To the Editors,
We read with interest the article by Axelrad et al,1 which reported that patients with inflammatory bowel disease (IBD) are not at increased risk of COVID-19 and that immunosuppression did not modify the risk of severe outcomes, as previously suggested.2,3 Some authors have even hypothesized that biologics have a protective effect against SARS-CoV-2 infection,4,5 although it is also possible that the lack of increased risk is because patients with IBD spontaneously adopt more rigorous self-protecting measures. So far, no study has specifically addressed the social behaviors of patients with IBD who are treated or not treated using biologics. Therefore, we assessed the incidence of COVID-19 in patients with IBD according to whether they used biologics and their feelings and behaviors about the infectious risk.
We considered all patients with IBD scheduled for an outpatient visit at our hospital during the COVID-19 lockdown (March 10 to May 3, 2020). Patients who attended the clinic for intravenous or subcutaneous administration of biologic therapy formed Group 1, and patients not treated with biologics who attended the clinic for active IBD or had a virtual telephone examination for IBD in remission formed Group 2. A COVID-19 diagnosis was considered “sure” for a polymerase chain reaction (PCR)–confirmed SARS-CoV-2 genome in a nasopharyngeal swab and “likely” for patients who had at least 3 typical symptoms or signs of COVID-19 (fever, cough, dyspnea, anosmia, dysgeusia, diarrhea) and contact with another patient with COVID-19. The following data were collected from medical charts: age, sex, employment status, comorbidities, and IBD type, duration, activity, and therapies. To understand the patients’ perceived risk of COVID-19 and their social behavior, we administered an ad hoc questionnaire asking if they felt a greater-than-normal risk of infection and, if so, whether this was because of their disease, treatments, or both. Moreover, we asked if patients spontaneously stopped working or increased social restrictions (beyond government-imposed restrictions) to avoid infection.
The study enrolled 243 patients with IBD, of whom 124 were being treated with biologics (Group 1) and 119 were not (Group 2). The 2 groups were similar in age, employment status, comorbidities, and disease activity, but they differed significantly in disease duration. Significantly fewer Group 1 patients were taking 5-aminosalicylates or thiopurines (Table 1). Overall, there were 11 diagnoses of COVID-19 (1 sure, 10 likely), with 2 diagnoses in Group 1 and 9 in Group 2 (1.6% vs 7.6%; odds ratio = 0.20; 95% confidence interval, 0.04-0.95; P = 0.031). The 1 PCR-confirmed patient with COVID-19 was in Group 2. Of the 10 likely diagnoses, 5 patients (4 in Group 2) had radiographically confirmed pneumonia, and 2 of them (1 per group) were subsequently hospitalized. No patient with COVID-19 was taking thiopurines.
TABLE 1.
Demographic and Clinical Features of Enrolled Patients
| Group 1: Biologic Therapy | Group 2: No Biologic Therapy | P† | |
|---|---|---|---|
| Patients, n | 124 | 119 | — |
| Male, n (%) | 79 (63.7) | 63 (52.9) | 0.093 |
| Age, y (mean ± SD) | 45.9 ± 14.5 | 49.0 ± 16.1 | 0.121 |
| Employed, n (%) | 101 (81.5) | 84 (70.6) | 0.052 |
| Comorbidities, n (%)* | 43 (34.7) | 50 (42.0) | 0.291 |
| Duration of disease, y (mean ± SD) | 10.8 ± 8.6 | 14.5 ± 11.3 | 0.004 |
| Active disease, n (%) | 30 (24.2) | 19 (16.0) | 0.150 |
| Therapy, n (%) | |||
| None | 0 (0.0) | 26 (21.8) | NA |
| 5-aminosalicylates | 50 (40.3) | 77 (64.7) | 0.001 |
| Low-bioavailability steroids | 2 (1.6) | 4 (3.4) | 0.439 |
| Systemic steroids | 3 (2.4) | 5 (4.2) | 0.493 |
| Thiopurines | 2 (1.6) | 15 (12.6) | 0.001 |
| Infliximab | 25 (20.2) | — | NA |
| Adalimumab | 38 (30.6) | — | NA |
| Golimumab | 12 (9.7) | — | NA |
| Vedolizumab | 26 (21.0) | — | NA |
| Ustekinumab | 13 (10.5) | — | NA |
| Etrolizumab | 2 (1.6) | — | NA |
| Mirikizumab | 3 (2.4) | — | NA |
| Risankizumab | 4 (3.2) | — | NA |
| Infliximab plus azathioprine | 1 (0.8) | — | NA |
| COVID-19, n (%) | 2 (1.6) | 9 (7.6) | 0.031 |
| Sure | 0 (0.0) | 1 (0.8) | 0.490 |
| Likely | 2 (1.6) | 8 (6.7) | 0.056 |
| Pneumonia | 1 (0.8) | 4 (3.4) | 0.206 |
| Hospitalization | 1 (0.8) | 1 (0.8) | 1.000 |
*At least 1: coronary artery disease, arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney failure, neoplasms, immune-mediated inflammatory disease.
†Student t test or Fisher exact test.
NA indicates not applicable.
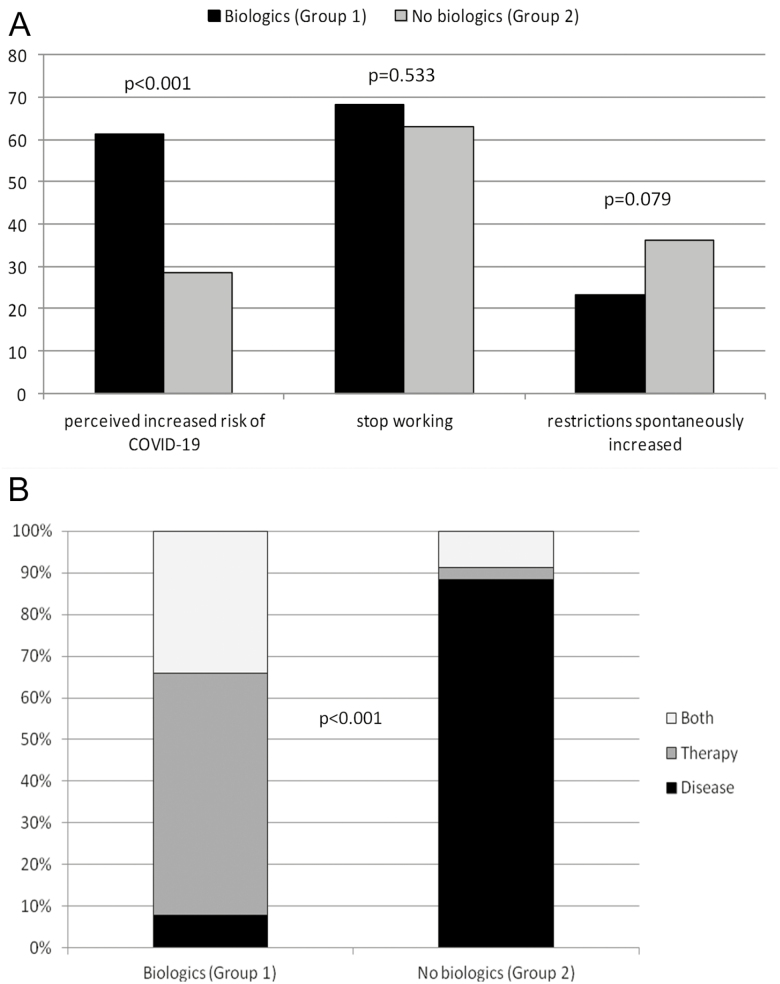
A higher proportion of patients in Group 1 than in Group 2 perceived themselves to be at greater-than-normal risk of infection: 76 (61.3%) vs 34 (28.6%) patients (odds ratio = 3.96; 95% confidence interval, 2.31-6.78; P < 0.001). However, similar proportions of employed patients in Groups 1 and 2 stopped working during the lockdown (69 [68.3%] vs 53 [63.1%] patients; P = 0.533), and similar proportions spontaneously increased social restrictions (29 [23.4%] vs 43 [36.1%] patients; P = 0.079) to avoid infection (Figure 1A). The causes of perceived increased risk varied with group assignment (P < 0.001). Of the 76 patients from Group 1 who perceived that they were at greater risk, 6 attributed this risk to IBD itself, 44 to the treatment, and 26 to both causes; of the 34 patients from Group 2, these numbers were 30, 1, and 3, respectively (Figure 1B).
FIGURE 1.
Feelings and behaviors of patients with IBD by study group. A, Responses to the 3 main questions (Fisher exact test). B, Causes of the perceived increased risk, by study group (χ 2 test).
This study provides evidence for a protective role of biologics against SARS-CoV-2 infection in IBD. In particular, patients taking biologics were 5 times less likely to be diagnosed with the infection. Limitations of the study are the relatively small sample size and the lack of PCR confirmation of most patients with COVID-19. Our study supports the hypothesis that immunomodulating drugs interfering with cytokine production or activity have a protective role against COVID-19.4,5 Moreover, our study excludes the possibility that younger age, fewer comorbidities, and greater self-protection—features theoretically associated with the use of biologics by patients with IBD—contribute to lowering the risk of SARS-CoV-2 infection. These findings provide a further reassuring message about the safety of biologics for patients with IBD during the COVID-19 pandemic.
Conflicts of interest: CB received lecture fees from Takeda, AbbVie, and Janssen. SS received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals and served as a consultant and a member of advisory boards for AbbVie and Janssen Pharmaceuticals. The other authors have no financial interests to disclose.
REFERENCES
- 1. Axelrad JE, Malter L, Hong S, et al. . From the American epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York City metropolitan area. Inflamm Bowel Dis. Accepted manuscript. Published online June 24, 2020. doi: 10.1093/ibd/izaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taxonera C, Sagastagoitia I, Alba C, et al. . 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bezzio C, Saibeni S, Variola A, et al. ; Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. [DOI] [PubMed] [Google Scholar]
- 4. Macaluso FS, Orlando A. Could patients with inflammatory bowel disease treated with immunomodulators or biologics be at lower risk for severe forms of COVID-19? Gastroenterology. Accepted manuscript. Published online ahead of print May 11, 2020. doi: 10.1053/j.gastro.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]