Abstract
Background
Remdesivir is a prodrug with in vitro activity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Its clinical efficacy in patients with COVID-19 under mechanical ventilation remains to be evaluated.
Methods
This study includes patients under mechanical ventilation with confirmed SARS-CoV-2 infection admitted to the ICU of Pesaro hospital between 29 February and 20 March 2020. During this period, remdesivir was provided on a compassionate use basis. Clinical characteristics and outcome of patients treated with remdesivir were collected retrospectively and compared with those of patients hospitalized in the same time period.
Results
A total of 51 patients were considered, of which 25 were treated with remdesivir. The median (IQR) age was 67 (59–75.5) years, 92% were men and symptom onset was 10 (8–12) days before admission to ICU. At baseline, there was no significant difference in demographic characteristics, comorbidities and laboratory values between patients treated and not treated with remdesivir. Median follow-up was 52 (46–57) days. Kaplan–Meier curves showed significantly lower mortality among patients who had been treated with remdesivir (56% versus 92%, P < 0.001). Cox regression analysis showed that the Charlson Comorbidity Index was the only factor that had a significant association with higher mortality (OR 1.184; 95% CI 1.027–1.365; P = 0.020), while the use of remdesivir was associated with better survival (OR 3.506; 95% CI 1.768–6.954; P < 0.001).
Conclusions
In this study the mortality rate of patients with COVID-19 under mechanical ventilation is confirmed to be high. The use of remdesivir was associated with a significant beneficial effect on survival.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a new coronavirus identified on 7 January 2020 in China, in the city of Wuhan (Hubei province).1 Starting from this region the SARS-CoV-2 has spread worldwide and on 2 May 2020 over 3 million cases of SARS-CoV-2 had been reported, causing over 200 000 deaths.
Between 16 and 24 February 2020 the WHO–China Joint Mission examined 44 672 infected people in China and estimated an overall crude fatality rate of 3.8%. Fatality was much higher in the elderly (21.9% in those over 80 years old) and in patients with pre-existing conditions, such as cardiovascular disease (13.2%), uncontrolled diabetes (9.2%), high blood pressure (8.4%) and chronic respiratory diseases (8%). Fatality was also strongly influenced by gender and by the response of the healthcare system. The rate of infected people who needed hospital treatment was high (20%), causing a significant stress on the health system’s capacity.2
The overcrowding of ICU capacity had dramatic consequences in all countries affected by the epidemic. Several studies have reported high mortality rates in ICUs3–7 and, in some cases, intubated patients’ mortality was above 80%.8 ICU saturation has also characterized the first weeks of the epidemic in Italy, representing one of the biggest problems for the health system.
The SARS-CoV-2 hit Italy on 20 February, when the first case not imported from China was identified in Codogno, a city in the Lombardy region in northern Italy. During the following days, new cases, unrelated to the first one, were reported in other regions of northern Italy.9
In Pesaro, a city located in the Marche region, we identified the first patient affected by SARS-CoV-2 on 25 February 2020. Since then, we experienced an exponential increase in cases in our province. The great majority of cases were unrelated to the outbreaks in northern Italy and frequently presented a very serious clinical condition at the time of diagnosis.
During the first 3 weeks of March, the capacity of Pesaro hospital ICU was exceeded very quickly, leading to a lack of fundamental resources such as ventilators, nurses and doctors. The lack of data and evidence of efficacy regarding treatments and patient management made this period additionally difficult.10,11
In this setting, we requested the compassionate use of remdesivir, a prodrug that inhibits viral RNA polymerases with broad-spectrum activity against several RNA virus families,12–15 including SARS-CoV-2.16,17 Remdesivir has already demonstrated effectiveness in trials related to Middle East respiratory syndrome CoV (MERS-CoV) and Ebola virus infection18,19 and its clinical efficacy against SARS-CoV-2 is now under investigation.11,20–24
In this study we report the clinical outcome of 25 critically ill patients under mechanical ventilation treated with remdesivir.
Methods
Patients and treatments
We included all patients (older than 18 years) admitted to the ICU of Pesaro hospital in the first 3 weeks of the SARS-CoV-2 outbreak, from 29 February to 20 March 2020. At the time of admission, all patients had SARS-CoV-2 infection confirmed by RT–PCR assay and severe respiratory failure with the need for mechanical ventilation.
Within 48 h after admission to the ICU, all patients were evaluated by an infectious disease specialist who decided whether to proceed with the request for compassionate use of remdesivir, based on specific criteria set by the pharmaceutical company. Patients who died within the first 48 h after ICU admission were excluded from this study.
All the requests for compassionate use of remdesivir were sent to the company through a dedicated online portal and they were evaluated for approval by the clinical operation team. At that time access to compassionate use was reserved for patients who had SARS-CoV-2 infection confirmed and needed mechanical ventilation. Exclusion criteria were creatinine clearance under 30 mL/min, serum levels of ALT or AST more than five times the upper limit of the normal range and need for inotropic support.
For approved cases, the treatment lasted 10 days and consisted of a first dose of 200 mg IV on Day 1, plus 100 mg daily from Day 2 on. Ethics committee approval was obtained for each patient treated with remdesivir, and consents were obtained in accordance with ethics committee dispositions. After Day 1 of remdesivir treatment, patients who were under treatment with hydroxychloroquine and/or lopinavir/ritonavir continued hydroxychloroquine and discontinued lopinavir/ritonavir.
Data collection
Data on patients treated with remdesivir were collected retrospectively and compared with data on patients who recovered in the same time period in the ICU. Patients treated with remdesivir were considered the study group; patients not treated with remdesivir were considered the control group.
All clinical and laboratory data were collected daily from the medical records of ICU patients. These included demographic data, clinical symptoms at onset, ongoing and previous medical conditions, laboratory and vital signs at ICU admission, need for inotropic support and/or continuous veno-venous haemofiltration during the ICU stay. All treatments carried out before and during the stay in ICU were also recorded.
For all patients the Charlson Comorbidity Index and SOFA score were calculated at the time of entry into the ICU. End of follow-up was 2 May 2020.
Statistical analysis
Normally distributed continuous data were reported as the mean ± SD and compared using the two-sided Student’s t-test. Non-normally distributed continuous data were reported as the median and IQR and compared using the Mann–Whitney test. Categorical variables were analysed with the χ2-test with Yates correction or Fisher’s exact test, whichever was most appropriate.
Our primary outcome was mortality at the end of follow-up. To identify variables that were independent predictors of outcome, a Cox regression analysis with backward stepwise selection was constructed employing those variables with a significance level of P < 0.20.
Patient survival was evaluated using the Kaplan–Meier method and compared using the log-rank test. IBM SPSS Statistics version 24 (SPSS, Chicago, IL, USA) was employed for statistical analysis. Statistical significance was set at P < 0.05.
Results
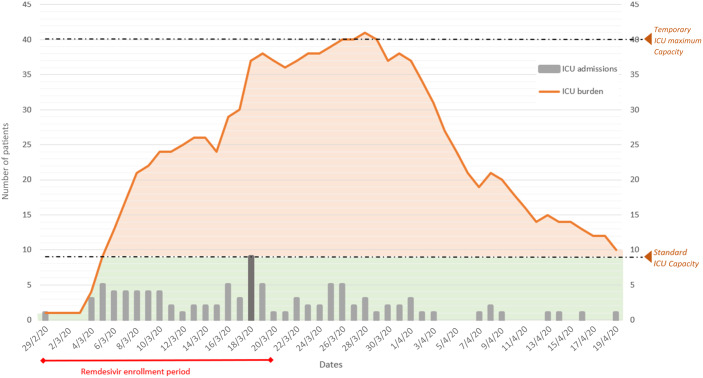
In the first 3 weeks of March, 60 patients were admitted to our ICU with confirmed severe acute respiratory syndrome from coronavirus SARS-CoV-2. The day with the highest number of ICU admissions was 18 March, with nine patients (Figure 1). All patients started mechanical ventilation on the day of admission. Nine patients were excluded from this study; three were transferred and lost during follow-up and six died in the first 48 h after hospitalization. The median (IQR) follow-up from the admission to ICU was 52 (46–56.5) days.
Figure 1.
Trend of ICU admissions during the first weeks of the SARS-CoV-2 epidemic in Pesaro. During this period, four new temporary ICUs were opened, increasing the number of beds from 9 to 40. Patients included in this study were admitted to the ICU between 29 February and 20 March 2020, when access to compassionate use of remdesivir was available. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Among the 51 patients analysed the median age was 67 (59–75.5) years, 92% were men and the median time of symptom onset was 10 (8–12) days before admission to the ICU. The most common comorbidities were high blood pressure (54.9%), diabetes mellitus (13.7%), ischaemic heart disease (13.7%) and moderate kidney failure (7.8%). The median Charlson Comorbidity Index was 3 (1–4), corresponding to an estimated 10 year survival of 77%.25 All patients were admitted because of respiratory failure and started mechanical ventilation. The SOFA score calculated at the time of admission to the ICU was 5 (4–6) (Table 1).
Table 1.
Demographic and clinical characteristics of the patients
| Characteristics | Total (N = 51) | Remdesivir group (N = 25) | No remdesivir group (N = 26) | P |
|---|---|---|---|---|
| Male sex, n (%) | 47 (92.2) | 23 (92) | 24 (92.3) | >0.999 |
| Median (IQR) age (years) | 67 (59–75.5) | 64 (57–75) | 70 (63.3–76) | 0.313 |
| Interval between symptom onset and ICU admission, median (IQR) (days) | 10 (8–12) | 11 (8–13) | 9 (8–11) | 0.197 |
| Comorbidities, n (%) | ||||
| ischaemic heart disease | 7 (13.7) | 3 (12) | 4 (15.4) | >0.999 |
| congestive heart failure | 4 (7.8) | 0 (0) | 4 (15.4) | 0.110 |
| COPD | 3 (5.9) | 0 (0) | 3 (11.5) | 0.235 |
| diabetes mellitus | 7 (13.7) | 3 (12) | 4 (15.4) | >0.999 |
| chronic kidney disease | 4 (7.8) | 2 (8) | 2 (7.7) | >0.999 |
| hypertension | 28 (54.9) | 14 (56) | 14 (53.8) | 0.877 |
| Median (IQR) Charlson Comorbidity Index | 3 (1–4) | 2 (1–3) | 3 (3–4) | 0.131 |
| Laboratory values | ||||
| mean ± SD WBC/mm3 | 9246 ± 3500 | 9172 ± 3203 | 9318 ± 3826 | 0.883 |
| mean ± SD neutrophils/mm3 | 8040 ± 3342 | 7902 ± 3224 | 8173 ± 3511 | 0.775 |
| median (IQR) lymphocytes/mm3 | 600 (400–900) | 600 (500–830) | 550 (300–900) | 0.263 |
| median (IQR) platelets × 103/mm3 | 190 (153–245) | 192 (162–242) | 184 (145–247) | 0.578 |
| median (IQR) creatinine (mg/dL) | 1.04 (0.86–1.45) | 0.97 (0.89–1.24) | 1.11 (0.85–1.57) | 0.468 |
| median (IQR) ALT (U/L) | 45 (32.5–65.5) | 45 (26–67) | 45 (37.3–61.8) | 0.402 |
| median (IQR) AST (U/L) | 34 (25.5–53) | 34 (23–55) | 33.5 (27.5–43.8) | 0.671 |
| median (IQR) total bilirubin (mg/dL) | 0.9 (0.65–1.25) | 0.9 (0.7–1.2) | 0.8 (0.6–1.28) | 0.564 |
| median (IQR) LDH (U/L) | 473 (387–559) | 450 (342–510) | 542 (416–559) | 0.070 |
| mean ± SD CRP (mg/dL) | 20.5 ± 11.8 | 20.9 ± 13.8 | 20.2 ± 9.7 | 0.833 |
| Median (IQR) SOFA score at admission | 5 (4–6) | 4 (3–5) | 5 (4–6) | 0.037 |
| CRRT, n (%) | 25 (49) | 10 (40) | 15 (57.7) | 0.206 |
| Concomitant therapies, n (%) | ||||
| hydroxychloroquine | 33 (64.7) | 17 (68) | 16 (61.5) | 0.771 |
| lopinavir/ritonavir | 29 (56.9) | 15 (60) | 14 (53.8) | 0.657 |
| tocilizumab | 9 (17.6) | 7 (28) | 2 (7.7) | 0.075 |
Normally distributed continuous data are reported as mean ± SD. Non-normally distributed continuous data are reported as median and IQR.
At admission to the ICU, laboratory parameters were as follows: mean (± SD) WBC count 9246 (± 3500)/mm3 ; median (IQR) lymphocytes 600 (400–900)/mm3; platelets 190 000 (153 000–245 000)/mm3; creatinine 1.04 (0.86–1.45) mg/dL; ALT 45 (32.5–65.5) U/L; AST 34 (25.5–53) U/L; total bilirubin 0.90 (0.65–1.25) mg/dL; lactate dehydrogenase (LDH) 473 (387–559) U/L; and C-reactive protein (CRP) 20.5 (± 11.8) mg/dL (Table 1).
During hospitalization most of the patients underwent other treatments with drugs under investigation for their clinical efficacy against SARS-CoV-2 disease. Thirty-three patients (64.7%) were treated with hydroxychloroquine, 29 (56.9%) with lopinavir/ritonavir and 9 (17.6%) with tocilizumab. In addition, 25 patients (49%) needed continuous renal replacement therapy (CRRT) because of kidney failure (Table 1).
Out of 51 patients, 25 underwent remdesivir treatment (study group), while the remaining 26 did not have access to the experimental drug (control group). The SOFA score at the time of entry to the ICU was higher in patients not treated than in patients treated with remdesivir (5 versus 4; P = 0.037). There was no further significant difference between these groups, either in clinical and laboratory characteristics or in the treatments carried out (Table 1). Tocilizumab was used more frequently in patients who had received remdesivir, but statistical significance was not achieved (28% versus 7.7%; P = 0.075) (Table 1).
Among the 25 patients treated with remdesivir, the median time between treatment initiation and symptom onset was 18 (15–20) days, and the time from admission to the ICU was 7 (4–8) days. Twenty patients completed the 10 day treatment, while five (20%) died of causes related to SARS-CoV-2 infection at a median of 5 (4–6) days after starting remdesivir.
At the end of follow-up 38 patients (74.5%) had died, 9 patients (17.6%) had been discharged from the hospital and 4 patients (7.8%) were still hospitalized but not ventilated. Survival analysis using Kaplan–Meier curves showed that mortality was significantly lower among patients treated with remdesivir than in untreated patients (56.0% versus 92.3% P < 0.001) (Figure 2). Death occurred a median (IQR) of 17 (13–20) days after ICU admission in the remdesivir group and 10 (8–13) days in the untreated group.
Figure 2.
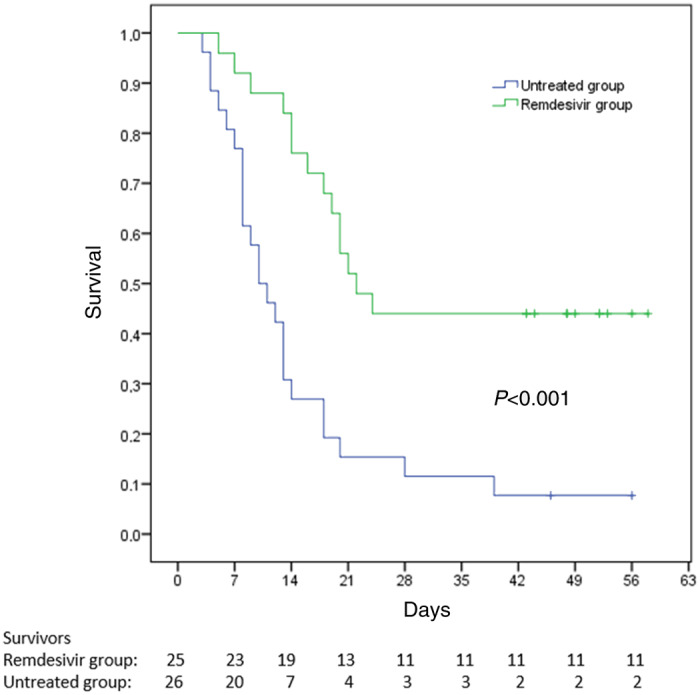
Survival analysis with Kaplan–Meier curves. Comparison between patients treated and not treated with remdesivir with a median (IQR) follow-up of 52 (46–56.5) days from admission to the ICU. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In the univariate analysis, the factors related to mortality were time between symptom onset and admission to the ICU, Charlson Comorbidity Index, platelet count and need for CRRT during the stay in the ICU. Treatment with remdesivir and treatment with tocilizumab were both associated with better survival. In the multivariate analysis we also included the following factors with P < 0.200: age; ALT; total bilirubin; and CRP value at admission to the ICU (Table 2).
Table 2.
Factors related to outcome in the study population
| Characteristics | Survivors (N = 13) | Not survivors (N = 38) | P | OR (95% CI) | P |
|---|---|---|---|---|---|
| Male sex, n (%) | 12 (92.3) | 35 (92.1) | >0.999 | ||
| Median (IQR) age (years) | 62 (57.5–69) | 71 (62–76) | 0.102 | 0.970 (0.924–1.018) | 0.218 |
| Interval between symptoms onset and ICU admission, median (IQR) days | 11 (10–13) | 9 (7.75–11.25) | 0.042 | 1.000 (0.879–1.139) | 0.995 |
| Comorbidities, n (%) | |||||
| ischaemic heart disease | 1 (7.7) | 6 (15.8) | 0.662 | ||
| congestive heart failure | 0 (0) | 4 (10.5) | 0.561 | ||
| COPD | 0 | 3 (7.9) | 0.561 | ||
| diabetes mellitus | 2 (15.4) | 5 (13.2) | 0.950 | ||
| chronic kidney disease | 0 | 4 (10.5) | 0.561 | ||
| hypertension | 9 (69.2) | 19 (50) | 0.379 | ||
| Median (IQR) Charlson Comorbidity Index | 2 (1–3) | 3 (1.75–4) | 0.045 | 1.184 (1.027–1.365) | 0.020 |
| Laboratory values | |||||
| mean ± SD WBC/mm3 | 9158±3180 | 9276±3642 | 0.918 | ||
| mean ± SD neutrophils/mm3 | 7838±3175 | 8109±3436 | 0.804 | ||
| median (IQR) lymphocytes/mm3 | 600 (500–900) | 600 (375–900) | 0.514 | ||
| median (IQR) platelets × 103/mm3 | 216 (170–269) | 184 (132–238) | 0.041 | 1 (1.000–1.000) | 0.304 |
| median (IQR) creatinine (mg/dL) | 0.91 (0.85–1.08) | 1.11 (0.84–1.56) | 0.336 | ||
| median (IQR) ALT (U/L) | 48 (28.5–79) | 44.5 (33–57.2) | 0.673 | ||
| median (IQR) AST (U/L) | 51 (26.5–64.5) | 33 (24.5–41.7) | 0.187 | 1.001 (0.998–1.004) | 0.524 |
| median (IQR) total bilirubin (mg/dL) | 1.2 (0.8–1.35) | 0.8 (0.6–1.25) | 0.182 | 1.358 (0.777–2.375) | 0.283 |
| median (IQR) LDH (U/L) | 457 (330–545) | 504 (388–575) | 0.310 | ||
| mean ± SD CRP (mg/dL) | 24.8±14.3 | 19.1±10.6 | 0.135 | 0.999 (0.967–1.031) | 0.936 |
| Median (IQR) SOFA score at admission | 4 (3.5–5) | 5 (4–6) | 0.488 | ||
| CRRT, n (%) | 2 (15.4) | 23 (60.5) | 0.013 | 0.837 (0.409–1.714) | 0.627 |
| Concomitant therapies, n (%) | |||||
| hydroxychloroquine | 10 (76.9) | 23 (60.5) | 0.336 | ||
| lopinavir/ritonavir | 8 (61.5) | 21 (55.3) | 0.944 | ||
| tocilizumab | 5 (38.5) | 4 (10.5) | 0.036 | 2.074 (0.714–6.024) | 0.180 |
| remdesivir | 11 (84.6) | 14 (36.8) | 0.008 | 3.506 (1.768–6.954) | <0.001 |
In the multivariate analysis, the Charlson Comorbidity Index was the only factor associated with mortality (OR 1.184; 95% CI 1.027–1.365; P = 0.020), while remdesivir treatment was the only one associated with survival (OR 3.506; 95% CI 1.768–6.954; P < 0.001) (Table 2).
Discussion
In this study, we evaluated the effects of remdesivir in critically ill patients under mechanical ventilation hospitalized in the ICU of Pesaro hospital during 3 weeks of the SARS-CoV-2 epidemic. During this period, we used remdesivir through compassionate use access. To date there are no clinical studies that strongly demonstrate the efficacy of a specific treatment in cases of SARS-CoV-2 infection.10,11 Remdesivir has broad-spectrum antiviral activity against several virus families, including filoviruses, paramyxoviruses, pneumoviruses and coronaviruses.12–15In vitro testing has also shown that remdesivir has activity against SARS-CoV-2.16,17 Although there are several clinical reports describing a beneficial effect of remdesivir in severely ill patients with COVID-19, literature data so far show conflicting results.3,22–24,26
A randomized multicentre trial involving 237 patients showed that remdesivir use was not associated with a difference in time to clinical improvement.22 Only one patient in the mentioned study was mechanically ventilated. A more recent randomized placebo-controlled trial with 1063 patients showed that remdesivir was superior to placebo in shortening the time to recovery.24 This beneficial effect was not seen in the subgroup of 272 patients who underwent mechanical ventilation.24 However, the authors clarified that median recovery time for this subgroup of patients could not be estimated, suggesting that the follow-up time may have been too short.24
Another report described outcomes in 53 patients with COVID-19 who were treated with remdesivir on a compassionate use basis and found that clinical improvement occurred in 68% of cases.23 In the latter study, 80% of the patients were mechanically ventilated, suggesting the potential of this drug in critical patients.
Our data confirm that remdesivir exerts a beneficial effect in terms of survival in patients with COVID-19 undergoing mechanical ventilation. Although the mortality rate of patients treated with remdesivir was as high as 56%, it was still significantly lower than that of patients who were not treated with the experimental drug.
The overall mortality found in our study (74.5%) is one of the highest reported in the literature. However, it should be noted that most of the studies showing lower mortality, between 24% and 65%, had much shorter follow-up and still had many patients under mechanical ventilation at the time of the analysis.3–7 One of the few studies reporting 28 day mortality in intubated patients showed a mortality rate of 81%.8 Furthermore, the data presented in our study refer only to the first 3 weeks of the epidemic, when ICU capacity was extremely stressed (Figure 1).
Although the poor knowledge of the management of this new infection has played a fundamental role in determining this high mortality rate, as well as the lack of effective antiviral regimens, the overcrowding of ICUs has certainly created an unfavourable condition.
Due to the large number of patients who needed hospitalization from 25 February, our hospital was completely reorganized within a few days. All SARS-CoV-2-negative patients were transferred to other facilities and our hospital was dedicated exclusively to confirmed SARS-CoV-2 patients. At the same time the number of ICU beds was increased 4-fold (from 9 to 40 beds) (Figure 1). Nevertheless, the initial need for ventilators, doctors and specialized nurses was largely unmet during the first 3 weeks of the epidemic.
Our study has several limitations. First, it is a retrospective, observational study. Nevertheless, all clinical data collected and analysed were complete and detailed for each patient. Second, being a single-centre study, the number of patients considered is low. The low number of participants has certainly weakened the statistical power of the study. However, we were able to detect a significant improvement in the mortality rate of patients treated with remdesivir. Third, there is a bias in patient selection. The selection was made in the first hours after entering the ICU, according to the criteria set for the compassionate use study. Patients with mild renal impairment or who had started inotropic support (e.g. low-dose norepinephrine) were not eligible for remdesivir treatment. This selection explains the significant difference in SOFA score between treated and untreated patients and it is certainly a criticism of this study. Despite this bias, the SOFA score at the time of ICU admission was low in both groups (4 versus 5), with a predicted mortality of 20%.27 Actually, clinical conditions at the time of admission did not influence the survival analysis and most of the patients presented a long, progressive clinical worsening resulting in multi-organ failure, with a median time of death of 13 days after entry to the ICU. Additionally, in six patients who met the inclusion criteria and for which remdesivir was requested, the drug was not delivered because of the interruption of the compassionate programme. All six patients died, thus indicating that patient selection did not bias towards a better outcome. Fourth, remdesivir was started late, at a median of 18 days after symptom onset. Although animal models have shown that administering remdesivir early in SARS-CoV and MERS-CoV infections reduces viral shedding,13 there is still no clinical evidence of better outcome with early treatment. Moreover, SARS-CoV-2 has been shown to persist for several weeks in critically ill patients28,29 and to play a role in maintaining activation of the immune system by binding to macrophages, thus reinforcing antiviral usefulness even in the advanced stages of the disease.30–32 Finally, we did not collect viral load data to confirm the antiviral effects of remdesivir. This was largely due to the scarcity of resources at the time of the epidemic peak.
In conclusion, in this study the mortality rate of patients with COVID-19 under mechanical ventilation is confirmed to be high. The use of remdesivir was associated with a significant beneficial effect on survival.
Funding
This study was supported by internal funding (Ricerca di Ateneo to F.B. n. 243/394).
Transparency declarations
None to declare.
References
- 1.WHO. Novel coronavirus—China. 2020. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
- 2.WHO–China Joint Mission. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 3. Bhatraju PK, Ghassemieh BJ, Nichols M. et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med 2020; 382: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arentz M, Yim E, Klaff L. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 4720: 2019–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 10022: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 2600: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazzerini M, Putoto G.. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Heal 2020; 8: e641–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poston JT, Patel BK, Davis AM.. Management of critically ill adults with COVID-19. JAMA 2020; doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 11. Alhazzani W, Møller MH, Arabi YM. et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–7. [DOI] [PMC free article] [PubMed]
- 12. Lo MK, Jordan R, Arvey A. et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep 2017; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheahan TP, Sims AC, Graham RL. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon CJ, Tchesnokov EP, Feng JY. et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 2020; 295: 4773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warren TK, Jordan R, Lo MK. et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531: 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon CJ, Tchesnokov EP, Woolner E. et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 2020; 295: 6785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30: 269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulangu S, Dodd LE, Davey RT. et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019; 381: 2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheahan TP, Sims AC, Leist SR. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020; doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen CY, Xin DQ, Xue DS.. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis 2020; 35: 101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ko WC, Rolain JM, Lee NY. et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 2020; 55: 105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Zhang D, Du G. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grein J, Ohmagari N, Shin D. et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020; 382: 2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigel JH, Tomashek KM, Dodd LE. et al. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med 2020; doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 26. Holshue ML, DeBolt C, Lindquist S. et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferreira FL, Bota DP, Bross A. et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286: 1754–8. [DOI] [PubMed] [Google Scholar]
- 28. Zheng S, Fan J, Yu F. et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369: m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu K, Chen Y, Yuan J. et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGonagle D, Sharif K, O’Regan A. et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19: 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merad M, Martin JC.. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020; 2: 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Feng Z, Diao B. et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv2020; doi: 10.1101/2020.03.27.20045427.