Abstract
Background
There are scarce data about SARS-CoV-2 infection in patients with inflammatory bowel disease (IBD). Our aim was to analyze the incidence, clinical presentation, and severity of SARS-CoV-2 infection in patients with IBD.
Methods
This is a cross-sectional, observational study. We contacted all the patients being treated at our IBD unit to identify those patients with suspected or confirmed SARS-CoV-2 infection, following the World Health Organization case definition. Data were obtained by patient electronical medical records and by phone interview.
Results
Eighty-two of 805 patients with IBD (10.2%; 95% confidence interval [CI], 8.3-12.5) were diagnosed as having confirmed (28 patients, 3.5%; 95% CI, 2.4-5.0) or suspected (54 patients, 6.7%) infection. Patient age was 46 ± 14 years, 44 patients were female (53.7%), 17.3% were smokers, 51.2% had Crohn disease (CD), and 39.0% had comorbidities. Digestive symptoms were reported in 41 patients (50.0%), with diarrhea as the most common (42.7%). One patient (1.2%) was diagnosed with IBD flare-up during SARS-CoV-2 infection. Twenty-two patients (26.8%) temporarily withdrew from their IBD treatment because of COVID-19. Most of the patients had mild disease (79.3%), and 1 patient died (1.2%). In the multivariate analysis, the presence of dyspnea was associated with moderate to severe infection (odds ratio, 5.3; 95% CI, 1.6-17.7; P = 0.01) and myalgias (odds ratio, 4.8; 95% CI, 1.3-17.9; P = 0.02) were related to a milder clinical course. Immunosuppression was not related to severity.
Conclusions
SARS-CoV-2 infection in patients with IBD is not rare. Dyspnea is associated with a more severe infection. Therapy for IBD, including immunomodulators and biologic therapy, is not related to a greater severity of COVID-19, and SARS-CoV-2 infections do not appear to be related to IBD flare-ups.
Keywords: COVID-19, immunosuppression, incidence, inflammatory bowel disease, SARS-CoV-2
INTRODUCTION
The severe acute respiratory syndrome coronavirus (SARS-CoV-2, previously known as 2019-nCoV) was identified in China in December 2019, and on March 11, 2020, the World Health Organization declared a global pandemic of coronavirus disease 2019 (COVID-19).1, 2 The more common symptoms of COVID-19 in adults are fever, cough, and shortness of breath, followed by a wide clinical spectrum including diarrhea, nausea, vomiting, headache, myalgias, sore throat, nasal congestion, fatigue, dysosmia, or dysgeusia, although some people have experienced an asymptomatic infection.3, 4 In more severe patients, COVID-19 has caused severe viral pneumonia and acute respiratory distress syndrome requiring respiratory support and resulting in a high rate of mortality. A hyperinflammatory cytokine response increasing the levels of cytokines and chemokines such as interleukin-6 has been related to the most severe presentations of COVID-19.5 It has been suggested that SARS-CoV-2 infection resembles a series of etiopathogenic mechanisms similar to other immune-mediated disorders. Therefore, some therapies used in immune-mediated disorders have been used in COVID-19 such as glucocorticoids, Janus kinase inhibitors, or tocilizumab (an interleukin-6 inhibitor).6, 7
Inflammatory bowel disease (IBD), including CD, ulcerative colitis, and unclassified colitis, is an immune-mediated disorder that may be treated with immunomodulators such as corticosteroids, thiopurines (azathioprine, mercaptopurine), methotrexate, calcineurin inhibitors, anti-tumor necrosis factor agents (infliximab, adalimumab, golimumab), other biologics (vedolizumab, ustekinumab), or Janus kinase inhibitors (tofacitinib). Immunosuppression and malnutrition in patients with IBD may increase the risk of opportunistic infections.8, 9 The angiotensin-converting enzyme 2 receptor expressed in the lung and enterocytes of the small intestine and colon is used by SARS-CoV-2 as a viral entry into the cell.10
In the inflamed bowels of patients with IBD, there may be a higher expression of angiotensin-converting enzyme 2 with a theoretical increased risk of SARS-CoV-2 infection via the gut.11 Despite this, no patients with IBD with COVID-19 were found in IBD centers in Wuhan (China) or in northern Italy in initial reports.12 The international database of COVID-19 in patients with IBD, SECURE-IBD, includes patients with confirmed COVID-19 cases worldwide with 1379 cases of infection reported by June 5, 2020.13 In Spain, a multicenter study from the Basque Country found 40 patients with IBD with a positive test for SARS-CoV-2 by April 8, 2020.14 With the current data, there does not appear to be an increased risk of COVID-19 or a more severe evolution of SARS-CoV-2 infection in patients with IBD.11
In any case, there is a need for more data about the risk, clinical characteristics, and evolution of COVID-19 in patients with IBD to avoid underestimating the risks and helping in treatment decisions.15 The aim of our study was to describe the incidence, clinical characteristics, and evolution of SARS-CoV-2 infection in patients with IBD being treated in our IBD unit, located in an area of high incidence of COVID-19 in Madrid, Spain.
METHODS
This was a single-center observational cross-sectional study evaluating the incidence of COVID-19 in patients with IBD being treated in our IBD unit in the south of Madrid, Spain. We identified all the patients with IBD undergoing treatment in our IBD unit up until April 2020, based on our prospectively maintained database, and we contacted all of them by e-mail or phone to inform them about the study between April 24 and May 27, 2020. As telephone and e-mail monitoring is commonly used in our clinical practice, almost all patients were contacted.
The patients who agreed to participate answered the following 3 questions: Have you been diagnosed with confirmed or suspected coronavirus infection? Have you had a fever or cough, or experienced shortness of breath during the coronavirus pandemic? Have you had close contact with anyone diagnosed with coronavirus infection? If a patient had been diagnosed with coronavirus infection or had had a fever or cough or experienced shortness of breath, we then reviewed electronic primary care and hospital clinical records and made another phone call to confirm all the data. With all this information, these patients were classified into confirmed, suspected, or low-suspicion COVID-19 case groups. If information provided by patients changed during the study, we urged them to contact the IBD unit again to update their data.
Confirmed and suspected cases of infection were defined following the World Health Organization case definitions.16 Thereby, patients with confirmed cases were defined as those with laboratory confirmation of COVID-19 infection. Patients with suspected cases were defined as those diagnosed with clinically suspected COVID-19 after evaluation by primary care, emergency services, and/or our IBD unit. Patients with low-suspicion cases were defined as those with symptoms not clearly related to COVID-19 after being evaluated by primary care, emergency services, and/or our IBD unit, and they were excluded from the study. The data collected from the electronic clinical records and the phone interview in the patients with confirmed and suspected COVID-19 were demographics data, IBD characteristics (subtype, location, phenotype, treatment, and disease activity), comorbidities, laboratory findings including testing of SARS-CoV-2 (polymerase chain reaction [PCR] and serology), chest imaging (all informed by a radiologist), symptoms (date of onset and duration), close contact with patients with COVID-19, medications, and outcomes. COVID-19 cases of infection were defined as mild when they were managed on an outpatient basis, as moderate if hospital admission was required, and severe where intensive care unit (ICU) admission, mechanic ventilation, or rescue therapy with bolus administration of steroids or tocilizumab was necessary.
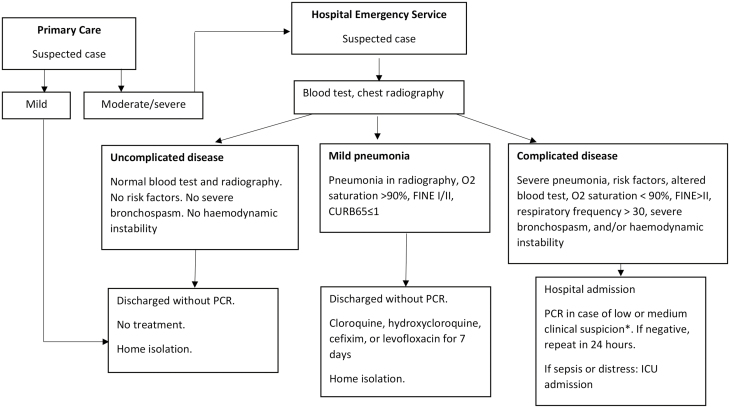
Following the diagnostic protocol of our area, and because of the limited availability of SARS-CoV-2 PCR tests, PCR was only performed in inpatients with a low to moderate clinical suspicion of COVID-19 until the end of April 2020, and serology tests were performed beginning in April 2020, alongside the PCR test (Fig. 1). We conducted PCR nasopharyngeal swab testing using the LightMix Modular SARS-CoV E-gene/RdRP gene (Roche Dx, Basel, Switzerland) LC 480 or the Viasure SARS-CoV-2 S gene (Becton Dickinson) BD MAX System (Certest BIOTEC, Zaragoza, Spain). Serology tests were conducted using MAGLUMI 2019-nCoV IgG and IgM (Shenzhen New Industries Biomedical Engineering Co, Shenzhen, People’s Republic of China).
FIGURE 1.
Algorithm for managing patients with suspected COVID-19 in Hospital Universitario de Fuenlabrada. Adapted from the April 9, 2020 version of the algorithm. *Since April 23, 2020, the PCR test was included for all inpatients.
Ethical Considerations
This study was approved by the ethics committee of Hospital Universitario de Fuenlabrada in Madrid. Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research. Our IBD unit has the quality certificate of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis.
Statistical Analysis
The qualitative variables were presented using percentages and 95% confidence intervals. The Kolmogorov-Smirnov test was carried out to evaluate the normality of the continuous variables. The quantitative variables were expressed using mean and SD or median and interquartile range when the variable did not follow a normal distribution. We calculated the cumulative incidence.
In the bivariate study, categorical variables were compared using the χ 2 test, and the comparisons between quantitative and qualitative variables were carried out using the Student t test or the Mann-Whitney U test if the variables did not follow a normal distribution. The multivariable analysis was conducted to study which variables were associated with the severity of infection using a logistic regression model. The statistical tool that was used to conduct the analysis was the IBM SPSS Statistics program.
RESULTS
Diagnosis
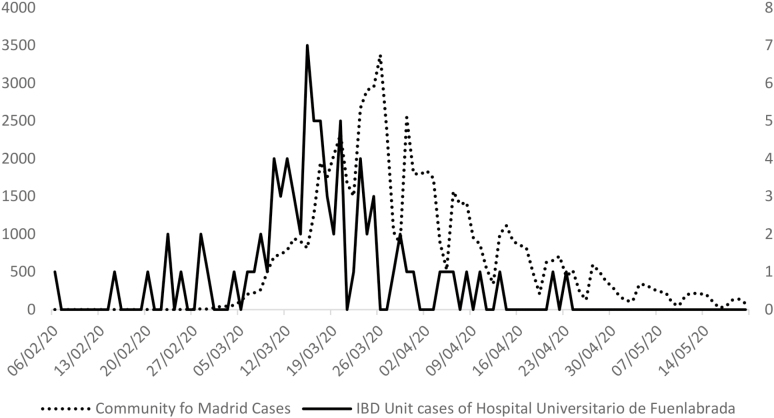
Of the 923 patients who are being treated at our IBD unit and were contacted by phone or e-mail, 805 (87.2%) were ultimately included in the study (Fig. 2). Of these patients, 82 (10.2%; 95% confidence interval [CI], 8.3%-12.5%) were diagnosed as confirmed (28 patients, 3.5%; 95% CI, 2.4%-5.0%) or suspected cases (54 patients, 6.7%). Table 1 shows the epidemiological characteristics of the patients included. The most commonly reported close contact with a COVID-19 patient was at work (25 patients, 30.5%) or a family member or friend (13 patients, 15.9%). Fig. 3 shows the number of patients with COVID-19 cases by date in our IBD unit in comparison with the cases of infection in the Community of Madrid. Fifty (61.0%) patients contacted their primary care physician because of symptoms, 32 (39.0%) went to the emergency services, 27 (32.9%) contacted the Community of Madrid COVID-19 emergency phone line, and 20 patients (24.4%) contacted our IBD unit while they had SARS-CoV-2 infection.
FIGURE 2.
Flowchart of the study.
Table 1.
Demographic and Clinical Characteristics of Patients Included in the Study
| Confirmed Cases of Infection (n = 28) | Suspected Cases of Infection (n = 54) | Total (N = 82) | |
|---|---|---|---|
| Sex (%) | |||
| Female | 13 (46.4) | 31 (57.4) | 44 (53.7) |
| Age, y | |||
| (mean ± SD) | 54 ± 14 | 43 ± 12 | 46 ± 14 |
| Smoking (%) | |||
| Yes | 1 (3.6) | 13 (24.5) | 14 (17.3) |
| Type of IBD (%) | |||
| CD | 11 (39.3) | 31 (57.4) | 42 (51.2) |
| Ulcerative colitis | 14 (50.0) | 21 (38.9) | 35 (42.7) |
| IBD unclassified | 3 (10.7) | 2 (3.7) | 5 (6.1) |
| Age at diagnosis, y, CD (%) | |||
| A1 (<16) | 2 (18.2) | 5 (16.1) | 7 (16.7) |
| A2 (between 17 and 40) | 5 (45.4) | 18 (58.1) | 23 (54.7) |
| A3 (>40) | 4 (36.4) | 8 (25.8) | 12 (28.6) |
| Location, CD (%) | |||
| L1 terminal ileum | 5 (45.4) | 10 (32.3) | 15 (35.7) |
| L2 colon | 3 (27.3) | 8 (25.8) | 11 (26.2) |
| L3 ileocolonic | 3 (27.3) | 10 (32.3) | 13 (30.9) |
| L1 + L4 upper gastrointestinal | — | 2 (6.4) | 2 (4.8) |
| L2 + L4 | — | 1 (3.2) | 1 (2.4) |
| Behavior, CD (%) | |||
| B1 nonstricturing/nonpenetrating | 8 (72.7) | 27 (87.0) | 35 (83.3) |
| B2 stricturing | 3 (27.3) | 2 (6.5) | 5 (11.9) |
| B3 penetrating | — | 2 (6.5) | 2 (4.8) |
| Location, ulcerative colitis (%) | |||
| Extensive colitis | 4 (28.6) | 4 (19.0) | 8 (22.9) |
| Left-sided colitis | 5 (35.7) | 13 (62.0) | 18 (51.4) |
| Proctitis | 5 (35.7) | 4 (19.0) | 9 (25.7) |
| IBD treatment (%) | |||
| Yes | 26 (92.9) | 48 (88.9) | 74 (90.2) |
| Type of IBD treatment (%) | |||
| Mesalazine | 16 (57.1) | 25 (46.3) | 41 (50.0) |
| Azathioprine | 9 (32.1) | 15 (27.8) | 24 (29.3) |
| Mercaptopurine | — | 3 (5.6) | 3 (3.7) |
| Methotrexate | 1 (3.6) | 1 (1.9) | 2 (2.4) |
| Infliximab | 2 (7.1) | 4 (7.4) | 6 (7.3) |
| Adalimumab | 2 (7.1) | 6 (11.1) | 8 (9.8) |
| Golimumab | 1 (3.6) | 2 (3.7) | 3 (3.7) |
| Ustekinumab | — | 3 (5.6) | 3 (3.7) |
| Comorbidities (%) | |||
| Yes | 17 (60.7) | 15 (27.8) | 32 (39.0) |
| Type of comorbidity (%) | |||
| Chronic kidney disease | 3 (10.7) | — | 3 (3.7) |
| Chronic obstructive pulmonary disease | 6 (21.4) | 2 (3.7) | 8 (9.8) |
| Congestive heart failure | 1 (3.6) | — | 1 (1.2) |
| Coronary heart disease | — | 1 (1.9) | 1 (1.2) |
| Cerebrovascular disease | 1 (3.6) | — | 1 (1.2) |
| Diabetes mellitus | 4 (14.3) | 1 (1.7) | 4 (4.9) |
| Hypertension | 10 (35.7) | 5 (9.3) | 15 (18.3) |
| Dyslipidemia | 7 (25.0) | 9 (17.0) | 16 (19.8) |
| Malignant neoplasm | 6 (21.4) | 4 (7.4) | 10 (12.2) |
| Chronic liver disease | 1 (3.6) | 1 (1.9) | 2 (2.5) |
FIGURE 3.
Number of patients with COVID-19 cases by date. Comparison of patients with COVID-19 cases by date between Community of Madrid (date of notification of patients with confirmed cases) and IBD unit of Hospital Universitario de Fuenlabrada (date of symptom onset in patients with confirmed and suspected cases). Left y axis: number of cases of infection in Community of Madrid. Right y axis: number of cases of infection in the IBD unit. Data from Community of Madrid extracted from the Spanish Ministry of Health, https://cnecovid.isciii.es/covid19, accessed May 23, 2020.
Clinical Manifestations
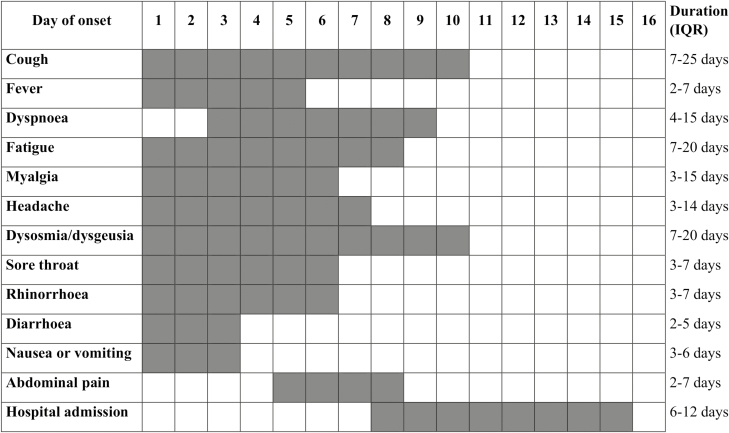
Clinical manifestations are shown in Table 2, and date of onset and duration of symptoms are shown in Fig. 4. Digestive symptoms were reported in 41 patients (50.0%). In 8 patients (9.8%), digestive symptoms reminded them of previous symptoms associated with their intestinal disease, with only 1 patient (1.2%) diagnosed with an IBD flare-up (Harvey-Bradshaw index score = 7). Most patients had multiple symptoms, with a median of 6 symptoms per patient (interquartile ratio [IQR], 4-7), with no differences between confirmed and suspected cases of COVID-19.
TABLE 2.
Clinical Manifestations of SARS-CoV-2 Infection in Patients With IBD
| Confirmed Cases of Infection (n = 28) | Suspected Cases of infection (n = 54) | Total (N = 82) | |
|---|---|---|---|
| Cough | 21 (75.0) | 44 (81.5) | 65 (79.3) |
| Fever | 24 (85.7) | 40 (74.1) | 64 (78.0) |
| Dyspnea | 13 (46.4) | 19 (35.2) | 32 (39.0) |
| Fatigue | 19 (67.9) | 34 (63.0) | 53 (64.6) |
| Myalgia | 11 (39.3) | 26 (48.1) | 37 (45.1) |
| Headache | 17 (60.7) | 28 (51.9) | 45 (54.9) |
| Dysosmia/ dysgeusia* | 17 (60.7) | 19 (35.2) | 36 (43.9) |
| Sore throat | 8 (28.6) | 27 (50.0) | 35 (42.7) |
| Rhinorrhea* | 5 (17.9) | 22 (40.7) | 27 (32.9) |
| Diarrhea | 13 (46.4) | 22 (40.7) | 35 (42.7) |
| Nausea or vomiting | 6 (21.4) | 10 (18.5) | 16 (19.5) |
| Abdominal pain | 5 (17.9) | 7 (13.0) | 12 (14.6) |
Numbers in parentheses are percentages.
*Statistically significant differences between confirmed and suspected COVID-19 cases of infection (P < 0.05).
FIGURE 4.
Date of onset and duration of symptoms in IBD patients with confirmed or suspected COVID-19. The first gray square in the left of each row represents the median time of onset of symptoms and the median days since the onset of symptoms to hospital admission. The number of gray squares in each row represents the median duration of symptoms and hospital admission. There were no statistically significant differences between confirmed and suspected COVID-19 patients in any item. Abbreviation: IQR, interquartile range.
Radiological and Laboratory Findings
Forty-five patients (54.9%) underwent a chest radiography or computed tomography, with bilateral lesions in 12 patients (26.7% of the patients with radiology exam), unilateral involvement in 7 patients (15.5%), and no findings in 26 patients (57.8%).
Laboratory findings are shown in Table 3. Fifty patients (61.0%) underwent a SARS-CoV-2 PCR test, with 18 patients (34.0% of patients with PCR test) testing positive. Three patients (16.7%) with a positive PCR test had a previous negative PCR test. The time from the onset of symptoms to the PCR test was significantly shorter in patients with a positive result (8.5 days; IQR, 4.5-19) than in patients with a negative PCR test (61 days; IQR, 39-70) (P = 0.01), likely because the serology testing was carried out alongside the PCR test in many patients after the acute infection, following laboratory procedures.
TABLE 3.
Laboratory Findings Comparison Between Mild and Moderate to Severe Cases of Infection
| Mild Cases of Infection (n = 65) | Moderate to Severe Cases of Infection (n = 17) | |
|---|---|---|
| Creatinine (mg/dL)* | 0.8 ± 0.2 | 1.1 ± 0.3 |
| LDH (U/l)* | 209 ± 54 | 357 ± 131 |
| CRP (mg/dL)* | 0.8 (0.6-4.5) | 9.9 (4.6-18.5) |
| D-dimer (ng/mL)* | 285 (248-395) | 955 (515-1548) |
| Hemoglobin (g/dL)* | 14.2 ± 1.6 | 11.6 ± 2.3 |
| Leukocytes (per µL) | 7290 ± 2149 | 6094 ± 3351 |
| Neutrophils (per µL) | 5085 (3952-7098) | 5710 (3830-13670) |
| Lymphocytes (per µL)* | 2196 ± 306 | 945 ± 633 |
Data are expressed using mean and SD or median and IQR when the variable did not follow a normal distribution. Creatinine, LDH, CRP, D-dimer, and neutrophil values are presented as the highest value, and hemoglobin, leukocyte, and lymphocyte values are presented as the lowest in case of several blood tests. Mild cases were patients who were managed on an outpatient basis. Moderate to severe cases were patients with hospital admission, ICU admission, mechanical ventilation, or rescue therapy with bolus administration of steroids or tocilizumab as necessary.
aStatistically significant differences between patients with mild and moderate to severe cases of infection (P < 0.05).
CRP indicates C-reactive protein; LDH, lactate dehydrogenase.
Thirty-six patients (43.9%) underwent a SARS-CoV-2 serology test after a median of 63 days (IQR, 52-70) after symptom onset. Only 2 patients with a positive PCR test underwent a serology test, being IgM-positive and IgG-positive in both. Most of the patients with a serology test were immunosuppressed (29 patients, 80.6%), with a positive serology test in 8 of these patients (27.6%) in contrast with 4 positive results in the 7 patients (57.1%) who were non-immunosuppressed with a serology test (P = 0.14).
Treatment
Twenty-four patients (29.3%) received treatment for SARS-CoV-2 infection. Hydroxychloroquine for 7 days was the most used treatment (20 patients, 24.4%), followed by lopinavir-ritonavir for 7 days (14 patients, 17.1%), cephalosporins for a median of 5 days (IQR, 4-8; 13 patients, 15.9%), bolus administration of steroids for a median of 6 days (IQR, 4-8; 6 patients, 7.3%), azithromycin for 5 days (IQR, 3-5; 3 patients, 3.7%), tocilizumab for 3 days (IQR, 1-3; 2 patients, 2.4%), levofloxacin for 2 days (2 patients, 2.4%), and chloroquine for 7 days (1 patient, 1.2%). Confirmed patients were more treated more often than patients with suspected COVID-19 (53.6% vs 16.7%; P = 0.01).
Twenty-two patients (26.8%) temporarily withdrew from their IBD treatment because of COVID-19. The decision to withdraw was mainly taken by consensus with the IBD unit (15 patients, 68.2%), although in 5 patients (22.7%) the IBD therapy was interrupted by another physician without contact with our IBD unit, and 2 patients (9.1%) withdrew from their IBD treatment on their own.
Evolution of the Infection
Most patients experienced mild disease (65 patients, 79.3%), 10 patients (12.2%) experienced moderate disease, and 7 patients (8.5%) had a severe SARS-CoV-2 infection. Patients with mild disease were younger (age 43 ± 11 years vs age 60 ± 14 years; P = 0.01), and more often experienced myalgias (50.8% vs 23.5%; P = 0.04) and rhinorrhea (40.0% vs 5.9%; P = 0.01) than the patients with moderate to severe disease. Dyspnea was more common in patients with moderate to severe disease than in patients with mild disease (64.7% vs 32.3%; P = 0.02). There was a tendency of more severe COVID-19 infection in patients with ulcerative colitis vs patients with CD (28.6% vs 11.9%; P = 0.07).
Most patients with moderate to severe disease had comorbidities (14 patients, 82.4%), but this was less common in patients with mild disease (27.7%; P < 0.01). The following comorbidities were statistically significantly more common in patients with moderate to severe disease than in patients with mild disease: hypertension (52.9% vs 9.2%), diabetes (23.5% vs 0%), dyslipidemia (41.2% vs 14.1%), chronic lung disease (29.4% vs 4.6%), and chronic kidney disease (17.6% vs 0%). Smoking, the number of symptoms, IBD therapy, immunosuppression, and close contact with someone with known COVID-19 were not related to severity. Fifteen patients (88.2%) with moderate to severe disease and 13 patients (20.0%) with mild disease were diagnosed as confirmed cases of COVID-19.
In the multivariate analysis, we found that the presence of dyspnea was a risk factor for the development of moderate to severe infection (odds ratio [OR], 5.3; 95% CI, 1.6-17.7; P = 0.01) and that on the contrary, myalgias (OR, 4.8; 95% CI, 1.3-17.9; P = 0.02) were associated with a milder clinical course of SARS-CoV-2 infection.
The median duration of hospitalization was 7 days (IQR, 3.5-10). Only 1 patient (1.2%), a man aged 59 years with comorbidities, was admitted to the ICU. His SARS-CoV-2 PCR test was positive, and he was admitted to the ICU 7 days after the onset of symptoms and was still in the ICU after 55 days at the end of the follow-up. A woman aged 74 years who was admitted because of thrombotic microangiopathy likely secondary to a signet ring cell carcinoma of unknown primary origin with bone marrow metastasis, and who had a positive SARS-CoV-2 PCR test, was the only patient who died during the follow-up (1.2%).
DISCUSSION
In contrast with initial reports, SARS-CoV-2 infection is not rare in patients with IBD: approximately 10% of the patients in our IBD unit were diagnosed with suspected or confirmed COVID-19. Data from 24 Italian IBD referral units found 79 patients with IBD with a diagnosis of COVID-19 through March 29, 2020, 49 of them with PCR confirmation.17 In the Nancy (France) and Milan (Italy) cohort of 6000 patients with IBD, 15 patients who were SARS-CoV-2 PCR–positive were found with a cumulative incidence of 0.25%, similar to the cumulative incidence in France and Italy at this time (0.17%).18 Discovering the real number of infected patients remains a global challenge because of the limited availability of PCR and serology tests during the pandemic, the possibility of false results, and the still uncertain interpretation of serology results. In our series we contacted all patients and reviewed their electronic medical reports to try to detect all the possible cases of infection. Our incidence is near to the seroprevalence of 11.4% in Madrid reported by the Spanish Health Ministry in the ENE-COVID study,19 and the evolution of the curve in our series is very close to those official data. The drop in the incidence of COVID-19 at the end of April could be likely explained by the end of the initial wave of the pandemic in our area in relation to the national lockdown in place since mid-March, although a more stringent case definition because of a wider availability of PCR tests at that time could had a role in this decrease.
Gubatan et al20 found 5 of 168 patients with IBD (3%) with positive SARS-CoV-2 testing in Northern California and found that advanced age (>age 66 years) is independently associated with an increased risk of contracting COVID-19. The most common symptom in the whole cohort, including 1160 individuals with or without IBD who were SARS-CoV-2-positive, was coughing (63%), followed by sore throat (41%), dyspnea (37.5%), fever (36%), and body pain (32%), with 19% of patients presenting with gastrointestinal symptoms (diarrhea 15.5%, abdominal pain 13%, and nausea/vomiting 9%). In the review of Mao et al21 combining data from 29 studies (N = 6064), the prevalence of digestive symptoms in patients with COVID-19 was 15% (95% CI, 10%-21%), with symptoms such as loss of appetite found in 21% of patients, diarrhea in 9%, nausea or vomiting in 7%, and abdominal pain in 3%. They also found that patients with gastrointestinal symptoms had an increased risk of severe COVID-19 compared with those without gastrointestinal symptoms (OR, 4.0; 95% CI, 1.5-10.6), although the risk of severe COVID-19 was not increased in patients with digestive comorbidities compared with patients without digestive comorbidities (OR, 0.6; 95% CI, 0.15-2.2). In our series half of patients had digestive symptoms, probably because we specifically asked about them, and as shown in other series, diarrhea is the most common digestive symptom of COVID-19. Contrary to Mao et al,21 diarrhea was not related to COVID-19 severity in our series. In the general population, the incidence of gastrointestinal symptoms widely varies between reports (3%-79%), requiring more data from larger series.22 Because digestive symptoms may precede pulmonary manifestations, if they are attributed to an IBD flare, then this conclusion can lead to delayed diagnosis of SARS-CoV-2 infection, with significant impact on the transmissibility of the virus.
Current recommendations for the management of patients with IBD during the COVID-19 pandemic from the different professional gastroenterological societies include continuing IBD therapies because the risk of active disease appears to be more significant than the role of immunosuppression in the increased risk of developing severe COVID-19.23 Minimizing corticosteroid exposure, avoiding initiation of immunomodulators, and preference for subcutaneous drugs for patients starting biologic therapy are some of the recommendations.24, 25 In our series, SARS-CoV-2 infection did not appear to be related to IBD flare-ups, and any IBD therapy was associated with a more severe evolution of COVID-19, supporting the current recommendations for our clinical practice and the advice that we provide to patients with IBD.
Younger age in patients with IBD with a lower rate of comorbidities could be associated with a lower risk of severe COVID-19. Taxonera et al26 reported 12 laboratory-confirmed patients with COVID-19 found in 1918 patients with IBD in an IBD center in Madrid, without an increased risk of infection or associated mortality ratio compared with the general population. Allocca et al18 found that 5 of 15 (33%) patients with IBD were hospitalized because of SARS-CoV-2 infection, but none of them required intensive care or died. In our series, patients with dyspnea experienced a more severe infection, and myalgias were associated with milder disease.
Our study has several limitations. First, although most of our patients did not undergo an analytical confirmation, all of them were treated as cases of SARS-CoV-2 infection following the current protocols, and we did not observe any relevant clinical difference between patients with suspected and confirmed cases. In any event, we have presented patients with confirmed and suspected cases separately for the most relevant data. The lack of population testing and the fact that asymptomatic patients and those with low-suspicion symptoms could be SARS-CoV-2–positive could underestimate the real incidence. For example, in the ENE-COVID study one-third of seropositive participants were asymptomatic, and only 20% of symptomatic participants who were seropositive reported a previous PCR test.19
Second, during the pandemic our management protocols have changed several times as new information became available, so initially the SARS-CoV-2 diagnoses were based on coughing, fever, and dyspnea symptoms, but later different symptoms like dysosmia/dysgeusia, headache, or myalgias were considered. Although rhinorrhea was more prevalent in suspected than in confirmed cases of infection and dysgeusia, a symptom considered as highly related to COVID-19, was less prevalent in this group of patients, most of the patients with suspected infection had cough and/or fever, 2 of the key symptoms of COVID-19. Regardless, because some of the patients with suspected infection could not be patients with COVID-19 we have presented the confirmed and suspected data separately.
Third, most of our patients with a serology test were immunosuppressed. Currently, data are lacking about seroconversion in patients with IBD after SARS-CoV-2 infection and about how immunosuppression could affect IgM and IgG detection. The ENE-COVID study19 found that 80.5% of patients with SARS-CoV-2 infection in the general population (95% CI, 73.2-86.2) have detectable IgG at least 2 weeks after PCR positivity. Thereby, immunogenicity after influenza vaccination in patients with IBD is low, mainly among patients who are immunosuppressed.27, 28 This is an interesting point to clarify in large cohort studies.
CONCLUSIONS
In conclusion, in our series from one of the areas of the world that is currently most affected by the SARS-CoV-2 infection, COVID-19 is not rare in patients with IBD. Most patients in the study experienced mild disease, although the appearance of dyspnea was associated with a more severe infection. Digestive symptoms are frequent in patients with IBD, mainly mild diarrhea, but COVID-19 does not appear to induce IBD flare-ups. Therapy for IBD is not related to infection severity. Larger studies with longer follow-up periods will be necessary to confirm these findings and to clarify the long-term evolution of SARS-CoV-2 infection and seroconversion in patients with IBD.
ACKNOWLEDGMENTS
We acknowledge all the workers at Fuenlabrada Health area who have contributed to the care of the patients in our IBD unit during the COVID-19 pandemic.
Supported by: None.
Author contributions: IG, AA, LJ, and FB conceived the study. IG, AA, LJ, DB, MA, DG, and FB completed the database of patients. LM conducted performance laboratory tests. IG and AA analyzed the data and wrote the manuscript. LJ, LM, and FB critically revised the manuscript. All authors approved the final manuscript.
Conflicts of interest: IG has served as a speaker, consultant, and advisory member for or has received research funding from Kern Pharma, Takeda, and Janssen. AA has served as a speaker and has received research grant or advisory fees from MSD, Lilly, Roche, Takeda, and Janssen. FB has served as a speaker, consultant, and advisory member for or has received research funding from MSD, AbbVie, Takeda, Janssen, Pfizer, Biogen, Amgen, Ferring, Faes Farma, Tillotts Pharma, Chiesi, and Gebro Pharma.
REFERENCES
- 1. Zhou P, Yang X, Wang X, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO Director-General’s remarks at the media briefing on COVID-19 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed May 27, 2020.
- 3. Guan W, Ni Z, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beltrán‐Corbellini Á, Chico‐García JL, Martínez‐Poles J, et al. . Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol. 2020;10.1111/ene.14273 [Online ahead of print, 2020 Apr 22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin C, Zhou L, Hu Z, et al. . Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang W, Zhao Y, Zhang F, et al. . The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toniati P, Piva S, Cattalini M, et al. . Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahier JF, Magro F, Abreu C, et al. ; European Crohn’s and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. [DOI] [PubMed] [Google Scholar]
- 9. Borman ZA, Côté-Daigneault J, Colombel JF. The risk for opportunistic infections in inflammatory bowel disease with biologics: an update. Expert Rev Gastroenterol Hepatol. 2018;12:1101–1108. [DOI] [PubMed] [Google Scholar]
- 10. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Ani AH, Prentice RE, Rentsch CA, et al. . Review article: prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment Pharmacol Ther. 2020;52:54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norsa L, Indriolo A, Sansotta N, et al. . Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology. 2020;159:371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brenner EJ, Ungaro RC, Colombel JF, et al. . SECURE-IBD Database public data update https://covidibd.org/current-data/. Accessed June 5, 2020.
- 14. Rodríguez-Lago I, Ramírez de la Piscina P, Elorza A, et al. . Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain). Gastroenterology. 2020;S0016–5085(20)30560-6 [Online ahead of print, 2020 Apr 21]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gower-Rousseau C, Fumery M, Pariente B, et al. . Inflammatory bowel disease and the SARS-CoV-2 pandemic: more speed, less haste. Gastroenterology. 2020;S0016–5085(20)30605-3 [Online ahead of print, 2020 May 7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance https://www.who.int/publications-detail/global-surveillance-for-covid-19-caused-by-human-infection-with-covid-19-virus-interim-guidance. Accessed May 27, 2020.
- 17. Bezzio C, Saibeni S, Variola A, et al. . Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. [DOI] [PubMed] [Google Scholar]
- 18. Allocca M, Fiorino G, Zallot C, et al. . Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. . Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;S0140-6736(20)31483-5 [Online ahead of print, 2020 Jul 3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gubatan J, Levitte S, Balabanis T, et al. . SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020;S0016-5085(20)30601-6 [Online ahead of print, 2020 May 6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao R, Qiu Y, He J-S, et al. . Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanzel J, Ma C, Marshall JK, et al. . Managing inflammatory bowel disease during COVID-19: summary of recommendations from gastrointestinal societies. Clin Gastroenterol Hepatol. 2020;18:2143–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy NA, Jones GR, Lamb CA, et al. . British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu L-R, Mao R, Fiorino G, et al. . 2nd expert interview of March 20, 2020 https://ecco-ibd.eu/publications/covid-19.html. Accessed May 29, 2020.
- 26. Taxonera C, Sagastagoitia I, Alba C, et al. . 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. deBruyn JC, Hilsden R, Fonseca K, et al. . Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:25–33. [DOI] [PubMed] [Google Scholar]
- 28. Cullen G, Bader C, Korzenik JR, et al. . Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. [DOI] [PubMed] [Google Scholar]