Abstract
Background
There is a limited understanding of the impact of coronavirus disease 2019 (COVID-19) on the Latinx population. We hypothesized that Latinx patients would be more likely to be hospitalized and admitted to the intensive care unit (ICU) than White patients.
Methods
We analyzed all patients with COVID-19 in 12 Massachusetts hospitals between February 1 and April 14, 2020. We examined the association between race, ethnicity, age, reported comorbidities, and hospitalization and ICU admission using multivariable regression.
Results
Of 5190 COVID-19 patients, 29% were hospitalized; 33% required the ICU, and 4.3% died. Forty-six percent of patients were White, 25% Latinx, 14% African American, and 3% Asian American. Ethnicity and race were significantly associated with hospitalization. More Latinx and African American patients in the younger age groups were hospitalized than whites. Latinxs and African Americans disproportionally required the ICU, with 39% of hospitalized Latinx patients requiring the ICU compared with 33% of African Americans, 24% of Asian Americans, and 30% of Whites (P < .007). Within each ethnic and racial group, age and male gender were independently predictive of hospitalization. Previously reported preexisting comorbidities contributed to the need for hospitalization in all racial and ethnic groups (P < .05). However, the observed disparities were less likely related to reported comorbidities, with Latinx and African American patients being admitted at twice the rate of Whites, regardless of such comorbidities.
Conclusions
Latinx and African American patients with COVID-19 have higher rates of hospitalization and ICU admission than White patients. The etiologies of such disparities are likely multifactorial and cannot be explained only by reported comorbidities.
Keywords: COVID-19, disparities, ethnicity, hospitalizations, race, SARS-CoV-2
The health, societal, and economic impacts of coronavirus disease 2019 (COVID-19) have been felt worldwide, with nearly 12 million confirmed infections, leading to more than 500 000 deaths by early July 2020, with numbers continuing to grow [1–3]. Studies from China, Italy, Spain, and the United States have identified several factors associated with symptomatic infection and hospitalization, with or without admission to intensive care units (ICUs) [4–9]. Older age has been shown to significantly increase the risk for hospitalization and death [10, 11]. Hypertension, heart disease, obesity, and diabetes are common comorbidities associated with hospitalization in COVID-19 patients [2, 5, 12, 13]. Most of these published reports either describe the disease in relatively homogenous ethnic and racial populations [5] or they do not compare COVID-19-infected patients who did and did not require hospitalization [2].
In the United States, where a racially and ethnically diverse population has been exposed to infection in the setting of known racial and ethnic health disparities [14], several news reports have suggested that ethnic and racial minorities, especially Latinx and non-Latin African American individuals, may bear a higher burden of disease during the COVID-19 pandemic [15–19]. These reports also propose that such disparities are due to higher rates of preexisting comorbidities in Latinx and non-Latin African American patients. Other than these limited reports, the association between ethnicity and race and reported preexisting comorbidities as risk factors for hospitalization and ICU admission in COVID-19 patients has yet to be examined in a large ethnically and racially diverse population. In this paper, we hypothesized that there are ethnicity- and race-related disparities in hospitalization and ICU admission for COVID-19 patients regardless of age and reported preexisting comorbidities. We used medical records available from the largest not-for-profit health care system in Massachusetts to examine the association between age, race and ethnicity, reported preexisting comorbidities, and the need for hospitalization and ICU admission in a large study population of COVID-19-positive patients.
METHODS
Data Source
Mass General Brigham is a not-for-profit health care system affiliated with Harvard Medical School that comprises 12 hospitals across eastern Massachusetts. These hospitals include Massachusetts General Hospital (Boston), Brigham and Women’s Hospital (Boston), Brigham and Women’s Faulkner Hospital (Boston), Massachusetts Eye and Ear Infirmary (Boston), Spaulding Rehabilitation Network (Boston, Cambridge), McLean Hospital (Belmont), Cooley Dickinson Hospital (Northampton), Martha’s Vineyard Hospital (Oak Bluffs), Nantucket Cottage Hospital (Nantucket), Newton-Wellesley Hospital (Newton), North Shore Medical Center (Salem, Lynn, Danvers), and Wentworth-Douglass Hospital (Dover). The system cares for about one-third of patients in Massachusetts.
Patient Consent Statement
The design of the study was approved by the Mass General Brigham Institutional Review Board (IRB), which deemed that the study does not include factors necessitating patient consent.
Data Collection
We used data reporting functions available through the electronic health record (Epic Systems, Verona, WI, USA) shared by all Mass General Brigham health care system institutions. We collected data on all patients 18 years or older who tested positive for COVID-19 during an inpatient, outpatient, or emergency room visit between February 1, 2020, and April 14, 2020. We revisited the records on April 25, 2020, to collect follow-up data on mortality and other outcomes.
Patients who presented to Mass General Brigham institutions with symptoms of fever, cough, sore throat, fatigue, muscle aches, or new anosmia; who were exposed to someone who tested positive for COVID-19; or who were referred by a health care provider were tested per specified testing criteria/guidelines set forth by the institution. Patients were diagnosed as infected with COVID-19 if SARS-CoV-2 RNA was detected in upper or lower respiratory specimens by nucleic acid testing (NAT) assays designated for emergency use authorization (EUA) by the Food and Drug Administration (FDA) and in accordance with the Centers for Disease Control and Prevention (CDC) guidelines [20, 21]. Each assay targets at least 1 SARS-CoV-2 gene region; positive results are reported for each assay, as defined by the manufacturer or reference laboratory.
End Points
Hospitalization at any time during the course of the illness and admission to an ICU at any time during hospitalization were primary end points. Patients who were discharged home initially but admitted later were categorized as hospitalized patients. Patients hospitalized for longer than the follow-up period were censored for study outcomes.
Covariates
We extracted the following covariates from the electronic health records for all patients: age, gender, patient-reported race (White, African American, Asian American or Pacific Islander, other, or unknown), patient-reported ethnicity (Latin or non-Latin), smoking status, and the presence of recorded metabolic diseases including obesity (as measured by body mass index [BMI]), diabetes mellitus, and hyperlipidemia. The presence of organ-specific disease included hypertension, coronary heart disease, congestive heart failure, chronic obstructive pulmonary disease, asthma, interstitial lung disease, cerebrovascular disease, chronic kidney disease, end-stage renal disease, malignancy including hematologic malignancy (lymphoma, leukemia), HIV, and history of organ or bone marrow transplantation. Missing data were imputed by multiple imputation using the Amelia package in R [22]. The multiple-imputation models used all baseline data. BMI and smoking status for all patients were also imputed by multiple-imputation models using R, and 10 imputations were carried out in total under the assumption that data were missing at random. Data for median household income and population density were obtained from Census data reported by the Census Bureau for the state of Massachusetts and were linked to patient by zip code of reported residential address [23]. Because immunocompromised patients generally demonstrate increased susceptibility to respiratory viral infections, we analyzed our study population of patients for history of solid organ transplantation (SOT), lymphoma, leukemia, or HIV to assess whether these factors were associated with hospitalization or ICU admission.
Statistical Analysis
As appropriate, descriptive analyses of variables are presented as proportions or medians with interquartile ranges (IQRs) for all end points (not hospitalized, hospitalized, admitted to the ICU). Categorical data were compared using chi-square tests, while the t test was used for continuous variables to identify univariable associations. Multivariable logistic regression models were constructed to identify factors associated with hospitalization and ICU admission. We tested our assumption that data were missing at random by constructing logistic regression models for missingness using all of the variables included in multiple imputations. We further examined the association between racial and ethnic background and hospital admission by comparing proportions of end points for each 5-year age interval from 18 to 90. We also examined the association between racial and ethnic background, preexisting comorbidities, and hospitalization or ICU admission by stratifying by number of baseline comorbidities (0 vs ≥1) and race and ethnicity. We further performed a sensitivity analysis with adjustment for socioeconomic status as a predictor for hospitalization/ICU admission for patients from Massachusetts state with median household income data available. Patients with median household income lower than the 20th percentile ($53 335) were classified as having low socioeconomic status as a dichotomous variable. Statistical significance was defined as P < .05 for all analyses, and all statistical analyses were completed using R, version 3.6.1 [24]. We graphed the geographic representation of the confirmed COVID patients during our study using Microsoft Excel, version 16.36.
RESULTS
Characteristics of all Hospitalized vs Nonhospitalized Patients Irrespective of Race or Ethnicity
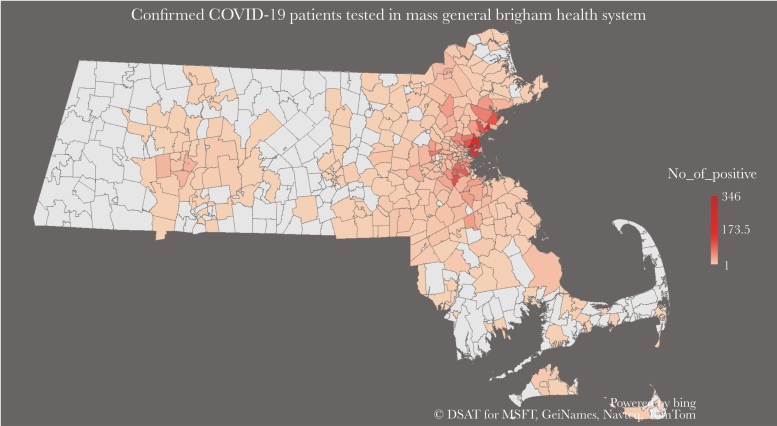
A total of 5190 patients were diagnosed with laboratory-confirmed COVID-19 in the time frame of the study and were included in our analysis (Figure 1). Out of the total study population, 1489 (28.6%) were hospitalized. Overall, hospitalized patients were more likely to be male (56% vs 42%; P < .001) and older (median, 62 vs 47 years; P < .001) compared with nonhospitalized patients. Hospitalized patients were also more likely to be obese (34% vs 17% BMI 30–40 kg/m2; P < .001), with on average more cardiovascular and pulmonary risk factors and more comorbid conditions compared with nonhospitalized patients (Table 1). The most common comorbidities in the hospitalized study population were hypertension (48%), hyperlipidemia (36%), diabetes (33%), and obstructive lung disease (15%). The mortality rate was higher in hospitalized compared with nonhospitalized patients (15% vs 0.2%; P < .001). Our test of the missing at random assumption demonstrated significant predictors of missingness for all variables for which imputation was conducted.
Figure 1.
Zip code distribution of laboratory-confirmed COVID-19 patients at the 12 hospitals comprising the Mass General Brigham health care system in Massachusetts.
Table 1.
Characteristics of All COVID-19-Positive Patients in the Mass General Brigham Health Care System
| All Patients (n = 5190) | Hospitalized (n = 1489) | Not Hospitalized (n = 3701) | P Value | |
|---|---|---|---|---|
| Median age (IQR), y | 52 (36–66) | 62 (50–76) | 47 (33–60) | <.001 |
| Age categories | <.001 | |||
| 18–40 y | 1617 (31) | 201 (13.5) | 1416 (38) | |
| 41–60 y | 1867 (36) | 487 (33) | 1380 (37) | |
| >60 y | 1706 (33) | 801 (54) | 905 (25) | |
| Male gender | 2378 (46) | 840 (56) | 1538 (42) | <.001 |
| Race | <.001 | |||
| White | 2404 (46) | 620 (42) | 1784 (48) | |
| Latinx | 1309 (25) | 470 (32) | 839 (23) | |
| African American | 719 (14) | 209 (14) | 510 (14) | |
| Others | 581 (11) | 140 (9) | 441 (12) | |
| Asian American | 177 (3) | 50 (3.4) | 127 (3.4) | |
| Comorbidities | ||||
| BMIa | <.001 | |||
| ≤30 kg/m2 | 1844 (35.5) | 823 (55) | 1020 (27.5) | |
| 30–40 kg/m2 | 1143 (22) | 511 (34) | 632 (17) | |
| >40 kg/m2 | 232 (4.5) | 111 (7.5) | 122 (3) | |
| Smoking statusb | <.001 | |||
| Current | 213 (4) | 64 (4.3) | 149 (4) | |
| Former | 1090 (21) | 447 (30) | 643 (17) | |
| Never | 3044 (59) | 801 (54) | 2243 (61) | |
| Diabetes mellitus | 969 (19) | 497 (33) | 472 (13) | <.001 |
| Hyperlipidemia | 1273 (25) | 534 (36) | 739 (20) | <.001 |
| Hypertension | 1617 (31) | 712 (48) | 905 (24.5) | <.001 |
| Obstructive lung disease | 624 (12) | 225 (15) | 399 (11) | <.001 |
| Interstitial lung disease | 13 (0.3) | 11 (0.7) | 2 (0.05) | <.001 |
| Coronary artery disease | 257 (5) | 145 (10) | 112 (3) | <.001 |
| CHF | 136 (3) | 86 (6) | 50 (1.4) | <.001 |
| Cerebrovascular disease | 141 (3) | 73 (5) | 68 (2) | <.001 |
| Obstructive sleep apnea | 201 (4) | 81 (5.4) | 120 (3) | .0003 |
| CKD | 281 (5) | 184 (12) | 97 (2.6) | <.001 |
| Transplantation | 22 (0.4) | 17 (1) | 5 (0.1) | <.001 |
| Auto-immune diseases | 158 (3) | 61 (4) | 97 (2.6) | .007 |
| Malignancy | 342 (7) | 115 (8) | 227 (6) | .04 |
| Total comorbidities | <.001 | |||
| 0 | 2402 (46) | 434 (29) | 1968 (72) | |
| 1–2 | 1668 (32) | 519 (35) | 1149 (31) | |
| >2 | 1120 (22) | 536 (36) | 584 (16) | |
| Death | 225 (4.3) | 218 (15) | 7 (0.2) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; IQR, interquartile range.
aMissing for 38% of population.
bMissing for 16% of population.
Distribution of Race and Ethnicity Among Hospitalized Patients
Among the total COVID-19-positive patient study population, 2404 (46%) were White, 1309 were Latinx (25%), 719 were African American (14%), and 177 were Asian American (3%). Ethnicity and race were significantly associated with the rate of hospitalization. Latinx and African American patients were more likely to be admitted to the hospital than White patients (35.9% and 29.1%, respectively, vs 25.8% for White patients). Overall Latinx, African American, and Asian American hospitalized patients were younger compared with White patients (median age, 52, 60, and 61, vs 72, respectively; P < .001) (Table 2).
Table 2.
Baseline Characteristics by Race and Ethnicity Among Admitted COVID-19 Patients
| Characteristics | White (n = 620) | Latinx (n = 470) | African American (n = 209) | Asian American (n = 50) | Others (n = 140) | P Value |
|---|---|---|---|---|---|---|
| Median age (IQR), y | 72 (60–83) | 52 (41–65) | 60 (50–70) | 61 (46–73) | 60 (47–75) | <.0001 |
| Age category | <.0001 | |||||
| 18–40 y | 39 (6) | 106 (22) | 28 (13) | 7 (14) | 21 (15) | |
| 41–60 y | 124 (20) | 214 (46) | 79 (38) | 18 (36) | 52 (37) | |
| >60 y | 457 (74) | 150 (32) | 102 (49) | 25 (50) | 67 (48) | |
| Male gender | 343 (55) | 265 (56) | 111 (53) | 31 (62) | 90 (64) | .25 |
| Comorbidities | ||||||
| Last BMIa | .0002 | |||||
| ≤30 kg/m2 | 373 (60) | 219 (47) | 115 (55) | 38 (76) | 78 (56) | |
| 30–40 kg/m2 | 193 (31) | 194 (41) | 70 (33) | 9 (18) | 45 (32) | |
| >40 kg/m2 | 47 (8) | 37 (8) | 18 (9) | 0 (0) | 9 (6) | |
| Smoking statusb | <.0001 | |||||
| Current | 32 (5) | 16 (3) | 13 (6) | 1 (2) | 2 (1) | |
| Former | 273 (44) | 76 (16) | 57 (27) | 10 (20) | 31 (22) | |
| Never | 280 (45) | 308 (66) | 116 (56) | 33 (66) | 64 (46) | |
| Comorbidities | ||||||
| Diabetes mellitus | 208 (34) | 151 (32) | 80 (38) | 18 (36) | 40 (28) | .38 |
| Hyperlipidemia | 296 (48) | 126 (27) | 59 (28) | 21 (42) | 32 (23) | <.0001 |
| Hypertension | 363 (59) | 159 (34) | 115 (55) | 26 (52) | 49 (35) | <.0001 |
| Obstructive lung disease | 124 (20) | 49 (10) | 32 (15) | 8 (16) | 12 (8) | <.0001 |
| Interstitial lung disease | 7 (1) | 3 (0.6) | 1 (0.5) | 0 (0) | 0 (0) | .56 |
| Coronary artery disease | 98 (16) | 23 (5) | 11 (5) | 6 (12) | 7 (5) | <.0001 |
| CHF | 55 (9) | 10 (2) | 16 (8) | 2 (4) | 3 (2) | <.0001 |
| Cerebrovascular disease | 42 (7) | 12 (3) | 12 (6) | 1 (2) | 6 (4) | .02 |
| Obstructive sleep apnea | 52 (8) | 11 (2) | 11 (5) | 1 (2) | 6 (4) | .0003 |
| CKD | 114 (18) | 34 (7) | 24 (11) | 5 (10) | 7 (5) | <.0001 |
| Transplantation | 7 (1) | 8 (2) | 7 (3) | 0 (0) | 0 (0) | .07 |
| Auto-immune diseases | 36 (6) | 15 (3) | 8 (4) | 0 (0) | 2 (1) | .04 |
| Malignancy | 77 (12) | 19 (4) | 12 (6) | 0 (0) | 7 (5) | <.0001 |
| Total comorbidities | <.0001 | |||||
| 0 | 109 (18) | 198 (42) | 55 (26) | 15 (30) | 57 (41) | |
| 1–2 | 224 (36) | 151 (32) | 73 (35) | 16 (32) | 55 (39) | |
| >2 | 287 (46) | 121 (26) | 81 (39) | 19 (38) | 28 (20) | |
| Requiring critical care | 184 (30) | 182 (39) | 68 (33) | 12 (24) | 38 (27) | .007 |
| Outcome | ||||||
| Death | 140 (23) | 33 (7) | 27 (13) | 3 (6) | 15 (11) | <.0001 |
| Discharged to home | 259 (42) | 299 (64) | 115 (55) | 32 (64) | 92 (66) | <.0001 |
| Discharged to SNF | 82 (13) | 19 (4) | 15 (7) | 2 (4) | 8 (6) | <.0001 |
| Discharged to rehab/STF | 33 (5) | 18 (4) | 8 (4) | 3 (6) | 5 (3) | .69 |
| Still in hospital | 106 (17) | 101 (21) | 44 (21) | 10 (20) | 20 (14) | .19 |
| Length of stay, median (IQR), d | 8 (5–13) | 7 (4–14) | 7 (4–13) | 7.5 (5–12) | 7 (4–12) | .57 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, nody mass index; SNF, skilled nursing facility; STF, short-term facility.
aData missing for 3% of population.
bData missing for 12% of population.
Age, Reported Preexisting Comorbidities, and Hospitalization by Race
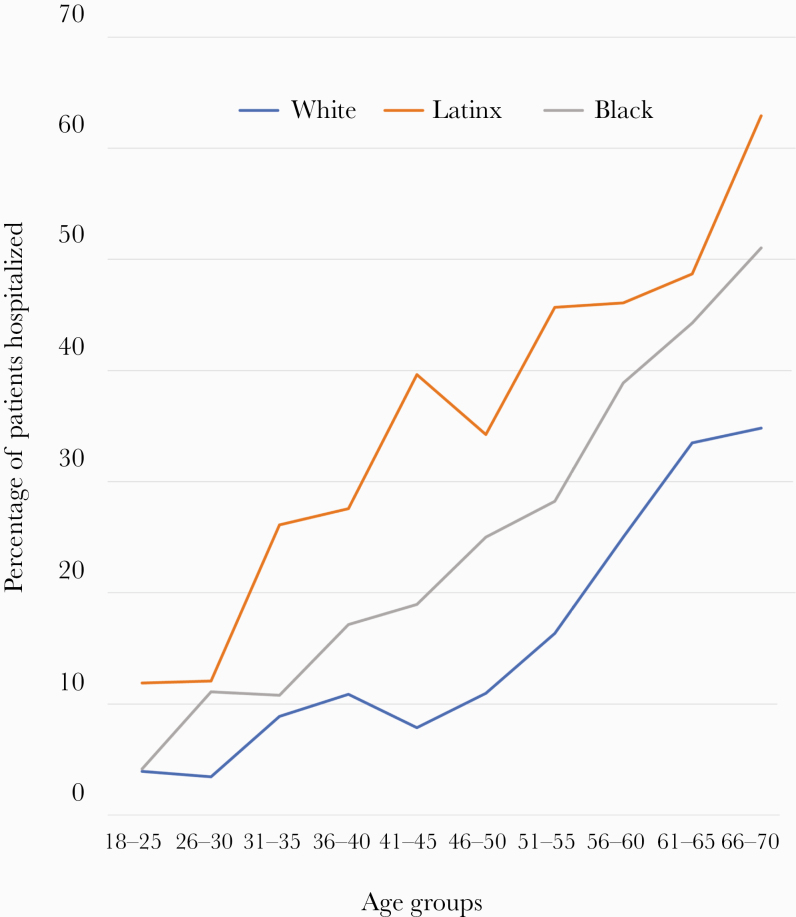
Subgroup analyses of the ages between 18 and 85 at 5-year intervals showed that in each age group, Latinx and African American patients were more likely to be admitted as a result of COVID-19 compared with White patients (P < .05 for each comparison) (Figure 2). For example, among those aged 18–40 and 40–60 years, Latinx and African American patients vs White patients were admitted to the hospital at the rates of 22% and 13% vs 6% and at 46% and 38% vs 20%, respectively (P < .001 for each comparison). We observed similar rates among those aged >60 years (Supplementary Table 1).
Figure 2.
Percentage of COVID-19 patients hospitalized by race and ethnicity and age.
With regard to reported comorbidities, hospitalized Latinx patients were more likely to be obese in the range of 30–40 kg/m2 (41% compared with 31% among White and 33% among African American patients; P < .001). However, the proportions of White, Latinx, and African American patients who were in the range of >40 kg/m2 were similar. White patients had higher rates of reported hyperlipidemia, hypertension, obstructive lung disease, coronary artery disease, congestive heart failure, chronic kidney disease, and malignancy compared with other groups (P < .05) (Table 2; Supplementary Table 2). When stratified by baseline reported comorbidities, Latinx and African American patients were admitted at twice the rate of Whites, regardless of whether they had reported preexisting comorbidities (P < .001) (Table 3).
Table 3.
Rate of Hospitalization Among all COVID-19 Patients by Baseline Comorbidities and Race and Ethnicity
| White (n = 2404) | Latinx (n = 1309) | African American (n = 719) | ||
|---|---|---|---|---|
| Comorbidity Count per Patient | Hospitalized | Hospitalized | Hospitalized | P Valuea |
| 0 | 109/939 (12) | 198/681 (29) | 55/319 (17) | <.0001 |
| ≥1 | 511/1465 (35) | 272/628 (43) | 154/400 (38) | .001 |
Data are presented as No. (%).
Abbreviation: COVID-19, coronavirus disease 2019.
aComparing the patients hospitalized by race.
Compared with nonhospitalized patients, univariable logistic regression stratified by race identified preexisting comorbidities including metabolic, cardiovascular, cerebrovascular, pulmonary, and kidney disease as predictors for hospitalization in all racial and ethnic groups (P < .05) (Supplementary Table 3). After adjustment for age, gender, baseline comorbidities, and racial and ethnic background in multivariable regression analysis, older age, male gender, diabetes (White: odds ratio [OR], 1.87; 95% CI, 1.40–2.50; Latinx: OR, 2.66; 95% CI, 1.70–4.14; African American: OR, 1.74; 95% CI, 1.01–3.01), chronic kidney disease (White: OR, 2.61; 95% CI, 1.78–3.83), interstitial lung disease (White: OR, 9.09; 95% CI, 1.02–81.32), and transplantation (African American: OR, 8.12; 95% CI, 1.46–45.21) were independently associated with hospitalization (Table 4). Most of these predictors remained significant after adjustment for socioeconomic status (Supplementary Table 4).
Table 4.
Multivariable Logistic Regression Model Predicting Hospital Admission Among Patients With COVID-19
| Variables | White (n = 2404) | Latinx (n = 1309) | African American (n = 719) |
|---|---|---|---|
| Age | |||
| 18–40 y | Reference | ||
| 41–60 y | 2.03 (1.37–3.01)* | 2.45 (1.83–3.28)* | 2.37 (1.41–4.00)* |
| >60 y | 5.81 (3.95–8.55)* | 6.89 (4.51–10.52)* | 6.09 (3.37–11.00)* |
| Gender | |||
| Female | Reference | ||
| Male | 1.44 (1.17–1.78)* | 1.73 (1.33–2.24)* | 1.77 (1.20–2.60)* |
| Smoking status | |||
| Never | Reference | ||
| Current | 1.10 (0.68–1.78) | 1.20 (0.66–2.19) | 1.23 (0.64–2.37) |
| Former | 1.20 (0.95–1.51) | 0.78 (0.54–1.12) | 2.01 (1.22–3.32)* |
| Last BMI | |||
| ≤30 kg/m2 | Reference | ||
| 30–40 kg/m2 | 0.85 (0.66–1.08) | 1.48 (1.12–1.97)* | 1.07 (0.68–1.67) |
| >40 kg/m2 | 1.41 (0.87–2.28) | 1.51 (0.87–2.61) | 2.07 (0.90–4.73) |
| Comorbidities | |||
| Diabetes mellitus | 1.87 (1.40–2.50)* | 2.66 (1.70–4.14)* | 1.74 (1.01–3.01)* |
| Hyperlipidemia | 0.93 (0.70–1.24) | 0.96 (0.61–1.52) | 0.63 (0.35–1.10) |
| Hypertension | 1.01 (0.75–1.37) | 1.20 (0.77–1.89) | 0.73 (0.45–1.32) |
| Obstructive lung disease | 1.30 (0.95–1.76) | 1.06 (0.65–1.71) | 1.01 (0.55–1.85) |
| Interstitial lung disease | 9.09 (1.02–81.32)* | 6.12 (0.56–66.47) | 3.3e + 6 (0.000–NA) |
| Coronary artery disease | 1.44 (0.99–2.09) | 1.16 (0.52–2.59) | 0.51 (0.14–1.78) |
| CHF | 1.13 (0.69–1.84) | 1.65 (0.49–5.61) | 2.22 (0.69–7.20) |
| Cerebrovascular disease | 1.22 (0.75–1.99) | 0.90 (0.34–2.37) | 1.10 (0.39–3.08) |
| Obstructive sleep apnea | 1.05 (0.68–1.63) | 0.45 (0.18–1.10) | 2.30 (0.78–6.84) |
| CKD | 2.61 (1.78–3.83)* | 1.93 (0.92–4.05) | 1.31 (0.61–2.83) |
| Transplantation | 0.77 (0.21–2.82) | 4.07 (0.89–18.52) | 8.12 (1.46–45.21)* |
| Auto-immune diseases | 1.12 (0.70–1.80) | 1.23 (0.53–2.83) | 0.99 (0.31–3.22) |
| Malignancy | 0.85 (0.60–1.21) | 1.08 (0.52–2.27) | 0.71 (0.28–1.76) |
| Total comorbidities | |||
| 0 | Reference | ||
| 1–2 | 1.69 (1.19–2.40)* | 0.90 (0.59–1.36) | 1.71 (0.92–3.22) |
| >2 | 1.50 (0.81–2.78) | 0.45 (0.18–1.10) | 2.67 (0.87–8.23) |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019.
*Statistically significant.
Median Household Income and Population Density by Patient Zip Code
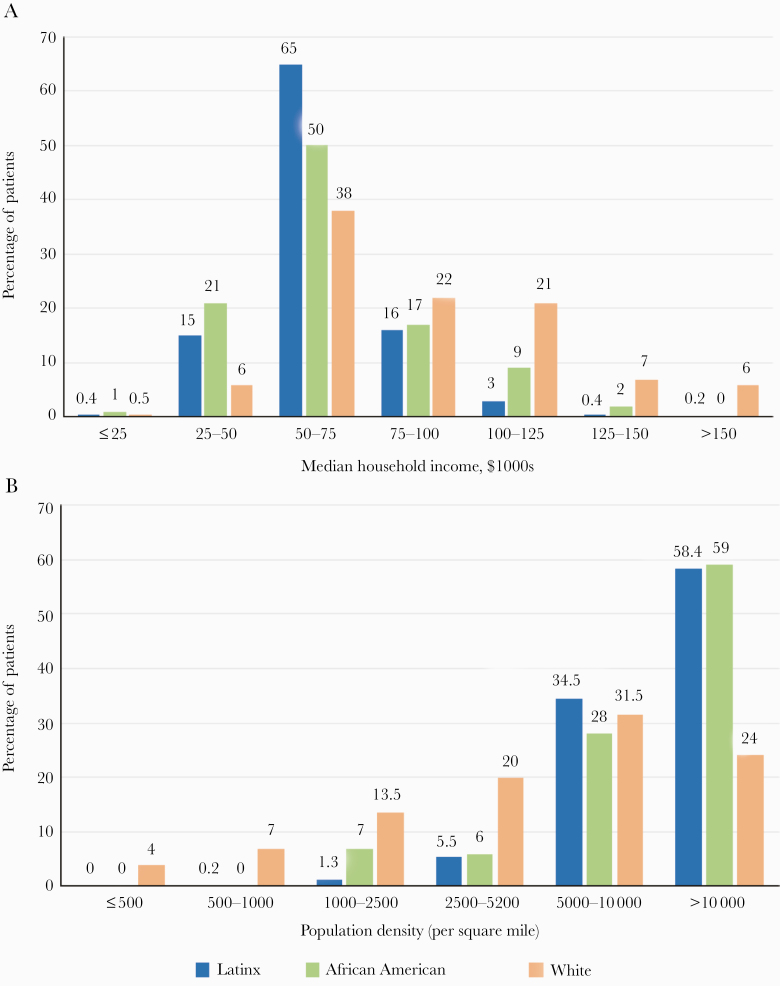
Based on patients’ zip code data, 43% of all the patients who tested positive lived in zip codes with a median income between $50 000 and $75 000; of these, 35% were hospitalized. A higher proportion of hospitalized Latinx and African American patients lived in these zip codes (65% and 50%, respectively, compared with 38% of White patients) (Figure 3A; Supplementary Table 7). Interestingly, smaller proportions of patients living in zip codes with income <$50 000 were hospitalized (Supplementary Table 6). In addition, residence in a zip code with greater density of living per household was corelated with a higher likelihood of hospitalization (Figure 3B; Supplementary Table 8). In our study population, 44% of admitted patients were from areas with a population density >10 000/square mile.
Figure 3.
A and B, Median household income and population density by patient zip code among COVID-19 patients admitted to the Mass General Brigham health care system in Massachusetts: The figure shows (A) household income and (B) population density per zip code distribution for Latinx, African American, and White patients who were admitted. The household income and population density reported were obtained from county-level census data.
Patients With a History of Solid Organ Transplant, Lymphoma, and HIV
A history of solid organ transplantation was associated with a significantly increased risk for hospitalization (P < .001). Of the 22 transplant patients who were COVID-19 positive (12 kidney recipients, 3 liver recipients, 4 heart recipients, 2 lung recipients, and 1 heart/lung recipient), 17 (77%) were admitted. In contrast, HIV, lymphoma, and a history of leukemia were not associated with increased risk for hospitalization or ICU admission (Supplementary Table 9). A possible difference between SOT patients and those with HIV or a history of lymphoma or leukemia is that SOT patients were universally immunosuppressed at the time of infection, while the degree of immune impairment for other groups was likely more heterogeneous.
Distribution of Race and Ethnicity Among ICU Admitted Patients
Latinx and African American patients disproportionally required admission to the ICU compared with White patients. Overall, 39% of hospitalized Latinx patients required admission to the ICU compared with 33% of African American patients, 24% of Asian American patients, and 30% of White patients (P < .007) (Table 2).
The presence of reported metabolic or organ-specific comorbidities was not significantly associated with need for ICU admission (Supplementary Table 5). In multivariable regression analysis, age >60 years (Latinx: OR, 2.71; 95% CI, 1.44–5.09; African American: OR, 5.49; 95% CI, 1.46–20.63), obesity with BMI >40 kg/m2 (Latinx: OR, 3.43; 95% CI, 1.45–7.67), and diabetes (African American: OR, 2.78; 95% CI, 1.08–7.11) were identified as significant predictors of ICU admission (Table 5). In addition to these predictors, low median household income was as a significant predictor for ICU admission in White patients (OR, 2.50; 95% CI, 1.39–4.52) after adjustment for socioeconomic status (Supplementary Table 6).
Table 5.
Multivariable Logistic Regression Model Predicting ICU Admission Among Hospitalized Patients With COVID-19
| Variables | White (n = 620) | Latinx (n = 470) | African American (n = 209) |
|---|---|---|---|
| Age | |||
| 18–40 y | Reference | ||
| 41–60 y | 0.89 (0.38–2.11) | 1.39 (0.81–2.37) | 2.75 (0.79–9.62) |
| >60 y | 1.14 (0.50–2.59) | 2.71 (1.44–5.09)* | 5.49 (1.46–20.63)* |
| Gender | |||
| Female | Reference | ||
| Male | 1.27 (0.87–1.85) | 1.45 (0.96–2.20) | 1.96 (0.96–4.00) |
| Smoking status | |||
| Never | Reference | ||
| Current | 1.17 (0.55–2.50) | 1.56 (0.63–3.89) | 1.46 (0.34–6.27) |
| Former | 1.38 (0.92–2.06) | 1.07 (0.59–1.93) | 1.83 (0.84–4.00) |
| Last BMI | |||
| ≤30 kg/m2 | Reference | ||
| 30–40 kg/m2 | 1.26 (0.84–1.88) | 1.43 (0.93–2.21) | 1.66 (0.75–3.70) |
| >40 kg/m2 | 1.26 (0.63–2.53) | 3.34 (1.45–7.67)* | 2.56 (0.65–10.18) |
| Comorbidities | |||
| Diabetes mellitus | 1.18 (0.76–1.85) | 0.80 (0.42–1.51) | 2.78 (1.08–7.11)* |
| Hyperlipidemia | 0.92 (0.58–1.45) | 1.40 (0.70–2.81) | 0.95 (0.40–24) |
| Hypertension | 0.78 (0.47–1.30) | 0.80 (0.41–1.56) | 0.34 (0.12–0.96)* |
| Obstructive lung disease | 0.94 (0.58–1.53) | 0.35 (0.15–0.81)* | 0.72 (0.25–2.05) |
| Interstitial lung disease | 1.14 (0.23–5.53) | 2.20 (0.16–31.08) | 1.47e + 7 (1.04e-203–NA) |
| Coronary artery disease | 0.51 (0.29–0.89)* | 0.51 (0.18–1.45) | 0.65 (0.12–3.58) |
| CHF | 1.42 (0.74–2.72) | 0.89 (0.19–4.20) | 2.01 (0.53–7.93) |
| Cerebrovascular disease | 0.64 (0.30–1.37) | 0.36 (0.08–1.57) | 0.52 (0.11–2.37) |
| Obstructive sleep apnea | 1.39 (0.73–2.66) | 0.14 (0.02–0.81)* | 0.75 (0.14–4.06) |
| CKD | 1.06 (0.64–1.75) | 0.48 (0.19–1.19) | 1.43 (0.48–4.31) |
| Transplantation | 0.51 (0.05–4.75) | 3.30 (0.63–17.36) | 3.01 (0.54–16.97) |
| Auto-immune diseases | 0.82 (0.38–1.79) | 1.37 (0.41–4.63) | 1.92 (0.32–11.68) |
| Malignancy | 0.75 (0.41–1.35) | 1.34 (0.47–3.82) | 0.82 (0.18–3.68) |
| Total comorbidities | |||
| 0 | Reference | ||
| 1–2 | 1.22 (0.64–2.34) | 0.90 (0.46–1.76) | 1.33 (0.43–4.08) |
| >2 | 1.83 (0.66–5.12) | 1.49 (0.39–5.70) | 1.12 (0.17–7.25) |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
*Statistically significant.
DISCUSSION
In spite of aggressive efforts by the medical and public health communities worldwide, understanding of the overall impact of the COVID-19 pandemic remains limited. Based on data from China, Italy, and Spain and preliminary data from the United States, patients who are 60 years or older are more vulnerable to COVID-19, with higher morbidity and mortality [4–9]. Furthermore, patients with other comorbidities, such as cardiovascular disease and hypertension, are more likely to be hospitalized and die from the infection [2, 5, 12, 13]. This evidence mostly derives from studies performed in racially and ethnically homogeneous populations [5, 10]. The impact of the disease in an ethnically and racially diverse population has not been fully explored.
Anecdotal and news reports and a report from the United Kingdom suggest that racial and ethnic minorities may be more likely to contract COVID-19 and more likely to suffer poor outcomes as a result of infection [15–19, 25]. Price-Haywood et al. and others have shown that the majority of patients hospitalized with COVID-19 and of those who died in a Louisiana study population were African American [15, 26]. Our firsthand clinical experience with COVID-19 patients indicates that in addition to African American patients, a higher percentage of COVID-19 Latinx patients required hospitalization and critical care admission. To examine this issue, we investigated the impact of COVID-19 on the patient population covered by our group of hospitals, which serve a diverse and broad population of Eastern Massachusetts similar to the racial and ethnic compositions of many large metropolitan areas of the United States [27]. We confirmed that age, male gender, and obesity are indeed important risk factors for worse outcomes after COVID-19 infection and that the presence of reported comorbid medical conditions is an important contributing factor to hospitalization among all ethnic and racial groups.
Three additional important findings emerged from our study. First, analysis of our large study population confirmed our firsthand clinical experience and showed indeed that Latinx and African American patients are at higher risk of being hospitalized and admitted to ICU level of care with COVID-19 than White patients. A second important finding is that the differences observed between Latinx and African American vs White patients occur at all age groups and are not only limited to the “higher-risk” older age groups identified in prior studies. A third important finding is that the observed disparities appear to be less likely related to reported preexisting medical comorbidities, as Latinx and African American patients who tested positive for COVID-19 were admitted at twice the rate of Whites, regardless of whether they had reported comorbidities or not. In addition, the proportion of White patients who had reported comorbidities such as hyperlipidemia, hypertension, obstructive lung disease, coronary heart disease, and cerebrovascular disease was at least as great as the proportions of Latinx and African American patients who had these comorbidities.
The underlying etiology of such disparity in hospitalization from COVID-19 between Latinx and African American vs White patients is likely multifactorial. First, patients from these historically disadvantaged racial and ethnic groups may be less likely to be insured than White patients. Immigration status could also play another role in restricting access of racial and ethnic minority patients to health insurance coverage and increasing their challenges in finding a source of care to get tested or accessing COVID-related treatments. Therefore, they may have presented at a later, more severe phase of the disease and therefore required hospitalization. In support of this, when we analyzed the zip code of residence of these patients, we found that both residence in a zip code of low median income and greater density of living in the same household were closely correlated with higher rate of hospitalization. In general, lower-income patients tend to defer seeking health care for fear of financial burden and/or limited health care access and quality [28].
Second, it is possible that Latinx and African American patients with COVID-19 have a higher severity of reported comorbidities. Our data do not show that such disparities in hospitalization can be explained by the presence of reported comorbidities. However, there are limitations to our interpretation of these data. Because our findings are based on reported data in the medical records, they do not take into account the severity of the preexisting conditions, which is difficult to quantify in such a large study population. It is likely that the severity of certain underlying comorbidities is higher in ethnic and racial minorities than in White patients, perhaps due to previously described health care disparities [29, 30] or to issues with medication use and adherence [31–33].
Third, other issues of stress and allostatic load that could impact health; these issues, which were out of the scope of our observational study, may also be contributing factors to the observed disparities and require further investigation [34, 35]. These include crowded housing conditions, as we alluded above, or the type of employment where exposure to COVID-19 could possibly be more common.
Our study has several possible limitations. First, this is a registry database study from a single mixed health system (with primary and tertiary institutions) using structured data captured in the electronic medical record. This study may also underrepresent COVID-19 patients who do not seek medical attention or whose medical data are stored at other facilities. However, the strengths of this study include a large, diverse study population of COVID-19-positive patients from a wide geographic region that allowed us to analyze a large number of Latinx, African American, Asian American, and White patients. We were also able to collect detailed sets of variables on each patient, including factors that predict hospitalization and ICU-level care, which allows for multivariable adjustment. Follow-up identified a number of outcome events, including deaths and ICU-level admissions. While we acknowledge the limitations of our study, reporting data based on societal understanding of race and ethnicity, using patient self-reported race and ethnicity, is an important step in highlighting existing disparities in COVID-19 treatment and trying to mitigate contributing factors for the future [38, 39].
Our findings also could have immediate policy implications, as it would be crucial to target the most vulnerable groups when testing or vaccination strategies are devised to limit further spread of COVID-19 and minimize its impact. This is especially relevant with the new surge in the numbers of COVID cases in states where Latinx patients constitute a significant portion of the population such as Florida and Texas.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank the staff of Mass General Brigham Hospitals who cared for the patients included in this study.
Potential conflicts of interest. Dr. Ross Zafonte serves on the Scientific Advisory Board of Oxeia Biopharma, Biodirection, ElMINDA, and Myomo. He also evaluates patients in the MGH Brain and Body-TRUST Program, which is funded by the NFL Players Association. The remaining authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McMichael TM, Currie DW, Clark S, et al. ; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med 2020; 382:2005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legido-Quigley H, Mateos-García JT, Campos VR, et al. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Public Health 2020; 5:e251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. In press. [DOI] [PubMed] [Google Scholar]
- 8. Rosenbaum L. Once upon a time…the hero sheltered in place. N Engl J Med 2020; 383:e5. [DOI] [PubMed] [Google Scholar]
- 9. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. In press. [DOI] [PubMed] [Google Scholar]
- 10. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. In press. [DOI] [PubMed] [Google Scholar]
- 11.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020; 12:6049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez AD, Hirschman C. The changing racial and ethnic composition of the US population: emerging American identities. Popul Dev Rev 2009; 35:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferdinand KC, Nasser SA. African-American COVID-19 mortality: a sentinel event. J Am Coll Cardiol 2020; 75:2746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yancy CW. COVID-19 and African Americans. JAMA. In press. [DOI] [PubMed] [Google Scholar]
- 17. Choi YJ, Lee HY, An S, et al. Predictors of cervical cancer screening awareness and literacy among Korean-American women. J Racial Ethn Health Disparities 2020; 7:1–9. [DOI] [PubMed] [Google Scholar]
- 18. How COVID-19 is a perfect storm for African American Americans Washington Post. April 26, 2020. Available at: https://www.washingtonpost.com/opinions/2020/04/26/we-must-address-social-determinants-affecting-black-community-defeat-covid-19/. Accessed 13 May 2020.
- 19. LatinXs and African Americans are hardest hit by COVID-19 in New York City. U.S. News April 8, 2020. Available at: https://www.usnews.com/news/national-news/articles/2020-04-08/coronavirus-disproportionally-kills-hispanics-and-blacks-in-new-york-city. Accessed 13 May 2020.
- 20.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) 2020 Available at: https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html. Accessed 21 April 2020.
- 21.Food and Drug Administration. Emergency use authorization 2020 Available at: https://www.fda.gov/media/136314/download. Accessed 21 April 2020.
- 22. Honaker J, King G, Blackwell M. Amelia II: a program for missing data. J Stat Softw 2011; 45:1–47. [Google Scholar]
- 23. Income statistics and demographics for states, counties, cities and zip codes 2020. Available at: https://cubit-data.myshopify.com/. Accessed 29 April 2020.
- 24. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing; 2019. Available at: https://www.R-project.org/. Accessed 1 May 2020. [Google Scholar]
- 25. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis 2020; 20:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among African American patients and white patients with Covid-19. N Engl J Med 2020; 382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mass.gov. Population health statistics: race and ethnicity reports. 2020. Available at: https://www.mass.gov/lists/population-health-statistics-race-and-ethnicity-reports. Accessed 13 May 2020.
- 28. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health 2010; 100(Suppl 1):S186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heisler M, Faul JD, Hayward RA, et al. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the Health and Retirement Study. Arch Intern Med 2007; 167:1853–60. [DOI] [PubMed] [Google Scholar]
- 30. Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med 2002; 347:1585–92. [DOI] [PubMed] [Google Scholar]
- 31. Xie Z, St Clair P, Goldman DP, Joyce G. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One 2019; 14:e0212117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alsabbagh MH, Lemstra M, Eurich D, et al. Socioeconomic status and nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Value Health 2014; 17:288–96. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Baik SH. Race/ethnicity, disability, and medication adherence among medicare beneficiaries with heart failure. J Gen Intern Med 2014; 29:602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc 2012; 104:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338:171–9. [DOI] [PubMed] [Google Scholar]
- 36. Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr 2020; 14:655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muscogiuri G, Pugliese G, Barrea L, et al. Commentary: obesity: the “Achilles heel” for COVID-19? Metabolism 2020; 108:154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chowkwanyun M, Reed AL Jr. Racial health disparities and Covid-19 - caution and context. N Engl J Med 2020; 383:201–3. [DOI] [PubMed] [Google Scholar]
- 39. Webb Hooper M, Napoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020; 323:2466–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.