Abstract
Efforts to recognize and minimize the risk to study participants will be necessary to safely and ethically resume scientific research in the context of the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. These efforts are uniquely challenging in the context of human immunodeficiency virus (HIV) cure clinical trials, which often involve complex experimental therapy regimens and perhaps analytic treatment interruption, in which participants pause antiretroviral therapy. In this viewpoint, we discuss our approach to reopening an HIV cure trial in this context, with a focus on key considerations regarding study design, informed consent and participant education, and study implementation. These recommendations might be informative to other groups seeking to resume HIV cure research in settings similar to ours.
Keywords: HIV cure–related trials, analytical treatment interruptions, SARS-CoV-2, COVID-19, risk mitigation
Approach to reopening a human immunodeficiency virus–cure trial that includes an analytic treatment interruption in the context of the severe acute respiratory syndrome coronavirus 2 pandemic, with considerations regarding study design, informed consent and participant education, and study implementation.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has had a major impact on research operations worldwide. As shelter-in-place guidelines were implemented throughout early 2020, many centers suspended research efforts to protect study participants and research staff while the scientific and medical communities raced to understand this novel infection.
Since that time, significant progress has been made in understanding the transmission, prevention, and management of SARS-CoV-2 infection and its associated illness, coronavirus disease 2019 (COVID-19) [1–6]. Just as the pandemic has taken a toll on patients without COVID-19 who delay or defer life-saving medical care [7], there is growing recognition that continued deferment of participant-based research has the potential to set back scientific progress and the advancement of health. For these reasons, institutions are beginning to relax SARS-CoV-2 restrictions while implementing new precautions, based on our now-substantial knowledge of routes of SARS-CoV-2 transmission, to maximize safety. Recognizing and mitigating the risk of SARS-CoV-2 infection is a critical step in ensuring that research studies can be conducted safely and ethically in the context of the ongoing pandemic.
RISKS AND CHALLENGES
Safety considerations are important in the context of human immunodeficiency virus (HIV) research studies, particularly trials of investigational agents that are meant to achieve sustained antiretroviral treatment (ART)-free suppression of HIV infection [8]. There are several reasons why this is the case. First, the level of intensity of such trials is likely to place participants at elevated risk for acquiring SARS-CoV-2 by increasing the number of potential exposure events due to the high frequency of study visits. Second, the impact of the interaction between HIV and SARS-CoV-2 coinfection remains incompletely understood, particularly in people living with HIV (PLWH), who have additional comorbidities associated with increased COVID-19 severity [9–12]. The risk is likely to be even more significant when comorbidities are incompletely controlled [13]. Third, such studies often require analytic treatment interruption (ATI), in which participants discontinue HIV medications under close medical supervision to determine the effect of interventions aimed at ART-free suppression [8, 14]. Participants are expected to experience HIV rebound during the ATI, and the impact of SARS-CoV-2 infection in PLWH off ART remains a major research question [10].
In light of these considerations, we developed and implemented recommendations prior to reopening a clinical trial that may be used as a model for similar HIV cure–related research operations that are attempting to open in the context of the ongoing SARS-CoV-2 pandemic. Our goal was to determine how to best operationalize an ATI in the era of COVID-19 and to generate an actionable plan that would allow this clinical trial to safely resume. Our process involved close, multi-disciplinary consultation between the biomedical study team; our community advisory boards, including racially, ethnically, and geographically diverse community members; and socio-behavioral scientists. The multi-disciplinary approach outlined here was developed over a series of meetings, during which the goal was to minimize the risk of SARS-CoV-2 acquisition and severe COVID-19 disease while empowering research participants to make informed decisions about their involvement in the study.
OVERARCHING PRINCIPLES
We began by defining 3 general principles to guide the research team during the study period:
1. We believe in the ongoing value of HIV cure–related research, notwithstanding the challenges posed by the SARS-CoV-2 pandemic. Our team articulated the position that engagement in studies employing ATIs is acceptable in cases in which the research goal is considered essential and a sufficient risk mitigation plan can be implemented.
2. We commit to reviewing all emerging data on general and HIV-specific risks of SARS-CoV-2 infection, as well as local epidemiology and public health recommendations, during the course of the study. This commitment acknowledges that recommendations related to SARS-CoV-2 prevention and treatment are likely to evolve over time. As a result, the team must be willing to update study procedures according to emerging findings and recommendations, with the overall goal of risk mitigation and rigorous participant engagement throughout the study.
3. We seek to balance the dual considerations of protocol-defined exclusions and robust informed consent regarding study participation risk. This was most clearly illustrated both by efforts to implement protocol-defined restrictions based on the current understanding of COVID-19 risk and by efforts to empower participants to make informed decisions about the acceptability of such risks in individual cases.
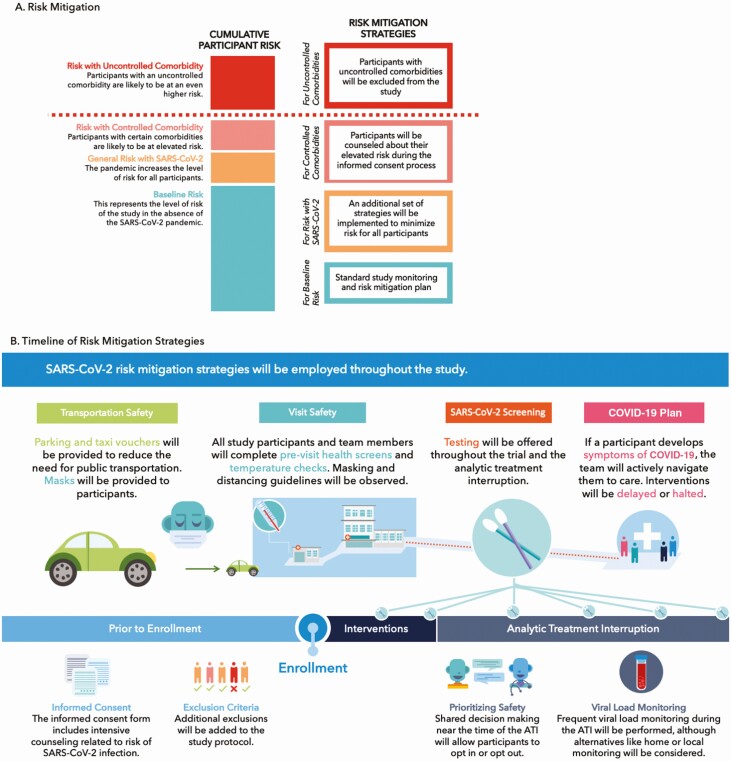
Following these principles, our team sought to answer critical questions related to study design, informed consent and participant education, and study implementation (Table 1). The goal of this effort was to identify and mitigate risk throughout the study, as summarized in Figure 1.
Table 1.
Key Questions
| Appropriateness of the study |
| Is the research question of sufficient importance that potential increased risks to participants are acceptable? |
| Should the study be implemented during the SARS-CoV-2 pandemic? |
| Study design |
| Are there structural changes to the study that might minimize risks to participants? |
| Should exclusion criteria be updated to minimize participant risk? |
| What should be the frequency of SARS-CoV-2 testing during the study? |
| What should be the frequency of HIV viral load monitoring during the ATI? |
| What should be the frequency of CD4 + T-cell monitoring during the ATI? |
| Informed consent and participant education |
| How should study risks related to SARS-CoV-2 be described in the informed consent form? |
| How can the study team ensure that participants are adequately informed and counseled about SARS-CoV-2–related risks during the study? |
| How should the study team account for emerging scientific information around SARS-CoV-2, COVID-19, and HIV, and evolving standards of prevention and treatment during the study? |
| Is there an independent clinician or health advisor with whom the participant can engage throughout the study? |
| Study implementation |
| How can risks related to the frequency of in-person trial visits be minimized? |
| What should SARS-CoV-2 standard-of-prevention precautions look like? |
| Analytical treatment interruption |
| What additional risk mitigation measures should be implemented around the ATI? |
| SARS-CoV-2 contingencies |
| What should happen if a participant has COVID-19–related symptoms? |
| What should happen if a participant tests positive for SARS-CoV-2 during the study? |
| What would happen if the local SARS-CoV-2 epidemic worsens? |
| How can an independent body such as a community advisory board or safety monitoring committee be engaged to advise the study team during the study? |
| How would the rollout of multiple SARS-CoV-2 vaccine trials or the potential implementation of an efficacious and approved vaccine be handled during the study? |
Questions are for consideration in the implementation of HIV cure studies during the SARS-CoV-2 pandemic.
Abbreviations: ATI, analytic treatment interruption; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
A, Risk mitigation approach adopted by the research team, demonstrating risk conferred by the study itself, the SARS-CoV-2 pandemic, controlled and uncontrolled comorbidities. B, Summary of risk mitigation strategy to be implemented by the study. Abbreviations: ATI, analytic treatment interruption; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
STUDY DESIGN
Justification for Study
We began by asking the question: “should this study be reopened during the SARS-CoV-2 pandemic?” To address this question, the study team and community advisory board convened a meeting and discussed whether the scientific questions addressed by the trial were of sufficient importance to justify the potential for the increased risk to study participants. This included discussion of the overall scientific value of the trial, the current risk-benefit assessment, and how this assessment might change if the study needed to be altered, paused, or halted in the future. We reached consensus that the study should proceed as long as an extensive risk mitigation plan could be implemented.
Necessity for ATI
Because the ATI in this study would occur 34 weeks after enrollment, we acknowledged that there would be insufficient information for participants to make an informed decision regarding the ATI at the time of the initial consent process. For this reason, we decided it would be necessary to allow participants to reconsent or opt out of the ATI based on an up-to-date risk-benefit assessment in closer proximity to that stage of the study. This opportunity for reassessment at the time of the ATI is likely to be critical for similar studies involving a delay between initial consent and treatment interruption, but may not be necessary when the ATI occurs in closer proximity to study enrollment. The decision was further informed by the study team’s determination that important scientific questions could still be answered even in cases in which a participant who completed the study interventions declined to interrupt HIV medications. In cases in which these facts are not present, investigators may choose to postpone the start of the study until the end of the pandemic.
Protocol-defined Exclusion Criteria
We deliberated on whether to employ strict study exclusion criteria in the context of the SARS-CoV-2 pandemic. This included a review of evidence regarding the factors associated with elevated risks of adverse outcomes related to SARS-CoV-2 infection in general and/or in people living with HIV [9, 10, 13, 15–17], as well as an acknowledgement that the implementation of strict exclusions based on some factors (ie, diabetes, hypertension, obesity) might preclude the eligibility of racial/ethnic minority populations who are disproportionately affected by HIV [18–20]. Thus, we adopted an approach that balanced strict exclusions versus more intensified exclusion thresholds that address safety concerns among those likely to be at the highest risk. In addition, we ensured that our informed consent process clearly describes the increased risk of participation.
Our preexisting protocol exclusion criteria would exclude candidates at risk for potentially severe COVID-19 outcomes (eg, advanced age greater than 65 years old; preexisting cardiovascular, kidney, or liver disease), based on previous ATI study norms. In addition, we added exclusions based on severe or uncontrolled diabetes, uncontrolled hypertension, obesity, and active tobacco smoking or vaping (Table 2). We balanced these exclusions by allowing the enrollment of individuals with less severe manifestations of these comorbidities and, in such individuals, chose to focus on strengthening risk mitigation strategies and the informed consent process regarding COVID-19 and other potential trial risks.
Table 2.
Exclusion Criteria
| Participants will be ineligible to participate in the ATI in the case of any of the below: |
| 1. Unable or unwilling to practice up-to-date CDC recommendations, including physical distancing and masking in situations where physical distancing is not possible |
| 2. Age greater than 65 years |
| 3. Active tobacco smoking or vaping, and unwilling to quit |
| 4. Uncontrolled asthma or chronic obstructive pulmonary disorder |
| 5. Uncontrolled hypertension |
| 6. Uncontrolled diabetes despite medical therapy |
| 7. Severe obesity |
| 8. Chronic kidney disease |
| 9. Other medical comorbidities deemed by the Principal Investigator to confer unacceptably elevated risk at the time of the ATI |
Data are for COVID-19–related ATI exclusion criteria as determined through an extensive, deliberative process with a community advisory board.
Abbreviations: ATI, analytic treatment interruption; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019.
INFORMED CONSENT AND PARTICIPANT EDUCATION
We decided to strengthen the informed consent process with an acknowledgement of the potential for increased risks of exposure to SARS-CoV-2 and/or severity of illness if infection occurs, as well as the acknowledgement that there are limited data on the impact of infection on PLWH in the absence of ART. Sample consent language is available in the Appendix. The informed consent process will include intense personal, case-by-case counseling, as well as the acknowledgement that there will be unknown risks during the study. To account for emerging scientific information around SARS-CoV-2, COVID-19, and HIV—as well as evolving standards of prevention and treatment—we plan to reconsent trial participants at key time points in the study, based on updated risk information and scientific evidence. The informed consent form and handouts provided to study participants will be updated regularly based on emerging data, and will include additional links to websites where up-to-date information can be found.
To further strengthen the informed consent process, we prepared written materials for trial participants to summarize the COVID-19 risk and the risk mitigation strategies to be employed during the study (Supplementary Appendices II–III). To ensure participants are adequately informed and counseled during the study, we are also making additional study staff available to discuss COVID-19–related risks throughout the study.
STUDY IMPLEMENTATION
Risks of Travel and Study Visits
Since an important risk from participation in the study was considered to be the frequency of in-person trial visits at the study site, we decided on a 3-fold strategy to mitigate this risk. First, we will offer telemedicine visits and/or home phlebotomy when feasible and desired. Second, we will maximize travel safety by incentivizing self-driving when possible, paying for private rideshare or taxi services to minimize the need for public transportation, and offering face masks to participants to use during travel. Third, we will maximize safety at the study site by following up-to-date medical center policies (Table 3). These include previsit telephone health screening (eg, symptom assessment prior to each visit); in-person health screening (eg, temperature checks), which will be required to gain access to the research center; physical distancing while at the research center (eg, remaining 6 feet apart); and masking during all study interactions.
Table 3.
Medical Center Policies Around Risk Mitigation
| All participants in the study will be required to observe current medical center policies regarding: |
| 1. Masking while in the research center |
| 2. Physical distancing while in the research center |
| 3. Previsit telephone health screening, which may include questions about symptoms of COVID-19 and documentation of the necessity of the research visit |
| 4. In-person health screening, which may include temperature and symptom checks, and may be required to gain access to the research center |
| 5. Any other medical center policies that are put in place during the study |
Abbreviation: COVID-19, coronavirus disease 2019.
Frequency of Laboratory Monitoring
Another important study design issue that emerged was whether to modify the frequency of HIV viral load monitoring during the ATI. Balancing the need for more intensive monitoring versus the risk of in-person attendance at the research site, we decided to maintain the same frequency of HIV viral load monitoring as before the SARS-CoV-2 pandemic, in an effort to ensure participant safety. This aspect of the pandemic further underscores the need to develop and validate a reliable, home-based viral load test for future remote monitoring during ATIs. Such an assay would greatly improve the ability of research teams to perform remote visits during the treatment interruption.
Because of the potential combined effects of ATI and SARS-CoV-2 infection on lymphocyte populations [10, 21], we also discussed the need for frequent CD4 + T-cell count monitoring during the study. In our study, this parameter is measured at each visit, including weekly monitoring during the first 24 weeks of the ATI; regardless of plasma HIV RNA levels, treatment will be reinitiated if the CD4 + T-cell count demonstrates a sustained decrease below 350 cells/uL. Other studies that do not otherwise require such intensive CD4 + T-cell monitoring might consider doing so during the SARS-CoV-2 pandemic. As with viral monitoring, this also comes with the need of balancing intensive monitoring against the risk of in-person attendance.
Implementation of a SARS-CoV-2 Testing Plan
There was consensus that due to the nature of the experimental therapies and ATI, the trial needed to include SARS-CoV-2 testing for both asymptomatic and symptomatic participants. To balance participant safety and minimize the testing burden, we identified key trial time points for SARS-CoV-2 testing of asymptomatic participants. This included testing prior to the start of the immune-based interventions, prior to the start of the ATI, and every 4 weeks during the ATI period. In addition, SARS-CoV-2 testing will be performed upon participant request (eg, opt-in procedure due to possible known exposure). Asymptomatic participants will also have the option to opt out of testing to reduce their burden and discomfort. To further minimize the burden to participants, our trial team implemented a plan to provide on-site testing free of cost. We will also provide participants with written COVID-19 testing location information if they wish to be tested at other sites.
Active SARS-CoV-2 Response Plan
We formulated a response plan for situations related to COVID-19 symptoms or a confirmed diagnosis of SARS-CoV-2 infection.
Participants who report symptoms potentially attributable to COVID-19 will receive testing center navigation assistance, which in most cases will include connection to testing through primary care providers or the local Department of Public Health. Study visits will be deferred at the discretion of the Principal Investigator until negative testing is confirmed or symptoms resolve. We will also follow up-to-date medical center policies regarding the timing of visits following development of symptoms.
In the event that a participant tests positive for SARS-CoV-2, the response will depend upon the stage of the protocol in which the positive test occurs. During the intervention portion of the study, upcoming interventions will be delayed until deemed safe by the Principal Investigator. In general, this will be for a period of 14 days, according to the current guidance at the time of diagnosis. During the ATI period, the Principal Investigator will consult with the study participant regarding ongoing participation in the ATI or ART reinitiation. Study continuation will be based upon the current guidance at the time, and will consider factors such as (1) the presence or absence of symptoms; (2) current scientific knowledge about SARS-CoV-2 infection in people living with HIV; (3) current US Center for Diseases Control and Prevention and/or World Health Organization guidance. Participants will also be encouraged to keep a supply of ART at home in case of a positive SARS-CoV-2 test during the ATI, to facilitate rapid treatment reinitiation should it be necessary.
Context of the Local Epidemic
Finally, we considered a situation in which the broader local epidemic worsens, given the challenges in predicting epidemiologic trends over the full duration of the study. We acknowledged that once the trial is underway, there will need to be ongoing risk assessments in consultation with the community advisory board and the trial’s safety monitoring committee, which has the capacity to serve as an external advisory body to make recommendations regarding continuing, pausing, or halting the study. This might include pausing enrollment or, if necessary, postponing certain study interventions if determined to not affect participant safety or compromise the scientific integrity of the study. In cases in which the local epidemiology changes and shelter-in-place recommendations are initiated, we plan to convene the safety monitoring committee to determine whether the study interventions and/or ATI should be delayed or halted for all participants.
Other Considerations
While one of our primary efforts is to provide education to participants on the risks associated with COVID-19, we acknowledge the complexity of both the study and the SARS-CoV-2 pandemic. For this reason, in addition to providing trial-related resources to participants, we also encourage them to engage with their primary care and/or HIV clinician as an external advisor throughout the study. This model has served us and our study volunteers well over decades of HIV research, and includes encouraging the participant to discuss the study’s risk-benefit ratio before enrollment and throughout their participation, as well as making all study-related laboratory results available to the primary clinician. In the case of the SARS-CoV-2 pandemic, this provider represents an external party invested in the participant’s well-being who can provide advice on ongoing participation in the context of both clinical and local epidemiologic knowledge. In other settings, a community health worker or other community advisor might serve in a similar role.
Although hypothetical, we also discussed our approach should a SARS-CoV-2 vaccine become available during the trial period. Participants in our study would be excluded from participating in another, concurrent trial of a SARS-CoV-2 vaccine due to a combination of scientific and safety issues. However, should a SARS-CoV-2 vaccine become available and approved during the course of our study, we would encourage participants who wished to receive the vaccine to do so; study measurements would be adjusted accordingly.
In addition, our socio-behavioral sub-study allows the trial team to track COVID-19 as an emergent theme. Of note, local epidemiologic trends mirror state- and country-level data demonstrating higher rates of SARS-CoV-2 infection among Black and Latinx populations. Factors affecting the participation of these groups and other marginalized populations (ie, women, transgender individuals, HIV serodiscordant/mixed-status couples) in HIV cure–related research are not well understood. Given this, and the study team’s efforts at broad inclusion, our study could be among the first to capture rigorous data from such populations participating in HIV cure–related research involving an ATI during the SARS-CoV-2 pandemic. We are also working with trial participants to be responsive to their needs and concerns throughout the trial [22]. The SARS-CoV-2 plan supplements our other risk mitigation strategies for HIV transmission during the ATI [23].
Limitations
Our COVID-19 risk mitigation plan was developed in a region with ample testing capacity, which currently has a relatively small epidemic. Other clinical research sites implementing ATI trials will need to fully consider their local epidemiologic situation. We also recognize that the risk of acquiring COVID-19 or developing severe outcomes may differ between various population groups, particularly racial/ethnic minority populations in the United States [24]. We are actively monitoring emerging SARS-CoV-2 scientific findings during the study and updating our trial protocol and informed consent as needed.
CONCLUSION
In addition to the risks it poses to health worldwide, the SARS-CoV-2 pandemic is a major threat to the scientific progress that is necessary to drive forward research priorities, such as the HIV cure agenda. In the setting of rapid developments in understanding the transmission, pathogenesis, and prevention of SARS-CoV-2 since the beginning of the pandemic, our multidisciplinary team has chosen to follow a harm- or risk-reduction approach regarding HIV cure–related studies including ATIs during the COVID-19 pandemic. We will continue to follow universal precautions for SARS-CoV-2, emerging participant risk level data, local COVID-19 epidemiology, and public health recommendations. At this time, we believe our multi-pronged mitigation and rigorous informed consent approaches provide reasonable methods to limit evolving SARS-CoV-2 infection risk while allowing scientific progress in the search towards sustained, ART-free suppression of HIV.
Supplementary Material
Notes
Acknowledgements. The authors thank the amfAR and Delaney AIDS Research Enterprise Community Advisory Boards.
Financial support. This work was supported the National Institute for Allergy and Infectious Disease at the National Institutes of Health (grant number T32 AI60530–12 to M. J. P.), the amfAR Institute for Human Immunodeficiency Virus Cure Research (grant number amfAR 109301), the Delaney AIDS Research Enterprise (grant number UM1AI126611), and the National Institute of Mental Health (grant number R21MH118120 to K. D.).
Potential conflicts of interest. M. J. P. receives grant funding from Gilead Sciences through the University of California, San Francisco, Resource Allocation Program. S. G. D. has received grants and/or personal fees from Gilead Sciences, Merck & Co, and Viiv; has received consulting fees from AbbVie; and serves on the Scientific Advisory Board of Enochian Biosciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US [manuscript published online ahead of print 16 June 2020]. Health Aff (Millwood) 2020. doi:101377hlthaff202000818 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz KL, Murti M, Finkelstein M, et al. . Lack of COVID-19 transmission on an international flight. CMAJ 2020; 192:E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung NHL, Chu DKW, Shiu EYC, et al. . Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ; Coronavirus Disease 2019 (COVID-19) Systematic Urgent Review Group Effort (SURGE) Study Authors . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395:1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of COVID-19—preliminary report [manuscript published online ahead of print 22 May 2020]. N Engl J Med 2020. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 6.Randomized Evaluation of Covid-19 Therapy (RECOVERY) Group, Horby P, Lim WS, et al. . Dexamethasone in hospitalized patients with COVID-19 - preliminary report [manuscript published online ahead of print 17 July 2020]. N Engl J Med 2020. doi: 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 7.Rosenbaum L. The untold toll - the pandemic’s effects on patients without COVID-19. N Engl J Med 2020; 382:2368–71. [DOI] [PubMed] [Google Scholar]
- 8.Julg B, Dee L, Ananworanich J, et al. . Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV 2019; 6:e259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigel K, Swartz T, Golden E, et al. . COVID-19 and people with HIV infection: outcomes for hospitalized patients in New York City [manuscript published online ahead of print 28 June 2020]. Clin Infect Dis 2020. Available at: 10.1093/cid/ciaa880. [DOI] [Google Scholar]
- 10.Ho HE, Peluso MJ, Margus C, et al. . Clinical outcomes and immunologic characteristics of COVID-19 in people with HIV [manuscript published online ahead of print 30 June 2020]. J Infect Dis 2020. Available at: 10.1093/infdis/jiaa380. [DOI] [Google Scholar]
- 11.Del Amo J, Polo R, Moreno S, et al. . Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study [manuscript published online ahead of print 26 June 2020]. Ann Intern Med 2020. doi: 10.7326/M20-3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. JAMA 2020; 323:19–20. doi: 10.1001/jama.2019.19775 [DOI] [PubMed] [Google Scholar]
- 13.Williamson EJ, Walker AJ, Bhaskaran K, et al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis DM, Deeks SG. How unavoidable are analytical treatment interruptions in HIV cure-related studies? J Infect Dis 2019; 220:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco JL, Ambrosioni J, Garcia F, et al. . COVID-19 in patients with HIV: clinical case series [manuscript published online ahead of print 15 April 2020]. Lancet HIV 2020. doi: 10.1016/S2352-3018(20)30111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harter G, Spinner CD, Roider J, et al. . COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection 2020; 48:681–6. doi: 10.1007/s15010-020-01438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. ; Coronavirus Disease 2019 (COVID-19) Infectious Diseases Team. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV 2020; 7:e554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyerowitz EA, Kim AY, Ard KL, et al. . Disproportionate burden of COVID-19 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS 2020; 34:1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadhera RK, Wadhera P, Gaba P, et al. . Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA 2020; 323:2192–5. doi: 10.1001/jama.2020.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (updated); vol.31. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 27 July 2020.
- 21.Clarridge KE, Blazkova J, Einkauf K, et al. . Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog 2018; 14:e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubé K, Barr L, Palm D, Brown B, Taylor J. Putting participants at the centre of HIV cure research. Lancet HIV 2019; 6:e147–9. [DOI] [PubMed] [Google Scholar]
- 23.Peluso MJ, Dee L, Campbell D, et al. . A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad 2020; 6:34–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes EK, Zambrano LD, Anderson KN, et al. . Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.