustments. Other studies have found for-profit hospitals to have more variable quality of care [21, 22]; however, this has not been seen previously with hospitalized patients with pneumonia in HMS hospitals [15]. The fact that empiric antibacterial use was low in some hospitals suggests that unnecessary antibacterial use can be reduced even during pandemics—for example, by fast-moving, responsive, and well-supported antibiotic stewardship programs [23].
The high rate of empiric antibacterial use seen in patients hospitalized with COVID-19 must be considered in the context of the low prevalence of confirmed community-onset bacterial coinfection (3.5% in all patients, 4.9% in those who received antimicrobials). For every patient we identified as having a bacterial infection, nearly 20 without an identified infection also received empiric antibacterial therapy. These findings are similar to a 2-hospital study in the United Kingdom, which noted that 3.2% of patients with COVID-19 had early confirmed bacterial infections (increasing to 6.1% later during hospitalization) [7], and a study in New York, which identified 3.6% of hospitalized patients with COVID-19 who had bacterial or fungal coinfections [8]. Other studies with systematic sampling have found bacterial coinfections in up to 30% of patients with COVID-19, although the clinical significance is unclear as only 4% were severely or critically ill [6].
As with all retrospective studies of bacterial infection, we were limited by the poor sensitivity and incomplete use of diagnostic tests for bacterial infections. In particular, there was very low use of respiratory cultures in our cohort: only 7.7% of patients had a respiratory culture performed in the first 3 days of hospitalization. This is much lower than a prior study of patients hospitalized with pneumonia in HMS hospitals where 32.3% had respiratory cultures [15]. There are 2 potential reasons for the lack of respiratory cultures. First, it is likely that fewer respiratory cultures—specifically more sensitive tests, such as induced sputum or bronchoalveolar lavage—were ordered due to concerns regarding aerosolization (and therefore healthcare worker safety) in patients with COVID-19. This may explain why we found other tests that do not create aerosols were used at similar or higher levels than in prior studies. For example, 54.8% of patients had nonculture respiratory pathogen testing (eg, urine legionella antigen) compared with 15.9% in our prior study of patients hospitalized with pneumonia in HMS hospitals [15]. Second, respiratory cultures may be difficult to obtain in patients with COVID-19 due to the nature of their coughs: only 13.1% of patients in our cohort had documented sputum production compared with 51.6% in a historical sample of HMS patients with pneumonia [15]. This deficiency in diagnostic testing is a critical barrier to coinfection detection and antibiotic stewardship. Regardless, because patients with COVID-19 have a known viral pathogen (SARS-CoV-2) that explains their infectious symptoms, more judicious antibacterial use may be necessary in the absence of other signs of bacterial infection.
Our findings suggest some potential ways to improve antibacterial use and point to continued need for investigation. First, diagnostic uncertainty caused by delays in the turnaround time for COVID-19 PCR testing may have contributed to antibacterial use, highlighting the need for more testing capacity and faster COVID-19 turnaround times. We found that over half (54.3%) of patients positive for COVID-19 had antibacterial therapy stopped within 1 day of testing returning positive. As the turnaround time for COVID-19 decreased, early empiric antibacterial use decreased. Second, we found patients who were more severely ill had more empiric antibacterial treatment. In a subset of patients with severe COVID-19, a cytokine storm rather than bacterial sepsis may be responsible for early decompensation [24]. Further studies are needed to aid clinicians in distinguishing the 2 conditions. For example, stewardship programs could help guide antibacterial de-escalation and cessation for critically ill patients who have a negative workup for bacterial pathogens. Similarly, biomarkers have a theoretical role in distinguishing patients who have versus do not have bacterial infection, although it is unclear if they would truly change clinical practice: we found procalcitonin values of less than 0.1 ng/mL to have a negative-predictive value of 98.3%, yet nearly a third of patients treated empirically with antibacterials had procalcitonin values this low.
Our study represents a diverse look at early empiric antibacterial therapy across multiple hospitals. However, our findings must be considered in the context of limitations. First, we do not have complete data on secondary bacterial infections, which may develop later during hospitalization. It is likely that, for some patients, bacterial coinfections develop later in hospitalization [25]. Second, we have limited data on patient outcomes given insufficient time since most were discharged, limiting our ability to assess the effect of early empiric antibacterial use on outcomes. Larger or prospective studies are needed to help determine who would benefit from empiric antibacterial therapy. Third, as noted above, we were limited by a lack of systematic diagnostic testing. Fourth, we excluded azithromycin as an “antibacterial” because we were unable to distinguish between azithromycin use as an antibacterial versus targeted therapy for COVID-19; thus, we likely underestimate the true prevalence of antibacterial overuse.
Study strengths include data from multiple, diverse hospitals across a state that was surging at the time of data collection. Furthermore, the existing infrastructure from our prior pneumonia quality-improvement work [13–15] allowed us to rapidly, but rigorously, collect and analyze data with experienced abstractors and analysts.
Our findings have important implications. The known risks associated with unnecessary antibacterial use and the low rate of confirmed early bacterial coinfection in patients with COVID-19 suggest against routinely prescribing antibacterial therapy to patients with COVID-19 pneumonia who present without other risk factors or signs of bacterial infection. Second, the variation in antibacterial use across hospitals suggests an imperfect response to limited data. It will be key to understand whether existing stewardship or quality infrastructure may also help hospitals make better treatment decisions during pandemics. Third, our findings suggest that faster testing turnaround time is imperative to help inform empiric treatment decisions, including antibacterial therapy. Notably, antibacterials were stopped after COVID-19 tests returned positive at higher rates than with other viral pneumonias [26]. Finally, we need better training and understanding of how to incorporate imperfect tests and diagnostic uncertainty into decision making [27].
In conclusion, we found high use of early empiric antibacterial therapy in patients hospitalized with COVID-19, despite a low prevalence of confirmed community-onset bacterial coinfections. Given the potential harms to patients and society from unnecessary antibacterial use—plus the additional burden on staff use and PPE (personal protective equipment) required for antibacterial administration—it is imperative that we develop strategies to help clinicians prescribe antibacterials judiciously to hospitalized patients with COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all participating collaborative quality initiatives (CQIs) and their members. Participating CQIs include the following: Hospital Medicine Safety (HMS) Consortium, Michigan Bariatric Surgery Collaborative (MBSC), Michigan Surgical Quality Collaborative (MSQC), Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI), Michigan Value Collaborative (MVC), Michigan Emergency Department Improvement Collaborative (MEDIC), Michigan Radiation Oncology Quality Collaborative (MROQC), Michigan Anticoagulation Quality Improvement Initiative (MAQI2), Michigan Spine Surgery Improvement Collaborative (MSSIC), Integrated Michigan Patient-Centered Alliance in Care Transitions (IMPACT), and the Michigan Trauma Quality Improvement Program (MTQIP).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. This manuscript does not represent the views of the Department of Veterans Affairs or the US government.
Financial support. This work was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network, as part of their Value Partnerships program. V. M. V. is supported by a career development award from the Agency for Healthcare Research and Quality (1-K08-HS26530-01). H. C. P. is supported by grant number R01 HS026725 from the Agency for Healthcare Research and Quality. This material is the result of work supported with resources and use of facilities at the Ann Arbor VA Medical Center.
Potential conflicts of interest. S. A. F. reports expert testimony fees, grants from Blue Cross Blue Shield of Michigan and the Agency for Healthcare Research and Quality (AHRQ), and personal fees from Wiley Publishing, outside the submitted work. H. C. P. reports grants from AHRQ, the National Institutes of Health, and the Department of Veterans Affairs, outside the submitted work. H. C. P. also serves on the Surviving Sepsis Campaign Guidelines. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook DJ, Marshall JC, Fowler RA. Critical illness in patients with COVID-19: mounting an effective clinical and research response. JAMA 2020. [DOI] [PubMed] [Google Scholar]
- 4. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020; 81:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clancy CJ, Nguyen MH. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis 2020; ciaa524. doi: 10.1093/cid/ciaa524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu X, Ge Y, Wu T, et al. . Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res 2020; 285:198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes S, Troise O, Donaldson H, Mughal N, Moore L. Bacterial and fungal coinfection among hospitalised patients with COVID-19: a retrospective cohort study in a UK secondary care setting. Clin Microbiol Infect 2020; S1198-743X(20)30369-4. doi: 10.1016/j.cmi.2020.06.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nori P, Cowman K, Chen V, et al. . Bacterial and fungal co-infections in COVID-19 patients hospitalized during the New York city pandemic surge. Infect Control Hosp Epidemiol 2020:1–13. doi: 10.1017/ice.2020.368. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langford BJ, So M, Raybardhan S, et al. . Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; S1198-743X(20)30423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehmann CJ, Pho MT, Pitrak D, Ridgway JP, Pettit NN. Community acquired co-infection in COVID-19: a retrospective observational experience. Clin Infect Dis 2020; ciaa902. doi: 10.1093/cid/ciaa902. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawson TM, Moore LSP, Zhu N, et al. . Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; ciaa530. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michigan Medicine, Universit of Michigan. Michigan Medicine teams with Blue Cross Blue Shield of Michigan and 25 Michigan hospitals for unique COVID-19 data collection to help determine treatments and best care practices. Published 2020. Available at: https://www.uofmhealth.org/news/archive/202004/michigan-medicine-teams-blue-cross-blue-shield-michigan-and. Accessed 11 June 2020.
- 13. Vaughn VM, Petty LA, Flanders SA, et al. . A deeper dive into antibiotic stewardship needs: a multihospital survey. Open Forum Infect Dis 2020; 7:ofaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in Michigan hospitals: a multi-hospital cohort study. Clin Infect Dis 2019; 69:1269-77. doi: 10.1093/cid/ciy1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaughn VM, Flanders SA, Snyder A, et al. . Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 16. Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in Michigan hospitals: a multi-hospital cohort study. Clin Infect Dis 2019; 69:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gautret P, Lagier JC, Parola P, et al. . Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 20. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brock DW, Buchanan A. Ethical issues in for-profit health care. For-Profit Enterprise in Health Care J Med Philos 1987; 12:1-35. doi: 10.1093/jmp/12.1.1. [DOI] [Google Scholar]
- 22. Horwitz JR. Making profits and providing care: comparing nonprofit, for-profit, and government hospitals. Health Aff (Millwood) 2005; 24:790–801. [DOI] [PubMed] [Google Scholar]
- 23. Stevens MP, Patel PK, Nori P. Involving antimicrobial stewardship programs in COVID-19 response efforts: all hands on deck. Infect Control Hosp Epidemiol 2020; 41:744–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Specialty Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somers EC, Eschenauer GA, Troost JP, et al. . Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020; ciaa954. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yee C, Suarthana E, Dendukuri N, Nicolau I, Semret M, Frenette C. Evaluating the impact of the multiplex respiratory virus panel polymerase chain reaction test on the clinical management of suspected respiratory viral infections in adult patients in a hospital setting. Am J Infect Control 2016; 44:1396–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prescott HC, Iwashyna TJ. Improving sepsis treatment by embracing diagnostic uncertainty. Ann Am Thorac Soc 2019; 16:426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.