Abstract
Background
Convalescent plasma therapy is a leading treatment for conferring temporary immunity to COVID-19–susceptible individuals or for use as post-exposure prophylaxis. However, not all recovered patients develop adequate antibody titers for donation and the relationship between avidity and neutralizing titers is currently not well understood.
Methods
SARS-CoV-2 anti-spike and anti-nucleocapsid IgG titers and avidity were measured in a longitudinal cohort of COVID-19 hospitalized patients (n = 16 individuals) and a cross-sectional sample of convalescent plasma donors (n = 130). Epidemiologic correlates of avidity were examined in donors by linear regression. The association of avidity and a high neutralizing titer (NT) were also assessed in donors using modified Poisson regression.
Results
Antibody avidity increased over duration of infection and remained elevated. In convalescent plasma donors, higher levels of anti-spike avidity were associated with older age, male sex, and hospitalization. Higher NTs had a stronger positive correlation with anti-spike IgG avidity (Spearman ρ = 0.386; P < .001) than with anti-nucleocapsid IgG avidity (Spearman ρ = 0.211; P = .026). Increasing levels of anti-spike IgG avidity were associated with high NT (≥160) (adjusted prevalence ratio = 1.58 [95% confidence interval = 1.19–2.12]), independent of age, sex, and hospitalization.
Conclusions
SARS-CoV-2 antibody avidity correlated with duration of infection and higher neutralizing titers, suggesting a potential alternative screening parameter for identifying optimal convalescent plasma donors.
Keywords: SARS-CoV-2, avidity, anti-spike, anti-nucleocapsid, convalescent plasma
Evaluation of antibody avidity from potential convalescent plasma donors and hospitalized COVID-19 patients suggests increased SARS-CoV-2 IgG avidity is associated with being older, male, and hospitalized. Avidity is correlated with neutralizing titers offering a potential screening parameter for convalescent donors.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease (COVID-19), was declared a pandemic in early 2020 by the World Health Organization [1, 2]. As of 1 September 2020, there have been over 25 million confirmed COVID-19 cases and over 840 000 deaths [2, 3]. Symptoms of COVID-19 include cough, fever, fatigue, muscle pain, and shortness of breath, with hospitalized patients at higher risk of death [2–4].
Currently, there remains no prophylactic vaccine for SARS-CoV-2 infection and pharmaceutical therapeutics are limited. Convalescent plasma therapy is presently the best option to confer temporary immediate passive immunity for infection prevention or early treatment of individuals [5]. Historically, passive immune therapy has been used as post-exposure prophylaxis or hospital treatment for a variety of viral infections [5–9]. Observational studies have evaluated the use of convalescent plasma to treat COVID-19, suggesting both safety and efficacy, as reflected by shortened duration of hospitalizations and lower mortality (ie, as compared to nontransfused controls) [6–11].
Neutralizing antibodies in convalescent individuals are of major interest as they bind to various viral epitopes, inhibiting infectivity by blocking attachment or entry into host cells [12]. While the US Food and Drug Administration (FDA) initially did not require neutralizing antibody titer testing for potential convalescent plasma donors at the beginning of the pandemic, they recommended a target titer of > 1:320 for ideal donors if testing was available; this target titer was subsequently lowered [5, 13]. Additionally, 30% of recovered patients have low titers of SARS-CoV-2–specific neutralizing antibodies and > 5% have undetectable levels [5, 14]. Determinants of SARS-CoV-2 neutralizing antibody responses are largely unknown.
Of the 4 main structural SARS-CoV-2 proteins, spike (S1 subunit and receptor binding domain) and nucleocapsid proteins are the most immunogenic [15, 16]. In recent studies, potent antiviral receptor binding domain specific antibodies were found in all COVID-19 convalescent plasma donors despite low titers of neutralizing antibodies [17]. While the overall antibody titer is most likely an important factor in determining the viral neutralization potential of a given convalescent plasma donor, multiple other factors likely play a role. These include antibody binding avidity, diversity of response, and amounts of differing antibody classes. While antibody avidity, combined strength of the antibody-antigen complex, can have substantial impact on the humoral immune response, there are limited data on temporal dynamics and correlates of SARS-CoV-2 antibody avidity responses, and whether a stronger avidity response is associated with higher neutralizing titers. An association between antibody avidity and neutralizing antibody titer may also help to identify optimal convalescent plasma donors.
The aim of this study was to characterize SARS-CoV-2 immunoglobulin G (IgG) antibody titers and avidity responses in acute and convalescent patients, and compare their association with neutralizing antibody titers.
METHODS
Study Sample
Longitudinal COVID-19 Sample Patients
All patients had confirmed SARS-CoV-2 infection via a positive RNA nasopharyngeal swab and a known date of symptom onset. Discarded blood serum samples were collected from specimens sent for clinical testing of patients over the duration of their inpatient stay. Samples were selected from individuals with multiple time points available after observed seroconversion. In total 16 distinct hospitalized patients at the Johns Hopkins Hospital contributed 84 serum samples with a median of 5 samples per patient (range, 2–8; interquartile range [IQR], 4.5–6.0).
Convalescent Plasma Donors
Recovered COVID-19 patients were contacted by study personnel to determine interest in donating convalescent plasma. All subjects had confirmed infection of SARS-CoV-2 determined by a positive reverse transcription polymerase chain reaction (RT-PCR) of a nasopharyngeal swab. All potential donors had to be at least 18 years old and meet criteria for blood donation (ie, never been diagnosed with human immunodeficiency virus [HIV], not pregnant, etc.). Potential donors had 25 mL of whole blood collected. Blood was separated into plasma and peripheral blood mononuclear cells within 12 hours of collection, and plasma samples were aliquoted and stored at −80°C. All donors provided informed written consent.
Ethics Statement
Studies of both cohorts were approved by The Johns Hopkins University School of Medicine Institutional Review Board.
Laboratory Testing
Neutralizing Antibody Titers
Quantitation of neutralizing antibody titers against 100 fifty percent tissue culture infectious doses was performed using a microneutralization (NT) assay, as previously described [18, 19]. Endpoint NT titers were calculated as the highest serum dilution that eliminated cytopathic effect in 50% of the wells (ie, 3 of 6 replicate wells). NT area under the curve (AUC) values were estimated using the exact number of wells protected from infection at every plasma dilution; samples that had no NT activity were assigned a value of one-half of the lowest NT AUC. For analytic purposes in this study, NT AUC values ≥ 40 were considered to indicate moderate neutralization potency and NT AUC values ≥ 160 were considered to indicate high neutralization potency [13].
Enzyme-Linked Immunosorbent Assays
Euroimmun anti-SARS-CoV-2 IgG enzyme-linked immunosorbent assay (ELISA) for the S1 domain of spike protein and EDI Novel Coronavirus COVID-19 IgG ELISA (Epitope Diagnostics Inc.) for nucleocapsid protein were performed per the manufacturers’ protocols. For Euroimmun, optical density (OD) of the sample divided by calibrator provided arbitrary unit (AU) ratio for which ≥ 1.1 were considered positive and ≥ 0.8–1.1 were considered indeterminate. For EDI, normalized optical density (ODn) ≥ 0.22 were considered positive and ≥ 0.18 were considered indeterminate.
Avidity Enzyme-Linked Immunosorbent Assays
Avidity assays were performed for samples that had Euroimmun AU ratios ≥ 0.8 and EDI ODns ≥ 0.18 (ie, indeterminates and seropositive specimens). Euroimmun anti-SARS-CoV-2 ELISA IgG kits and EDI Novel Coronavirus COVID-19 IgG ELISA kits were used with modified protocols for avidity testing [19]. Each reaction utilized the following components: 100 µL of diluted plasma (1:101 dilution for Euroimmun or 1:100 dilution for EDI per manufacturers’ protocols), and 100 µL of undiluted positive, negative, or calibrator controls. Plates containing reaction components were incubated either for 1 hour at 37°C followed by 3 washes (Euroimmun) or 30 minutes at room temperature followed by 5 washes (EDI). Urea, 300 µL, diluted in the appropriate wash buffer at varying concentrations (0, 1, 2, 4, 6, and 8 M) were added to the plates and incubated at 37°C for 10 minutes [20]. Plates were washed 3 or 5 times followed by manufacturer’s protocol for addition of conjugate and substrate. Ratios of sample with urea concentration to sample without urea (either AU or ODn) were used to calculate dissociation constant (DC50). The DC50 was the concentration of urea where 50% of the signal was lost (Supplementary Figure 1). In samples where 50% of the signal was not lost by 8 M urea solution, samples DC50 were truncated at 8. DC50 calculations were performed using AAT Bioquest IC50 calculator using 4 parameter logistic regression model [21].
Statistical Analysis
Descriptive statistics were used to characterize both study populations: the longitudinal cohort of hospitalized COVID-19 patients and the cross-sectional sample of COVID-19 convalescent plasma donors. Percentages were calculated for categorical variables and continuous variables were described with medians and corresponding interquartile ranges (IQR). The primary study outcomes included anti-spike IgG avidity and anti-nucleocapsid IgG avidity as measured by the Euroimmun and EDI assays, respectively. Longitudinal trajectories in IgG titers and avidity outcomes were visualized by spaghetti plots and examined descriptively by time since symptom onset in the cohort of hospitalized COVID-19 patients. The remaining statistical analyses were conducted among the sample of convalescent plasma donors.
The distribution of anti-spike IgG avidity and anti-nucleocapsid IgG avidity was examined by epidemiologic characteristics, including sex, age, race/ethnicity, and hospitalization status (ie, COVID-19 illness severity). Differences between groups were calculated by Wilcoxon rank-sum tests or Kruskal-Wallis tests, as appropriate. Correlations between age and avidity were assessed by Pearson correlation coefficient. Ordinary least-squares linear regression was used to examine the association of age, sex, and hospitalization status with avidity DC50 values. Multivariable models included age, sex, and hospitalization status regardless of statistical significance, as they have previously been shown to be important for other IgG antibody responses in this study population [19]. The multivariable analysis also included adjustment for time since symptom onset. Subgroup analyses were performed stratified by sex using similar methodology. The primary analysis included avidity DC50 values for individuals who were considered indeterminate for a given IgG ELISA based on the manufacturer’s cutoff. Thus, sensitivity analyses were performed that excluded donors who had an indeterminate sample.
As a secondary analysis, evaluations of whether anti-spike IgG titers and avidity as well as anti-nucleocapsid IgG titers and avidity correlated with NT AUC values. Data were visualized with nonparametric locally weighted scatterplot smoothing (LOWESS) curves and correlations were assessed with Spearman rank correlation coefficients. To determine whether IgG titers and avidity are associated with elevated neutralizing antibodies, modified Poisson regression with robust variance was used to estimate prevalence ratios (PR) and corresponding 95% confidence intervals (CI) of an NT AUC value ≥160. Multivariable analyses were performed including the serologic biomarker of interest, age, sex, hospitalization status, and time since symptom onset. This analysis was also repeated using an NT AUC value ≥40 as the outcome of interest.
Sensitivity analyses were also performed including days since initial positive PCR diagnosis in multivariable models as opposed to self-reported days symptom onset; time since initial positive PCR-positive diagnosis, which was confirmed with medical documentation, may be a more reliable indicator of duration of infection than a self-reported measure.
RESULTS
Characteristics of the Study Population
There were 16 hospitalized COVID-19 patients in the longitudinal cohort who contributed an average of 5 samples per person (n = 84 total samples). Plasma samples were collected from patients at a median of 13 days (range, 4–35; IQR, 10–16) after symptom onset (Table 1). Using the manufacturers’ cutoffs, 76% (n = 64) of the samples were considered seropositive for anti-spike IgG by the Euroimmun assay and 89% (n = 75) were considered seropositive for anti-nucleocapsid IgG using the EDI assay. Of note, 19 (23%) samples were seronegative for anti-spike IgG and 7 (8%) samples were seronegative for anti-nucleocapsid IgG. However, all specimens collected after 14 days since symptom onset were considered to be seropositive by the Euroimmun assay and the EDI assay (Supplementary Table 1). IgG avidity was measured in seropositive and indeterminate specimens; anti-spike IgG avidity was measured in 65 samples (16 patients) and anti-nucleocapsid IgG avidity was measured in 76 samples (16 patients).
Table 1.
Characteristics of Participants Overall and by Sex
| Characteristics | No. of Samples (%) (n = 84 Samples; N = 16 Participants) | ||
|---|---|---|---|
| Longitudinal Cohort | |||
| Days post symptom onset (IQR) | 13 (10–16) | ||
| Euroimmun anti-spike IgG | |||
| Seropositive | 64 (76) | ||
| Indeterminate | 1 (1) | ||
| Seronegative | 19 (23) | ||
| EDI anti-nucleocapsid IgG | |||
| Seropositive | 75 (89) | ||
| Indeterminate | 2 (2) | ||
| Seronegative | 7 (8) | ||
| Convalescent Plasma Cohort | |||
| No. of participants (%) | |||
| Overall (n = 130) | Female (n = 60) | Male (n = 70) | |
| Age group, y | |||
| 18–29 | 34 (26) | 17 (28) | 17 (24) |
| 30–39 | 23 (18) | 9 (15) | 14 (20) |
| 40–49 | 28 (22) | 13 (22) | 15 (21) |
| 50–59 | 23 (18) | 11 (18) | 12 (17) |
| ≥60 | 22 (17) | 10 (17) | 12 (17) |
| Race/ethnicity | |||
| Non-Hispanic white | 98 (75) | 47 (78) | 51 (73) |
| Non-Hispanic black | 4 (3) | 2 (3) | 2 (3) |
| Hispanic | 5 (4) | 4 (7) | 1 (1) |
| Non-Hispanic Asian | 14 (11) | 2 (3) | 12 (17) |
| Other/multiracial/unknown | 9 (7) | 5 (8) | 4 (6) |
| Median days post symptom onset (IQR)a | 49 (43–55) | 50 (43–56) | 48 (43–54) |
| Hospitalization status severity | |||
| No | 117 (90) | 52 (87) | 65 (93) |
| Yes | 11 (8) | 6 (10) | 5 (7) |
| Missing | 2 (2) | 2 (3) | 0 (0.0) |
| Euroimmun anti-spike IgG | |||
| Seropositive | 117 (90) | 50 (83) | 67 (96) |
| Indeterminate | 7 (5) | 5 (8) | 2 (3) |
| Seronegative | 5 (4) | 4 (7) | 1 (1) |
| Missing | 1 (1) | 1 (2) | 0 (0) |
| EDI anti-nucleocapsid IgG | |||
| Seropositive | 105 (81) | 46 (77) | 59 (84) |
| Indeterminate | 9 (7) | 5 (8) | 4 (6) |
| Seronegative | 16 (12) | 9 (15) | 7 (10) |
Abbreviations: EDI, Epitope Diagnostics Inc.; IgG, immunoglobulin G; IQR, interquartile range.
aData were missing for 1 participant.
There were 130 participants in the cross-sectional sample of convalescent plasma donors; the median age was 42 years (IQR, 29–55), 46% (n = 60) were female, and 75% (n = 98) were non-Hispanic white (Table 1). Plasma samples were collected from donors a median of 49 days (IQR, 43–55) after symptom onset. Only 11 (8%) donors were previously hospitalized during the course of their infection, although 2 donors were missing data on hospitalization status. Using the manufacturers’ cutoffs, 90% (n = 117) of donors were considered seropositive for anti-spike IgG by the Euroimmun assay, while 81% (n = 105) of donors were considered seropositive for anti-nucleocapsid IgG using the EDI assay. There were some samples that were considered indeterminate for anti-spike IgG serostatus (5% [n = 7]) and anti-nucleocapsid IgG serostatus (7% [n = 9]); plasma from 4 donors were indeterminate by both assays. A greater proportion of donors were seronegative for anti-nucleocapsid IgG (12% [n = 16]) than for anti-spike IgG (4% [n = 5]). Characteristics of donors were similar by sex; however, males were more likely than females to be non-Hispanic Asian (17% vs 3%) and seropositive for anti-spike IgG (96% vs 83%). Prevalence of a high neutralizing titer AUC value greater than 160 was 25% (32/126) overall, 21% (12/58) in females, and 29% (20/68) in males.
Longitudinal Trajectories in SARS-CoV-2 IgG Titers and Avidity Responses
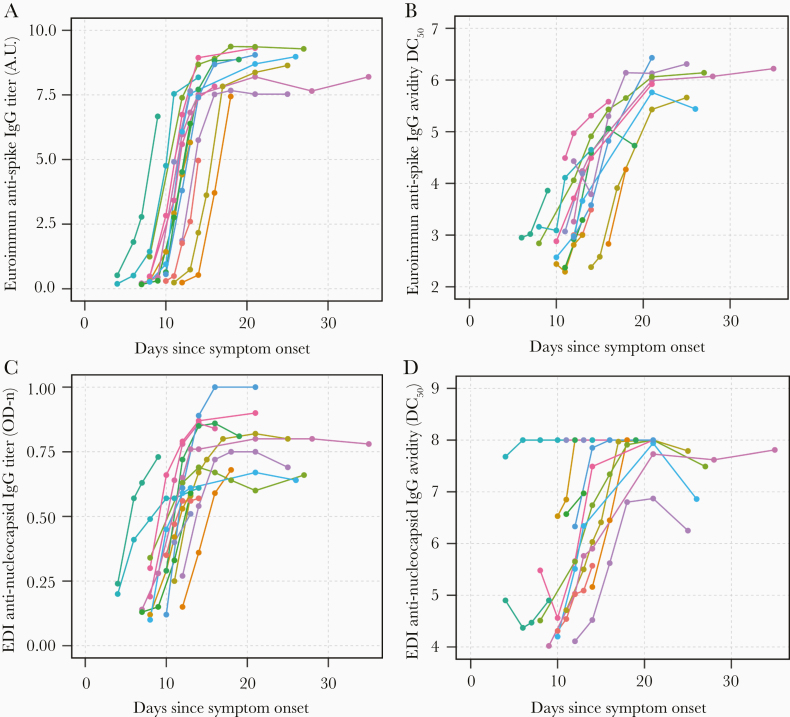
Longitudinal trajectories in SARS-CoV-2 IgG titers and avidity responses were examined by time since symptom onset among the cohort of hospitalized COVID-19 patients (Figure 1). Anti-spike IgG titers increased over days post symptom onset and appeared to peak around day 21 before beginning to plateau (Figure 1A). This same trend was observed with anti-spike IgG avidity (Figure 1B). Similarly, anti-nucleocapsid IgG titers increased with duration of illness, but appeared to peak earlier than anti-spike IgG (around day 15; Figure 1C). However, anti-nucleocapsid IgG avidity followed the same trend as anti-spike IgG avidity and appeared to peak around day 21 (Figure 1D).
Figure 1.
Longitudinal trajectories in anti-spike and anti-nucleocapsid SARS-CoV-2 IgG titers and avidity responses in hospitalized COVID-19 patients by time from symptom onset. Plasma samples taken over course of hospitalization were used to evaluate total antibody titers and antibody avidity (DC50). Each patient is represented by a different color. (A) Anti-spike IgG titers; (B) anti-spike IgG avidity; (C) anti-nucleocapsid titers, and (D) anti-nucleocapsid IgG avidity were measured over days since symptom onset. Antibody avidity is indicated by calculated dissociation constant (DC50) where concentration of urea results in 50% of signal loss. Antibody titers are indicated by semiquantitative arbitrary units (AU) calculated from corrected OD (Euroimmun) or direct ODn values (EDI). Abbreviations: COVID-19, coronavirus disease 2019; EDI, Epitope Diagnostics Inc.; IgG, immunoglobulin G; OD, optical density; ODn, normalized optical density; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Epidemiologic Correlates of SARS-CoV-2 IgG Avidity Among Convalescent Plasma Donors
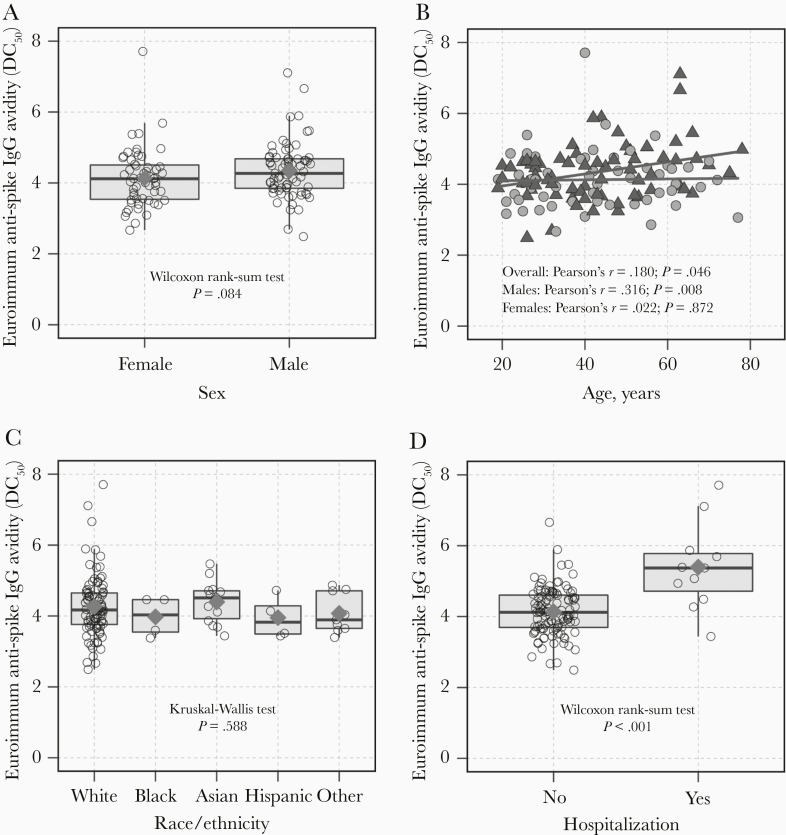
The distribution of anti-spike IgG avidity was examined by sex, age, race/ethnicity, and hospitalization status among convalescent plasma donors (Figure 2). The median avidity value was 4.12 (IQR, 3.52–4.53) among females and 4.27 (IQR, 3.85–4.68) among males (P = .084; Figure 2A). Age was positively correlated with anti-spike IgG avidity among males (r = 0.316; P = .008) but not among females (r = 0.022; P = .872; Figure 2B). There was no significant difference in anti-spike IgG avidity by race/ethnicity (P = .588; Figure 2C). Donors who were hospitalized during the course of their infection had stronger anti-spike IgG avidity than donors who were not hospitalized (P < .001; Figure 2D). After adjustment for age, hospitalization, and time since symptom onset, males had on average greater anti-spike IgG avidity than females but this association was not statistically significant (βadjusted = .248 [95% CI, −.014 to .510]; Table 2). In multivariable analyses, hospitalization was a consistent predictor of stronger anti-spike IgG avidity overall, among females and males. In contrast, age was significantly associated with anti-spike IgG avidity among males (age per 10 years, βadjusted = .150 [95% CI, .032–.267]) but not among females (βadjusted = .018 [95% CI, −.114 to .150]).
Figure 2.
Anti-spike SARS-CoV-2 IgG avidity responses (DC50) by epidemiologic characteristics among potential COVID-19 convalescent plasma donors. Anti-spike IgG avidity was evaluated compared to (A) sex, (B) age, (C) race/ethnicity, and (D) hospitalization. Red diamonds (A, C, D) indicate the arithmetic mean of avidity DC50 values for a given group and (B) avidity DC50 values are denoted by green triangles for males and grey circles for females. Abbreviations: COVID-19, coronavirus disease 2019; DC50, 50% dissociation constant; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Epidemiologic Correlates of Anti-spike and Anti-nucleocapsid SARS-CoV-2 IgG Avidity (DC50) Among Potential COVID-19 Convalescent Plasma Donors
| Outcome | ||||
|---|---|---|---|---|
| Univariable Models | Multivariable Models | |||
| Epidemiologic Characteristic | β (95% CI) | P | β (95% CI) | P |
| Euroimmun antispike IgG avidity DC 50 | ||||
| Overall population | ||||
| Age, per 10 y | .096 (.002 to .191) | .046 | .091 (.004 to .178) | .040 |
| Male sex | .213 (−.075 to .500) | .146 | .248 (−.014 to .510) | .063 |
| Hospitalization | 1.257 (.799 to 1.715) | < .001 | 1.258 (.800 to 1.717) | < .001 |
| Time post symptom onset, per 5 d | .047 (−.031 to .124) | .234 | .010 (−.061 to .080) | .790 |
| Female population | ||||
| Age, per 10 y | .012 (−.134 to .158) | .872 | .018 (−.114 to .150) | .783 |
| Hospitalization | 1.327 (.693 to 1.962) | < .001 | 1.224 (.571 to 1.877) | < .001 |
| Time post symptom onset, per 5 d | .122 (.011 to .232) | .031 | .075 (−.031 to .181) | .161) |
| Male population | ||||
| Age, per 10 y | .169 (.045 to .293) | .008 | .150 (.032 to .267) | .013 |
| Hospitalization | 1.244 (.574 to 1.915) | < .001 | 1.180 (.519 to 1.840) | .001 |
| Time post symptom onset, per 5 d | −.020 (−.129 to .089) | .715 | 4.044 (2.980 to 5.108) | .348 |
| EDI antinucleocapsid IgG avidity DC 50 | ||||
| Overall population | ||||
| Age, per 10 y | .139 (.032 to .247) | .011 | .123 (.017 to .229) | .024 |
| Male sex | −.099 (−.432 to .235) | .558 | −.040 (−.361 to .280) | .804 |
| Hospitalization | .606 (.064 to 1.148) | .029 | .565 (.016 to 1.113) | .044 |
| Time post symptom onset, per 5 d | .033 (−.055 to .122) | .459 | .014 (−.073 to .101) | .755 |
| Female population | ||||
| Age, per 10 y | .142 (−.000 to .284) | .050 | .117 (−.023 to .257) | .099 |
| Hospitalization | .352 (−.316 to 1.020) | .295 | .357 (−.327 to 1.041) | .299 |
| Time post symptom onset, per 5 d | .028 (−.087 to .142) | .627 | .019 (−.092 to .131) | .731 |
| Male population | ||||
| Age, per 10 y | .138 (−.024 to .301) | .094 | .119 (−.043 to .281) | .146 |
| Hospitalization | .889 (.016 to 1.761) | .046 | .796 (−.093 to 1.685) | .078 |
| Time post symptom onset, per 5 d | .035 (−.103 to .174) | .612 | .012 (−.125 to .148) | .865 |
Ordinary least-squares linear regression models were used to examine associations with anti-spike and anti-nucleocapsid IgG avidity DC50 values. The multivariable regression models included all other covariates shown. Separate models were constructed for each outcome and subgroup analyses were performed stratified by sex. Values bolded indicate statistical significance (P < .05).
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; DC50, 50% dissociation constant; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
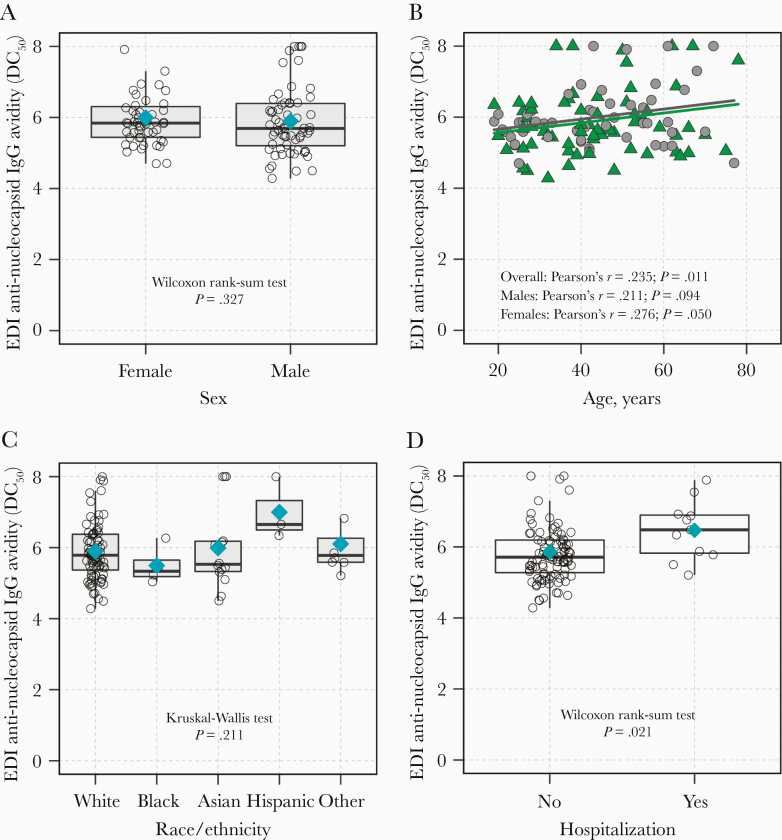
The distribution of anti-nucleocapsid IgG avidity was also examined by sex, age, race/ethnicity, and hospitalization status (Figure 3), with similar findings as anti-spike IgG avidity, except sex did not modify the correlation between age and anti-nucleocapsid IgG avidity (Figure 3B). In multivariable analysis, age was associated with anti-nucleocapsid IgG avidity in the overall model (age per 10 years, βadjusted = .123 [95% CI, .017–.229]), as was hospitalization status (βadjusted = .565 [95% CI, .016–1.113]; Table 2).
Figure 3.
Anti-nucleocapsid SARS-CoV-2 IgG avidity responses (DC50) by epidemiologic characteristics among potential COVID-19 convalescent plasma donors. Anti-nucleocapsid IgG avidity was evaluated compared to (A) sex, (B) age, (C) race/ethnicity, and (D) hospitalization. Blue diamonds (A, C, D) indicate the arithmetic mean of avidity DC50 values for a given group and triangles (B) denote avidity DC50 values for males and circles denote avidity DC50 values for females. Abbreviations: COVID-19, coronavirus disease 2019; EDI, Epitope Diagnostics Inc.; DC50, 50% dissociation constant; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In sensitivity analyses excluding indeterminate samples, effect estimates were slightly attenuated but inferences remained similar (Supplementary Tables 2 and 3). Inferences were also similar for time since diagnosis when included in the multivariable models as opposed to time from symptom onset (Supplementary Tables 4 and 5).
Associations Between SARS-CoV-2 IgG Responses and Neutralizing Antibody Titers Among Convalescent Plasma Donors
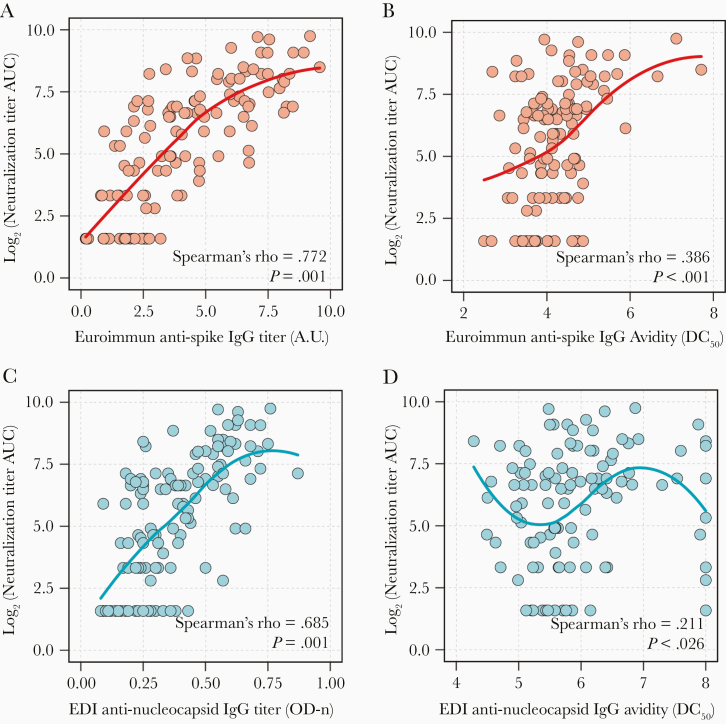
There was a strong positive correlation between anti-spike IgG titers and anti-spike IgG avidity (Spearman ρ = 0.541; P < .001); however, there was no significant correlation between anti-nucleocapsid IgG titers and anti-nucleocapsid IgG avidity (Spearman ρ = 0.165; P = .078; Supplementary Figure 2). Both anti-spike IgG titers and anti-spike IgG avidity were positively correlated with neutralizing antibody titer AUC values (Spearman ρ = 0.772 and 0.386, respectively; P < .001 for both comparisons; Figure 4A and 4B). Higher levels of anti-spike IgG titers (PR = 1.57 [95% CI, 1.42–1.74]) and higher levels of anti-spike IgG avidity (PR = 1.80 [95% CI, 1.45–2.22]) were significantly associated with a higher prevalence of a neutralizing antibody titer AUC value > 160 (Table 3). These associations were also significant after adjustment for age, sex, hospitalization status, and time from symptom onset in multivariable analysis. Similar results were obtained when days since diagnosis was incorporated into the model rather than time from symptom onset (Supplementary Table 6).
Table 3.
Associations Between SARS-CoV-2 IgG Antibody Responses and Elevated SARS-CoV-2 Neutralizing Antibody Titers
| Outcome | ||||
|---|---|---|---|---|
| Univariable Models | Multivariable Models | |||
| Serologic Biomarker | PR (95% CI) | P Value | aPR (95% CI) | P Value |
| Neutralizing Antibody Titer AUC value ≥ 160 | ||||
| Anti-spike IgG titers, AU | 1.57 (1.42–1.74) | < .001 | 1.61 (1.43–1.81) | < .001 |
| Anti-spike IgG avidity, DC50 | 1.80 (1.45–2.22) | < .001 | 1.58 (1.19–2.12) | .002 |
| log2 anti-nucleocapsid IgG titer, ODn | 7.02 (3.64–13.56) | < .001 | 10.43 (4.65–23.41) | < .001 |
| log2 anti-nucleocapsid IgG avidity, DC50 | 2.80 (.73–10.75) | .133 | 1.57 (.40–6.18) | .516 |
| Neutralizing Antibody Titer AUC value ≥ 40 | ||||
| Anti-spike IgG titers, AU | 1.24 (1.17–1.31) | < .001 | 1.24 (1.15–1.33) | < .001 |
| Anti-spike IgG avidity, DC50 | 1.31 (1.16–1.48) | < .001 | 1.18 (.99–1.41) | .064 |
| log2 anti-nucleocapsid IgG titer, ODn | 2.01 (1.56–2.49) | < .001 | 2.25 (1.66–3.04) | < .001 |
| log2 anti-nucleocapsid IgG avidity, DC50 | 1.67 (.84–3.32) | .140 | 1.42 (.77–2.64) | .264 |
Prevalence ratios of a neutralizing titer AUC value ≥160 and ≥40 were estimated from Poisson regression models with robust standard errors. A different model was used for each serologic biomarker shown. Multivariable models were used to estimate adjusted prevalence ratios which included adjustment for age, sex, hospitalization, and time from symptom onset. Values bolded indicate statistical significance (P < .05).
Abbreviations: aPR, adjusted prevalence ratio; AU, arbitrary unit; AUC, area under curve; CI, confidence interval; DC50, 50% dissociation constant; ODn, normalized optical density; PR, prevalence ratio.
While there was a strong positive correlation between anti-nucleocapsid IgG titers and neutralizing antibody titer AUC values (Spearman ρ = 0.685; P < .001; Figure 4C), the correlation between anti-nucleocapsid IgG avidity and neutralizing antibody titer AUC values was weak (Spearman ρ = 0.211; P = .026) and nonlinear (Figure 4D). Anti-nucleocapsid IgG titers were strongly associated with a higher prevalence of a neutralizing antibody titer AUC value ≥160 in univariable, as well as multivariable analysis (Table 3). Anti-nucleocapsid IgG avidity did not have a statistically significant association with neutralizing antibody titer AUC value ≥160 (adjusted PR = 1.57 [95% CI, .40–6.18]). Associations were attenuated when considering a neutralizing antibody titer AUC value ≥40 but remained in the same direction of association.
Figure 4.
Correlations of anti-spike and anti-nucleocapsid SARS-CoV-2 IgG responses with neutralizing antibody titers among potential COVID-19 convalescent plasma donors. Correlations between neutralizing antibody titer AUC values and (A) anti-spike IgG titers, (B) anti-spike IgG avidity, (C) anti-nucleocapsid IgG titers, and (D) anti-nucleocapsid avidity were examined using Spearman correlation coefficient and nonparametric LOWESS curves. Antibody avidity is indicated by calculated dissociation constant (DC50) where concentration of urea results in 50% of signal loss. Antibody titers are indicated by arbitrary units (AU) calculated from corrected OD (Euroimmun) or direct ODn values (EDI). Abbreviations: AUC, area under the curve; COVID-19, coronavirus disease 2019; EDI, Epitope Diagnostics Inc.; IgG, immunoglobulin G; ODn, normalized optical density; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
SARS-CoV-2 remains a critical health threat. Better understanding of antibody development is necessary. While antibody response is used to evaluate both the early and late stages of immune responses, the relationship between antibody titers and avidity has yet to be established in COVID-19 [22, 23]. Antibody avidity testing has been used to aid in diagnosis of recent infections, including Epstein-Barr virus, HIV, West Nile Virus, and other SARS CoV infections [24–27]. As some recovered patients do not develop high antibody titers following infection, not everyone is an ideal candidate for plasma donation and those who are may find it burdensome to return to the clinic following titer testing to donate plasma. Thus, based on these data that anti-spike IgG avidity is better correlated with neutralizing titers, testing antibody avidity has potential use as another screening parameter to identify optimal convalescent plasma donors. However, further research is needed to examine the potential benefit of this strategy.
As was expected, antibody avidity and titers were low during initial infection, but increased as infection progressed. In previous SARS outbreaks, low antibody avidity was observed early in infection and increased within the first month of symptom onset [22, 23]. Additionally, high IgG avidity was observed 3 weeks following symptom onset in recovered COVID-19 individuals [28]. Consistent with those findings, anti-spike and anti-nucleocapsid IgG avidity increased with antibody titers within the first month of symptom onset and remained elevated following viral clearance. Of the 2 major immunogenic proteins, nucleocapsid proteins are more abundantly expressed, which could help to explain why titers for anti-nucleocapsid IgG appears earlier than anti-spike IgG [22, 29]. Avidity for both antibodies appears to peak around 3 weeks following symptom onset, which corresponds to previous reports of IgG seroconversion in COVID-19 patients occurring between 8 and 21 days following symptom onset [30, 31].
Male bias has been documented in COVID-19 patients, with males having increased disease severity and mortality compared to females [32–34]. Additionally, increased risk for severity of disease is associated with advancing age, even though males and females both have the same susceptibility risk [33, 35]. In the convalescent plasma cohort, antibody avidity was increased in older males compared to younger males. However, no statistically significant differences in anti-spike or anti-nucleocapsid avidity between males and females was observed, which may potentially be due to lack of power, particularly for anti-spike avidity. All participants donating convalescent plasma had recovered from COVID-19, and even though males tend to have a higher mortality rate than females, those parameters were not established in these studies.
Avidity was significantly higher among hospitalized patients compared to those who were not hospitalized. Those hospitalized may have had higher viral loads and/or increased exposure, exacerbating symptoms, increasing immune response and antibody production to combat increased viral loads. Moreover, anti-spike avidity was correlated with higher neutralizing antibody titers. Neutralizing antibodies prevent viral replication by binding to viral proteins and blocking the interaction with cellular receptors or cell surface attachment to inhibit host membrane fusion [36]. There was a correlation between anti-nucleocapsid titers and neutralizing antibody titer AUC, but this may reflect the overall anti-SARS-CoV-2 antibody levels, as anti-nucleocapsid antibodies are not believed to have neutralizing activity. However, nucleocapsid antibodies may be necessary for viral clearance via antibody-dependent cellular cytotoxicity, antibody-dependent cell-mediated phagocytosis, and/or complement activation [33, 35].
Asymptomatic individuals have lower antibody titers as compared to symptomatic patients and those individuals who recover from COVID-19 without becoming hospitalized typically do not have high neutralizing antibody activity [36]. In these studies, persons infected with SARS-CoV-2 who were symptomatic but not hospitalized had lower antibody avidity and 4%–12% had no detectable antibody titers. Those with low titers and avidity may have either robust innate immune or adaptive T-cell responses or strongly binding antibodies early during exposure that results in viral clearance, and it will be important to examine these additional aspects of the anti-COVID immune response moving forward. The mechanisms behind why some individuals do not reach detectable antibody titers following viral clearance needs further investigation.
This study had limitations. Antibody titers were analyzed using 2 different methods, and although increasing values of AU and/or ODn may indicate increase in antibody titers, these values are proxies for quantitative measures. Plateaus observed in both assays may be due, in part, to a small dynamic range of the assay, and as a result antibodies do not appear to continue to rise. Only hospitalized patients were assessed in the longitudinal study and the antibody titers or avidity findings may not be generalizable to those who have mild or subclinical disease. Longitudinal samples did not have demographic characteristics collected, so avidity cannot be attributed to sex or age differences in that cohort. In the convalescent plasma donors, less than 10% were hospitalized and only 1 time point was collected per individual.
Additionally, the usage of modified ELISA avidity assays have not been approved by the FDA for convalescent plasma qualification. However, alternative antibody assays are under evaluation for convalescent plasma testing [37]. Although this study did not analyze longitudinal convalescent plasma, it should be further investigated as reports of declining antibody titers are observed 2–3 months following infection [38]. This would help to identify the optimal window of plasma donation. Furthermore, future studies should explore convalescent plasma in relation to ABO blood groups [39, 40].
The use of avidity in conjunction with other serologic testing may be useful in establishing parameters for optimal convalescent plasma donor screening. Increase in avidity following viral clearance is a good indication that strong antibodies and/or neutralizing antibodies are still present. Those with higher anti-spike antibody avidity also have higher neutralizing titers and can aid in identification of those who establish robust adaptive immune response to infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. We would like to thank all participants who enrolled in this study without whom this study could not take place. We thank the National Institute of Infectious Diseases, Japan, for providing VeroE6TMPRSS2 cells and acknowledge the Centers for Disease Control and Prevention, BEI Resources, NIAID, NIH for SARS-Related Coronavirus 2, Isolate USA-WA1/2020, NR-5228.
Disclaimer. EMB is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Any view or opinions that are expressed in this manuscript are that of the author’s based on his own scientific expertise and professional judgment; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of FDA, and do not bind or otherwise obligate or commit either Advisory Committee or the Agency to the views expressed.
Financial support. This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases; extramural support from the National Institute of Allergy and Infectious Diseases (grant numbers R01AI120938, R01AI120938S1, and R01AI128779 to A. A. R. T.); National Institutes of Health (NIH) Center of Excellence in Influenza Research and Surveillance (grant numbers HHSN272201400007C to A. P. and T32AI102623 to E. U. P.); National Heart Lung and Blood Institute (grant numbers K23HL151826 to E. M. B.); and Bloomberg Philanthropies (A. C.); Department of Defense (grant number W911QY2090012 to D. S.). R. E. F. and E. K. were supported in part by National Institutes of Health (NIH) (grant numbers UM1-AI068613 and R01-095068). A. C. was supported in part by National Institutes of Health (NIH) (grant numbers AI052733, AI15207 and HL059842).
Potential conflicts of interest. E. M. B. reports personal fees and nonfinancial support from Terumo BCT, and personal fees and nonfinancial support from Grifols Diagnostics Solutions outside the submitted work. S. S. reports personal fees from Janssen, Karyopharm, and Intermountain Health; and grants and personal fees from Merck and ReViral, outside the submitted work.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus disease (COVID-19) pandemic https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 1 July 2020.
- 3. Centers for Disease Control and Prevention. Coronavirus Disease 2019 https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 28 June 2020.
- 4. Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med 2020; 46:1472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020; 130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salazar E, Perez KK, Ashraf M, et al. Treatment of COVID-19 patients with convalescent plasma [published online ahead of print 11 August 2020]. Am J Clin Pathol doi: 10.1016/j.ajpath.2020.08.001. [DOI] [Google Scholar]
- 8. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 2020; 130:4791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020; 117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv 20036145 [Preprint] 16. March 2020. [cited 14 September 2020]. Available from: 10.1101/2020.03.16.20036145. [DOI] [Google Scholar]
- 11. Tobian AAR, Shaz BH. Earlier the better: convalescent plasma. Blood 2020; 136:652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou G, Zhao Q. Perspectives on therapeutic neutralizing antibodies against the novel coronavirus SARS-CoV-2. Int J Biol Sci 2020; 16:1718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed 9 April 2020.
- 14. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv [Preprint] 30. March 2020. [cited 14 September 2020]. Available from: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 15. Meyer B, Drosten C, Müller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res 2014; 194:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020; 17:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaecher SR, Stabenow J, Oberle C, et al. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology 2008; 380:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein SL, Pekosz A, Park H-S, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population [published online ahead of print 7 August 2020]. J Clin Invest doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Q, Du Q, Guo B, et al. A method to prevent SARS-CoV-2 IgM false positives in gold immunochromatography and enzyme-linked immunosorbent assays. J Clin Microbiol 2020; 58:e00375-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. AAT Bioquest. IC50 calculator https://www.aatbio.com/tools/ic50-calculator. Accessed 28 June 2020.
- 22. Chan PKS, Lim P, Liu EYM, Cheung JLK, Leung DTM, Sung JJY. Antibody avidity maturation during severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis 2005; 192:166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan KH, Sonnenberg K, Niedrig M, et al. Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection. Clin Vaccine Immunol 2007; 14:1433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chawla A, Murphy G, Donnelly C, et al. Human immunodeficiency virus (HIV) antibody avidity testing to identify recent infection in newly diagnosed HIV type 1 (HIV-1)-seropositive persons infected with diverse HIV-1 subtypes. J Clin Microbiol 2007; 45:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan KH, Ng MH, Seto WH, Peiris JSM. Epstein-Barr virus (EBV) DNA in sera of patients with primary EBV infection. J Clin Microbiol 2001; 39:4152–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levett PN, Sonnenberg K, Sidaway F, et al. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol 2005; 43:5873–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan KH, Sonnenberg K, Niedrig M, et al. Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection. Clin Vaccine Immunol 2007; 14:1433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valdivia A, Torres I, Huntley D, et al. Qualitative assessment of SARS-CoV-2-specific antibody avidity by lateral flow immunochromatographic IgG/IgM antibody assay [published online ahead of print 24 July 2020]. J Med Virol doi: 10.1002/jmv.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Liu L, Kou G, et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol 2020; 58:e00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis 2020; 26:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020; 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vanblargan LA, Goo L, Pierson TC. Deconstructing the antiviral neutralizing-antibody response: implications for vaccine development and immunity. Microbiol Mol Biol Rev 2016; 80:989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klasse PJ. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol 2014; 2014:157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janeway C, Travers P, Walport M, Shlomchik M Immunobiology. 5th edn. London: Churchill Livingstone, 2001. [Google Scholar]
- 36. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention. COVID-19 convalescent plasma EUA decision memo https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed 24 August 2020.
- 38. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 39. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe covid-19 with respiratory failure [published online ahead of print 17 June 2020]. N Engl J Med doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Latz CA, DeCarlo C, Boitano L, et al. Blood type and outcomes in patients with COVID-19. Ann Hematol 2020; 99:2113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.