Abstract
PURPOSE
To develop a clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation (HSCT) recipients.
METHODS
Recommendations were developed by an international multidisciplinary panel that included a patient advocate. We conducted a systematic review of systemic antifungal prophylaxis in children and adults with cancer and HSCT recipients. The Grading of Recommendations Assessment, Development, and Evaluation approach was used to make strong or weak recommendations and to classify level of evidence as high, moderate, low, or very low. The panel considered directness of the data to pediatric patients.
RESULTS
There were 68 randomized trials included in the systematic review, of which 6 (9%) were conducted in a solely pediatric population. Strong recommendations were made to administer systemic antifungal prophylaxis to children and adolescents receiving treatment of acute myeloid leukemia, to those undergoing allogeneic HSCT pre-engraftment, and to those receiving systemic immunosuppression for graft-versus-host disease treatment. A strong recommendation was made to administer a mold-active agent with an echinocandin or a mold-active azole when systemic antifungal prophylaxis is warranted. For children younger than 13 years of age, an echinocandin, voriconazole, or itraconazole is suggested. Posaconazole may also be used in those age 13 years or older. A strong recommendation against routine administration of amphotericin as systemic antifungal prophylaxis was made.
CONCLUSION
We developed a clinical practice guideline for systemic antifungal prophylaxis administration in pediatric patients with cancer and HSCT recipients. Implementation and assessment of guideline-concordant rates and impacts are important future steps.
INTRODUCTION
Children and adolescents receiving intensive myelosuppressive chemotherapy and some pediatric hematopoietic stem-cell transplantation (HSCT) recipients are at high risk for invasive fungal disease (IFD) caused by yeasts and molds.1-4 In these patients, infections with Candida and Aspergillus species are most common.1-3 IFDs are important because they are associated with substantial morbidity, delayed cancer treatment, increased health services utilization, and treatment-related mortality.5
CONTEXT
Key Objectives
Which pediatric patients with cancer and hematopoietic stem-cell transplantation (HSCT) recipients should routinely receive systemic antifungal prophylaxis?
Knowledge Generated
Based on a systematic review, an international multidisciplinary guideline panel recommended systemic antifungal prophylaxis be administered to children and adolescents receiving treatment of acute myeloid leukemia, to those undergoing allogeneic HSCT pre-engraftment, and to those receiving systemic immunosuppression for graft-versus-host disease treatment. When systemic antifungal prophylaxis is warranted, an echinocandin or a mold-active azole should be used.
Relevance
This clinical practice guideline for systemic antifungal prophylaxis is important because of the impact of invasive fungal disease in pediatric patients with cancer and HSCT recipients and because of the presence of multiple approaches to invasive fungal disease prophylaxis, including no prophylaxis.
Systemic antifungal prophylaxis can be an effective approach to reducing IFD. A clinical practice guideline (CPG) facilitates evidence-based clinical care by describing risks and benefits of different management options based on a systematic review of the literature. Risks and benefits are weighed against each other by a panel of experts to arrive at care recommendations. Previously published CPGs6,7 addressing systemic antifungal prophylaxis in pediatric patients are > 5 years old and thus do not consider results of recent trials. In addition, those panels had limited regional and disease representation. Key representatives of two previously published CPGs for pediatric patients (T.L., E.C., L.L.D., A.H.G., E.R., M.S., A.W., P.D.R., and L.S.)6,7 were brought together to arrive at a single harmonized CPG, thus improving consistency of recommendations internationally. The objective was to develop a CPG for systemic antifungal prophylaxis in pediatric patients with cancer and HSCT recipients.
METHODS
Panel Constitution
The panel included representatives from the fields of pediatric hematology/oncology, pediatric HSCT, pediatric infectious diseases, nursing, and pharmacy; a patient advocate; and a CPG methodologist (Data Supplement). Panel members were selected based on clinical and methodologic expertise and geographic representation. All panel members declared potential conflicts of interest, and none precluded participation in this CPG (Data Supplement).
General CPG Development Approach
We used standard approaches to create this CPG,8 including the Appraisal of Guidelines for Research and Evaluation II instrument, to direct development.9 Financial support for CPG creation was provided by the Pediatric Oncology Group of Ontario. However, CPG development, drafting of recommendations and the manuscript, and the decision to submit for publication were independent from the funder.
The key clinical questions were developed by the panel and are listed in Table 1. The target population is children and adolescents (age 0-18 years) receiving chemotherapy for cancer or undergoing HSCT. Target users are physicians, microbiologists, nurse practitioners, nurses, pharmacists, antibiotic stewards, and other health care professionals who are concerned with infectious complications in pediatric patients receiving chemotherapy or undergoing HSCT.
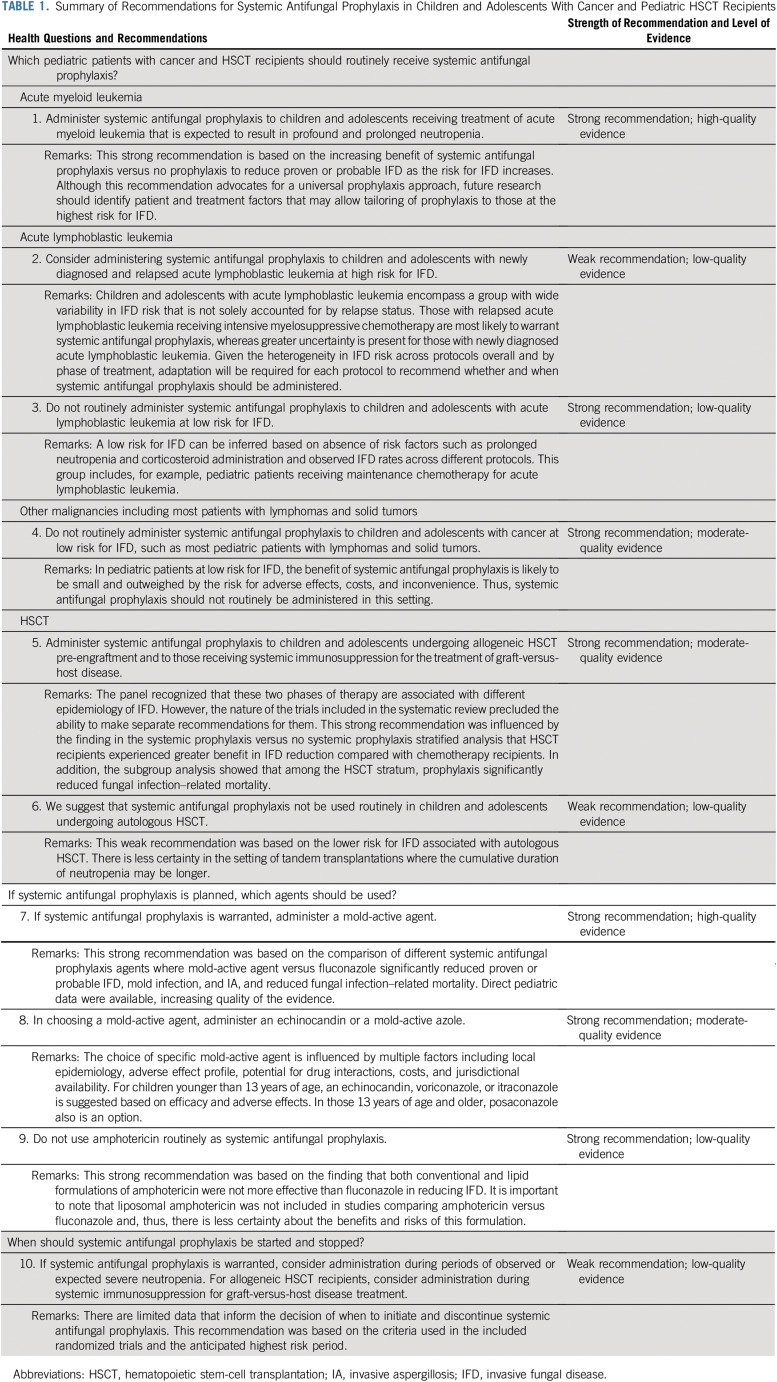
TABLE 1.
Summary of Recommendations for Systemic Antifungal Prophylaxis in Children and Adolescents With Cancer and Pediatric HSCT Recipients
Panel members identified and rated the importance of outcomes by consensus. Outcomes that were considered critical for decision making were proven or probable IFD, mold infection or yeast infection, fungal infection–related mortality, and overall mortality. Outcomes considered important were drug-related adverse effects and antifungal resistance. Empirical antifungal therapy was not considered important and, thus, not evaluated. Because changing diagnostic technologies can influence possible IFD events, possible IFD was included post hoc as an outcome.
To rate the level of evidence and to formulate recommendations, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used.10 The level of evidence indicates the degree of certainty that estimates from the systematic review reflect effects of prophylaxis in our target population, namely pediatric patients with cancer and HSCT recipients. Evidence was rated as high, moderate, low, or very low. Rating was downgraded if there were limitations in study design, lack of consistency, or imprecision or if direct data were lacking. Considering the level of evidence, strong or weak recommendations were made. Strong recommendations were made where benefits clearly outweighed the risks or vice versa and, thus, patients should receive or not receive the intervention as a general policy. Weak recommendations were made where the benefits and risks were closely matched or where there was uncertainty in their estimates. Efficacy, toxicity, and resources, including costs, influenced recommendation formulation.
Searching, Selecting, and Describing the Evidence
The evidence base used to create this CPG was founded on randomized clinical trials because they are generally less susceptible to bias in comparison with observational trials.11 The literature search was performed with the assistance of a library scientist in the following databases: MEDLINE, MEDLINE In-Process, MEDLINE Epub Ahead of Print, and Embase. The Data Supplement shows the full search strategy. Inclusion criteria were fully published randomized trials with a parallel group design that compared the administration of a systemic antifungal agent to any control group as prophylaxis. At least 90% of study participants had to be patients with cancer receiving chemotherapy or HSCT recipients. There was no restriction by language. We excluded studies of ketoconazole because both the US Food and Drug Administration and the European Medicines Agency have warned against its systemic use as a result of the risk for hepatic toxicities, adrenal suppression, and drug interactions.12,13 The search included studies published from January 1, 1980, to November 18, 2019.
The primary outcome of the systematic review was proven or probable IFD. Other outcomes were proven or probable mold infection, proven or probable invasive aspergillosis (IA), proven or probable yeast infection, overall mortality, fungal infection–related mortality, and discontinuation of antifungal prophylaxis as a result of an adverse effect. For all IFD outcomes (namely IFD, mold infection, IA, and yeast infection), if the study used the European Organisation for Research and Treatment in Cancer (EORTC)/Mycosis Study Group criteria for categorization, proven or probable IFD outcomes were abstracted. If a study did not use the EORTC criteria, IFD outcomes were mapped to EORTC categories (2008 revised version)14 by four investigators (T.L., P.P., P.D.R., and L.S.) by consensus where possible. These outcomes were considered missing if mapping was not possible.
We compared systemic antifungal prophylaxis, mold-active prophylaxis, and non–mold-active prophylaxis (fluconazole) versus no systemic prophylaxis, both by group and then stratified by specific agent evaluated. We next compared different systemic antifungal prophylaxis agents focusing on mold-active agents (amphotericin, mold-active azole, or echinocandin) versus fluconazole as a group and then broken down by subcategories and specific agents. We also compared mold-active azole versus echinocandin.
Study and demographic characteristics were year of publication, country of study conduct, age of participants, cancer diagnosis or HSCT type, and number of randomly assigned participants. Study-level covariates were participant age group (adult, pediatric, or both), treatment group (chemotherapy only, HSCT only, or both chemotherapy and HSCT), and EORTC criteria use to classify IFD (yes or no). Age group was categorized as adult when all participants were older than 15 years of age, and it was categorized as pediatric when all participants were younger than 25 years of age or when the median or mean age was younger than 15 years. Agent dose and schedule, start and stop criteria, and use of therapeutic drug monitoring were also collected. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials was used.15
Titles and abstracts of articles identified by the systematic review were screened, and articles potentially meeting eligibility criteria were evaluated at full text. All steps, including screening, full text review, and data abstraction, were performed in duplicate (V.F., P.P., or P.D.R.). If disagreement occurred, it was resolved by consensus or with arbitration by a third reviewer (T.L. or L.S.). Agreement in study inclusion was described using the kappa statistic.16
Statistical Analysis
Data synthesis used the risk ratio (RR) with the 95% CI to describe prophylaxis effects. In this analysis, RR < 1 indicates that the intervention is better than control. The Mantel-Haenszel random effects model in Review Manager 5.3 (Cochrane Collaboration, Nordic Cochrane Centre, London, United Kingdom)17 was used to estimate treatment effects. Outcomes were synthesized where there were at least three studies for main analysis and where there were at least two studies for each stratum in stratified analysis. If the number of events was zero in both groups, that study was not included in synthesis, which is considered a standard approach in meta-analyses.15 If a study included more than two randomized groups, the control group weight was proportionately divided between the different intervention groups, and all intervention versus control comparisons were included in the meta-analysis.15 We also calculated the I2 value, which is the percentage of total variation across studies as a result of heterogeneity rather than chance.15
Stratified analysis focused on two comparisons and four outcomes to limit multiple testing. The two comparisons were systemic antifungal prophylaxis versus no systemic antifungal prophylaxis, and mold-active agent versus fluconazole. The four outcomes were proven or probable IFD, proven or probable mold infection, fungal infection–related mortality, and antifungal discontinuation as a result of adverse effect (only for mold-active agent v fluconazole comparison). Strata evaluated were study-level covariates and, in addition, the risk for IFD and mold in the control group (above and below the median value). P value for interaction (P int) was used to determine whether heterogeneity in the prophylaxis effect could be explained by study-level covariates; we did not focus on stratum-specific P values.15
Funnel plots were used to explore the possibility of publication bias when at least 10 studies were available for an analysis.15 Funnel plots are graphical displays of the effect measure on the x-axis and precision on the y-axis. An absence of studies in the right lower quadrant (for this specific analysis) may indicate publication bias. If there was a suggestion of publication bias, we used the trim and fill technique to describe its potential impact. In this event, we removed outlying studies (trim) and added hypothetical negative studies with equal weight (fill).15 Analysis used Review Manager 5.3.17
Formulating Recommendations, Assigning Quality of Evidence, and Manuscript Preparation
We drafted evidence tables based on the systematic review results. Recommendations were developed during a series of conference calls and an in-person meeting held in Lyon, France, on October 25, 2019. Deliberations also used the results of a recently published systematic review of risk factors for IFD.4 Draft versions of the recommendations and manuscript were circulated until approved by all authors. We used the peer-review mechanism as an efficient approach to external review. We plan to update this CPG in 5 years or sooner in the event of important new information.
RESULTS
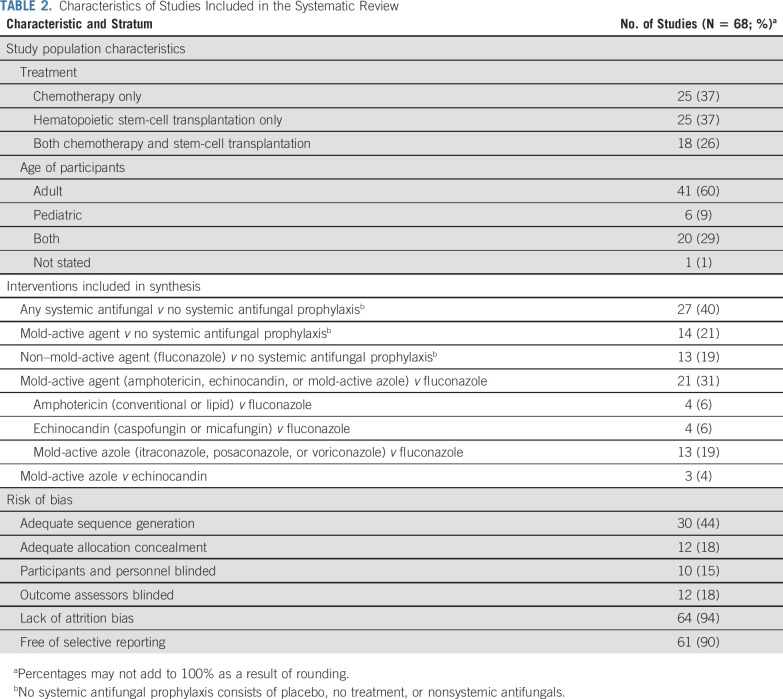
The search strategy identified 68 randomized trials that were included in the systematic review. Agreement in study inclusion between reviewers was perfect (κ = 1.00). The Data Supplement illustrates the flow diagram of study identification, selection, and reasons for exclusion. Health questions, recommendations, strength of recommendation, level of evidence, and remarks are summarized in Table 1. Characteristics of the included trials are listed in Table 2, and study-level details are provided in the Data Supplement. Among the five trials of voriconazole and six trials of posaconazole, none used therapeutic drug monitoring to systematically guide dosing. Six studies were conducted solely in a pediatric population (Data Supplement).
TABLE 2.
Characteristics of Studies Included in the Systematic Review
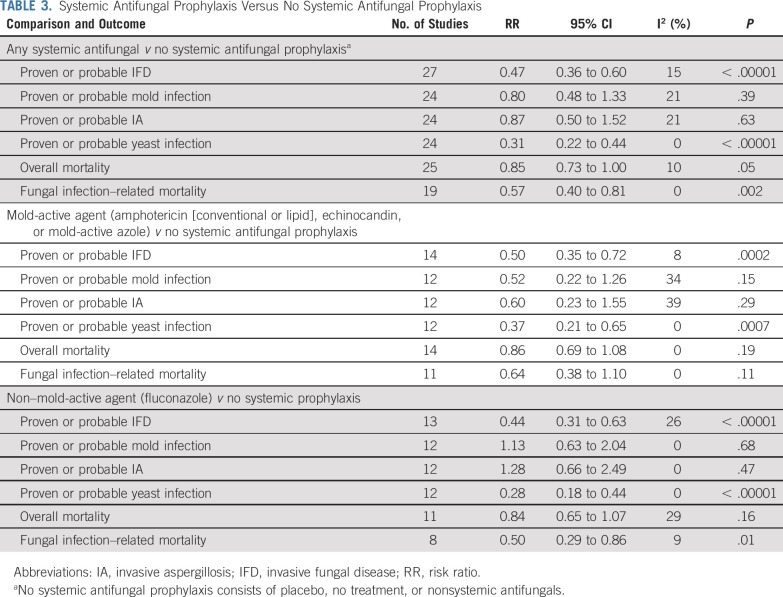
Table 3 provides comparisons between systemic antifungal prophylaxis versus no systemic antifungal prophylaxis for all studies and stratified by mold-active agent and non–mold-active agent (fluconazole). The Data Supplement further stratifies these analyses by agent evaluated, namely amphotericin (all formulations), lipid amphotericin formulations, and itraconazole. Compared with no systemic prophylaxis, systemic antifungal prophylaxis significantly reduced proven or probable IFD (RR, 0.47; 95% CI, 0.36 to 0.60; P < .00001), proven or probable yeast infection (RR, 0.31; 95% CI, 0.22 to 0.44; P < .00001), and fungal infection–related mortality (RR, 0.57; 95% CI, 0.40 to 0.81; P = .002). The effects of mold-active agent and non–mold-active agent versus no systemic antifungal prophylaxis were similar to the overall analysis for the reduction of IFD, yeast infection, and fungal infection–related mortality. The Data Supplement shows stratified analyses of comparisons between systemic antifungal prophylaxis versus no systemic prophylaxis for the outcomes of proven or probable IFD, proven or probable mold infection, and fungal infection–related mortality. For the outcome of proven or probable IFD, significantly greater benefit was observed with increasing risk for IFD in the control group, both when the risk was dichotomized (P int = .03; Data Supplement) and when the risk was divided into quartiles (P int = .0009; Data Supplement). More specifically, the effect of prophylaxis was an RR of 0.72 (95% CI, 0.43 to 1.20) for those in the lowest quartile (smallest risk for IFD) compared with an RR of 0.25 (95% CI, 0.17 to 0.36) for those in the highest quartile (greatest risk for IFD). Benefit was also significantly greater in patients receiving HSCT only compared with chemotherapy only or chemotherapy plus HSCT (P int = .03). The effect of prophylaxis did not differ based on risk for mold infection in the control group (P int = .90). We also performed an analysis of amphotericin (conventional or lipid formulations) versus no systemic antifungal prophylaxis stratified by daily dosing versus nondaily dosing. Effects on IFD and fungal infection–related mortality were similar when comparing daily and nondaily dosing (Data Supplement).
TABLE 3.
Systemic Antifungal Prophylaxis Versus No Systemic Antifungal Prophylaxis
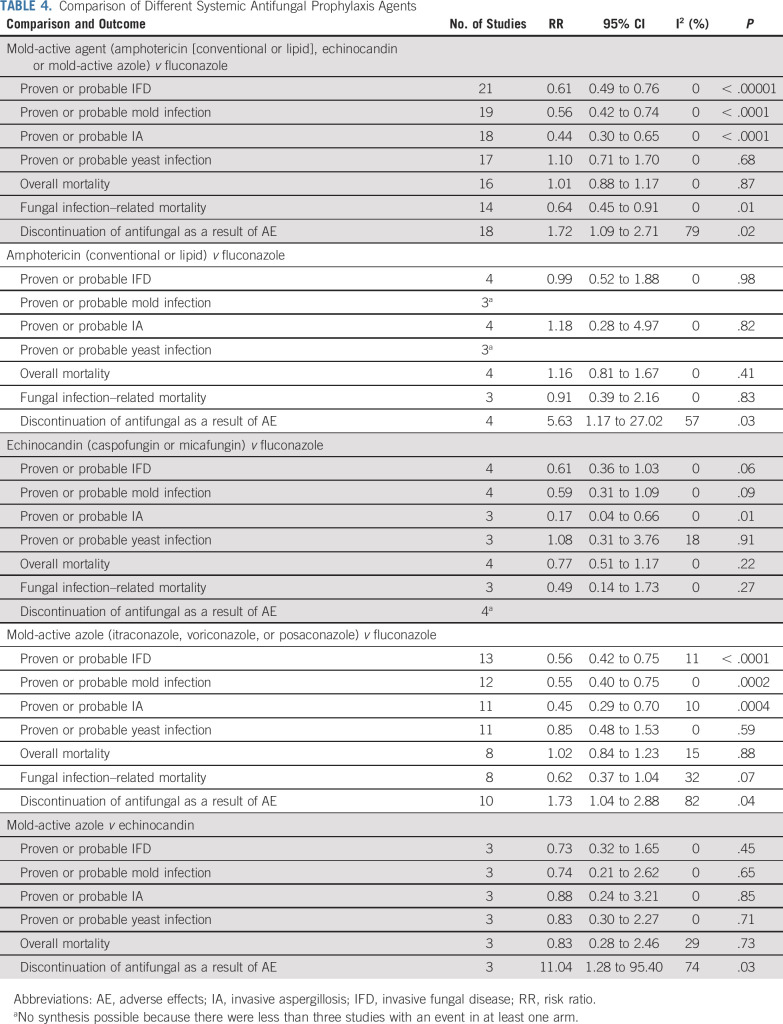
Table 4 summarizes the comparisons of different systemic antifungal prophylaxis groups, with additional comparisons by subcategory and agents shown in the Data Supplement. Use of a mold-active agent (amphotericin [conventional or lipid formulations], echinocandin, or mold-active azole), when compared with fluconazole, significantly reduced proven or probable IFD (RR, 0.61; 95% CI, 0.49 to 0.76; P < .00001), proven or probable mold infection (RR, 0.56; 95% CI, 0.42 to 0.74; P < .0001), proven or probable IA (RR, 0.44; 95% CI, 0.30 to 0.65; P < .0001), and fungal infection–related mortality (RR, 0.64; 95% CI, 0.45 to 0.91; P = .01). However, use of a mold-active agent significantly increased discontinuation of antifungal prophylaxis as a result of an adverse effect (RR, 1.72; 95% CI, 1.09 to 2.71; P = .02). When stratified by type of antifungal, amphotericin (conventional or lipid formulations) did not reduce proven or probable IFD (RR, 0.99; 95% CI, 0.52 to 1.88; P = .98) but did significantly increase discontinuation of antifungal prophylaxis as a result of an adverse effect (RR, 5.63; 95% CI, 1.17 to 27.02; P = .03). In contrast, the benefits of echinocandin versus fluconazole and mold-active azole versus fluconazole in reducing proven or probable IFD, proven or probable mold infection, and proven or probable IA were similar to the overall comparison of mold-active agent versus fluconazole. Table 4 also shows that mold-active azole, when compared with echinocandin, did not have a statistically significant different effect on IFD, mold infection, IA, or yeast infection, but did significantly increase discontinuation of antifungal prophylaxis as a result of an adverse effect. The Data Supplement shows stratified analyses of mold-active agent versus fluconazole; no significant interactions were observed.
TABLE 4.
Comparison of Different Systemic Antifungal Prophylaxis Agents
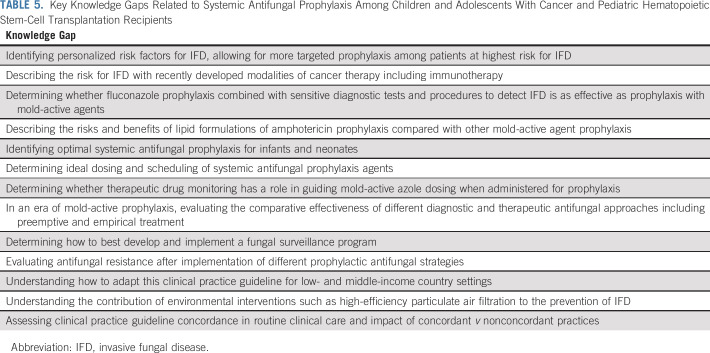
The Data Supplement shows systemic antifungal prophylaxis initiation and discontinuation criteria by diagnosis or treatment group and also illustrates sensitivity analyses where publication bias was suggested in funnel plots. None substantially altered interpretation of the base analyses. In addition, the Data Supplement shows synthesis in which possible IFD, mold, or IA was reported. These did not substantially alter interpretation of the base analysis. Research gaps are outlined in Table 5.
TABLE 5.
Key Knowledge Gaps Related to Systemic Antifungal Prophylaxis Among Children and Adolescents With Cancer and Pediatric Hematopoietic Stem-Cell Transplantation Recipients
Recommendation 1
Administer systemic antifungal prophylaxis to children and adolescents receiving treatment of acute myeloid leukemia that is expected to result in profound and prolonged neutropenia (Evidence quality: high; Strength of recommendation: strong).
Explanation.
This recommendation was informed by the systematic review of risk factors identifying that patients with acute myeloid leukemia are at high risk for IFD. The benefit of systemic antifungal prophylaxis was greater for those at higher risk for proven or probable IFD, leading to this strong recommendation.
Recommendation 2
Consider administering systemic antifungal prophylaxis to children and adolescents with newly diagnosed and relapsed acute lymphoblastic leukemia at high risk for IFD (Evidence quality: low; Strength of recommendation: weak).
Explanation.
The risk for IFD in pediatric acute lymphoblastic leukemia is protocol and phase specific. This risk is also dependent on remission status; chemotherapy-related neutropenia; and corticosteroid formulation, dose, and duration of administration. On the basis of the systematic review of risk factors for IFD,4 the panel believed that there are likely to be subgroups of pediatric patients with acute lymphoblastic leukemia who would benefit from systemic antifungal prophylaxis. However, the panel was unable to identify comprehensive baseline data on IFD incidence in the various acute lymphoblastic leukemia populations that would permit more specific recommendations. The panel also acknowledged that treatments for poor-risk acute lymphoblastic leukemia are changing. For example, immunotherapies are being used increasingly and may be associated with a lower risk for IFD compared with conventional, myelosuppressive chemotherapy. Given these factors, the panel did not make a strong recommendation for antifungal prophylaxis in pediatric patients with acute lymphoblastic leukemia. Rather, the panel made a weak recommendation with the understanding that protocol-specific recommendations adjusted to specific phases of therapy such as induction or reinduction are required.
Recommendation 3
Do not routinely administer systemic antifungal prophylaxis to children and adolescents with acute lymphoblastic leukemia at low risk for IFD (Evidence quality: low; Strength of recommendation: strong).
Explanation.
For children and adolescents with acute lymphoblastic leukemia, a low risk for IFD can be inferred based on absence of risk factors such as prolonged neutropenia and corticosteroid administration, combined with observed IFD rates across different protocols and phases of therapy. This group includes, for example, pediatric patients receiving maintenance chemotherapy for acute lymphoblastic leukemia.
Recommendation 4
Do not routinely administer systemic antifungal prophylaxis to children and adolescents with cancer at low risk for IFD, such as most pediatric patients with lymphomas and solid tumors (Evidence quality: moderate; Strength of recommendation: strong).
Explanation.
In pediatric patients at low risk for IFD, benefit of systemic antifungal prophylaxis is likely to be small and outweighed by the risk for adverse effects, costs, and inconvenience. Thus, systemic antifungal prophylaxis should not routinely be administered in this setting. It is important to emphasize that some patients with lymphomas and solid tumors are at high risk for IFD, such as those with advanced Burkitt lymphoma and some infants with brain tumors. Thus, implementation must consider patient- and treatment-specific risk factors rather than relying on diagnosis alone in deciding which populations merit antifungal prophylaxis.
Recommendation 5
Administer systemic antifungal prophylaxis to children and adolescents undergoing allogeneic HSCT pre-engraftment and to those receiving systemic immunosuppression for the treatment of graft-versus-host disease (GVHD; Evidence quality: moderate; Strength of recommendation: strong).
Explanation.
The panel recognized that pre-engraftment and during systemic immunosuppression for the treatment of GVHD were two distinct periods, each increasing the risk for IFD but with different epidemiology. The Data Supplement shows that many studies of allogeneic HSCT recipients included both periods, with few studies focusing on the period of immunosuppression for GVHD treatment. Thus, the panel felt this lack of granularity precluded separate recommendations for these two different periods.
This strong recommendation was influenced by the finding in the systemic prophylaxis versus no systemic prophylaxis stratified analysis that HSCT recipients experienced greater benefit in proven or probable IFD reduction compared with chemotherapy recipients (Data Supplement). In addition, the subgroup analysis showed that among the HSCT stratum, antifungal prophylaxis significantly reduced fungal infection–related mortality (Data Supplement). Although the adult data were clearer, these data may be less generalizable to pediatric patients because of different transplantation approaches such as stem-cell source. As a result, the evidence quality was reduced. The panel suggested that administration in patients receiving systemic treatment of GVHD was reasonable based on risk factors for IFD but could not provide more granularity around whether there is a subgroup in which GVHD treatment is sufficiently short as to not warrant antifungal prophylaxis.
Recommendation 6
We suggest that systemic antifungal prophylaxis not be used routinely in children and adolescents undergoing autologous HSCT (Evidence quality: low; Strength of recommendation: weak).
Explanation.
A lower risk for IFD associated with autologous HSCT can be inferred from the systematic review of IFD risk factors.4 Consequently, systemic antifungal prophylaxis was not recommended for this group. However, there was less certainty in the setting of tandem transplantations where the cumulative duration of neutropenia may be longer. The degree of mucositis associated with specific conditioning regimens may also influence IFD risk and may affect the decision to administer antifungal prophylaxis.
Recommendation 7
If systemic antifungal prophylaxis is warranted, administer a mold-active agent (Evidence quality: high; Strength of recommendation: strong).
Explanation.
This strong recommendation was based on the comparison of different systemic antifungal prophylaxis agents (Table 4) where a mold-active agent versus fluconazole significantly reduced proven or probable IFD, mold infection, and IA, and reduced fungal infection–related mortality. Although a mold-active agent also increased adverse effects, the panel felt the benefits outweighed the negative aspects. Direct pediatric data were available, increasing quality of the evidence.
Trials comparing a mold-active agent versus fluconazole were presumably conducted in settings where there is an appreciable risk for mold infection. In settings where the risk for mold is sufficiently low, fluconazole may be an appropriate choice for prophylaxis.
Recommendation 8
In choosing a mold-active agent, administer an echinocandin or a mold-active azole (Evidence quality: moderate; Strength of recommendation: strong).
Explanation.
If systemic antifungal prophylaxis is warranted, the choice of which specific mold-active agent is influenced by multiple factors including local epidemiology, adverse effect profile, drug interaction potential, costs, and jurisdictional availability. All mold-active agents have disadvantages. For example, echinocandins must be administered intravenously daily, which may make this option less desirable for ambulatory populations. Use of mold-active azoles may be limited by drug interactions, hepatotoxicity, and adverse effects resulting in discontinuation of prophylaxis. For children younger than 13 years of age, an echinocandin, voriconazole, or itraconazole is suggested based on efficacy, adverse effects, and availability of pediatric dosing information. In settings where all agents are available, either an echinocandin or voriconazole is favored based on toxicity profile. However, itraconazole may be used if other options are not available. Posaconazole may also be used in those 13 years of age and older. When using mold-active azoles, best practices with respect to therapeutic drug monitoring should be applied.
Recommendation 9
Do not use amphotericin routinely as systemic antifungal prophylaxis (Evidence quality: low; Strength of recommendation: strong).
Explanation.
This strong recommendation was based on the finding that amphotericin was not more effective than fluconazole in reducing proven or probable IFD but was associated with more adverse effects (Table 4). Stratified analysis did not reveal differential efficacy based on whether amphotericin was administered on a daily or nondaily schedule (Data Supplement).
Recommendation 10
If systemic antifungal prophylaxis is warranted, consider administration during periods of observed or expected severe neutropenia. For allogeneic HSCT recipients, consider administration during systemic immunosuppression for GVHD treatment (Evidence quality: low; Strength of recommendation: weak).
Explanation.
There are limited data that inform the decision of when to initiate and discontinue systemic antifungal prophylaxis. This recommendation was based both on criteria used in the included randomized trials and the anticipated highest risk periods.
DISCUSSION
This CPG of systemic antifungal prophylaxis is important because of the impact of IFD in pediatric patients with cancer and HSCT recipients and uncertainty in the ideal approach. If systemic antifungal prophylaxis is warranted, the panel made a strong recommendation to administer a mold-active agent with an echinocandin or a mold-active azole. In choosing a specific agent, local epidemiology, adverse effect profile, potential for drug interactions, costs, and jurisdictional availability must be weighed against each other for specific settings. If all agents are available and appropriate from a microbiologic perspective, echinocandins may be favored when limiting adverse effects and hepatotoxicity are valued. Conversely, mold-active azoles may be favored when oral administration and convenience are favored.
It is possible that more broad-spectrum antifungal prophylaxis will change the choice of empirical antifungal therapy, and if the new choice has more adverse effects, the strategy could result in more toxicity overall. Future comparative effectiveness studies should evaluate the overall impact of antifungal prophylaxis selection on empirical antifungal choice and overall toxicities.
We found that fluconazole, when compared with no systemic antifungal prophylaxis, was associated with a reduction in fungal infection–related mortality but not overall mortality. It is possible that the reduction in fungal infection–related mortality occurred because these trials were conducted at a time when there were fewer IFD treatment options. It is also possible that this reduction is related to classification bias given the challenges in assigning cause of death.5
As with all CPGs, a structured approach to local adaptation, implementation, and evaluation is a key consideration. Important factors in this process include a baseline understanding of local and disease-specific epidemiology, establishing a leadership team that will oversee the process, and appropriate engagement and education of key stakeholders. Pediatric acute lymphoblastic leukemia is a particularly challenging population in which to make universal recommendations regarding systemic antifungal prophylaxis because of the wide variability in treatment regimens, each with differing IFD risk patterns and limitations to specific antifungal agents. Implementation of this CPG will likely require protocol-specific recommendations for patients with acute lymphoblastic leukemia.
In summary, we created a CPG for systemic antifungal prophylaxis in pediatric patients with cancer and HSCT recipients. Implementation and assessment of guideline-concordant rates and impacts are important future steps.
ACKNOWLEDGMENT
We thank Elizabeth Uleryk for conducting the literature search, Chris Dvorak, MD, and Maria Santolaya, MD, for their input, and Sandra Cabral for her invaluable research assistance throughout the process. We also thank Ivy Zou and Yuko Shimamoto for help with the article translation.
SUPPORT
Supported by the Pediatric Oncology Group of Ontario. Also supported by the Wellcome Trust Strategic Award (Grant No. 097377; A.W.), the Medical Research Council Center for Medical Mycology (Grant No. MR/N006364/2; A.W.) at the University of Exeter, and a Canada Research Chair in Pediatric Oncology Supportive Care (L.S.).
The Pediatric Oncology Group of Ontario did not have any influence over the content of this article or the decision to submit for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas Lehrnbecher, Brian T. Fisher, Bob Phillips, L. Lee Dupuis, Paula D. Robinson, Lillian Sung
Collection and assembly of data: Vicky Fioravantti, Priya Patel, Paula D. Robinson, Lillian Sung
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Practice Guideline for Systemic Antifungal Prophylaxis in Pediatric Patients With Cancer and Hematopoietic Stem-Cell Transplantation Recipients
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Lehrnbecher
Honoraria: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Consulting or Advisory Role: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Speakers' Bureau: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Brian T. Fisher
Employment: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst)
Fabianne Carlesse
Honoraria: Pfizer, United Medical, Merck Sharp & Dohme, Teva
Consulting or Advisory Role: Pfizer
Speakers' Bureau: UnitedHealthcare, Teva, Pfizer
Travel, Accommodations, Expenses: Pfizer
Elio Castagnola
Consulting or Advisory Role: Ferrer
Speakers' Bureau: Astellas Pharma
Andreas H. Groll
Consulting or Advisory Role: Pfizer, Gilead, F2G, Astellas Pharma
Speakers' Bureau: Gilead Sciences, Merck Sharp & Dohme, Pfizer, Basilea, F2G
Research Funding: Gilead Sciences (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst)
Gabrielle M. Haeusler
Research Funding: Gilead Sciences
Emmanuel Roilides
Research Funding: Gilead Sciences (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Astellas Pharma (Inst)
Travel, Accommodations, Expenses: Pfizer
William J. Steinbach
Consulting or Advisory Role: Sfunga
Research Funding: Merck Sharp & Dohme (Inst)
Adilia Warris
Honoraria: Gilead Sciences Belgium
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung L, Lange BJ, Gerbing RB, et al. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–3539. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 2.Dix D, Cellot S, Price V, et al. Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): Results from the Canadian Infections in AML Research Group. Clin Infect Dis. 2012;55:1608–1614. doi: 10.1093/cid/cis774. [DOI] [PubMed] [Google Scholar]
- 3.Sung L, Gamis A, Alonzo TA, et al. Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer. 2009;115:1100–1108. doi: 10.1002/cncr.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher BT, Robinson PD, Lehrnbecher T, et al. Risk factors for invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: A systematic review. J Pediatric Infect Dis Soc. 2018;7:191–198. doi: 10.1093/jpids/pix030. [DOI] [PubMed] [Google Scholar]
- 5.Alexander S, Pole JD, Gibson P, et al. Classification of treatment-related mortality in children with cancer: A systematic assessment. Lancet Oncol. 2015;16:e604–e610. doi: 10.1016/S1470-2045(15)00197-7. [DOI] [PubMed] [Google Scholar]
- 6.Science M, Robinson PD, MacDonald T, et al. Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2014;61:393–400. doi: 10.1002/pbc.24847. [DOI] [PubMed] [Google Scholar]
- 7.Groll AH, Castagnola E, Cesaro S, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 2014;15:e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 8.Oxman AD, Fretheim A, Schünemann HJ. Improving the use of research evidence in guideline development: Introduction. Health Res Policy Syst. 2006;4:12. doi: 10.1186/1478-4505-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: Performance, usefulness and areas for improvement. CMAJ. 2010;182:1045–1052. doi: 10.1503/cmaj.091714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–677. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 11.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency: European Medicines Agency recommends suspension of marketing authorization for oral ketoconazole, July 2013. https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-recommends-suspension-marketing-authorisations-oral-ketoconazole_en.
- 13. US Food and Drug Administration: FDA Drug Safety Communication: FDA warns that prescribing of Nizoral (ketoconazole) oral tablets for unapproved uses including skin and nail infections continues; linked to patient death. June 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-prescribing-nizoral-ketoconazole-oral-tablets-unapproved.
- 14.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S, (eds): Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011), The Cochrane Collaboration, 2011. http://handbook-5-1.cochrane.org/
- 16.Koch GG, Landis JR, Freeman JL, et al. A general methodology for the analysis of experiments with repeated measurement of categorical data. Biometrics. 1977;33:133–158. [PubMed] [Google Scholar]
- 17. The Nordic Cochrane Centre, The Cochrane Collaboration: RevMan (computer program). Version 5.3. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download.