Abstract
Objective
Evaluate the risk of pre-existing comorbidities on COVID-19 mortality, and provide clinical suggestions accordingly.
Setting
A nested case–control design using confirmed case reports released from the news or the national/provincial/municipal health commissions of China between 18 December 2019 and 8 March 2020.
Participants
Patients with confirmed SARS-CoV-2 infection, excluding asymptomatic patients, in mainland China outside of Hubei Province.
Outcome measures
Patient demographics, survival time and status, and history of comorbidities.
Method
A total of 94 publicly reported deaths in locations outside of Hubei Province, mainland China, were included as cases. Each case was matched with up to three controls, based on gender and age 1 year old (94 cases and 181 controls). The inverse probability-weighted Cox proportional hazard model was performed, controlling for age, gender and the early period of the outbreak.
Results
Of the 94 cases, the median age was 72.5 years old (IQR=16), and 59.6% were men, while in the control group the median age was 67 years old (IQR=22), and 64.6% were men. Adjusting for age, gender and the early period of the outbreak, poor health conditions were associated with a higher risk of COVID-19 mortality (HR of comorbidity score, 1.31 [95% CI 1.11 to 1.54]; p=0.001). The estimated mortality risk in patients with pre-existing coronary heart disease (CHD) was three times that of those without CHD (p<0.001). The estimated 30-day survival probability for a profile patient with pre-existing CHD (65-year-old woman with no other comorbidities) was 0.53 (95% CI 0.34 to 0.82), while it was 0.85 (95% CI 0.79 to 0.91) for those without CHD. Older age was also associated with increased mortality risk: every 1-year increase in age was associated with a 4% increased risk of mortality (p<0.001).
Conclusion
Extra care and early medical interventions are needed for patients with pre-existing comorbidities, especially CHD.
Keywords: epidemiology, coronary heart disease, survival analysis
Strengths and limitations of this study.
Since we used data outside of the epicentre of the outbreak in mainland China, we avoided the competing mortality risk caused by insufficient healthcare resources.
The study controlled for the confounding effects of age, gender and the early period of the pandemic, which were, in general, not considered in the existing literatures.
The survival-time-related statistical results can help guide the early clinical intervention to reduce mortality.
The lack of medical test results in the publicly reported data restricted further investigation on the association between comorbidity-related clinical index and mortality.
The missing at random assumption could not be verified.
Introduction
Since the first report of COVID-19 in December 2019 in Wuhan, Hubei Province, China, the novel virus infection has rapidly spread to other cities in China and has now been detected in 215 countries and locations internationally.1 On 11 March 2020, the WHO declared COVID-19 a pandemic and has called for aggressive actions from all countries to fight the disease. Current research has indicated that COVID-19 is caused by SARS-CoV-2, a beta-coronavirus similar to the SARS-CoV in its genetic sequence.2 Epidemiological evidence suggests that the initial reported cases in China had a history of exposure to the Huanan seafood market.3 4 With the escalated spread of the infection, there has been clear evidence of human-to-human transmission.5 6 The most common symptoms include fever, dry cough and fatigue,5–8 and there are asymptomatic yet contagious cases.9
According to the COVID-19 situation reports of World Health Organization (WHO)1, as of 31 July 2020, there were 87 956 confirmed cases in mainland China, including 4666 deaths. Internationally, a total of 17 million confirmed cases and 664 244 deaths have been reported outside of China. Considering the global public health threat posed by COVID-19, unravelling the prognostic factors for patients, especially the risk factors of mortality associated with COVID-19, has important implications for clinical practice and is urgently warranted.
Studies have indicated that in severe cases, patients tend to be older in age6 8 and are more likely to have had pre-existing medical conditions, including but not limited to hypertension,3 6 8 diabetes,6 8 cardiovascular diseases,3 6 8 10–12 cerebrovascular diseases,6 chronic obstructive pulmonary disease (COPD),3 8 cancer13 and digestive diseases,14 in comparison to those with non-severe cases.3 6 8–15
Recently, scholarly attention has focused on identifying the risk factors for death from COVID-19. Some evidence suggests that pre-existing medical conditions are likely risk factors for death from COVID-19. For example, a study based on 72 314 cases in China indicated that the case-fatality rate (CFR) tends to be higher among those who are older in age and who have pre-existing cardiovascular disease, diabetes and/or hypertension.9 Similarly, by conducting logistic regression on odds of in-hospital deaths among 54 diseased patients and 137 recovered patients in Wuhan City, Hubei Province, Zhou et al16 found that older age, higher Sequential Organ Failure Assessment score and D-dimer greater than 1 μg/mL at hospital admission were associated with increased odds of in-hospital death. Chen et al4 found that pre-existing hypertension and other cardiovascular complications were more common among diseased patients than recovered patients.
However, several gaps remain in the understanding of risk factors for mortality of COVID-19. First, most current research on pre-existing comorbidities of COVID-19 was based on univariate comparison, which did not account for important confounders such as age and gender.17–20 Second, no studies have investigated the hazard of the identified risk factors over time or the probability of survival at a given time. Under the rapidly changing pandemic situation, it is crucial to provide timely survival-time guidance for implementing the targeted treatment to the high-risk patients in clinical practice. Third, most existing studies on mortality risk factors were focused on patients diagnosed in Wuhan, Hubei Province, with little understanding about the mortality risk factors outside of Hubei Province. The risk factors are likely different inside and outside of Hubei Province since current research has found the clinical symptom severity5 and the CFR9 21 to be higher in Hubei Province (the centre of outbreak) than in cities outside of Hubei Province in China. Fourth, no studies thus far have taken into account the pandemic stage when evaluating mortality risk factors. It has been found that the average daily infection rate in China was different before and after 22 January 2020, since non-pharmaceutical interventions were taken by the government before this date.22 The change of time in the pandemic stage may also influence the risk factors for fatality associated with COVID-19.
To fill the above research gaps in the existing literature about the mortality risk factors for COVID-19, the present study was conducted using a nested case–control (NCC) study, which aimed to evaluate the risk of the common pre-existing comorbidities (hypertension, CHD, diabetes, etc) for mortality associated with COVID-19 in mainland China outside of Hubei Province. NCC, also called risk set sampling, has been widely used in studying fatal disease risk effect in large pharmacoepidemiological studies23–28 and risk prediction in pandemic influenza A (H1N1) 2009 (pH1N1).29 NCC is cost-effective in data collection and is especially suitable for research on the mortality risk of diseases such as COVID-19, where the number of event-free people largely exceeds those who are symptomatic.30 To attain this goal, we employed survival analysis on 275 publicly reported confirmed cases, adjusting for age, gender and the change in the pandemic stage in China (ie, before and after 22 January 2020).
Method
Study design and rationale
This study performed survival analysis under an NCC design to assess the roles of common comorbidities (cardiocerebrovascular, endocrine and respiratory disease, etc) in predicting mortality for COVID-19 among patients in mainland China outside of Hubei Province. The study period was from 18 December 2019, when the first laboratory-confirmed case was announced in China, to 8 March 2020.
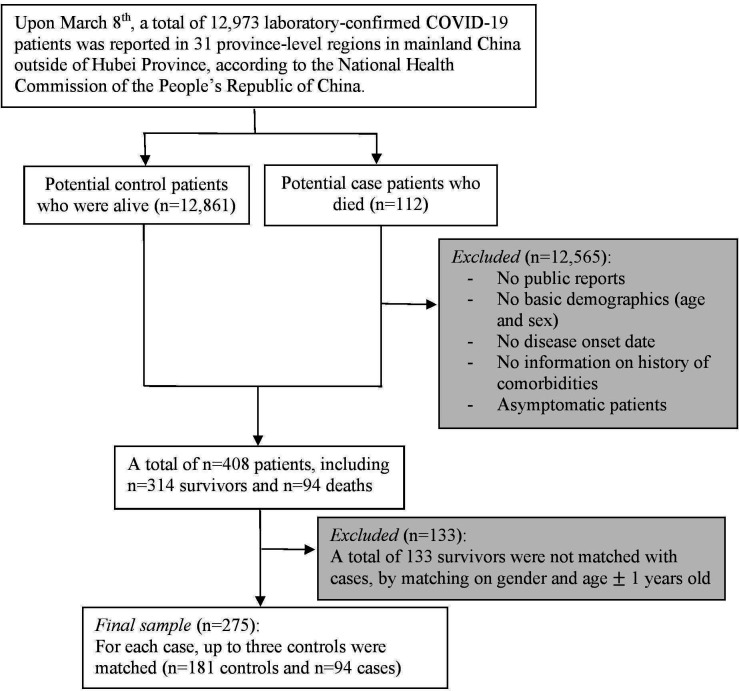
The study cohort was defined as all the publicly reported confirmed patients with COVID-19 outside of Hubei Province in mainland China during the study period. During this period, 112 deaths outside of Hubei Province were reported by the National Health Committee of China, and 18 were excluded from the present study due to unavailablility of case reports. A total of 448 publicly reported laboratory-confirmed COVID-19 cases (94 deaths and 354 survivors) were initially collected. To avoid selection bias due to intentionally collecting patients with certain pre-existing comorbidities, two authors independently collected, compared and reviewed the full text of each case report. Following the typical NCC design setting where all deaths were included as cases, and each case was matched with up to three controls on gender and age 1 year old (94 cases and 181 controls). The sample distribution across all 32 province-level regions in mainland China is presented in online supplemental table S1.
bmjopen-2020-038976supp001.pdf (118.1KB, pdf)
Data collection procedure
We routinely searched for daily news and public health reports on confirmed COVID-19 cases in all areas in mainland China outside of Hubei Province. Patients’ clinical and comorbidity characteristics were recorded and doubly confirmed by national/provincial/municipal health commission websites, the official COVID-19 data reporting websites in China. Follow-up time was defined as the duration from the date of disease onset until the end of observation on 8 March 2020, or when the participant died, whichever came first. For each eligible patient, we followed local reports to update their survival status until the end of follow-up time. Pre-existing comorbidities were recorded based on the descriptions in case reports.
As illustrated in figure 1, the inclusion criteria were publicly reported patients with COVID-19 who had complete information on basic demographics (age, gender and region), disease onset date (the first time they became symptomatic) and the history of comorbidities (including hypertension, CHD, cardiac failure, cerebral infarction, diabetes, chronic bronchitis, COPD, renal failure and history of surgery). Asymptomatic patients were not included in this study. In addition, we defined ‘comorbidity-free patients’ as those who were specifically described as ‘no pre-existing medical condition/comorbidity’ on the national/provincial/municipal health commission websites.
Figure 1.
Patient flow diagram detailing included subjects and exclusion criteria.
In the following three steps, we used patient no. 213 in the sample as an example to introduce the dynamic tracking method we used to identify any missing dates.
Step 1. We conducted an internet search on confirmed cases on baidu.com, the largest search engine in China, using the keywords “confirmed COVID-19 cases report” and “pre-existing comorbidities”. A search result pertained to one confirmed case reported on the website of the Municipal Health Commission of Binzhou (Shandong Province) on 17 February, described as ‘the 15th confirmed case: 30-year-old man without pre-existing morbidities, who lives in the neighbourhood of Xincun Village. This patient was diagnosed positive on 16 February and is being treated with precaution in Bincheng hospital’. We recorded the age, gender and region of this patient and that he was comorbidity-free.
Step 2. We then determined the onset date of COVID-19 for this patient based on another announcement on the same website. In the announcement titled ‘Possible exposure locations and times of the 15th confirmed case’, it says, ‘the patient was symptomatic on 14 February’.
Step 3. Finally, following the updates on the website, we confirmed the event status of this patient as discharged on 3 March 2020.
Statistical analysis
Analyses were performed in R V.3.6.2 (R Foundation for Statistical Computing) through RStudio V.1.2.5042. Data and code are available online at GitHub (https://github.com/GuTian-TianGu/COVID-19_NCCstudy). Baseline clinical characteristics were shown as mean (SD), median (range) or number (%), with a comparison of characteristics in subjects stratified by case and control via the non-parametric Mann-Whitney U test for continuous variables and χ2 or Fisher’s exact test for binary variables.
In order to use the time-to-event information under the NCC design, the inverse probability-weighted Cox proportional hazard regression model was employed.31 The matching between cases and controls, as well as the relative weights were simultaneously obtained via KMprob function in multiple NCC R package32 by specifying the Kaplan-Meier type weights with additional matching on gender and age 1 year old. Only survivors were assigned weights since all cases (deaths) were included as designed with a weight of one. A total of 113 survivors (mean age 46.5) with sampling probabilities of zero were considered ‘fail to match’ and excluded from the study, mainly due to younger age than cases. A majority of the excluded patients were from Shandong Province (38.1%) due to the relatively high representation of the sample (detailed information of excluded survivors is available online at GitHub). In a sensitivity analysis adjusting for Shandong Province (results not shown here), we observed the consistent results as the main analysis.
The comorbidity score, ranging from zero to nine, was defined as the summation of nine comorbidities that have been specifically mentioned in relation to COVID-19 outcomes (CHD, hypertension, cardiac failure, cerebral infarction, chronic bronchitis, COPD, diabetes, renal failure and history of surgery). The Kaplan-Meier curve was plotted to check the proportional hazard assumption, and the Pearson correlation test was used to rule out the multicollinearity concern before fitting any model. Univariate weighted Cox models were performed for each comorbidity. The multivariate weighted Cox model was used to determine if pre-existing comorbidity yielded prognostic hazard information. We included those comorbidities that were marginally significant (p<0.1) in the univariate analysis to the multivariate model. Other than the common risk factors (age and gender), the multivariate model also adjusted for the early period of the pandemic (after vs before 22 January 2020, when no intervention was taken by the government).22 Although matching was based on age and gender, we adjusted for the matching covariates since the matching was broken with inverse probability weighting.31 A separate multivariate model was built by using the comorbidity score as a continuous predictor, adjusting for the same covariates. Hazard ratios (HRs) from the weighted Cox model were reported along with 95% CIs and p values. Sensitivity analysis was performed using multivariate logistic regression to provide estimated odds ratio (ORs), which included the same covariates as the multivariate weighted Cox model.
Weighted Cox model-based survival estimates were plotted for a sample patient profile (65-year-old woman with no other comorbidities) to compare the survival probability over time with and without CHD. The log-rank test was used to compare the median survival difference.
Results
Sample description
Table 1 summarises patient demographics and pre-existing medical conditions. Results are presented for all patients in the study (n=275) as well as for cases (n=94) and controls (n=181), respectively. Patients were 24 to 94 years old (Meanage=66.4, SDage=14.5). The average age tended to be older in the case group (70.7 years old) than in the control group (64.2 years old). Median ages were similar to mean ages in both groups. A majority (62.9%) of the patients were men. Overall, 25.5% of the total patients had clinical symptoms associated with COVID-19 before 22 January 2020. A relatively small proportion of the total sample had COPD, renal failure, history of surgery and hepatic failure (4.4%, 4.4%, 3.6% and 1.1%, respectively). Among all pre-existing comorbidities with over 5% of the total sample, hypertension was the most common (39.6%), followed by diabetes (26.2%), CHD (14.5%), cardiac failure (8%), cerebral infarction (6.9%) and chronic bronchitis (6.9%). Patients in the case group had more CHD (p<0.001) and more cerebral infarction (p=0.05) than those in the control group. The mean comorbidity score was 1.22 (SD=1.21) in the overall sample and 1.6 (SD=1.32) in the case and 1.02 (SD=1.10) in the control group (p<0.001).
Table 1.
Patientcharacteristics, stratified by survival status*
| Overall | Case (deaths) | Control (survivors) | P value | |
| (N=275), n (%) | (N=94), n (%) | (N=181), n (%) | ||
| Matching variables | ||||
| Age | ||||
| Mean (SD) | 66.4 (14.5) | 70.7 (13.3) | 64.2 (14.7) | <0.001 |
| Median (IQR) | 68.0 (22) | 72.5 (16) | 67.0 (22) | NA |
| Male | 173 (62.9) | 56 (59.6) | 117 (64.6) | 0.49 |
| Other covariates | ||||
| Before 22 January 2020 | 70 (25.5) | 27 (28.7%) | 43 (23.8) | 0.52 |
| History of surgery | 10 (3.6) | 4 (4.3) | 6 (3.3) | 0.74 |
| Cardiocerebrovascular diseases | ||||
| Hypertension | 109 (39.6) | 42 (44.7) | 67 (37.0) | 0.27 |
| CHD | 40 (14.5) | 25 (26.6) | 15 (8.3) | <0.001 |
| Cardiac failure | 22 (8.0) | 10 (10.6) | 12 (6.6) | 0.35 |
| Cerebral infarction | 19 (6.9) | 11 (11.7) | 8 (4.4) | 0.05 |
| Endocrine diseases | ||||
| Diabetes | 72 (26.2) | 26 (25.4) | 46 (27.7) | 0.80 |
| Respiratory diseases | ||||
| Chronic bronchitis | 19 (6.9) | 7 (7.4) | 12 (6.6) | 1.00 |
| COPD | 12 (4.4) | 7 (7.4) | 5 (2.8) | 0.14 |
| Other diseases | ||||
| Renal failure | 12 (4.4) | 6 (6.4) | 6 (3.3) | 0.38 |
| Hepatic failure | 3 (1.1) | 3 (3.2) | 0 (0) | 0.07 |
| Comorbidity score | ||||
| Mean (SD) | 1.22 (1.21) | 1.60 (1.32) | 1.02 (1.10) | <0.001 |
Bold: statistically significant using threshold p<=0.05.
*Mean (SD) is reported for the continuous variables and the counts (%) for categorical variables. P values were calculated by the Mann-Whitney U test, χ2 test or Fisher’s exact test, as appropriate.
CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; NA, not applicable.
Model results
Results of the Pearson correlation test showed no significant correlations with the presence of comorbidities of interest, and the assumption of the proportional hazard was not violated.
Table 2 presents the results of univariate and multivariate weighted Cox models. Older age was associated with significantly higher risk of death with similar magnitude in univariate and multivariate models. In the adjusted model, every 1-year increase in age was associated with an estimated 4% higher risk of death (p<0.001). No significant hazard difference was found between male and female patients. Disease infection during the early no-intervention period was associated with a higher risk of death but was not statistically significant.
Table 2.
Univariate and multivariate model result from weighted Cox proportional hazard regression
| Characteristic | Univariate | Multivariate | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.05 (1.0 to 1.1) | <0.001 | 1.04 (1.02 to 1.07) | <0.001 | 1.04 (1.02 to 1.07) | <0.001 | 1.04 (1.02 to 1.06) | <0.001 |
| Male | 0.76 (0.5 to 1.2) | 0.24 | 1.05 (0.67 to 1.65) | 0.74 | 1.09 (0.70 to 1.70) | 0.71 | 0.997 (0.62 to 1.60) | 0.99 |
| Before 22 January 2020 | 1.12 (0.7 to 1.8) | 0.66 | 1.28 (0.79 to 2.07) | 0.29 | 1.20 (0.73 to 1.95) | 0.48 | 1.21 (0.74 to 1.98) | 0.45 |
| Comorbidity score | 1.50 (1.27 to 1.75) | <0.001 | 1.31 (1.11 to 1.54) | 0.001 | NA | NA | NA | NA |
| Cardiocerebrovascular | ||||||||
| CHD | 4.19 (2.5 to 7.1) | <0.001 | NA | NA | 2.93 (1.74 to 4.92) | <0.001 | 3.01 (1.82 to 4.98) | <0.001 |
| Hypertension | 1.37 (0.9 to 2.2) | 0.17 | NA | NA | NA | NA | NA | NA |
| Cardiac failure | 1.85 (0.9 to 3.9) | 0.10 | NA | NA | NA | NA | NA | NA |
| Cerebral infarction | 2.86 (1.4 to 5.8) | 0.004 | NA | NA | NA | NA | 1.90 (0.94 to 3.8) | 0.07 |
| Respiratory | ||||||||
| Chronic bronchitis | 1.05 (0.4 to 2.5) | 0.55 | NA | NA | NA | NA | NA | NA |
| COPD | 2.61 (1.2 to 5.6) | 0.01 | NA | NA | NA | NA | 1.85 (0.89 to 3.85) | 0.10 |
| Endocrine | ||||||||
| Diabetes | 1.14 (0.7 to 1.9) | 0.61 | NA | NA | NA | NA | NA | NA |
| Others | ||||||||
| Renal failure | 2.30 (0.9 to 6.0) | 0.09 | NA | NA | NA | NA | 2.02 (0.81 to 5.07) | 0.13 |
| History of surgery | 1.71 (0.6 to 5.1) | 0.34 | NA | NA | NA | NA | NA | NA |
HR=hazard ratio
CHD=coronary heart disease
COPD=chronic obstructive pulmonary disease
Bold: statistically significant using threshold p<=0.05
CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; NA, not applicable.
In a separate model using the comorbidity score as a predictor, we observed that a higher comorbidity score was associated with a higher mortality risk in both unadjusted and adjusted models (p=0.009 and p=0.03, respectively). All pre-existing comorbidities had an HR over 1 in the univariate model, of which CHD had the largest HR of 4.2 (p<0.001), followed by cerebral infarction (HR=2.9, p=0.004), COPD (HR=2.6, p=0.01), renal failure (HR=2.3, p=0.09), cardiac failure (HR=1.9, p=0.1), history of surgery (HR=1.7, p=0.34), hypertension (HR=1.4, p=0.17), diabetes (HR=1.1, p=0.61) and chronic bronchitis (HR=1.1, p=0.55), but not all were statistically significant. After adjusting for age, gender and the early period of the pandemic in China, CHD was the only comorbidity that yielded a significant mortality risk: patients who had COVID-19 with pre-existing CHD had an estimated 2.9 times higher risk of death than those without CHD. In addition, cerebral infarction, COPD and renal failure all had an estimated HR of around 2.0. Similar results were observed by using unweighted logistic regression in a sensitivity analysis (online supplemental table S2).
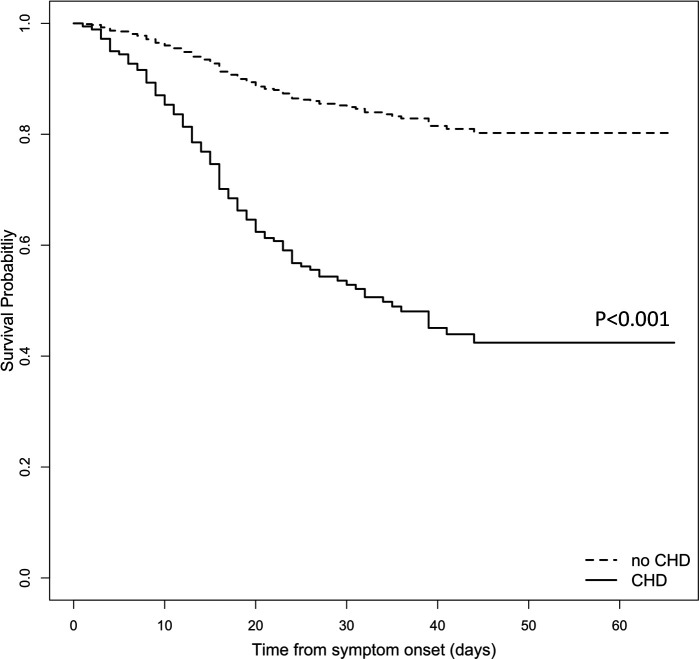
The overall median follow-up was 40 days, during which 94 deaths were observed. Figure 2 shows the estimated survival probability over 70 days for a sample patient profile with and without CHD (65-year-old woman with no other comorbidities). For such a patient profile, having pre-existing CHD led to a significantly shorter survival probability over time compared with those without CHD (p<0.001). For those with CHD, the estimated 30-day survival probability was 0.53 (95% CI 0.34 to 0.82); on the other hand, this number was 0.85 (95% CI 0.79 to 0.91) for those without CHD.
Figure 2.
Estimated survival probability over time from the adjusted Cox proportional hazard model for an example patient profile (65-year-old woman without CHD (dashed line) or with CHD (solid line), who had no other comorbidities). The estimated 30-day survival probability was 0.53 (95% CI 0.34 to 0.82) for patients with pre-existing CHD, while 0.85 (95% CI 0.79 to 0.91) for those without (p<0.001). CHD, coronary heart disease.
Discussion
In our research, based on publicly reported confirmed cases and adjusted for the confounding effect of age, gender and the early period of the pandemic when no intervention was taken, we used survival analysis to estimate the fatality risk of pre-existing comorbidities in COVID-19. There were three major findings: first, poor health condition was associated with higher mortality risk of COVID-19. Second, after adjusting for confounders, CHD was the only significant risk factor for COVID-19 mortality. Patients with pre-existing CHD were 3.11 times more likely to die than those without CHD (p<0.001). For one patient profile (65-year-old woman without other comorbidities), we saw an estimated 30-day survival probability of 0.85 (p<0.001). However, for those with CHD, the 30-day survival probability was 0.53. Third, older age was associated with an increased risk of death. Specifically, every 1-year increase in age was associated with a 4% increased risk of mortality (p<0.001).
To the best of our knowledge, the present study is the first to provide substantial statistical evidence showing the effect of CHD in predicting mortality for COVID-19. This result is consistent with previous studies that found higher CFR among patients with cardiovascular disease.9 15 It is worth pointing out that the existing studies9 16 that investigate prognostic factors for death used χ2 tests or univariate logistic regression that does not control for potential confounders. In contrast, by conducting weighted Cox proportional hazard regression models, our study used time-to-event outcomes, which offer more survival-time-related information to help guide the clinical intervention and more statistical power to detect risk factors.33 34
Previous studies have indicated that cardiovascular events following pneumonia may increase the risk of mortality,4 35–40 which explained our findings from the viewpoint of pathophysiological mechanisms. One potential mechanism underlying the association between pneumonia and cardiovascular events is inflammation.37 Specifically, the inflammatory reaction following pneumonia can result in plaque instability and damage in the blood vessels. Evidence of elevated local inflammation in the atherosclerotic coronary arteries following acute systemic infections has been shown in many studies.37 39 Thus, infections may result in heightened loading that is imposed on cardiomyocytes and lead to sympathetic hyperactivity or ischaemia, which may increase the risk of arrhythmia and heart failure in patients who had COVID-19 with pre-existing CHD.36
Given the limited understanding of the prognostic factors for COVID-19, more research, including potentially prospective studies, is needed to investigate the mechanism by which pre-existing CHD may influence the survival probability of patients with COVID-19. From the clinical point of view, early evaluation of a patient’s medical history is necessary to implement early medical interventions and decrease the mortality risk. We suggest monitoring the dynamic heart rate for patients with pre-existing CHD. For those severe symptomatic patients who had pre-existing heart ischaemia and abnormal heart function, early medical intervention may be needed.40
Furthermore, our results indicated that the risk of death from COVID-19 was significantly higher in older patients. Adjusting for others, every 1-year increase in age was associated with a 4% increased risk of death, which is similar to what was found in previous studies.8 9 16 Alongside the evidence of prognostic risk in CHD, we suggest that extra care is needed for those with CHD, especially for elderly patients.
Previous studies yielded mixed results regarding gender differences in mortality risk of COVID-19. Some studies found male sex was associated with a higher risk of death from COVID-19,41 whereas other studies did not find gender to be a significant factor predicting the mortality risk of COVID-19.10 16 In the current study, gender was not a significant mortality risk factor for COVID-19. More research is needed to further our understanding of gender differences in the outcome of COVID-19 and underlying mechanisms.
The design of excluding patients from Hubei Province was based on the concerns of unknown confounders caused by insufficient medical resources in the epicentre. Centers for Disease Control and Precention (CDC)’s report has pointed out that the rapidly increasing number of infections could easily crash the healthcare system by exceeding its maximum capacity.42 Therefore, analysing patient data outside of Hubei Province avoided the competing mortality risk caused by insufficient healthcare resources and revealed the true underlying impact of pre-existing comorbidities on COVID-19 mortality.43
This study has several limitations. It is worth noting that the data were collected when COVID-19 was spreading rapidly in China and the health authorities and researchers had limited understanding of the incubation period, modes of viral transmission and effective treatment. Whether our findings can be generalised to later epidemic phases warrants future research. One limitation of the present study lies in the nature of publicly reported data. Researchers have pointed out that severe cases may be over-represented in publicly reported data.44 Nevertheless, we have managed to reduce the potential bias caused by severe case over-representation by appropriately matching the cases and controls in the NCC design. Following the NCC design, we allowed the controls to be matched with cases on age 1 year old instead of the exact matching, which caused the significant age difference between two groups (table 1). Thus, we adjusted for the matching covariates in all the models to address this.31 The auto-matching procedure via the statistical programme also prevented the possibility of tendentiously selecting survivors with comorbidity-free history during data collection. In addition, NCC design is favoured in our situation where the risk factor data and event of interest can be identified opportunistically from publicly reported confirmed cases.30 Therefore, NCC was the optimal choice, given the restricted availability of public data. Moreover, due to the lack of information of treatment in the health reports published by the local health commission websites, we did not include treatment information into analysis, which may produce confounding effects. It calls for future research to investigate the mortality risk effect of pre-existing comorbidities by adding treatment as a covariate in the model. Finally, we were not able to verify the missing at random assumption of the 18 missing deaths (four from Heilongjiang, five from Henan, five from Beijing, two from Hunan, one from Liaoning and one from Yunnan) as well as the survivors.
In conclusion, our findings provided preliminary yet strong evidence supporting the association between pre-existing CHD and mortality risk for patients with COVID-19. Based on our findings, close monitoring, extra care and early medical intervention are needed for patients with pre-existing CHD to reduce the mortality risk associated with COVID-19.
Supplementary Material
Footnotes
Twitter: @GuTian_TianGu
Contributors: TG conducted the analysis, interpreted the data, drafted the article and rechecked the transcribed manuscript. QC conducted the literature review and drafted the article. ZY guided and supervised the statistical analysis. BF helped with data analysis. AL helped with literature review, data collection, management and the data quality check, and was responsible for reference organization. LX provided clinical support and interpretation of the results. RW collected, preprocessed and managed data, and conducted preliminary data analysis. YH guided and supervised the research process. RW and YH provided funding support. All authors critically revised the manuscript for intellectual content, approved the final draft and agreed to accountability for all aspects of the work.
Funding: This project was funded by the National Natural Science Foundation of China (No. 71874111), China Ministry of Education Key Research Institute of Humanities and Social Sciences at Universities (No. 17JJD790008), Shanghai Municipal Health Bureau Foundation (No. 201740116), and Shanghai Jiao Tong University Scientific and Technological Innovation Funds (YG2020YQ01, YG2020YQ06).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patient consent for publication: Exempt.
Ethics approval: The data published in news reports and websites were open to the public and free of identifiers. The study was approved by Shanghai Jiao Tong University Public Health and Nursing Medical Research Ethics Committee (SJUPN-202001).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Raw data are available at https://github.com/GuTian-TianGu/COVID-19_NCCstudy.git.
References
- 1.Coronavirus disease 2019 (COVID-19) situation report – 193. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200731-covid-19-sitrep-193.pdf?sfvrsn=42a0221d_4
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao-Wei X, Xiao-Xin W, JiangXian-Gao XK-J, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m792. 10.1136/bmj.m792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 8.Guan W-jie, Ni Z-yi, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med Overseas Ed 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020. 10.1001/jama.2020.2648. [Epub ahead of print: 24 Feb 2020]. [DOI] [PubMed] [Google Scholar]
- 10.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Tu C, Zhu M, et al. Exploring the law of development and prognostic factors of common and severe COVID-19: a retrospective case-control study in 122 patients with complete course of disease. SSRN Journal 2020. 10.2139/ssrn.3555209 [DOI] [Google Scholar]
- 13.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol 2020;5:425–7. 10.1016/S2468-1253(20)30076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng S-Q, Peng H-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med 2020;9:575 10.3390/jcm9020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009;40:1082–90. 10.1161/STROKEAHA.108.540781 [DOI] [PubMed] [Google Scholar]
- 18.Camp PG, Goring SM. Gender and the diagnosis, management, and surveillance of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:686–91. 10.1513/pats.200706-081SD [DOI] [PubMed] [Google Scholar]
- 19.Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 2018;320:1319–20. 10.1001/jama.2018.12440 [DOI] [PubMed] [Google Scholar]
- 20.Prasad S, Sung B, Aggarwal BB. Age-Associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med 2012;54 Suppl:S29–37. 10.1016/j.ypmed.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinese COVID-19 outbreak distribution system. Available: http://2019ncov.chinacdc.cn/2019-nCoV/
- 22.Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA 2020;323:1915–23. 10.1001/jama.2020.6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azoulay L, Dell'Aniello S, Gagnon B, et al. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiology Biomarkers & Prevention 2011;20:337–44. 10.1158/1055-9965.EPI-10-0940 [DOI] [PubMed] [Google Scholar]
- 24.Schlienger RG, Fedson DS, Jick SS, et al. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy 2007;27:325–32. 10.1592/phco.27.3.325 [DOI] [PubMed] [Google Scholar]
- 25.Lipscombe LL, Lévesque LE, Gruneir A, et al. Antipsychotic drugs and the risk of hyperglycemia in older adults without diabetes: a population-based observational study. Am J Geriatr Psychiatry 2011;19:1026–33. 10.1097/JGP.0b013e318209dd24 [DOI] [PubMed] [Google Scholar]
- 26.Lipscombe LL, Gomes T, Lévesque LE, et al. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 2007;298:2634–43. 10.1001/jama.298.22.2634 [DOI] [PubMed] [Google Scholar]
- 27.Lévesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med 2005;142:481–9. 10.7326/0003-4819-142-7-200504050-00113 [DOI] [PubMed] [Google Scholar]
- 28.Cerfolio RJ, Bryant AS. Survival and outcomes of pulmonary resection for non-small cell lung cancer in the elderly: a nested case-control study. Ann Thorac Surg 2006;82:424–30. 10.1016/j.athoracsur.2006.02.085 [DOI] [PubMed] [Google Scholar]
- 29.Khandaker G, Rashid H, Zurynski Y, et al. Nosocomial vs community-acquired pandemic influenza A (H1N1) 2009: a nested case-control study. J Hosp Infect 2012;82:94–100. 10.1016/j.jhin.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 30.Langholz B, Clayton D. Sampling strategies in nested case-control studies. Environ Health Perspect 1994;102 Suppl 8:47–51. 10.1289/ehp.94102s847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Støer NC, Samuelsen SO. Inverse probability weighting in nested case-control studies with additional matching--a simulation study. Stat Med 2013;32:5328–39. 10.1002/sim.6019 [DOI] [PubMed] [Google Scholar]
- 32.Stoer N, Samuelsen S, multipleNCC . Weighted Cox-Regression for nested case-control data. R package version 1.2-2.2020. Available: https://CRAN.R-project.org/package=multipleNCC
- 33.George B, Seals S, Aban I. Survival analysis and regression models. J Nucl Cardiol 2014;21:686–94. 10.1007/s12350-014-9908-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annesi I, Moreau T, Lellouch J. Efficiency of the logistic regression and COX proportional hazards models in longitudinal studies. Stat Med 1989;8:1515–21. 10.1002/sim.4780081211 [DOI] [PubMed] [Google Scholar]
- 35.Tralhão A, Póvoa P. Cardiovascular events after community-acquired pneumonia: a global perspective with systematic review and meta-analysis of observational studies. J Clin Med 2020;9:414. 10.3390/jcm9020414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 37.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–13. 10.1056/NEJMra1216063 [DOI] [PubMed] [Google Scholar]
- 38.Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J 2007;34:11–18. [PMC free article] [PubMed] [Google Scholar]
- 39.Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005;45:1585–93. 10.1016/j.jacc.2005.01.054 [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Zhang X, Shi G, et al. Atrial fibrillation is an independent risk factor for hospital-acquired pneumonia. PLoS One 2015;10:e0131782. 10.1371/journal.pone.0131782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146:110–8. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qualls N, Levitt A, Kanade N, et al. Community mitigation guidelines to prevent pandemic influenza - United States, 2017. MMWR Recomm Rep 2017;66:1–34. 10.15585/mmwr.rr6601a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji Y, Ma Z, Peppelenbosch MP, et al. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health 2020;8:e480. 10.1016/S2214-109X(20)30068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020;172:577–82. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038976supp001.pdf (118.1KB, pdf)