Abstract
SARS-CoV-2 infection is associated with significant lung and cardiac morbidity but there is a limited understanding of the endocrine manifestations of coronavirus disease 2019 (COVID-19). Although thyrotoxicosis due to subacute thyroiditis has been reported in COVID-19, it is unknown whether SARS-CoV-2 infection can also lead to decompensated hypothyroidism. We present the first case of myxedema coma (MC) in COVID-19 and we discuss how SARS-CoV-2 may have precipitated multiorgan damage and sudden cardiac arrest in our patient.
A 69-year-old woman with a history of small cell lung cancer presented with hypothermia, hypotension, decreased respiratory rate, and a Glasgow Coma Scale score of 5. The patient was intubated and administered vasopressors. Laboratory investigation showed elevated thyrotropin, very low free thyroxine, elevated thyroid peroxidase antibody, and markedly elevated inflammatory markers. SARS-CoV-2 test was positive. Computed tomography showed pulmonary embolism and peripheral ground-glass opacities in the lungs. The patient was diagnosed with myxedema coma with concomitant COVID-19. While treatment with intravenous hydrocortisone and levothyroxine were begun the patient developed a junctional escape rhythm. Eight minutes later, the patient became pulseless and was eventually resuscitated. Echocardiogram following the arrest showed evidence of right heart dysfunction. She died 2 days later of multiorgan failure. This is the first report of SARS-CoV-2 infection with MC. Sudden cardiac arrest likely resulted from the presence of viral pneumonia, cardiac arrhythmia, pulmonary emboli, and MC—all of which were associated with the patient’s SARS-CoV-2 infection.
Keywords: hypothyroidism, thyroid, Coronavirus, COVID-19, immunotherapy
There is a limited understanding of the endocrine manifestations of infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. Thyrotoxicosis has been reported in 3 cases of subacute thyroiditis in coronavirus disease 2019 (COVID-19) [2-4]. To our knowledge, there have been no cases of decompensated hypothyroidism in the setting of COVID-19. Myxedema coma (MC) is a rare presentation of decompensated hypothyroidism often precipitated by infection [5]. We present the first case of MC in COVID-19. We discuss how SARS-CoV-2 infection may have precipitated multiorgan damage and sudden cardiac arrest in the setting of MC.
1. Case Description
A 69-year-old woman with a history of small cell lung cancer (SCLC) was brought into the emergency room after being found unresponsive at home. She lived alone and recent clinical history was limited. The vital signs at initial presentation were temperature 30°C, blood pressure 70/50 mm Hg, heart rate 83 beats per minute, respiratory rate 8 breaths per minute, and oxygen saturation 60% on room air. She was unresponsive to stimuli with agonal respirations and a Glasgow Coma Scale score of 5. Exam showed no evidence of goiter, prior surgical incision in the neck, periorbital edema, or generalized edema. She was intubated and was given intravenous (IV) fluids, vasopressors, and broad-spectrum antibiotics. Home medications included rivaroxaban, amlodipine, metoprolol, aspirin, gabapentin, and an albuterol inhaler. She had received induction with atezolizumab, a humanized monoclonal antibody, for treatment of SCLC 1 year prior. She had been lost to follow-up until she was seen by her oncologist about 1 month prior to admission when thyroid studies were recommended but not performed. She had no known personal or family history of thyroid disease.
The patient was admitted to the intensive care unit for undifferentiated shock. Initial laboratory studies (Table 1) were notable for hypoglycemia, leukocytosis, lymphopenia, and elevated inflammatory markers. Thyroid studies showed a thyrotropin (TSH) of 61.3 μU/mL (0.3-4.7 μU/mL), free thyroxine (fT4) of 0.2 ng/dL (0.8-1.7 ng/dL), and thyroid peroxidase antibody of 33.4 IU/mL (≤ 20 IU/mL). Initial SARS-CoV-2 testing via polymerase chain reaction of a nasopharyngeal sample was negative. Computed tomography pulmonary angiogram showed diffuse, severe pulmonary fibrosis bilaterally with multiple nodular and peripheral ground-glass opacities, and pulmonary emboli in the right upper lobe. Anticoagulation was not initiated because of hematemesis.
Table 1.
Pertinent laboratory findings on admission
| Laboratory value | Reference range | |
|---|---|---|
| White blood cell count, cells/µL | 22 × 10E3 | 4.16-9.95 × 10E3 |
| Absolute lymphocyte count, cells/µL | 0.64 × 10E3 | 1.3-3.4 × 10E3 |
| Hemoglobin, g/dL | 10.7 | 11.6-15.2 |
| Sodium, mmol/L | 151 | 135-146 |
| Potassium, mmol/L | 4.0 | 3.6-5.3 |
| Bicarbonate, mmol/L | 20 | 20-30 |
| Calcium, mg/dL | 8.1 | 8.6-10.4 |
| Serum creatinine, mg/dL | 1.80 | 0.6-1.3 |
| TSH, µU/mL | 61.3 | 0.3-4.7 |
| Free T4, ng/dL | 0.2 | 0.8-1.7 |
| Free T3, pg/dL | 66 | 222-387 |
| Total T3, ng/dL | 26 | 85-185 |
| Thyroid peroxidase antibody, IU/mL | 33.4 | ≤ 20 |
| Cortisola, mcg/dL | 49 | > ~10 |
| Lactate, mg/dL | 55 | 5-25 |
| Troponin, ng/mL | 0.14 | < 0.1 |
| Creatinine kinase, U/L | 1908 | 38-282 |
| IL-6, pg/mL | 21 | ≤ 5 |
| D-dimer, ng/mL | > 10 000 | ≤ 499 |
| Ferritin, ng/mL | 4759 | 8-180 |
| Lactate dehydrogenase, U/L | 614 | 125-256 |
| C-reactive protein, mg/dL | 13.8 | < 0.8 |
Abbreviations: IL-6, interleukin-6; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TSH, thyrotropin.
aCollected prior to hydrocortisone replacement at 19:44 h.
A clinical diagnosis of myxedema coma (MC) was made and treatment with 100 mg IV hydrocortisone followed by 200 mcg IV levothyroxine was ordered. Although initial electrocardiogram was normal, she became bradycardic with development of a junctional escape rhythm as the medications were being prepared for administration. Eight minutes after the completion of the levothyroxine infusion, the patient arrested in pulseless electrical activity (PEA). Following resuscitation efforts, the patient was managed with therapeutic hypothermia. A right bundle branch block (RBBB) and right ventricular (RV) dysfunction were noted on follow-up studies. She continued to receive 100 mcg of IV levothyroxine daily. Hydrocortisone was not continued because the cortisol level on presentation, collected prior to steroid therapy administration, effectively ruled out the risk of concurrent adrenal insufficiency. A tracheal aspirate sample was re-sent for SARS-CoV-2 polymerase chain reaction testing, which returned positive. She developed refractory status epilepticus and multiorgan failure and was transitioned to comfort-focused care. She died on hospital day 3.
2. Discussion
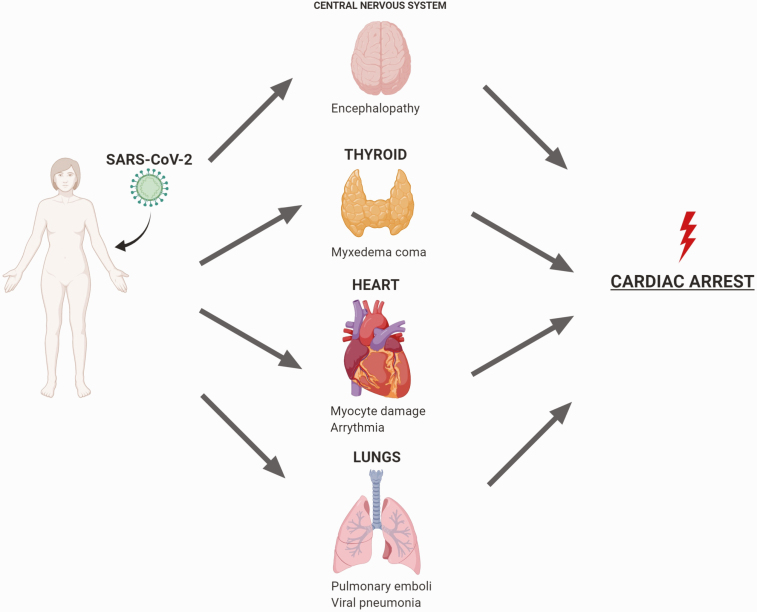
We present the first case of sudden cardiac arrest (SCA) in the setting of MC in COVID-19. We hypothesize our patient had preexisting clinically compensated hypothyroidism caused by undiagnosed immunotherapy-triggered autoimmune thyroiditis who then presented with decompensated hypothyroidism (ie, MC) in the setting of severe COVID-19. We discuss how SARS-CoV-2 may have precipitated SCA and multiorgan damage in our patient (Fig. 1).
Figure 1.
Multiorgan damage inflicted by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection resulting in cardiac arrest in our patient. SARS-CoV-2 infection and the resulting inflammatory state have been implicated in multisystem dysfunction including cardiac injury, pulmonary emboli, viral pneumonia, and now myxedema coma.
MC is an exceedingly rare form of decompensated hypothyroidism, with an estimated incidence of 1.08 cases per million people [6]. Our patient demonstrated many of the expected clinical findings of MC, including coma, hypothermia, hypoglycemia, hypoventilation, and bradycardia [5]. The presence of hypernatremia is unusual in MC but in this case may have reflected severe dehydration due to altered mental status. MC occurs when a precipitating factor disrupts thyroid hormone regulation in an already hypothyroid patient [7]. Thyroid hormone deficiency is often first detected at the time of crisis, as in our case [8]. An elevated thyroid peroxidase antibody suggests preexisting undiagnosed autoimmune thyroiditis, which may have occurred as a result of treatment for SCLC. Available evidence on the timing of onset of thyroid disease induced by anti-CTLA-4 and anti-PD-1 antibodies suggests that thyroid disease is often asymptomatic and is usually induced by at least 2 cycles of therapy [9-11]. One year prior to admission and during induction treatment for SCLC, the patient had received 3 doses of atezolizumab (a programmed death-ligand 1 inhibitor), which is associated with a 4% incidence of hypothyroidism after induction [12].
Although triggers of MC are numerous, including abrupt withdrawal of thyroid hormone replacement and recent trauma or surgery, infection is one of the most common precipitants [6]. Thyroid hormone is an important regulator of both the innate and adaptive immune systems, modulating lymphocyte, macrophage, and dendritic cell proliferation and function [13]. Consequently, patients with hypothyroidism may be at a higher risk of contracting infections and subsequently suffering from complications like MC [13]. Viral infections are frequently cited as a major environmental factor implicated in subacute thyroiditis and autoimmune thyroid diseases that are associated with thyrotoxicosis [14]. Increased circulating antibodies in the setting of autoimmunity and molecular mimicry may explain this phenomenon [14, 15]. Transient hypothyroidism often occurs in the recovery phase of subacute thyroiditis and less commonly permanent hypothyroidism can occur [16]. However, a direct association between hypothyroidism and viral infections has not been established [17]. Autopsy results of patients infected with the SARS-CoV-1 virus in the early 2000s found destruction of thyroid follicular epithelium and evidence of apoptosis [18].
We hypothesize that our patient had a decompensation of preexisting undiagnosed hypothyroidism precipitated by SARS-CoV-2 infection. Our patient exhibited the classic characteristics of severe COVID-19: lymphopenia, markedly elevated inflammatory markers, and peripheral ground-glass opacities. SARS-CoV-2 invades cells via the angiotensin-converting enzyme 2 (ACE2) receptor [19]. The ACE2 receptor is highly expressed in thyroid tissue, which may result in direct thyroid damage by SARS-CoV-2, although this is yet to be confirmed in autopsy studies [20]. Thyrotoxicosis has previously been reported in 3 cases of subacute thyroiditis occurring with or following COVID-19 [2-4]. Additionally, thyrotoxicosis occurs more frequently in severe COVID-19 cases, possibly related to an atypical mild thyroiditis [21]. Decompensated hypothyroidism has yet to be reported in COVID-19.
Several cardiac complications of MC have been reported, including cardiogenic shock with or without myocardial infarction, cardiac arrhythmias including sinus bradycardia, atrioventricular block, QT prolongation, and SCA [22, 23]. However, SCA is unusual with only 3 other cases to our knowledge [24-26]. Our case is the first SCA with infection as the precipitant of MC.
Cardiac damage in severe COVID-19 may occur in about 12% to 28% of patients [27]. Cardiac damage in COVID-19 may result from direct myocyte destruction, viral myocarditis, cytokine storm, microangiopathy, and stress in the setting of unmasked coronary artery disease [28]. In our patient, pulmonary embolism was noted along with a high D-dimer, despite the patient presumably being on rivaroxaban prior to hospitalization. Additionally, troponin elevation, new RBBB (often an indicator of RV strain) noted on electrocardiogram, and RV dysfunction evident on transthoracic echocardiogram, suggest that ongoing pulmonary thrombotic embolization may have led to a dramatic increase in RV afterload contributing to PEA arrest.
Owing to a high mortality rate, prompt infusion of thyroid hormone is the consensus treatment for MC [5]. High doses (eg, levothyroxine > 500 mcg/d or liothyronine > 75 mcg/d) are associated with higher mortality in patients older than 65 years and with preexisting cardiac disease [22]. One prior case of SCA occurred 15 minutes after a 300-mcg levothyroxine infusion [24]. This is similar to our case in that PEA arrest occurred 8 minutes after levothyroxine infusion. However, by contrast, we administered a more conservative initial levothyroxine loading dose of 200 mcg. We also intentionally avoided the administration of liothyronine to ensure slower hormonal onset. Given that the conversion of T4 to metabolically active T3 occurs over the course of hours, we suspect it is unlikely the levothyroxine infusion precipitated SCA in our case [29].
3. Conclusions
We report, to our knowledge, the first case of MC with SARS-CoV-2 infection complicated by sudden cardiac arrest. We discuss how SARS-CoV-2 may have precipitated multiorgan damage and SCA in our patient (Fig. 1). Further studies are needed to elucidate the direct effects of SARS-CoV-2 infection on heart and thyroid tissues. We suggest prompt treatment of MC with conservative doses of IV levothyroxine for patients with preexisting cardiovascular disease or in acute illnesses known to cause cardiac damage such as COVID-19.
Acknowledgments
Financial Support: The authors received no financial support for the research, authorship, and/or publication of this article.
Glossary
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- COVID-19
coronavirus disease 2019
- IV
intravenous
- MC
myxedema coma
- PEA
pulseless electrical activity
- RBBB
right bundle branch block
- RV
right ventricular
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
- SCA
sudden cardiac arrest
- SCLC
small cell lung cancer
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TSH
thyrotropin
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7):dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43(8):1171-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. 2020;43(8):1173-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathew V, Misgar RA, Ghosh S, et al. . Myxedema coma: a new look into an old crisis. J Thyroid Res. 2011;2011:493462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wall CR. Myxedema coma: diagnosis and treatment. Am Fam Physician. 2000;62(11):2485-2490. [PubMed] [Google Scholar]
- 8. Dutta P, Bhansali A, Masoodi SR, Bhadada S, Sharma N, Rajput R. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit Care. 2008;12(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ntali G, Kassi E, Alevizaki M. Endocrine sequelae of immune checkpoint inhibitors. Hormones (Athens). 2017;16(4):341-350. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari SM, Fallahi P, Elia G, et al. . Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int J Mol Sci. 2019;20(10):2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30(2):177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansfield AS, Każarnowicz A, Karaseva N, et al. . Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31(2):310-317. [DOI] [PubMed] [Google Scholar]
- 13. Jara EL, Muñoz-Durango N, Llanos C, et al. . Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol Lett. 2017;184:76-83. [DOI] [PubMed] [Google Scholar]
- 14. Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150(5):605-618. [DOI] [PubMed] [Google Scholar]
- 15. Benvenga S, Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev Endocr Metab Disord. 2016;17(4):485-498. [DOI] [PubMed] [Google Scholar]
- 16. Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya MM. Subacute thyroiditis: clinical presentation and long term outcome. Int J Endocrinol. 2014;2014:794943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei L, Sun S, Xu CH, et al. . Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol. 2007;38(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller I, Cannavaro D, Dazzi D, et al. . SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid. 1999;9(12):1167-1174. [DOI] [PubMed] [Google Scholar]
- 23. Benvenga S, Squadrito S, Saporito F, Cimino A, Arrigo F, Trimarchi F. Myxedema coma of both primary and secondary origin, with non-classic presentation and extremely elevated creatine kinase. Horm Metab Res. 2000;32(9):364-366. [DOI] [PubMed] [Google Scholar]
- 24. Bacci V, Schussler GC, Bhogal RS, Carter AC. Cardiac arrest after intravenous administration of levothyroxine. JAMA. 1981;245(9):920. [PubMed] [Google Scholar]
- 25. Muthu V, Luna B. Sudden cardiac death due to untreated hypothyroidism. J Innovations Card Rhythm Manag. 2013;4:1097-1099. [Google Scholar]
- 26. Salhan D, Sapkota D, Verma P, et al. . Sudden cardiac arrest as a rare presentation of myxedema coma: case report. J Community Hosp Intern Med Perspect. 2017;7(5):318-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with Coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26(6):470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Individualised requirements for optimum treatment of hypothyroidism: complex needs, limited options. Drugs Context. 2019;8:212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf). 2014;81(5):633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.