Abstract
Background
In the ongoing pandemic of COVID-19, respiratory failure has been reported as the main cause of death in those who develop critical illness. A few cases of concurrent myocarditis have been reported, but the extent of cardiac complications with the SARS-CoV-2 strain of coronavirus is still largely unknown.
Case summary
A 53-year-old man, suspected to have COVID-19 due to a new-onset cough, shortness of breath, and hypoxia, was referred to Cardiology with sudden symptomatic bradycardia. Initial rhythm analysis revealed Type 2 atrioventricular block (Mobitz II). On arrival at the coronary care unit, he was found to be in complete heart block (Type 3). Routine blood tests showed normal electrolytes and renal function, and no elevation in troponin-I levels. Echocardiography showed mild impairment in left ventricular systolic function, with no regional wall motion abnormalities or valvular lesions. He then developed high-degree AV block lasting 6.2 s, prompting the need for an urgent permanent pacemaker implantation.
Discussion
Just over a third of patients with myocarditis reportedly develop a rise in cardiac troponin. Clinically suspected myocarditis can occur in the absence of a troponin rise and rarely can cause high-grade bradyarrhythmias. Myocarditis and non-specific cardiac arrhythmias have been reported in a few cases of COVID-19, but this is the first reported case of a high-grade atrioventricular conduction block with SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV 2, Myocarditis, Complete heart block, Case report
Learning points
Just over a third of cases of myocarditis develop a rise in troponin levels.
Myocarditis can be associated with arrhythmias, often as an initial presentation.
Myocarditis is being increasingly recognized as a potential complication of COVID-19 as well.
Cardiac arrhythmias can be a presentation in COVID-19, even in the absence of significant cardiac injury.
Introduction
The first cases of infection from SARS-CoV-2 were reported in December 2019, originating in Wuhan, the capital of the Hubei Province of China.1 The disease caught the attention of the World Health Organization (WHO) and was soon reported to be of international concern in January 2020,2 before being declared a pandemic in March 2020.3
Previous zoonotic coronavirus infections such as the 2002–2003 severe acute respiratory syndrome (SARS-CoV) and the 2016 Middle East respiratory syndrome (MeRS-CoV), were associated with higher case fatality rates (9.6% and 34.4%, respectively) than the current COVID-19 pandemic (SARS-CoV-2), but the latter has resulted in more overall global deaths. This has been attributed in part to the strain’s higher infectivity and attack rate.4
While the clinical course is mostly characterized by respiratory symptoms, with respiratory arrest being the leading cause of death, data regarding cardiovascular involvement in COVID-19 are still rapidly emerging.5
Timeline
| Day | Medical information, investigation, treatment |
|---|---|
| Day 0 |
|
| Day 1 | Ultrasound abdomen showed thickened and oedematous gall bladder with normal common bile duct. |
| Day 2 |
|
| Day 4 |
|
| Day 5 |
|
| Day 6 | CT thorax showing large areas of bilateral ground-glass opacities and peripheral consolidation, consistent with features of COVID-19. |
| Day 7 | Worsening bradycardia: heart rate of 30–40 b.p.m. Initial rhythm on referral to Cardiology Type 2 atrioventricular block (Mobitz Type II). |
| Day 8 |
|
| Day 8 |
|
| Day 9 |
|
| Day 10 | Completed course of i.v. antibiotics and discharged. |
| Follow-up at 6 weeks |
|
Case presentation
A 53-year-old man, with no prior medical or cardiac conditions, and no relevant medications or drug history, self-presented to the Emergency Department with fever and right upper quadrant (RUQ) abdominal pain. Abdominal examination revealed RUQ tenderness, but cardiovascular and respiratory examination was within normal limits. Inflammatory markers were significantly elevated [C-reactive protein (CRP) 529 mg/L], and abdominal CT showed features of acute calculous cholecystitis. He was admitted under the surgical team and started on intravenous antibiotics.
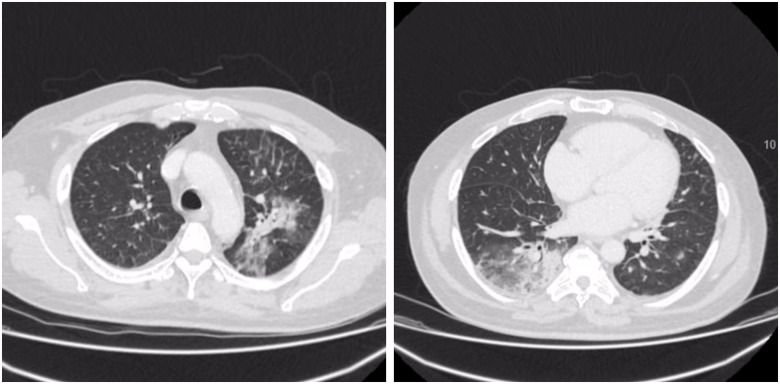
On Day 2, he developed new onset of hypoxia, requiring 1–2 L/min of supplementary oxygen. Baseline ECG showed normal sinus rhythm and no features of atrioventicular (AV) dissociation. Over the next 36 h, while his inflammatory markers improved (CRP 251 mg/L), his oxygen requirement increased to 8 L/min. CT thorax showed large areas of patchy bilateral ground-glass opacities and peripheral consolidation consistent with COVID-19 of at least moderate severity (Figure 1).
Figure 1.
Computed tomography scan of the thorax showing bilateral peripheral consolidation with ground-glass shadowing consistent with COVID-19.
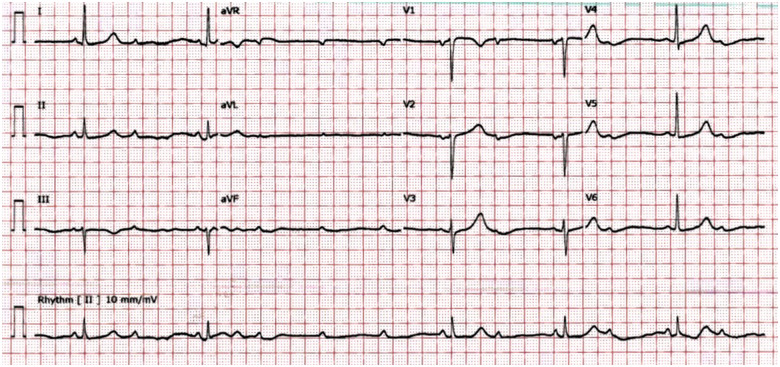
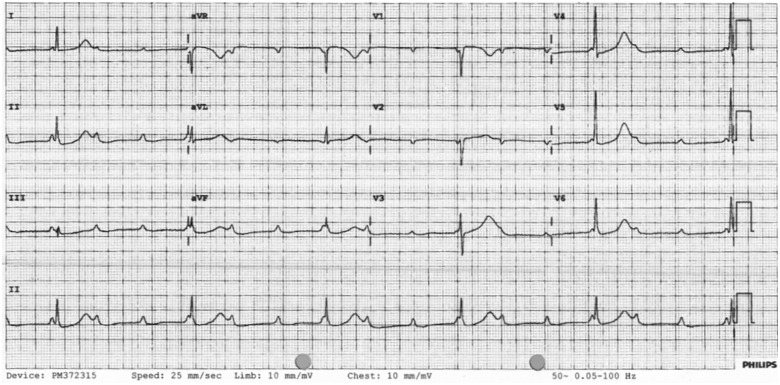
On Day 7, he had a symptomatic drop in heart rate (30–40 b.p.m.); ECG showed Type 2 AV block (Mobitz type II) (Figure 2). He was referred to Cardiology with outstanding swab results for SARS-CoV-2. On arrival at the coronary care unit (CCU), he was in complete heart block (Type 3) with junctional escape rhythm at 35 b.p.m. (Figure 3). Echocardiogram showed mild left ventricular (LV) systolic impairment with no obvious regional wall motion abnormalities (see Supplementary material online, Video 1). Blood tests revealed CRP of 90 mg/L, normal electrolytes, normal thyroid function [thyroid-stimulating hormone (TSH) 2.2 mU/L], and no rise in troponin (2.6 ng/L).
Figure 2.
12-lead ECG showing Mobitz II AV block.
Figure 3.
12-lead ECG showing complete AV block (Type 3) with junctional escape rhythm.
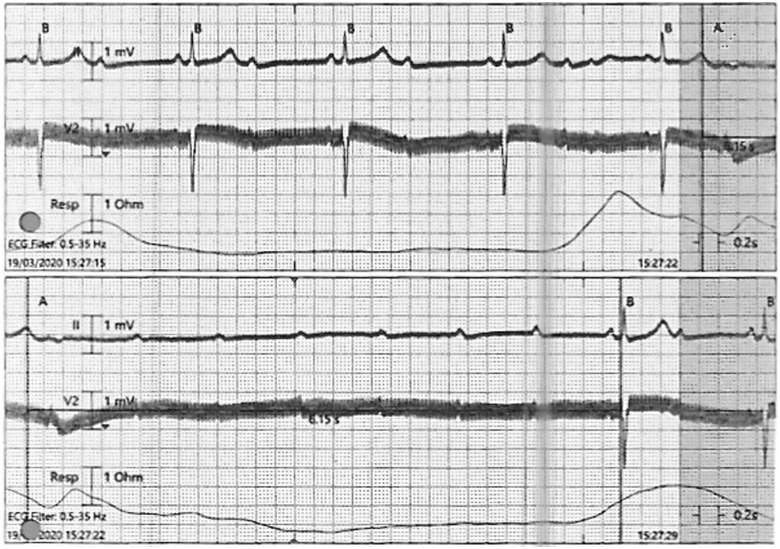
Apart from periods of AV block, his resting ECG otherwise remained normal. As he was asymptomatic and haemodynamically stable, we decided to await swab results, continue antibiotics, and monitor for further reduction in inflammatory markers. However, later that evening, the patient developed a prolonged episode of high-degree AV block lasting 6.2 s (Figure 4). We also received confirmation then that he had tested positive for SARS-CoV-2.
Figure 4.
ECG strip showing high-degree AV block lasting 6.2 s.
The period of prolonged AV block added urgency for pacing. However, the COVID-19 status and the fact that it was out of hours prompted debate over the ideal time for the procedure. We started the patient on an infusion of isoprenaline to prevent further episodes overnight, and planned for permanent pacemaker (PPM) implantation the following afternoon. As his respiratory symptoms had resolved, a PPM enabled us to discharge him the next day and minimize further risk of exposure for the healthcare staff.
During the procedure, the Wenckebach point was noted to be 75 b.p.m. He was discharged within 24 h with advice to isolate as per the national guidelines. Follow-up at 6 weeks showed pacing dependence with >95% V-pacing and 6.1% A-pacing. He will be followed up by the surgical team as an outpatient for further management of his gall bladder.
Discussion
Myocarditis is an inflammatory disease of the heart that often poses a diagnostic challenge due to the heterogeneity of presentation and a wide range of aetiologies. Additionally, the actual incidence of myocarditis is difficult to estimate as a tissue diagnosis with an endomyocardial biopsy is rarely obtained despite it being the gold standard for diagnosis.6
Arrhythmias can often be the first presenting feature of myocarditis. Acute and chronic myocarditis are considered to be among the most important causes of advanced AV block in young and middle-aged patients.7 In the European Study of the Epidemiology and Treatment of Inflammatory Heart Disease, 18% of the 3055 patients in the study had high-grade arrhythmias including complete heart block.8
Viral infections have been widely credited as causative factors of myocarditis. Since the onset of the current pandemic, cases of myocarditis in patients with COVID-19 have been reported. In a case series of 150 patients with COVID-19 conducted in Wuhan City, China, 7% of the reported 68 deaths (5 deaths) were attributed to myocarditis with circulatory failure; however, their pre-morbid cardiac status was unclear.9 Just recently, a case of myocarditis in COVID-19 was reported in a young, previously well adult.10
Our patient had no underlying medical, cardiac, or drug history. His presentation with complete heart block led us to a clinical diagnosis of suspected viral myocarditis as per the 2013 ESC Task Force criteria.11 His cardiac biomarkers were not elevated, but just over a third of cases of myocarditis present with a notable troponin rise.12 While a rise in troponin has been reported in COVID-19, a meta-analysis of four studies (a total of 341 patients) showed that a significant troponin rise was associated with more severe COVID-19-related illness as opposed to those with mild disease.13 While cardiac magnetic resonance imaging (MRI) might have offered more confirmation to the diagnosis, we felt it would not have altered our management in this case as our priority was to ensure a safe and early discharge. Our patient denied recent travel or household contacts with COVID-19 symptoms. We suspect he was exposed to the virus in hospital. He had presented to the walk-in Emergency Department with abdominal pain and, once diagnosed with cholecystitis, he had to be transferred to another hospital in the Trust to be cared for by the surgical team. The new-onset hypoxia and CT features consistent with COVID-19 prompted isolation on the ward and a serological test for confirmation.
Cardiac arrhythmias have been reported in COVID-19, but with a largely non-specific description. Cardiac arrhythmias were reported in 16.7% out of 138 hospitalized patients in a Chinese cohort, but predominantly in those requiring intensive care (44.4% vs. 6.9%), and the exact nature of arrhythmias was not documented or published.14 More recently, sinus node dysfunction has been reported in two isolated cases of COVID-19,15 but high-grade AV block has not yet been described.
Conclusion
Data concerning the impact of COVID-19 on the cardiovascular system are sparse, more so in patients with no prior cardiovascular disease or risk factors. We believe that identifying and acknowledging high-grade arrhythmias as a potential complication of COVID-19 would aid professionals in better managing the condition. This case highlights the importance of monitoring cardiac status in affected patients, and addresses the possibility of cardiac involvement in the absence of significant troponin rise. With limited availability and usage of endomyocardial biopsies for histological diagnosis, myocarditis remains largely a clinical diagnosis. More accounts of cardiac complications with SARS-CoV-2 will help professionals better understand the depth and scope of the disease.
Lead author biography

Dr. Vishnu Ashok is a Specialty Registrar in Cardiology at Glenfield Hospital in Leicester, United Kingdom. He also works as a Medical Registrar at Leicester Royal Infirmary, and he is a Collegiate Member of the Royal College of Physicians of London. He hopes to follow his special interest and develop a career in Cardiac Electrophysiology and Arrhythmias.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Consent: The authors confirm that consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1.World Health Organization. Pneumonia of unknown cause—China. 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (5 January 2020)
- 2.World Health Organization. Novel coronavirus—China. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (12 January 2020)
- 3.World Health Organization. WHO announces COVID-19 outbreak a pandemic. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (12 March 2020)
- 4. Wu Z, McGoogan JM.. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janardhanan R. Myocarditis with very high troponins: risk stratification by cardiac magnetic resonance. J Thorac Dis 2016;8:E1333–E1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barra SN, Providência R, Paiva L, Nascimento J, Marques AL.. A review on advanced atrioventricular block in young or middle‐aged adults. Pacing Clin Electrophysiol 2013. 2;35:1395–1405. [DOI] [PubMed] [Google Scholar]
- 8. Hufnagel G, Pankuwei S, Richter A, Schönian U.. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID): first epidemiological results. Herz 2000;25:279–285. [DOI] [PubMed] [Google Scholar]
- 9. Ruan Q, Yang K, Wang W, Jiang L, Song J.. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monmeneu Jose V, Mafe Eloy Dominguez, Soler Jorge Andres, Perez Bruno Ventura, Caravaca Javier Solsona, Torres Ricardo Broseta, García-Gonzalez Pilar, Ortega Laura Higueras, Lopez-Lereu Maria P, Maceira Alicia M. Subacute perimyocarditis in a young patient with COVID-19 infection. Eur Heart J Case Rep. 2020;doi: org/10.1093/ehjcr/ytaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 12. Lauer B, Niederau C, Kühl U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP.. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997;30:1354–1359 [DOI] [PubMed] [Google Scholar]
- 13. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peigh G, Leya MV, Baman JR, Cantey EP, Knight BP, Flaherty JD.. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep 2020;doi: org/10.1093/ehjcr/ytaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.