Abstract
PURPOSE
Women with gestational trophoblastic tumors (GTT) resistant to single-agent chemotherapy receive alternative chemotherapy regimens, which, although effective, cause considerable toxicity. All GTT subtypes express programmed death-ligand 1 (PD-L1), and natural killer (NK) cells are involved in trophoblast immunosurveillance. Avelumab (anti–PD-L1) induces NK cell–mediated cytotoxicity. The TROPHIMMUN trial assessed avelumab in women with chemotherapy-resistant GTT.
METHODS
In this phase II multicenter trial (ClinicalTrials.gov identifier: NCT03135769), women with GTT who experienced disease progression after single-agent chemotherapy received avelumab 10 mg/kg intravenously every 2 weeks until human chorionic gonadotropin (hCG) normalization, followed by 3 consolidation cycles. Rate of hCG normalization was the primary endpoint (2-step Simon design).
RESULTS
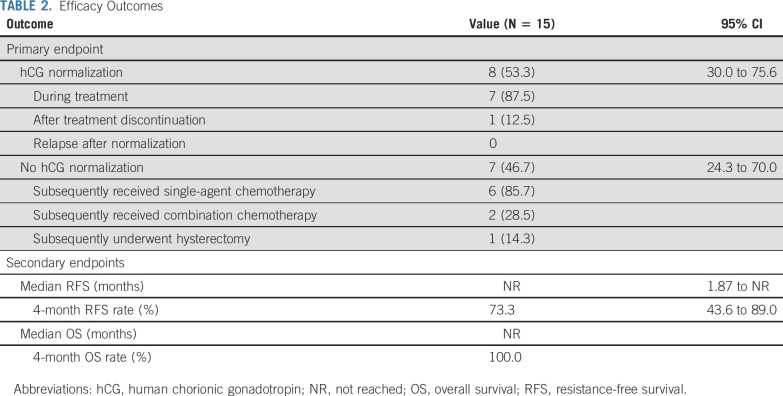
Between December 2016 and September 2018, 15 patients were treated. Median age was 34 years; disease stage was I or III in 53.3% and 46.7% of women, respectively; and International Federation of Gynecology and Obstetrics (FIGO) score was 0-4 in 33.3%, 5-6 in 46.7%, and ≥ 7 in 20% of patients. Prior treatment included methotrexate (100%) and actinomycin D (7%). Median follow-up was 25 months, and median number of avelumab cycles was 8 (range, 2-11). Grade 1-2 treatment-related adverse events occurred in 93% of patients, most commonly (≥ 25%) fatigue (33.3%), nausea/vomiting (33.3%), and infusion-related reaction (26.7%). One patient had grade 3 uterine bleeding (treatment unrelated). Eight patients (53.3%) had hCG normalization after a median of 9 avelumab cycles; none subsequently relapsed. Probability of normalization was not associated with disease stage, FIGO score, or baseline hCG. One patient subsequently had a healthy pregnancy. In avelumab-resistant patients (46.7%), hCG was normalized with actinomycin D (42.3%) or combination chemotherapy/surgery (57.1%).
CONCLUSION
In patients with single-agent chemotherapy-resistant GTT, avelumab had a favorable safety profile and cured approximately 50% of patients. Avelumab could be a new therapeutic option, particularly in patients who would otherwise receive combination chemotherapy.
INTRODUCTION
Patients diagnosed with low-risk gestational trophoblastic tumors (GTT), which represent approximately 95% of malignant gestational trophoblastic disease forms (including invasive mole, choriocarcinoma, epithelioid trophoblastic tumors, and placental site trophoblastic tumors), have International Federation of Gynecology and Obstetrics (FIGO) 2000 risk scores ranging from 0 to 6 and are treated with single-agent chemotherapy.1,2 In Europe, the 8-day methotrexate protocol (modified by Bagshawe et al3) is the most commonly used regimen.1,2,4 Methotrexate treatment is continued until either normalization of serum human chorionic gonadotropin (hCG) concentration (followed by 2 to 3 consolidation cycles) or methotrexate resistance is detected.1 In patients with methotrexate resistance (approximately 25%-69% of low-risk patients), subsequent treatment options are actinomycin D, which is associated with a cure rate of approximately 70%, or EMA-CO regimens (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine), which have a 100% cure rate but carry a high risk of long-term tox-icities.2,5-14
CONTEXT
Key Objective Could immunotherapy with avelumab (anti–programmed death-ligand 1 monoclonal antibody) be an alternative to poorly tolerated chemotherapy regimens in patients with gestational trophoblastic tumors resistant to single-agent chemotherapy?
Knowledge Generated Overall, 8 (53%) of 15 patients had human chorionic gonadotropin normalization and discontinued avelumab without additional relapse after a median of 29 months of follow-up, meaning they are likely to be cured. This included 5 patients (33%) who would have otherwise received combination chemotherapy and who therefore avoided immediate and long-term toxicities. One patient successfully treated with avelumab subsequently had a healthy pregnancy and delivery.
Relevance Avelumab represents a new therapeutic option in patients with gestational trophoblastic tumors resistant to single-agent chemotherapy, particularly in those who would otherwise receive combination chemotherapy.
Several lines of evidence suggest that the immune system plays an important role in the outcome of gestational trophoblastic diseases. Patients with spontaneous regression of metastatic disease have been reported.15-17 Additionally, programmed death-ligand 1 (PD-L1) is constitutively expressed in all premalignant and malignant trophoblast subtypes, independent of FIGO score, chemoresistance, or fatal outcome.18-20 This suggests that PD-L1 may have a crucial role in immune tolerance in these diseases. Trophoblastic disease arising from normal pregnancy and choriocarcinoma does not express classic class I (A and B) or class II HLA molecules, but instead expresses nonclassic class I HLA molecules, including HLA-C, HLA-E, and HLA-G.21,22 Natural killer (NK) cells comprise 70% of immune cells in the normal decidua during the first trimester.23,24 Moreover, granzyme-positive NK cells constitute the majority of the leukocyte population in peritumoral immune infiltrates of postmolar choriocarcinoma.25 This suggests that NK cells play a key role in the tolerance of normal trophoblast and could be responsible for cytotoxic antitumor responses in GTT. Considering these observations together, immune blockade of the PD-L1/programmed death-1 (PD-1) pathway may have the potential to reverse trophoblast tolerance both in normal pregnancy and in GTT,26 and supporting this hypothesis, clinical activity has been reported with pembrolizumab (anti–PD-1 antibody) in GTT case reports.27,28 Furthermore, an immunotherapy that can also induce tumor cell recognition through NK cells may have enhanced therapeutic potential in GTT.
Avelumab is a fully human immunoglobulin G1 anti–PD-L1 monoclonal antibody.29 Unlike other approved anti–PD-L1/PD-1 antibodies, avelumab can both reactivate adaptive immune responses by inhibiting the PD-L1/PD-1 interaction and also induce NK cell–associated antibody-dependent cell-mediated cytotoxicity of tumor cells.30,31 We hypothesized that in patients with GTT resistant to single-agent chemotherapy, avelumab may have similar clinical activity to chemotherapy but may be better tolerated. We report findings from a phase II study of avelumab monotherapy in patients with chemotherapy-resistant GTT.
METHODS
Study Design and Participants
TROPHIMMUN is an investigator-initiated, open-label, multicohort, phase II trial of avelumab in patients with chemotherapy-resistant GTT. The trial includes 2 cohorts of patients with GTT resistant to either single-agent chemotherapy (cohort A) or combination chemotherapy regimens (cohort B); outcomes from cohort A are reported in this article. The trial was sponsored by Lyon University Hospital (Hospices Civils de Lyon) and was conducted across 7 centers participating in the network of the French Gestational Trophoblastic Center (Centre de Référence des Maladies Trophoblastiques, Lyon, France). Consistent with the recommendations from The European Organization for Treatment of Trophoblastic Diseases, resistance to single-agent chemotherapy was defined as an increase in hCG by > 10% in 3 consecutive hCG values over a 2-week interval or hCG plateau with a change of < 10% in 4 consecutive hCG values over a 3-week interval.32
Eligible patients were women aged ≥ 18 years with a gestational trophoblastic neoplasia resistant to single-agent chemotherapy (methotrexate and/or actinomycin D); any number of previous lines of chemotherapy; Eastern Cooperative Oncology Group performance status ≤ 2; adequate bone marrow function (absolute granulocyte count of ≥ 1.5 × 109/L, platelet count of ≥ 100 × 109/L, and hemoglobin of ≥ 9.0 g/dL [blood transfusions were permitted]); adequate hepatic function (serum bilirubin ≤ 1.5 × upper limit of normal [ULN] and AST/ALT ≤ 2.5 × ULN [≤ 5 × ULN for patients with liver metastases]); and adequate renal function (creatinine clearance ≥ 30 mL/min per Cockcroft-Gault formula or local institutional standard method). Patients with type 1 diabetes, vitiligo, psoriasis, or hypothyroid/hyperthyroid disease that did not require immunosuppressive treatment were also eligible. Exclusion criteria included any prior immune checkpoint inhibitor treatment (including antibodies targeted to CTLA-4, PD-1, PD-L1, PD-L2, or CD137, or any other agent targeting T-cell costimulation or immune checkpoint pathways); receipt of any live vaccine ≤ 30 days before enrollment; treatment with an immunosuppressive medication within 7 days of start of study treatment (except for intranasal, inhaled, or topical steroids, or local steroid injections; systemic corticosteroids at physiologic doses of ≤ 10 mg/day prednisone or equivalent; or steroids as premedication for hypersensitivity reactions); presence of brain metastases (except for patients with brain metastases treated locally and subsequently clinically stable for ≥ 2 weeks before enrollment, with no ongoing neurologic symptoms related to the brain localization of the disease, and not receiving steroids except a stable or decreasing dose of < 10 mg/day prednisone or equivalent); active infections requiring systemic therapy; known HIV or AIDS-related illness; positive test for hepatitis B virus surface antigen and/or confirmatory hepatitis C virus (HCV) RNA (if anti–HCV antibody tested positive); or active autoimmune disease that may deteriorate with an immunostimulatory agent.
The study protocol (Clinical Trials.gov identifier: NCT03135769) was approved by the French independent ethics committee Comité de Protection des Personnes and the French health authorities Autorité Nationale de Sureté du Médicament. Patients were enrolled per international standards of good clinical practice and institutional safety monitoring. All patients provided written informed consent before study enrollment.
Procedures
Baseline assessments included thoracic-abdominal-pelvic and brain contrast-enhanced computed tomography (CT) scans, or thoracic CT scans and abdominal-pelvic and brain contrast-enhanced magnetic resonance imaging scans in patients with a contraindication to iodinated intravenous contrast for CT scan or a target lesion not assessable with CT scan; left ventricular ejection fraction assessment; archival curettage or tumoral tissue biopsy; patient interview and clinical examination, including electrocardiogram; and standard blood/urine assessments.
Patients received avelumab intravenously at 10 mg/kg every 2 weeks. Avelumab treatment was continued until the institutional normal serum hCG concentration was reached, followed by 3 additional cycles; hCG resistance occurred (> 20% rise in hCG level from baseline maintained for 3 consecutive weekly assessments or hCG plateau with < 10% decrease in 3 of 4 consecutive weekly assessments); unacceptable toxicity and/or death; intercurrent illness that prevented additional treatment; or patient withdrawal. However, because the hCG response profile with immunotherapy remains poorly understood, avelumab could be continued despite an hCG increase during the first 3 months of treatment to account for potential pseudoprogression, as observed in other tumor types.33
Safety was assessed every 2 weeks during cycles 1-4 and then every 2 cycles until cycle 24. Treatment-related adverse events (TRAEs) were classified according to the National Cancer Institute Common Terminology Criteria, version 4.0. Serum hCG levels were measured every week. Tumors were assessed using the baseline assessment method every 4 cycles until cycle 24.
Outcomes and Statistical Analysis
The primary endpoint was the proportion of patients with normalization of hCG allowing treatment discontinuation. Secondary endpoints included resistance-free survival (RFS), overall survival (OS), and safety. RFS was measured from the date of inclusion to the date of resistance to avelumab (as defined earlier). OS was measured from the date of inclusion to the date of death or end of follow-up, whichever occurred first. The study had a 2-step design aligned with optimal designs reported by Simon34 (1-sided 5% alpha risk and 90% power).34 In the cohort reported here, treatment was not considered to be effective if the success rate (hCG normalization) was ≤ P0 = 30% (null hypothesis). The alternative for efficacy was a success rate ≥ P1 = 70%. Enrollment of 6 assessable patients was planned in the first step. If ≥ 3 successes were observed, recruitment of up to 15 patients was planned. If ≥ 8 successes were observed among the 15 patients, the trial would be considered positive. Secondary endpoints of RFS and OS were analyzed using the Kaplan-Meier method.
RESULTS
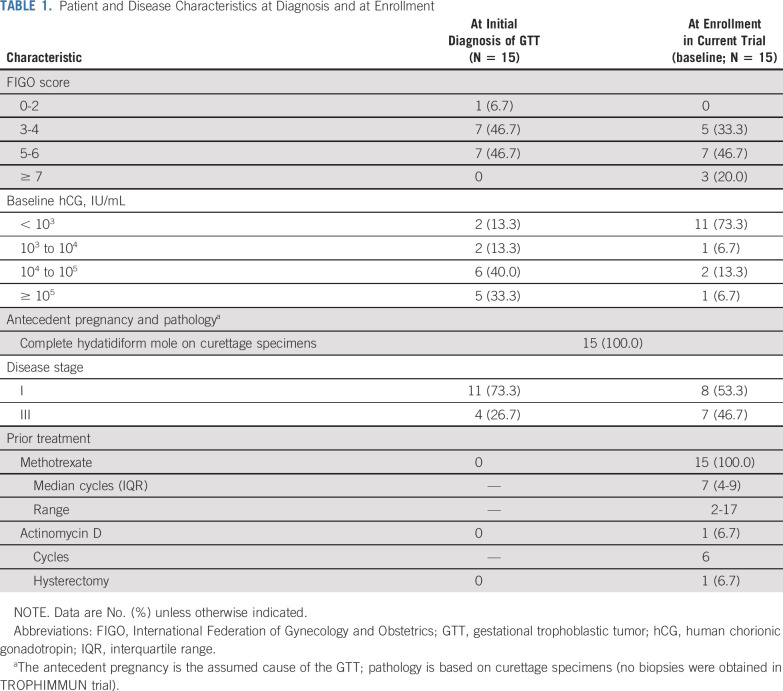
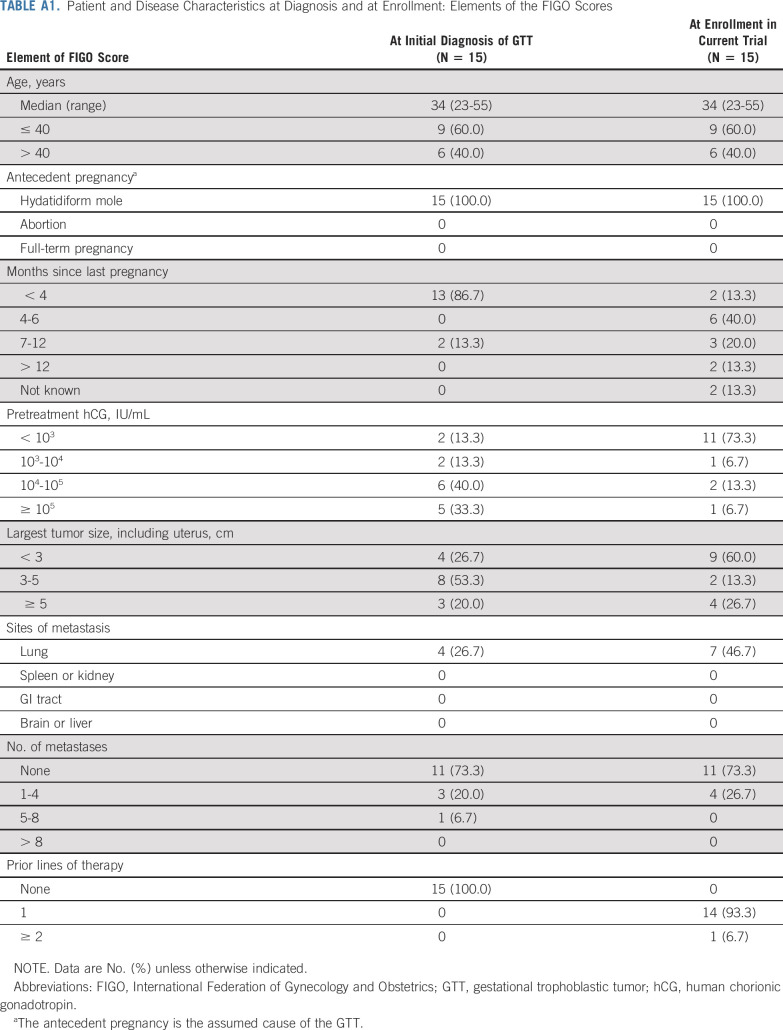
Between December 2016 and December 2018, 17 patients were screened, of whom 15 were enrolled and received ≥ 1 dose of avelumab (6 and 9 patients in the first and second steps, respectively). All 15 were included in efficacy and safety analyses (Fig 1). Median age was 34 years (range, 23-55 years). At enrollment, disease stage was I in 8 patients (53.3%) and III in 7 patients (46.7%); FIGO score was 0-4 in 5 patients (33.3%), 5-6 in 7 patients (46.7%), and ≥ 7 in 3 patients (20.0%; Table 1; Appendix Table A1, online only). All patients had received prior methotrexate treatment, and 1 patient (7%) had also received prior actinomycin D treatment. At the time of enrollment, 4 patients had baseline hCG ≥ 1,000 IU/L. At data cutoff (May 2020), median duration of follow-up was 25 months. The median number of avelumab cycles administered was 8 (range, 2-11).
FIG 1.
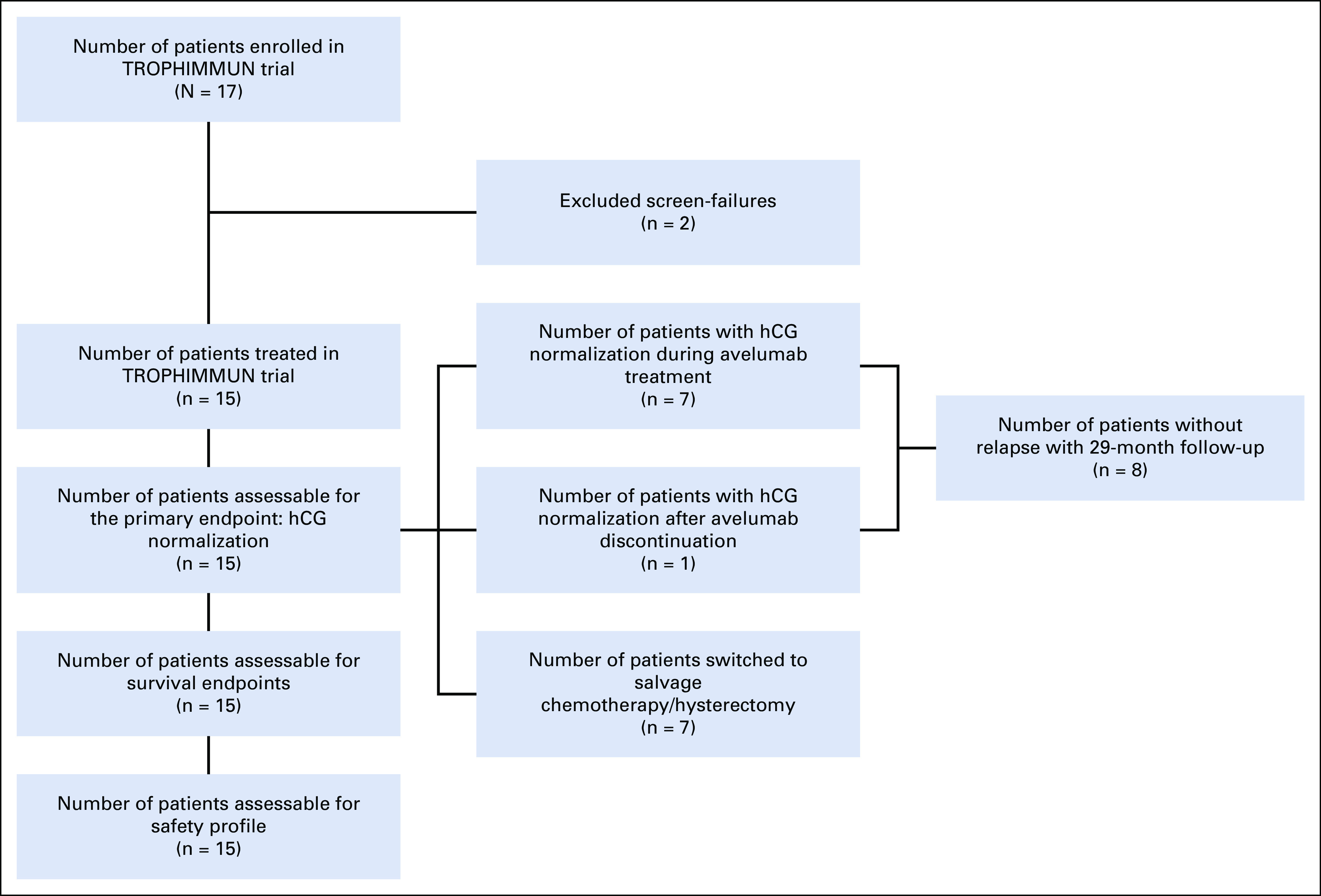
Patient flowchart during the study. hCG, human chorionic gonadotropin.
TABLE 1.
Patient and Disease Characteristics at Diagnosis and at Enrollment
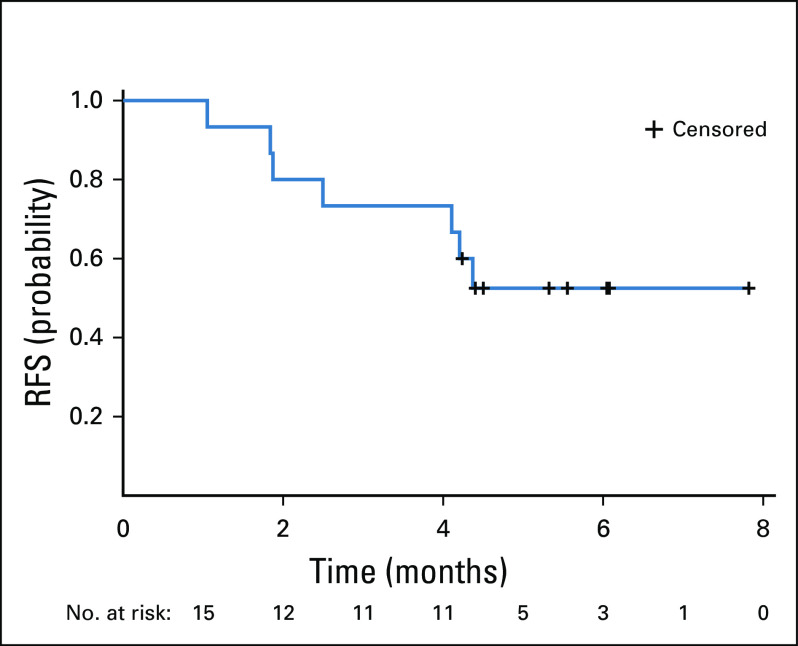
Seven (46.7%) of 15 patients had hCG normalization during avelumab treatment (Table 2); in these patients, the median number of avelumab cycles received was 9 (range, 6-11). One patient subsequently had a successful pregnancy (Fig 2).35 One patient (6.7%) had a normalized hCG level after discontinuing avelumab (9 cycles received). After a median follow-up of 29 months, no patient whose hCG level was normalized had a relapse after avelumab was discontinued, consistent with disease cure. Additionally, no patient had an initial increase in hCG (ie, pseudoprogression) before the hCG decline and subsequent normalization. In 7 patients (46.7%) whose hCG level was not normalized with avelumab (median number of cycles, 4.5 [range, 2-8]), 3 (42.3%) were subsequently cured with actinomycin D, 3 (42.3%) were cured with combination chemotherapy, and 1 (14.3%) underwent hysterectomy. In patients who were cured with avelumab or resistant to avelumab, baseline FIGO scores were similar (median 5 [range, 3-14] v 5 [range, 4-8], respectively), whereas there was a nonsignificant trend for a lower proportion of patients with metastatic (stage III) disease among those cured by avelumab (37.5%) versus those with resistance (57.1%). Although all 4 patients with baseline hCG > 1,000 IU/L experienced hCG normalization with avelumab, no relationship was observed between baseline hCG and the probability of normalization. Median RFS was not reached (95% CI, 1.9 months to not reached), and the 4-month RFS rate was 73.3% (95% CI, 43.6% to 89.0%), with all occurrences of resistance observed within 5 months of treatment (Fig 3). No deaths occurred during the study.
TABLE 2.
Efficacy Outcomes
FIG 2.
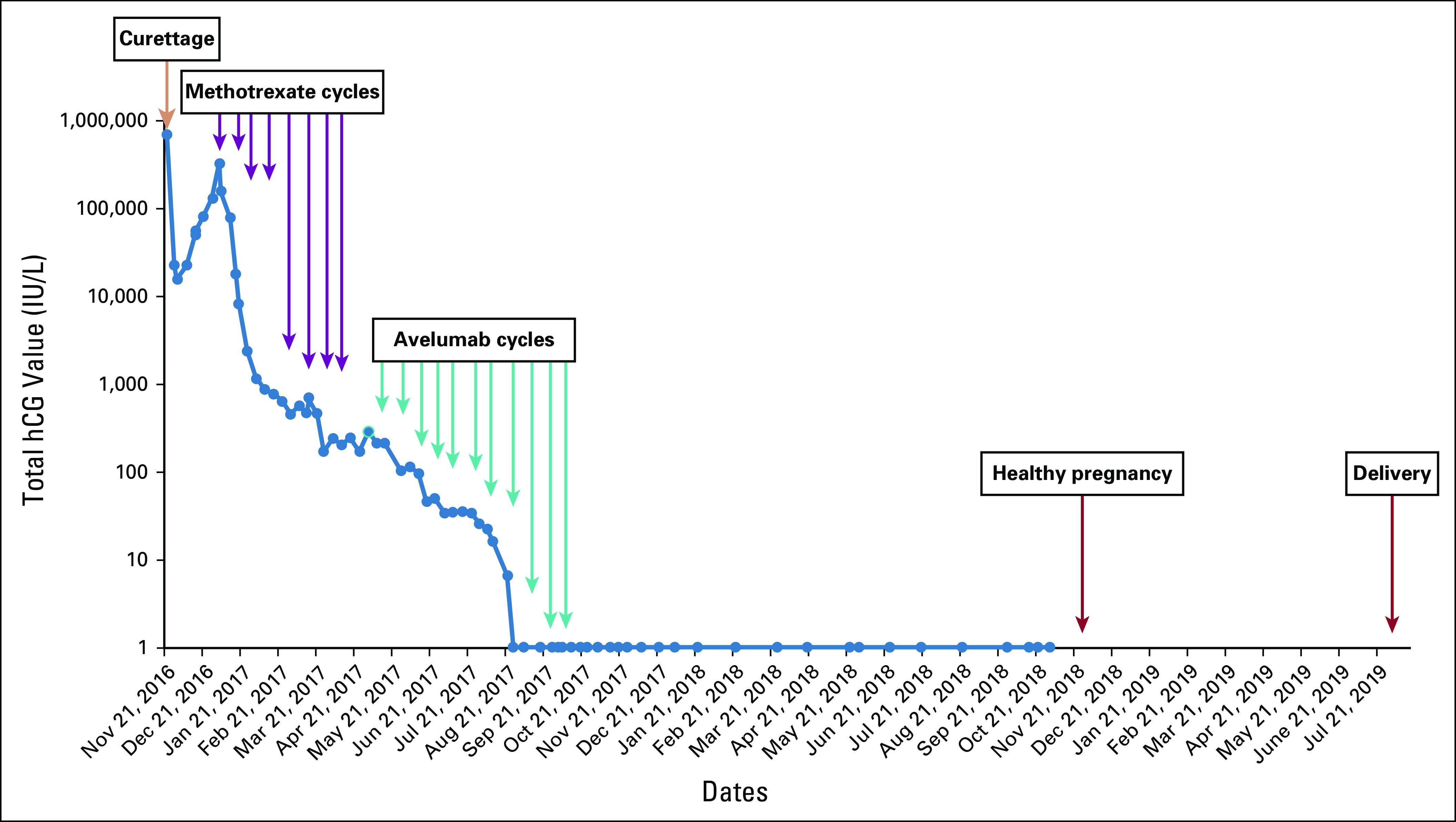
Change in human chorionic gonadotropin (hCG) over time in a patient who was cured with 11 cycles of avelumab and subsequently had a normal pregnancy.35
FIG 3.
Resistance-free survival (RFS) in TROPHIMMUN trial cohort A.
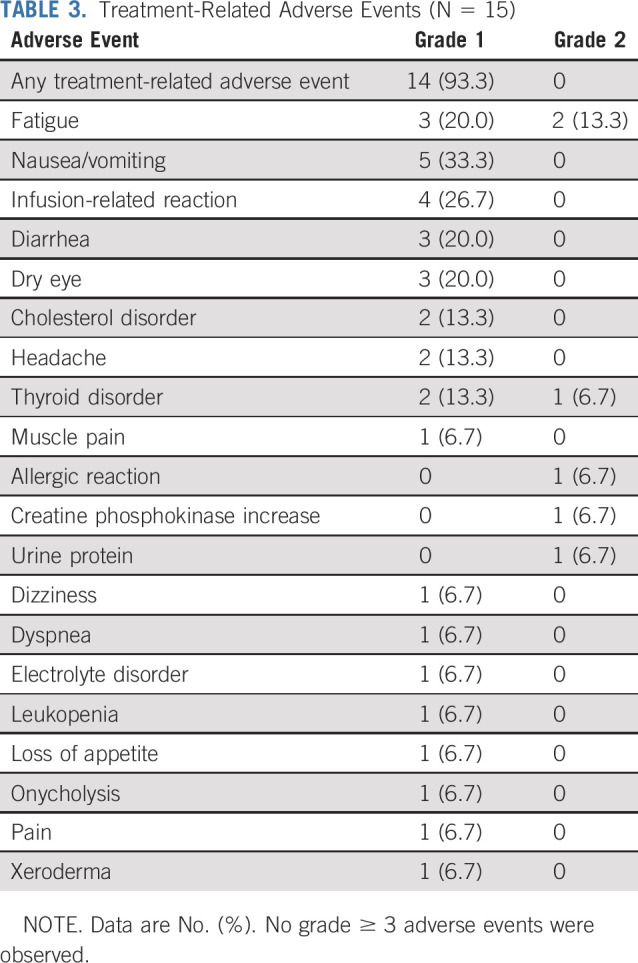
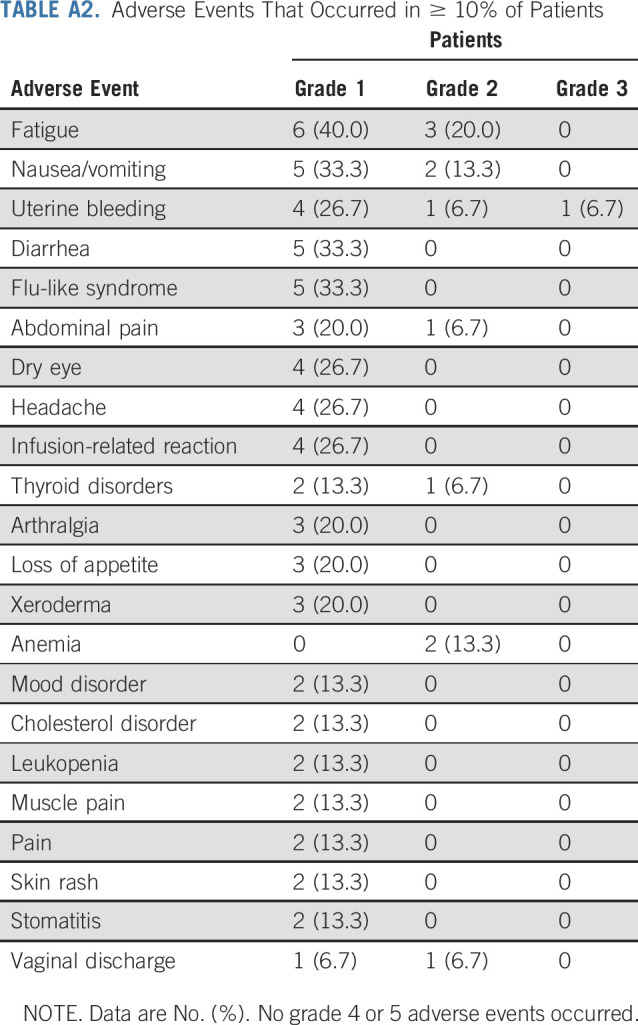
No patient had an avelumab dose reduction or delay for > 48 hours, and no patient discontinued avelumab because of toxicity. In total, 14 patients (93.3%) had a TRAE of any grade, which were all grade 1 or 2 (Table 3). The most common TRAEs (those occurring in ≥ 25% of patients) were fatigue (n = 5 [33.3%]), nausea/vomiting (n = 5 [33.3%]), and infusion-related reaction (n = 4 [26.7%]). Treatment-emergent adverse events (AEs; related or unrelated) are summarized in Appendix Table A2 (online only). Two patients (13.3%) had a serious AE: grade 2 ovarian cyst (n = 1 [6.7%]) and grade 3 uterine bleeding (n = 1 [6.7%]), which were both unrelated to treatment. Immune-related AEs of any grade occurred in 3 patients (20.0%): hyperthyroidism (n = 2 [13.3%]) and hypothyroidism (n = 1 [6.7%]).
TABLE 3.
Treatment-Related Adverse Events (N = 15)
DISCUSSION
To our knowledge, TROPHIMMUN is the first prospective trial of an immunotherapy in patients with GTT, for which chemotherapy is currently the mainstay of treatment. Findings are remarkable for several reasons. First, the trial was initiated and conducted in a short timeframe for such a rare cancer (approximately 1 in every 10,000 pregnancies, approximately 200 patients per year in France),36 which was enabled by the network of specialized gestational trophoblastic disease centers. Second, the trial demonstrated high efficacy for avelumab, a nonchemotherapy option, in patients with resistance to single-agent chemotherapy. In total, 53% of patients achieved a normalized hCG level during or after avelumab treatment, and none of these patients subsequently relapsed after a median follow-up of 29 months, consistent with disease cure. By comparison, in previous studies of chemotherapy in patients with GTT, relapse occurred in 85% within 24 months and 100% within 37 months.36-38 The 4-month RFS rate with avelumab was 73.3% (median, not reached). Disease stage, FIGO score, and baseline hCG level did not predict benefit with avelumab.
As a result of avelumab treatment, at least 5 patients avoided combination chemotherapy regimens, including 4 patients with a baseline hCG > 1,000 IU/L (an indication for combination chemotherapy, per current guidelines),32 and 1 patient who was enrolled after resistance to 2 lines of single-agent chemotherapy, and this does not consider patients who would have had resistance to actinomycin D. Consequently, avelumab treatment prevented short- and long-term toxicities associated with combination chemotherapy regimens in at least 33% of enrolled patients. Although 47% of patients did not have normalized hCG levels with avelumab, all patients were cured with subsequent therapy.
The frequency of responses to avelumab corroborates the hypothesis that immune tolerance has an important role in the biology of GTT, which was suggested by previous observations of consistent PD-L1 overexpression in GTT tissues18-20 and clinical responses to pembrolizumab in case reports.27,28 Because of the importance of NK cells in the immunology of pregnancy and GTT, it is possible that the clinical activity of avelumab was due in part to its ability to induce NK cell–mediated, antibody-dependent, cell-mediated cytotoxicity,30,31 although this cannot be confirmed with data from the current study. Avelumab showed a favorable safety profile that was consistent with previous studies of avelumab.39 Only grade 1-2 TRAEs were reported, and the rate of immune-related AEs, which are a recognized occurrence with immune checkpoint inhibitors,40,41 was low. Data are not available on long-term adverse effects with anti–PD-L1/PD-1 antibodies in GTT, although trials in other tumors have shown better tolerability than chemotherapy.42-45 Importantly, 1 patient in the current study had a healthy pregnancy and delivery after avelumab treatment,35 providing reassurance about the lack of impact on fertility in this patient population, which includes many women of child-bearing potential. However, additional data and longer follow-up are needed to allow more definitive conclusions on the safety of avelumab regarding subsequent fertility.
The trial has obvious limitations. The number of patients treated was low (N = 15), which reflects the low prevalence of GTT.36 There was also no direct comparison with a standard treatment arm. However, a phase III trial comparing pulse actinomycin D treatment versus multiday methotrexate in patients with low-risk GTT (GOG0275; ClinicalTrials.gov identifier: NCT01535053) was closed prematurely because of insufficient recruitment, highlighting the difficulty of performing randomized trials in this disease setting. The delayed hCG normalization that occurred in 1 patient after discontinuing avelumab suggests a potential delayed effect of immunotherapy, which requires additional study. Future studies will provide additional information on the potential role of avelumab in the treatment of GTT, including potential predictive markers, long-term safety, efficacy, and cost effectiveness. In the meantime, avelumab could represent a new therapeutic option for patients with GTT resistant to single-agent chemotherapy who would otherwise receive more toxic combination chemotherapy regimens, that is, patients with baseline hCG > 1,000 IU/L32, patients whose disease is resistant to both single-agent chemotherapy options, and patients with intolerance or a contraindication to second-line single-agent chemotherapy. In cohort B from this trial, we will assess the efficacy of avelumab in patients with resistance to combination chemotherapy. Furthermore, an ongoing trial (TROPHAMET, NCT04396223) combines avelumab with methotrexate as first-line treatment in patients with GTT, which aims to cure 95% of patients and further reduce the emergence of resistance and life-threatening disease evolution. In summary, TROPHIMMUN is a proof-of-concept trial showing that immunotherapy with avelumab could potentially cure approximately 50% of patients with GTT who have resistance to single-agent chemotherapy while avoiding the toxic effects of chemotherapy, expanding treatment options in this disease.
ACKNOWLEDGMENT
The authors thank all the patients and their families, the investigators, study nurses, pharmacists, pathologists, and all study teams. This research was financially supported by Merck KGaA, Darmstadt Germany, as part of an alliance between Merck KGaA and Pfizer.
APPENDIX
TABLE A1.
Patient and Disease Characteristics at Diagnosis and at Enrollment: Elements of the FIGO Scores
TABLE A2.
Adverse Events That Occurred in ≥ 10% of Patients
PRIOR PRESENTATION
Presented at ASCO Annual Meeting 2020, Chicago, IL, May 29-31, 2020; and presented in part at the European Society for Medical Oncology 2018 Congress, October 19-23, 2018.
SUPPORT
Supported by Merck KGaA, Darmstadt Germany, as part of an alliance between Merck KGaA and Pfizer.
AUTHOR CONTRIBUTIONS
Conception and design: Benoit You, Pierre-Adrien Bolze, Jean-Pierre Lotz, Delphine Maucort-Boulch, Adeline Roux, Catherine Mercier, Laurent Villeneuve, Francois Golfier
Administrative support: Laurent Villeneuve
Provision of study materials or patients: Benoit You, Mojgan Devouassoux-Shisheboran, Daniele Grazziotin-Soares
Collection and assembly of data: Jérome Massardier, Laurence Gladieff, Florence Joly, Touria Hajri, Sylvie Bin, Adeline Roux, Marine Alves-Ferreira, Daniele Grazziotin-Soares
Data analysis and interpretation: Florence Joly, Delphine Maucort-Boulch, Sylvie Bin, Pascal Rousset, Mojgan Devouassoux-Shisheboran, Carole Langlois-Jacques, Catherine Mercier, Gilles Freyer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Avelumab in Patients With Gestational Trophoblastic Tumors With Resistance to Single-Agent Chemotherapy: Cohort A of the TROPHIMMUN Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benoit You
Consulting or Advisory Role: Genentech, AstraZeneca, Novartis, Lek, Tesaro, Bayer, Amgen, Clovis Oncology, GSK, ECS Progastrin
Research Funding: Merck Serono (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Genentech, AstraZeneca, BMS, MSD Oncology, Bayer
Jérome Massardier
Stock and Other Ownership Interests: DTF
Laurence Gladieff
Honoraria: AstraZeneca, Roche, Clovis Oncology, Tesaro, MSD Oncology (Inst)
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, Tesaro
Travel, Accommodations, Expenses: PharmaMar, Tesaro, AstraZeneca
Florence Joly
Consulting or Advisory Role: AstraZeneca, Janssen, Roche, Sanofi, Ipsen, Pfizer, MSD Oncology, Bristol Myers Squibb, GSK, Astellas Pharma
Research Funding: Astellas Pharma, Janssen
Travel, Accommodations, Expenses: Roche, Janssen, AstraZeneca, Ipsen, GSK, BMS
Delphine Maucort-Boulch
Consulting or Advisory Role: Maat Pharma
Gilles Freyer
Honoraria: Roche, GSK, Clovis Oncology, Lilly, Pfizer, Novartis, MSD Oncology, Pierre Fabre, Genomic Health, Myriad Genetics, NanoString Technologies, Amgen, Biogaran Pharmaceuticals
Consulting or Advisory Role: Lilly, Pierre Fabre, AstraZeneca
Research Funding: Chugai Pharma (Inst), Roche (Inst), Mylan (Inst), Biogaran Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Novartis, Pierre Fabre, MSD Oncology, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 2.Bolze PA, Attia J, Massardier J, et al. Formalised consensus of the European Organisation for Treatment of Trophoblastic Diseases on management of gestational trophoblastic diseases. Eur J Cancer. 2015;51:1725–1731. doi: 10.1016/j.ejca.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Bagshawe KD, Dent J, Newlands ES, et al. The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumours (GTT) Br J Obstet Gynaecol. 1989;96:795–802. doi: 10.1111/j.1471-0528.1989.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 4.Seckl MJ, Sebire NJ, Fisher RA, et al. Gestational trophoblastic disease: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi39–vi50. doi: 10.1093/annonc/mdt345. [DOI] [PubMed] [Google Scholar]
- 5.Sita-Lumsden A, Short D, Lindsay I, et al. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009. Br J Cancer. 2012;107:1810–1814. doi: 10.1038/bjc.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prouvot C, Golfier F, Massardier J, et al. Efficacy and safety of second-line 5-day actinomycin-D in case of methotrexate failure for gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2018;28:1038–1044. doi: 10.1097/IGC.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 7.Lawrie TA, Alazzam M, Tidy J, et al. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;(6):CD007102. doi: 10.1002/14651858.CD007102.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman-Davis E, Hoekstra AV, Rademaker AW, et al. Treatment of nonmetastatic and metastatic low-risk gestational trophoblastic neoplasia: Factors associated with resistance to single-agent methotrexate chemotherapy. Gynecol Oncol. 2012;125:572–575. doi: 10.1016/j.ygyno.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 9.McNeish IA, Strickland S, Holden L, et al. Low-risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol. 2002;20:1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DP, Winig P, Shirley RL. Actinomycin D as initial therapy of gestational trophoblastic disease. A reevaluation. Obstet Gynecol. 1972;39:341–345. [PubMed] [Google Scholar]
- 11.Alazzam M, Tidy J, Osborne R, et al. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;(1):CD008891. doi: 10.1002/14651858.CD008891.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savage P, Cooke R, O’Nions J, et al. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol. 2015;33:472–478. doi: 10.1200/JCO.2014.57.5332. [DOI] [PubMed] [Google Scholar]
- 13.Gadducci A, Lanfredini N, Cosio S. Reproductive outcomes after hydatiform mole and gestational trophoblastic neoplasia. Gynecol Endocrinol. 2015;31:673–678. doi: 10.3109/09513590.2015.1054803. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1111/1471-0528.16198. Hoeijmakers YM, Sweep F, Lok C, et al: Risk factors for second-line dactinomycin failure after methotrexate treatment for low-risk gestational trophoblastic neoplasia: A retrospective study. BJOG 127:1139-1145, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niimi K, Yamamoto E, Nishino K, et al. Spontaneous regression of gestational trophoblastic neoplasia. Gynecol Oncol Rep. 2017;21:98–100. doi: 10.1016/j.gore.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabi Y, Hajri T, Massardier J, et al. Outcome of first-line hysterectomy for gestational trophoblastic neoplasia in patients no longer wishing to conceive and considered with isolated lung metastases: A series of 30 patients. Int J Gynecol Cancer. 2018;28:1766–1771. doi: 10.1097/IGC.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Teoh S, Short D, et al. Chemotherapy and human chorionic gonadotropin concentrations 6 months after uterine evacuation of molar pregnancy: A retrospective cohort study. Lancet. 2012;379:130–135. doi: 10.1016/S0140-6736(11)61265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veras E, Kurman RJ, Wang T-L, et al. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017;36:146–153. doi: 10.1097/PGP.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaguma S, Wang Z, Lasota J, et al. Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1): Analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol. 2016;40:1133–1142. doi: 10.1097/PAS.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolze PA, Patrier S, Massardier J, et al. PD-L1 expression in premalignant and malignant trophoblasts from gestational trophoblastic diseases is ubiquitous and independent of clinical outcomes. Int J Gynecol Cancer. 2017;27:554–561. doi: 10.1097/IGC.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 21.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Apps R, Murphy SP, Fernando R, et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulmer JN, Morrison L, Longfellow M, et al. Granulated lymphocytes in human endometrium: Histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 24.King A, Wellings V, Gardner L, et al. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989;24:195–205. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagymanyoki Z, Callahan MJ, Parast MM, et al. Immune cell profiling in intraplacental and postmolar choriocarcinomas. J Reprod Med. 2008;53:558–564. [PubMed] [Google Scholar]
- 26.Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J. 2015;38:25–31. doi: 10.4103/2319-4170.143511. [DOI] [PubMed] [Google Scholar]
- 27.Ghorani E, Kaur B, Fisher RA, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390:2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- 28.Choi MC, Oh J, Lee C. Effective anti-programmed cell death 1 treatment for chemoresistant gestational trophoblastic neoplasia. Eur J Cancer. 2019;121:94–97. doi: 10.1016/j.ejca.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Collins JM, Gulley JL. Product review: Avelumab, an anti-PD-L1 antibody. Hum Vaccin Immunother. 2019;15:891–908. doi: 10.1080/21645515.2018.1551671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks KC, Fantini M, Donahue RN, et al. Epigenetic priming of both tumor and NK cells augments antibody-dependent cellular cytotoxicity elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. OncoImmunology. 2018;7:e1466018. doi: 10.1080/2162402X.2018.1466018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lok C, van Trommel N, Massuger L, et al. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228–240. doi: 10.1016/j.ejca.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 35. doi: 10.1016/j.annonc.2020.02.015. Bolze PA, You B, Lotz JP, et al: Successful pregnancy in a cancer patient previously cured of a gestational trophoblastic tumor by immunotherapy. Ann Oncol 31:P823-825, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Golfier F, Raudrant D, Frappart L, et al. First epidemiological data from the French Trophoblastic Disease Reference Center. Am J Obstet Gynecol. 2007;196:172.e1–172.e5. doi: 10.1016/j.ajog.2006.10.867. [DOI] [PubMed] [Google Scholar]
- 37.Powles T, Savage PM, Stebbing J, et al. A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia. Br J Cancer. 2007;96:732–737. doi: 10.1038/sj.bjc.6603608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couder F, Massardier J, You B, et al. Predictive factors of relapse in low-risk gestational trophoblastic neoplasia patients successfully treated with methotrexate alone. Am J Obstet Gynecol. 2016;215:80.e1–80.e7. doi: 10.1016/j.ajog.2016.01.183. [DOI] [PubMed] [Google Scholar]
- 39.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124:2010–2017. doi: 10.1002/cncr.31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 41.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 43.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 44.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 45.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]