Abstract
PURPOSE
Immune checkpoint inhibitors (ICIs) are standard therapy in metastatic renal cell carcinoma (RCC). The safety and activity of the combination of ipilimumab and nivolumab in patients who have received prior ICI targeting the programmed death 1 (PD-1) pathway remains unknown. We evaluated ipilimumab and nivolumab in patients with metastatic RCC after prior treatment with anti–PD-1 pathway–targeted therapy.
PATIENTS AND METHODS
Patients with metastatic RCC who received prior anti–PD-1 pathway-targeted therapy and subsequently received ipilimumab and nivolumab were reviewed. Objective response rate and progression-free survival per investigator assessment were recorded. Toxicity of ipilimumab and nivolumab was also assessed.
RESULTS
Forty-five patients with metastatic RCC were included. All patients (100%) received prior ICIs targeting the PD-1 pathway. The median age was 62 years (range, 21-82 years). At a median follow-up of 12 months, the objective response rate to ipilimumab and nivolumab was 20%. The median progression-free survival while on ipilimumab and nivolumab was 4 months (range, 0.8-19 months). Immune-related adverse events (irAEs) of any grade with ipilimumab and nivolumab were recorded in 29 (64%) of the 45 patients; grade 3 irAEs were recorded in 6 (13%) of the 45 patients.
CONCLUSION
Ipilimumab and nivolumab demonstrated antitumor activity with acceptable toxicity in patients with metastatic RCC who had prior treatment with checkpoint inhibition.
INTRODUCTION
Renal cell carcinoma (RCC) is an immune-responsive tumor, with immune-modulating agents as a component of standard systemic therapy. Before approval of vascular endothelial growth factor (VEGF) pathway inhibitors, cytokines, including interferon (IFN) and interleukin-2 (IL-2), were standard of care in patients with metastatic RCC, despite modest efficacy and significant toxicities.1,2 An improved understanding of immune response and the tumor microenvironment has led to the resurgence of immunotherapy in RCC.3 The recent development of immune checkpoint inhibitors (ICIs) targeting the programmed death 1 (PD-1) and cytotoxic T-cell lymphocyte antigen-4 (CTLA-4) pathways has led to significant advances in the treatment of metastatic RCC.3
CONTEXT
Key Objective
Immune checkpoint inhibitors are standard initial treatment in metastatic renal cell carcinoma (RCC). The clinical efficacy of subsequent checkpoint inhibitors in patients with RCC with prior exposure to programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors is unknown. This analysis reports the clinical outcome of patients with metastatic RCC who had prior treatment with PD-1/PD-L1 inhibitors and subsequently were treated with combination ipilimumab and nivolumab.
Knowledge Generated
Ipilimumab and nivolumab combination demonstrated objective responses in a subset of patients with metastatic RCC who had prior exposure to PD-1/PD-L1 inhibitors.
Relevance
Ipilimumab and nivolumab may be an effective salvage treatment option for select patients with metastatic RCC with prior treatment with PD-1/PD-L1 inhibitors. Additional investigation into this approach to define durability of response and benefit/risk is needed.
Nivolumab, an anti–PD-1 antibody, was initially approved as monotherapy after failure of prior VEGF pathway–directed therapy.4 More recently, the combination of nivolumab and ipilimumab, an anti–CTLA-4 antibody, was approved in the first-line setting for patients with International Metastatic Database Consortium (IMDC) intermediate- and poor-risk disease on the basis of objective response rate (ORR) and overall survival advantages compared with sunitinib.5 Furthermore, pembrolizumab, an anti–PD-1 antibody, as well as avelumab, an anti–programmed death-ligand 1 (PD-L1) antibody, each in combination with axitinib, a VEGF receptor inhibitor, were approved for the frontline treatment of metastatic RCC.6,7 Thus, ICI-based therapy is now standard initial therapy in patients with metastatic RCC. Despite these advances, most patients with metastatic RCC will progress. Upon progression, options include agents directed against the VEGF or mammalian target of rapamycin pathways. The benefit of subsequent checkpoint inhibition with ipilimumab and nivolumab in patients who had prior exposure to anti–PD-1 or anti–PD-L1 antibody and no prior exposure to an anti–CTLA-4 antibody is not well defined in RCC. A retrospective analysis of patients with metastatic RCC who received prior ICI that targeted the PD-1 pathway and no prior treatment with an anti–CTLA-4 antibody who were treated with salvage ipilimumab and nivolumab was conducted.
PATIENTS AND METHODS
Key eligibility criteria included metastatic RCC of any histology, age ≥ 18 years, at least 1 dose of prior ICI targeting the PD-1 pathway, and treatment with at least 1 subsequent dose of ipilimumab and nivolumab. Medical records were reviewed for the following characteristics: age, sex, histology, prior treatments, best response to prior ICI (defined as best response on any previous line of ICI therapy), median time on prior ICI (defined as the median of the cumulative time of all lines of ICI from the start of therapy until disease progression, discontinued because of unacceptable toxicity, or treatment change), and toxicities to prior ICI. Eastern Cooperative Oncology Group performance status, IMDC risk group, and sites of metastasis at the time of initiation of ipilimumab and nivolumab were also collected.
Study Design and Methods
The primary objective of this study was to estimate an ORR to salvage ipilimumab and nivolumab in patients with metastatic RCC who received ICI as prior treatment. The ORR was defined as the percentage of patients having a confirmed investigator-assessed best response of complete response or partial response (PR) according to RECIST version 1.1.8 Best objective response (BOR) was defined as the best response designation recorded compared with baseline between the first dose of ipilimumab and nivolumab and the date of documented progression per RECIST version 1.1 or last recorded follow-up. Secondary objectives of interest were progression-free survival (PFS), duration of response (DOR), duration of follow-up, and toxicities to ipilimumab and nivolumab. PFS was calculated from the date of ipilimumab and nivolumab initiation to investigator-assessed clinical or radiographic progression by RECIST version 1.1 or death as a result of any cause (whichever occurred first). Patients who were alive with no disease progression were censored at the date of the last visit. DOR was calculated for all treated patients who achieved a complete response or PR and defined as the time between dates of first response and disease progression or death, whichever occurred first. The patients who did not have disease progression or death at the time of data cutoff were censored at the time of last follow-up. Duration of follow-up was defined as the time from the date of first ipilimumab and nivolumab dose to the date of the last follow-up or documented date of death. Safety was assessed throughout the treatment with ipilimumab and nivolumab, and the severity of adverse events (AEs) was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). AEs that led to ipilimumab and nivolumab discontinuation, treatment delays, specific intervention treatment, and need for corticosteroids were collected. Numerical variables were summarized with median and range; categorical variables were summarized with frequencies and percentages.
RESULTS
Baseline Characteristics
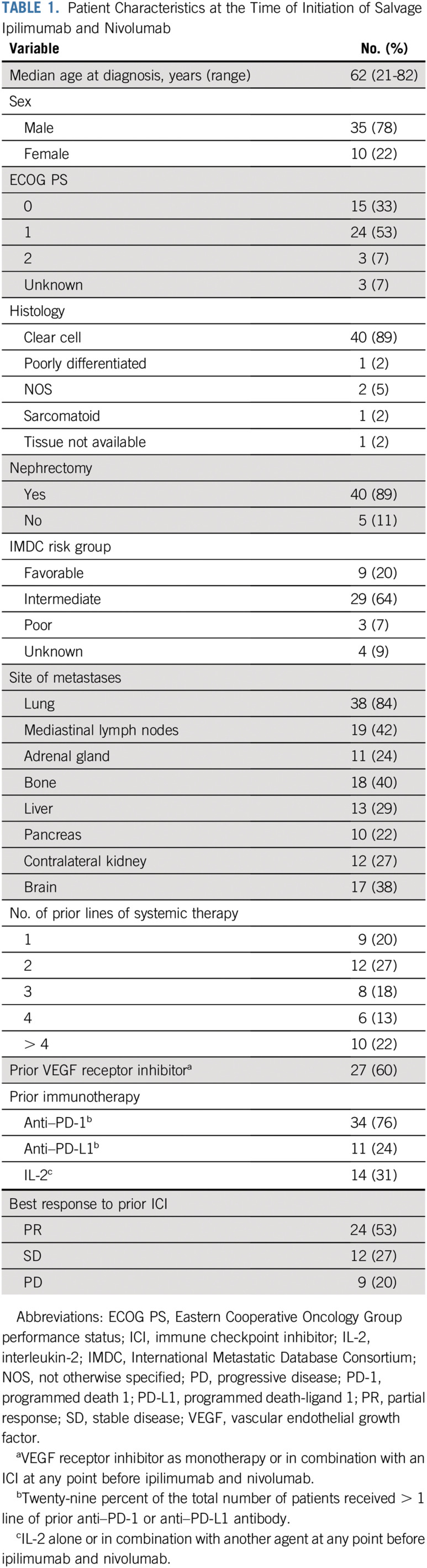
Forty-five patients with metastatic RCC from 5 medical centers in the United States who received prior ICI targeting the PD-1 pathway and who received at least one dose of salvage ipilimumab and nivolumab were included. The median age at the time of initiation of ipilimumab and nivolumab was 62 years (range, 21-82 years; Table 1). All patients had more than one metastatic site, and 38% had brain metastasis. Twenty percent of the patients were favorable risk on the basis of IMDC criteria, 64% were intermediate risk, 7% were poor risk, and 9% were unknown risk because of missing data.
TABLE 1.
Patient Characteristics at the Time of Initiation of Salvage Ipilimumab and Nivolumab
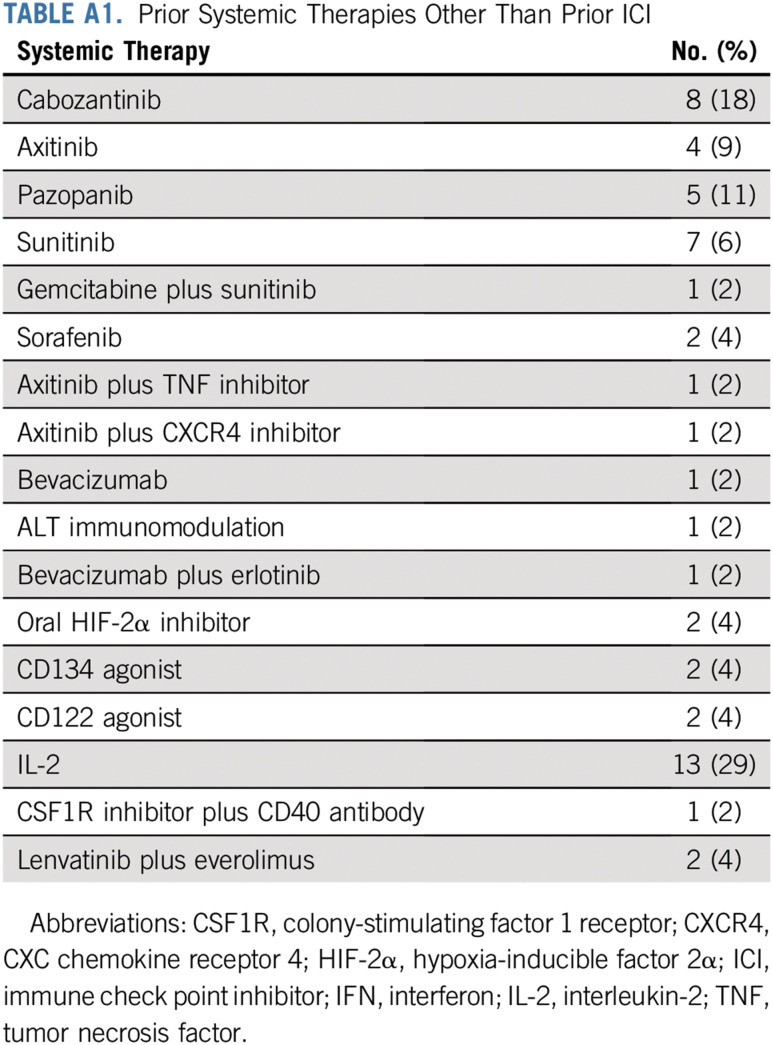
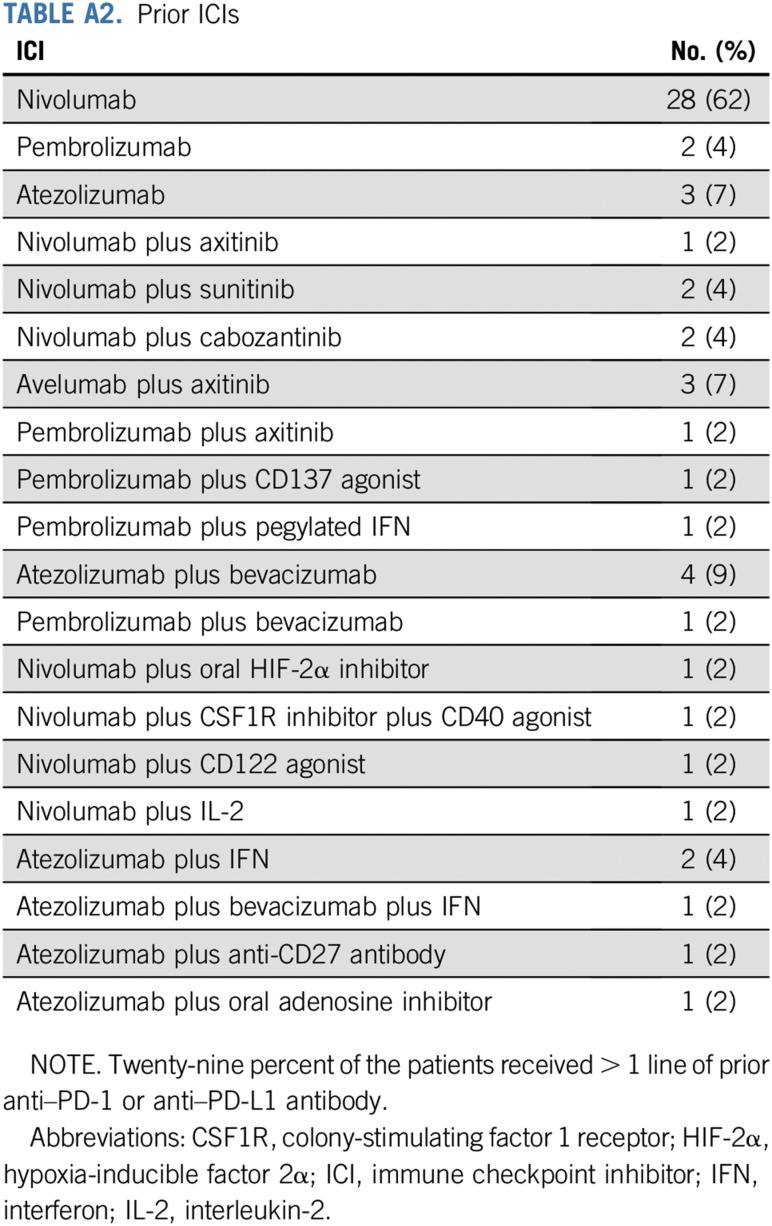
The median number of prior lines of therapy was 3 (range, 1-7 lines; Appendix Table A1, online only). All patients (100%) received at least one prior therapy targeting the PD-1 pathway; of these, 76% received an anti–PD-1 antibody, and 24% received an anti–PDL-1 antibody before ipilimumab and nivolumab. Of the 45 patients, 27 (60%) received monotherapy with prior anti–PD-1 or anti–PDL-1 antibody, 8 (18%) received an ICI that targeted the PD-1 pathway in combination with a VEGF receptor inhibitor (axitinib, sunitinib, or cabozantinib), 4 (9%) received an ICI in combination with bevacizumab, and 6 (13%) received an ICI in combination with another agent (oral hypoxia-inducible factor 2α inhibitor, CD122 agonist, IFN, anti-CD27 antibody, oral adenosine inhibitor, or combination of bevacizumab and IFN). The median number of prior ICI therapies targeting the PD-1 pathway was 1, with 32 (71%) of the 45 patients receiving one line of prior ICI therapy and 13 (29%) of the 45 patients having received more than one prior ICI regimen (Appendix Table A2). The BOR to prior ICI was PR in 53%, stable disease (SD) in 27%, and progressive disease (PD) in 20% (Table 1). The median time on prior ICIs was 13 months (range, 1-75 months). Immune-related AEs (irAEs) of any grade on prior ICIs were recorded in 15 (33%) of the 45 patients. The most common irAEs of any grade were arthralgia (9%), hepatotoxicity (7%), and diarrhea (4%). Grade 3 irAEs, including hyponatremia, hepatotoxicity, and thrombocytopenia, were recorded in 3 (7%) of the 45 patients.
Clinical Outcome to Ipilimumab and Nivolumab
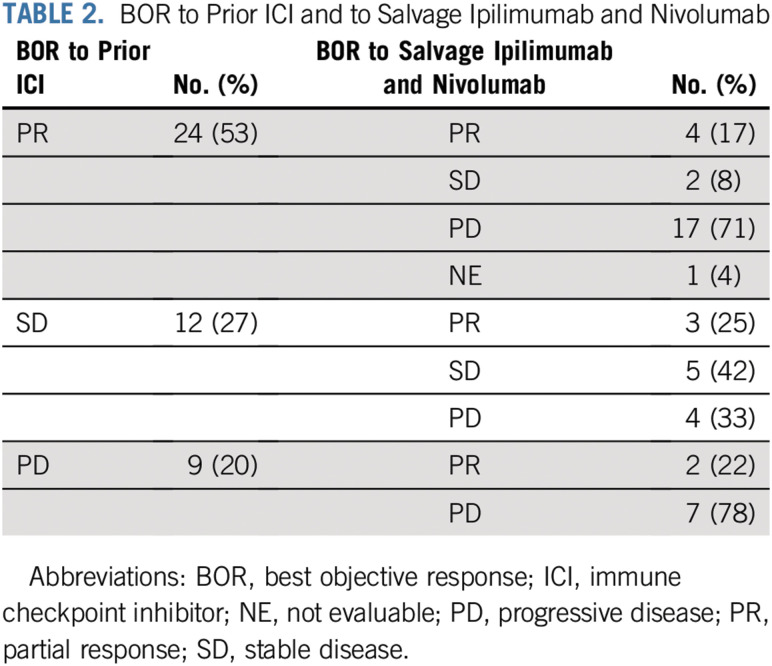
A total of 45 patients were considered evaluable for treatment response to salvage ipilimumab and nivolumab. The median duration of follow-up was 12 months (range, 0.8-21 months). The ORR to ipilimumab and nivolumab was 20%; 9 patients (20%) had PR, 7 (16%) had SD, and 28 (62%) had PD. One patient (2%) was lost to follow-up after receiving 4 cycles of induction ipilimumab and nivolumab and, therefore, was not evaluable (NE). The median PFS was 4 months (range, 0.8-19 months), and the median DOR was 7 months (range, 2-17 months). Among the 9 patients who had PR, 6 (67%) had an ongoing response, and 5 (55%) were receiving maintenance nivolumab at the time of data analysis. The best response to salvage ipilimumab and nivolumab according to best response to prior ICI is listed in Table 2.
TABLE 2.
BOR to Prior ICI and to Salvage Ipilimumab and Nivolumab
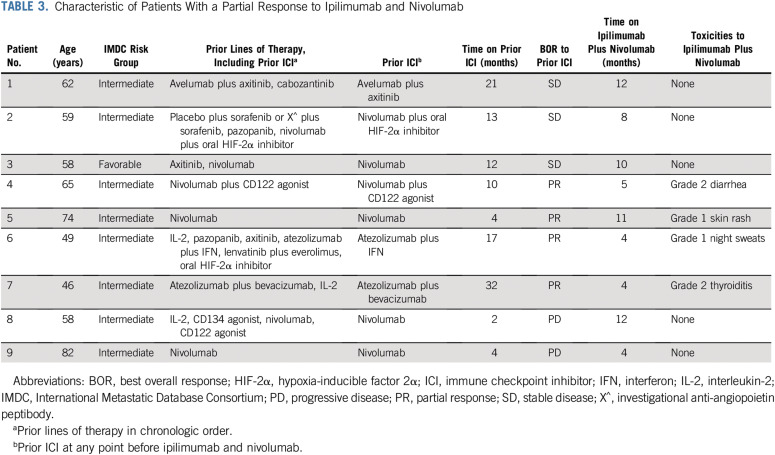
Twenty-three patients received monotherapy with ICI immediately before ipilimumab and nivolumab; of these, 3 (13%) had PR, 13 (57%) had PD, 6 (26%) had SD, and 1 (4%) was NE as best response to ipilimumab and nivolumab. All 5 patients (100%) who received ICI in combination with VEGF receptor inhibitor immediately before ipilimumab and nivolumab had PD as best response to ipilimumab and nivolumab. Of the 6 patients who received monotherapy with VEGF receptor inhibitor immediately before ipilimumab and nivolumab, 5 (83%) had PD, and 1 (17%) had PR as best response to salvage ipilimumab and nivolumab. The clinical characteristics of the patients who had a PR to salvage ipilimumab and nivolumab are listed in Table 3.
TABLE 3.
Characteristic of Patients With a Partial Response to Ipilimumab and Nivolumab
The median time between the last dose of prior ICI and initiation of salvage ipilimumab and nivolumab was 2.8 months (range, 0.7-21 months) in patients who had a PR, 5 months (range, 0.4-64 months) in those who had PD as best response, and 0.7 months (range, 0.5-13 months) who had SD as best response to salvage ipilimumab and nivolumab. Of the 31 patients who were IL-2 naïve before ipilimumab and nivolumab, the BOR to ipilimumab and nivolumab was PR in 7 (23%), SD in 5 (16%), PD in 18 (58%), and 1 patient (3%) was NE. Of the 14 patients who were exposed to IL-2 before ipilimumab and nivolumab, the BOR to ipilimumab and nivolumab was PR in 2 (14%), SD in 2 (14%), and PD in 10 (72%).
Safety of Ipilimumab and Nivolumab
The median time on ipilimumab and nivolumab was 4 months (range, 0.7-19 months). A total of 31 patients (69%) received all 4 doses of induction ipilimumab and nivolumab. The induction phase of ipilimumab and nivolumab was completed in 23 (51%) of the 45 patients without delay or treatment interruption. Treatment was delayed in 4 patients (9%) and discontinued in 6 (13%) because of irAEs. Four patients (9%) had interruption in treatment because of non–treatment-related AEs. One patient developed splenic rupture 1 week after the first cycle of ipilimumab and nivolumab secondary to a splenic artery aneurysm and underwent surgical management of the aneurysm; treatment with ipilimumab and nivolumab was later resumed without issue. One patient underwent stereotactic radiosurgery for metastatic brain lesions after the first cycle of ipilimumab and nivolumab, and treatment with ipilimumab and nivolumab was later resumed. Treatment was delayed in 2 patients because of infection. Treatment with ipilimumab and nivolumab was discontinued in 5 patients (11%) because of PD, and 3 patients (7%) died as a result of PD during the induction phase.
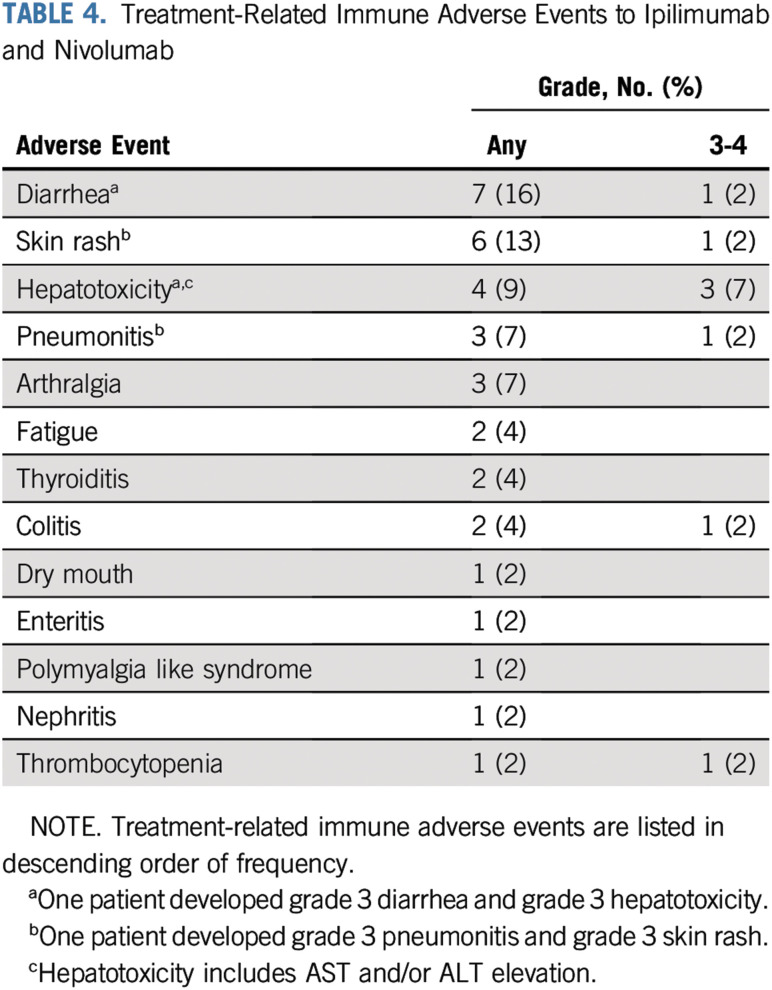
irAEs for ipilimumab and nivolumab of any grade were recorded in 29 (64%) of the 45 patients, with 6 (13%) reporting grade 3-4 irAEs (Table 4). The most common irAEs of any grade were diarrhea (16%), rash (13%), hepatotoxicity (9%), and pneumonitis (7%). The most common grade 3-4 irAEs were hepatotoxicity (7%) and pneumonitis, rash, diarrhea, thrombocytopenia, and colitis (2% each). Because of irAEs, 17 (38%) of the 45 patients received systemic corticosteroids (of any dose), and 9 (20%) received high-dose corticosteroids (defined as ≥ 40 mg of prednisone equivalent daily for 2 weeks). One patient (2%) was treated with infliximab for grade 3 colitis. There were no treatment-related deaths directly attributable to ipilimumab and nivolumab.
TABLE 4.
Treatment-Related Immune Adverse Events to Ipilimumab and Nivolumab
DISCUSSION
Ipilimumab and nivolumab is now standard initial therapy in IMDC intermediate- and poor-risk metastatic RCC. In addition, pembrolizumab as well as avelumab in combination with axitinib are approved initial options in first-line treatment of metastatic RCC. Despite significant clinical activity, a substantial percentage of patients who receive prior anti–PD-1 pathway–targeted therapy will eventually progress and require further systemic therapy. The role of additional checkpoint inhibition with ipilimumab and nivolumab in patients with prior exposure to anti–PD-1 pathway–targeted therapy but no prior exposure to anti–CTLA-4 pathway–targeted therapy remains undefined.
The current series demonstrates that objective PRs with salvage ipilimumab and nivolumab are possible in a subset of patients with metastatic RCC who are naïve to anti–CTLA-4 antibody and had prior exposure to therapy targeting the PD-1 pathway. Ipilimumab and nivolumab have been evaluated in previously treated patients with metastatic RCC.9 Approximately one third of the patients enrolled in CheckMate 016 had prior treatment with cytokines, and 19% had prior VEGF receptor inhibitor treatment, but none of the patients had prior exposure to ICIs. The ORR to ipilimumab and nivolumab was 40%, which demonstrates that ipilimumab and nivolumab are effective in patients previously treated with a cytokine or a VEGF receptor inhibitor.
Ipilimumab and nivolumab act in different phases of the immune response. CTLA-4 blockade affects the immune priming phase and induces the proliferation of effector T cells, regardless of T-cell receptor specificity.10 PD-1 blockade works during the effector phase and restores the immune function of T cells, which are exhausted as a result of high antigen exposure, as in advanced cancers.11,12 CTLA-4 blockade induces a proliferative gene expression signature predominantly in a subset of transitional memory T cells, whereas PD-1 blockade causes changes in genes involved in cytolysis and natural killer cell function.13 The differences in timing, site of action, and nonoverlapping changes in genes involved in antitumor response by anti–CTLA-4–targeted antibodies and anti–PD-1 antibodies have the potential of additive or synergistic effects in the treatment of advanced cancers.13 Thus, the mechanistic differences between an anti–CTLA-4 antibody and an anti–PD-1 antibody may allow activity of anti–CTLA-4 antibody in combination with anti–PD-1 antibody upon failure of prior anti–PD-1 pathway–targeted therapy.
Salvage ipilimumab and nivolumab therapy after single-agent nivolumab is currently being evaluated in multiple clinical trials. The TITAN trial enrolled patients with treatment-naïve advanced RCC with a clear cell component to upfront nivolumab therapy with the addition of ipilimumab in patients who have PD or SD.14 This study demonstrated an approximately 10% increase in ORR with the addition of ipilimumab to nivolumab. Two additional prospective trials, HCRN GU16-260 (ClinicalTrials.gov identifier: NCT03117309) and OMNIVORE (ClinicalTrials.gov identifier: NCT03203473) are evaluating the clinical outcome of the addition of ipilimumab in patients with metastatic RCC who do not achieve an objective response to nivolumab monotherapy. However, these studies enroll patients in the first-line setting and exclude patients exposed to prior ICIs. Of note, a subset of patients in this series with lack of an objective response to prior ICI-based therapy achieved a PR to salvage ipilimumab and nivolumab.
The percentage of patients who achieved a PR to salvage ipilimumab and nivolumab was not different on the basis of response to prior ICI regimens, which suggests that susceptibility to a CTLA-4 inhibitor–containing regimen is distinct from factors affecting response to regimens targeting the PD-1 pathway. A related observation was that median time between prior ICI and salvage ipilimumab and nivolumab initiation was shorter in responders to salvage ipilimumab and nivolumab compared with patients with PD, which raises the hypothesis that immediate treatment with a combination of anti–PD-1 pathway–targeted antibody and anti–CTLA-4 antibody may be associated with improved response.
Of note, the majority of patients in this study who received VEGF receptor inhibitor therapy immediately before ipilimumab and nivolumab did not have an objective response to salvage ipilimumab and nivolumab. Although limited by small numbers, this observation raises hypotheses about the immunomodulatory effects of immediate prior VEGF pathway inhibition in this setting.
This analysis has several limitations, including small sample size, the inherent bias in a retrospective analysis, lack of central blinded independent review to assess response to treatment, relatively short follow-up, and incomplete data collection in some patients. Of note, patients in this cohort were not exposed to prior ipilimumab and nivolumab, and few patients had ICI plus VEGF receptor inhibitor combination therapy, which are now the standard initial therapy in metastatic RCC. Thus, the utility of salvage ipilimumab and nivolumab in patients receiving one of these ICI-based combinations is largely unknown. The current data may be more relevant to patients who receive ICI monotherapy either after VEGF pathway targeted therapy in the frontline setting or as is being investigated in the adjuvant setting.
This series demonstrates that patients with metastatic RCC can achieve objective responses to salvage ipilimumab and nivolumab after prior anti–PD-1 pathway–targeted therapy. It is important to note that although a small proportion of patients had an objective response to salvage ipilimumab and nivolumab, the responses to salvage ipilimumab and nivolumab were durable in the majority of responders, and several patients derived clinical benefit even without achieving RECIST-defined PR. However, the lack of complete responses, high rate of PD, and the potential for toxicity provide caution to the application of these data to select patients. Prospective clinical trials are needed to further define the clinical activity of checkpoint inhibition after previous ICI exposure in metastatic RCC.
Appendix
TABLE A1.
Prior Systemic Therapies Other Than Prior ICI
TABLE A2.
Prior ICIs
Footnotes
Processed as a Rapid Communication manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Anita Gul, Neil J. Shah, Moshe C. Ornstein, Hans J. Hammers, Michael Hurwitz, Brian I. Rini
Financial support: Brian I. Rini
Administrative support: Brian I. Rini
Provision of study material or patients: Anita Gul, Charlene M. Mantia, Ying Long, Hans J. Hammers, David F. McDermott, Michael B. Atkins, Michael Hurwitz, Brian I. Rini
Collection and assembly of data: Anita Gul, Tyler F. Stewart, Charlene M. Mantia, Neil J. Shah, Emily Stern Gatof, Ying Long, Kimberly D. Allman, Hans J. Hammers, David F. McDermott, Michael B. Atkins, Michael Hurwitz, Brian I. Rini
Data analysis and interpretation: Anita Gul, Tyler F. Stewart, Charlene M. Mantia, Neil J. Shah, Moshe C. Ornstein, Hans J. Hammers, David F. McDermott, Michael B. Atkins, Brian I. Rini
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Salvage Ipilimumab and Nivolumab in Patients With Metastatic Renal Cell Carcinoma After Prior Immune Checkpoint Inhibitors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anita Gul
Speakers’ Bureau: Dava Oncology
Neil J. Shah
Honoraria: Castle Biosciences
Emily Stern Gatof
Stock and Other Ownership Interests: WS Investments Company (I)
Consulting or Advisory Role: Wilson Sonsini Goodrich & Rosati (I)
Travel, Accommodations, Expenses: Wilson Sonsini Goodrich & Rosati (I)
Kimberly D. Allman
Consulting or Advisory Role: Exelixis, Bayer AG, AstraZeneca
Speakers’ Bureau: Exelixis, Amgen
Travel, Accommodations, Expenses: Exelixis, Bayer AG, Amgen, AstraZeneca
Moshe C. Ornstein
Consulting or Advisory Role: Pfizer, Eisai, Exelixis
Speakers’ Bureau: Exelixis, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Exelixis, Bristol-Myers Squibb
Hans J. Hammers
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Exelixis, Bayer AG, Novartis, Merck, ARMO BioSciences, Corvus Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck, Pfizer, Eli Lilly, Novartis
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, Roche, Pfizer, Exelixis, Novartis, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Eli Lilly
Research Funding: Prometheus Laboratories (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Novartis (Inst), Alkermes (Inst), Peloton Therapeutics (Inst)
Other Relationship: Beth Israel Deaconess Medical Center
Uncompensated Relationships: X4 Pharmaceuticals, AVEO Oncology
Michael B. Atkins
Honoraria: Merck
Consulting or Advisory Role: Genentech, Pfizer, Novartis, X4 Pharma, Bristol-Myers Squibb, Merck, Exelixis, Eisai, Glactone Pharma, Agenus, Array BioPharma, Arrowhead Pharmaceuticals, Werewolf Pharma, Oncolys BioPharma, Surface Oncology, Iovance Biotherapeutics, Immunocore, Pyxis, Pneuma, Leads Biolabs, Fathom, AVEO Oncology, Cota Healthcare, Neoleukin
Research Funding: Bristol-Myers Squibb (Inst)
Michael Hurwitz
Employment: Pfizer (I), Gamida Cell (I), Arvinas (I)
Consulting or Advisory Role: Nektar, Janssen Pharmaceuticals, CRISPR Therapeutics
Research Funding: Apexigen, Astellas Pharma, AstraZeneca, MedImmune, Bayer AG, Bristol-Myers Squibb, Corvus Pharmaceuticals, Eli Lilly, Endocyte, Genentech, Genmab, Arvinas, Innocrin Pharma, Iovance Biotherapeutics, Merck, Nektar, Novartis, Pfizer, Progenics, Sanofi, Aventis, Seattle Genetics, Torque, Unum Therapeutics
Brian I. Rini
Leadership: MJH Life Sciences
Consulting or Advisory Role: Pfizer, Merck, Roche, Genentech, Synthorx, Bristol-Myers Squibb, AVEO Oncology, Surface Oncology, 3D Medicines
Research Funding: Pfizer (Inst), Roche (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), MedImmune (Inst), AVEO Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer, Bristol-Myers Squibb, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Raman R, Vaena D. Immunotherapy in metastatic renal cell carcinoma: A comprehensive review. BioMed Res Int. 2015;2015:367354. doi: 10.1155/2015/367354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009. [DOI] [PubMed]
- 9.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 Study. J Clin Oncol. 2017;35:3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 11.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 12. Chen DS, Irving BA, Hodi FS: Molecular pathways: Next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18:6580-6587, 2012. [DOI] [PubMed]
- 13. Das R, Verma R, Sznol M, et al: Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 194:950-959, 2015. [DOI] [PMC free article] [PubMed]
- 14. Grimm M-O, Schmidinger M, Duran Martinez I, et al: Tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Ann Oncol 30, 2019 (suppl; abstr LBA57)