Abstract
Background
Knowledge about neurological complications of COVID-19 in children is limited due to the paucity of data in the existing literature. Some systematic reviews are available describing overall clinical features of COVID-19 in children and neurological complications of COVID-19 in adults. But to the best of our knowledge, no systematic review has been performed to determine neurological manifestations of COVID-19.
Methods
Six different electronic databases (MEDLINE, EMBASE, Web of Science, CENTRAL, medRxiv and bioRxiv) were searched for articles related to COVID-19 and neurological complications in children. Studies/case series reporting neurological manifestations of COVID-19 in patients aged ≤18 years, as well as case reports, as neurological complications appear to be rare. The pooled estimate of various non-specific and specific neurological manifestations was performed using a random effect meta-analysis.
Results
Twenty-one studies/case series and five case reports (3707 patients) fulfilled the eligibility criteria and were included in this systematic review, from a total of 460 records. Headache, myalgia and fatigue were predominant non-specific neurological manifestations, presenting altogether in 16.7% cases. Total of 42 children (1%) were found to have been reported with definite neurological complications, more in those suffering from a severe illness (encephalopathy—25, seizure—12, meningeal signs—17). Rare neurological complications were intracranial hemorrhage, cranial nerve palsy, Guillain–Barré syndrome and vision problems. All children with acute symptomatic seizures survived suggesting a favorable short-term prognosis.
Conclusion
Neurological complications are rare in children suffering from COVID-19. Still, these children are at risk of developing seizures and encephalopathy, more in those suffering from severe illness.
Keywords: COVID-19, neurological symptoms, children, encephalopathy, seizures
BACKGROUND
The world is currently fighting the coronoa virus disease 19 (COVID-19) pandemic, for more than 6 months and there has been a massive impact on humanity, health care system and economy [1]. As compared to adults, a small number of pediatric cases are reported and the disease severity is also less in children. Still, gastrointestinal complications, cytokine storm leading to multisystem inflammatory disease or Kawasaki disease-like presentation have been reported in children suffering from COVID-19 [2, 3]. However, neurological complications like seizures and encephalopathy have only been reported in a few case series and single case reports [4]. Some systematic reviews are available describing overall clinical features of COVID-19 in children and neurological complications of COVID-19 in adults [5–11]. But to the best of our knowledge, no systematic review has been performed to determine neurological manifestations of COVID-19 in the pediatric population. The adult data regarding the prevalence of seizure, encephalopathy, stroke and Guillain–Barré syndrome (GBS) cannot be extrapolated to children, as the children have been reported to behave differently than adults.)
METHODS
We performed this systematic review to collate all reports of neurological complications in children with confirmed COVID-19 infection from the currently available literature. Accordingly, the primary aim of this systematic review was to provide a pooled estimate of neurological complications in children with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A meta-analysis of observational studies in epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed while conducting the study. A predefined search strategy was developed first. Three investigators performed independently a literature search in MEDLINE/PubMed, Web of Science, EMBASE, CENTRAL (Cochrane central register of controlled trials), medRxiv and bioRxiv for original articles, case series and case reports published between 1 December 2019 and 12 July 2020, without using any language restrictions. The search strategy was targeted to include children aged ≤18 years with confirmed COVID-19. The terminologies used were divided into three basic groups: study population (children/pediatric/infant/adolescent/child), terms describing or related to COVID-19 (also SARS-CoV-2, coronavirus, 2019 nCoV) and terms describing neurological symptoms (also seizure, encephalopathy/encephalitis, convulsion, confusion, and stroke, neuropsychiatric). Using these MeSH terms specific search strategies were developed for each search engine. The electronic search was later supplemented by a manual search of the references of the included articles, to identify additional cases.
STUDY SELECTION
As definite neurological complications were extremely rare in children with COVID-19 as based on preliminary reports, so all articles describing cases with any neurological complications were included in the systematic review. The other inclusion criteria were studies describing clinical manifestations and outcomes of children ≤18 years with COVID-19, in which at least one child had some neurological complications. All types of study design starting from randomized controlled trials, prospective cohort study, cross-sectional study, case–control study, retrospective case series including at least 10 participants describing neurological complications of children with COVID-19 were included in the analysis. Even correspondences or letters fulfilling the above criteria were considered eligible for review. Narrative or systematic reviews, editorials, perspectives, conference proceedings and studies describing other serotypes of coronaviruses were excluded from this study. Small case series were excluded if they described non-specific neurological complaints like myalgia, fatigue, headache or weakness, as they were likely to increase heterogeneity and impair the correct estimation of the pooled estimate of these clinical features. However, for specific neurological complications like seizure and encephalopathy, which seems to be rare, even single case reports were included, to provide a more comprehensive review on the topic. Children with preexisting neurological co-morbidities were excluded from the study, to exclude any confounding of results. Only for those neurological complications for which definite causal and temporal association with COVID-19 infection could be established were included in the study.
Each of the articles included underwent quality check as per a predefined set of criteria and validated guidelines. Independently two researchers first screened the title and abstract to select the articles describing COVID-19 cases in children. Subsequently, the reviewers went through the full text of all articles to screen for the description of any child with neurological complications.
The neurological manifestations in children with COVID-19 were divided into three categories. The first category involved specific neurological symptoms due to COVID-19 involving the neurological system directly, such as encephalopathy, seizures, etc. The second category included non-specific neurological symptoms like headache, myalgia and fatigue, predominantly caused by systemic involvement, rather than direct neuroinvasion. The third category involved preexisting co-morbid neurological disorders in children suffering from COVID-19. In the current review, only symptoms about the first and second categories were included.
DATA EXTRACTION AND QUALITY ASSESSMENT
A predesigned, standardized, well-structured proforma was developed for data extraction. Two investigators independently reviewed the eligible articles and extracted data from their full text. The extracted data included as much information available from the following: the number of children with COVID-19 having neurological complications, demographic details of these children, frequency and prevalence of each of these neurological complications, neuroimaging, electroencephalogram (EEG) and other investigation details, whether they are associated with a multisystem inflammatory syndrome or not, treatment received and final outcome, apart from the study site, study period, sample size, study design and other details of study method. A third independent investigator rechecked the completeness and accuracy of extracted data. If both investigators disagreed on some topic, then a consensus decision was achieved by discussing with this third investigator. Every effort was made to prevent duplication of data and every case included in the final analysis was ensured not to be part of another series.
Newcastle–Ottawa scale was used to assess the quality of the included studies. Table 1 shows how the studies were classified as good, fair, and poor quality. The risk of bias in the included study was determined by two investigators independently using GRADE’s approach and any dispute was settled by discussing with a third investigator.
Table 1.
Details of studies included in systematic review
| Author | Country | Patients (n) | Study design | Age (range) | Study quality |
|---|---|---|---|---|---|
| CDC COVID-19 response team | USA | 2572 | Retrospective case series | 0–18 years | Good |
| Garrazino, et al. | Italy | 168 | Cohort study | 1–17 years | Good |
| Li, et al. | China | 40 | Retrospective cohort | 16 days–14.2 years | Poor |
| Lu, et al. | China | 171 | Retrospective case series | 1 day–15 years | Fair |
| Verdoni, et al | Italy | 10 | Case–control study | 2.9–16 years | Fair |
| Whittaker, et al | England | 58 | Retrospective study | 3 months–17 years | Fair |
| Li, et al | China | 57 | Retrospective study | 0–5 years | Fair |
| Zhang, et al | China | 46 | Retrospective case series | 0–18 years | Poor |
| Sun Dan, et al | China | 36 | Retrospective study | 2–12 months | Fair |
| Oualha, et al | France | 27 | Retrospective study | 2 months–17.8 years | Fair |
| Parri, et al | Italy | 130 | Retrospective study | 0–18 years | Good |
| Wang, et al. | China | 31 | Retrospective case series | 6 months–17 years | Fair |
| Wu, et al. | China | 74 | Retrospective case series | 1 month–15 years | Good |
| Xia Wei, et al | China | 13 | Retrospective case series | 0–18 years | Fair |
| Parri, et al | Italy | 100 | Cohort study | 0–17.5 years | Fair |
| Bai, et al | China | 25 | Retrospective case series | 0.6–17.0 years | Fair |
| Miller, et al | USA | 44 | Retrospective case series | 7 months–18 years | Good |
| Yu pin Tan, et al | China | 10 | Retrospective case series | 2–12 years | Poor |
| Qiu, et al | China | 27 | Retrospective case series | 0–18 years | Fair |
| Ma Huijing, et al | China | 50 | Retrospective case series | 0–16 years | Fair |
| Dan Sun, et al | China | 10 | Retrospective case series | 0–18 years | Poor |
The following scoring system was used: good quality: at least 3/4 stars in selection domain, 1/2 stars in comparability domain and 2/3 stars in outcome/exposure domain; fair quality: 2 stars in selection domain, 1/2 stars in comparability domain, 2/3 stars in outcome/exposure domain; and rest of the studies as poor-quality studies. Similarly, quality of case reports was checked according to CARE guidelines and case reports were classified into good, fair and poor qualities, depending on whether they satisfied all 13 criteria, at least 10 or less than 10 criteria, respectively.
DATA SYNTHESIS AND STATISTICAL ANALYSIS
Appropriate descriptive statistics were used to represent various parameters and wherever feasible, pooled estimate, with 95% confidence intervals (CI) of these parameters were estimated. Categorical variables presented as frequency (percentage) and 95% CI, whereas continuous variables were presented as mean with standard deviation or median with interquartile range. Meta-analysis of data regarding various parameters was performed using STATA software. We utilized a random effect model assuming that frequency of different neurological complications and other parameters across different studies will be variable while pooling data of individual studies. Heterogeneity in studies was assessed by utilizing Higgins and Thompson’s I2 method and chi-square test on Cochran’s Q statistics. Egger’s test was used to assess the presence of publication bias.
RESULTS
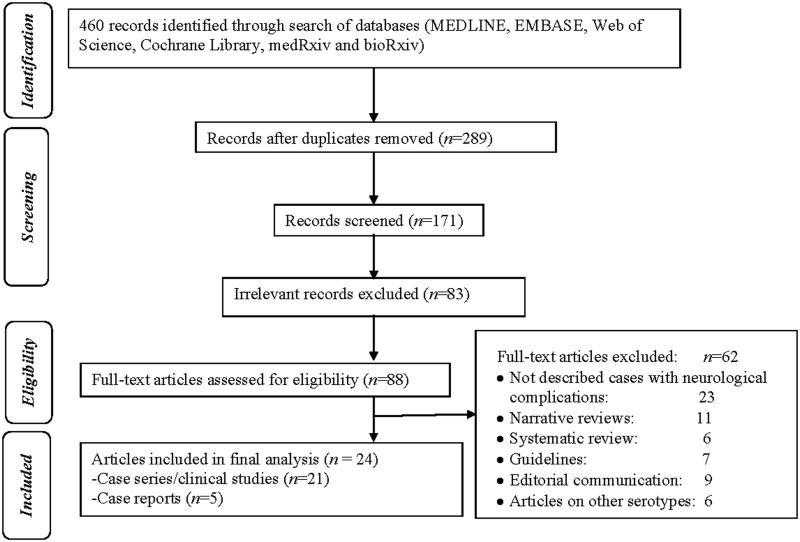
After a primary search, a total of 460 publications were retrieved. Among these, 289 were duplicates and hence removed accordingly. The eligibility of the remaining 171 papers was evaluated initially and 83 irrelevant articles were excluded according to the title, article type and abstract (Fig. 1). Eighty-eight articles were selected for full-text review. Ultimately, 21 prospective/retrospective studies/case series and 5 case reports were included in the final analysis [4, 12–36]. Out of the 21 studies, 11 were of fair quality, and 5 each were of good and poor quality (Table 1). Four case reports were of fair quality and one was of poor quality.
Fig. 1.
Flow diagram of the study selection process.
Out of these 21 studies, 14 studies described the prevalence of non-specific neurological manifestations, and rest 7 studies described specific neurological complications. Total of 3707 patients were included in the final analysis out of which 623 patients (16.7%, 95% CI 15.6–18.0%) had non-specific/specific neurological manifestations. Total of 581 patients (15.6%, 95% CI 14.7–17.1%) had non-specific neurological manifestations, while 42 children (1.0%, 95% CI 0.7–1.4%) had specific neurological manifestations. A total of 3260 patients were participants in 14 studies describing the non-specific neurological features (fatigue, headache, myalgia) and 444 patients were participants in 7 studies and 5 case reports describing specific neurological features (seizure, encephalopathy, meningeal signs, cranial nerve signs, vision changes). However, out of these seven studies, four studies described children with only severe COVID-19 symptoms, whereas all 15 studies in the first group described predominantly children with mild illness and in these studies, none of the children had any specific neurological features. Thus, specific neurological complaints like seizures, encephalopathy and meningeal signs are probably more common in children with severe illness (9.0%, 95% CI 6.5–12.1%).
We then the meta-analyzed individual prevalence of the three non-specific (headache, myalgia, fatigue, weakness) and two specific (encephalopathy, seizure, meningeal signs) neurologic manifestations, which have been described in Table 2. Although substantial heterogeneity was observed between studies describing these features, no significant publication bias was observed in Egger’s test (p > 0.05). Headache and fatigue were the most common non-specific neurological manifestations. Only one study described the prevalence of anosmia alone/along with ageusia 17/27 cases, 62%, apart from another case report of a child with thalassemia, who presented with anosmia only and later on diagnosed to have COVID-19.
Table 2.
Frequency of neurological symptoms in children with SARS-CoV-2 infection
| Symptoms | Number of studies/case reports (number of participants) | Predominant type of cases included | Number of patients with symptoms | Pooled estimates % (95% CI) | Heterogeneity (I2%) | p-Value for I2 |
|---|---|---|---|---|---|---|
| Fatigue/myalgia | 11 (3129) | Mild | 449 | 14.3 (13.1–15.6) | 35 | 0.006 |
| Headache | 8 (3036) | Mild | 114 | 3.7 (3.1–4.4) | 31 | 0.01 |
| Seizure | 7 (380) | Severe | 12 | 3.1 (1.8–5.4) | 27 | 0.02 |
| Encephalopathy | 5 (198) | Severe | 25 | 12.6 (8.7–17.9) | 59 | 0.001 |
Out of the total 41 children with COVID-19 who have been found to have definite neurological complications, 25 children had encephalopathy, 12 children had seizures and 17 children had meningeal signs. These specific neurological complications were found to be present along with multisystem inflammatory syndrome or Kawasaki-like presentation in two studies. Other rare definite neurological complications like cranial nerve palsy and vision changes also have been documented, in one series.
All the seizure episodes were acute symptomatic seizures and occurred during febrile episodes, only one being status epilepticus. Neuroimaging findings were documented in two cases and were normal in both of them. EEG abnormality was documented in two single case reports. Apart from single case reports and few small case series, definite demographic variables were not available separately for children with non-specific/specific neurological complications, as they were part of larger case series.
An 11-year-old boy with encephalitis had a generalized weakness for 2 days, refractory status epilepticus requiring four anticonvulsants, lymphocytic pleocytosis and elevated cerebrospinal fluid (CSF) protein, with normal CSF sugar. EEG showed intermittent frontal delta wave activity, while computerized tomography (CT) head was normal. Another 6-week-old infant had multiple episodes of brief tonic seizures, with normal neuroimaging and CSF examination, with an excess of temporal sharp transients and intermittent vertex delta slowing. The slow-wave pattern in EEG was also observed in one child with Kawasaki disease-like presentation during COVID-19. Although the semiology of seizures and antiepileptic drug used has not been mentioned in the reported cases, all children with seizures survived, without definite neurological sequel. However, 7 out of 10 children with seizures had severe or critical COVID-19 illness, suggesting seizures are probably more common with severe COVID-19 illness. Although encephalopathy has been reported in a few series/case reports, meningeal signs have been documented in only two series in children with a clinical picture suggestive of either multisystem inflammatory syndrome or Kawasaki disease-like illness. One child with encephalopathy and intracranial hemorrhage expired, while the rest of the children survived, although detailed information regarding neurocognitive deficit is lacking in the studies. Khalifa, et al. [35] have reported a case of GBS (acute inflammatory demyelinating polyneuropathy variant) in an 11-year-old boy and Frank, et al. [36] have reported another pediatric case of GBS (acute motor axonal neuropathy variant) in a 15-year-old boy with COVID-19, both of whom responded favorably to intravenous immunoglobulin.
DISCUSSION
This review summarizes the frequency and characteristics of neurological complications of pediatric COVID-19 in the age group up to 18 years from the available clinical studies. We could detect non-specific neurological symptoms in around 16% of children, while specific neurological complications were detected only around 1% of cases. Although only limited numbers of studies described neurological complications specifically, still neurological problems as such appear to be pretty rare. However, children with a severe and critical illness, especially those with multisystem inflammatory diseases appeared to have a higher prevalence of neurological symptoms.
In December 2019, there was the first description of SARS-CoV-2 in Wuhan, China. Since the beginning of the pandemic, data from a series of patients from Wuhan have pointed out the prevalence of neurological complications in 36.4% of hospitalized adult patients [37]. Therefore, the properties of virulence and invasiveness of the nervous system by the coronavirus deserve attention due to the neurotropism it presents. Several studies described a pleitropic diversity of neurological complications. Nonetheless, the exact mechanism remains to be determined since, initially, the direct involvement of the nervous system by the virus seemed to have the central role due to the early onset of neurological symptoms associated with SARS-CoV-2 infection. However, currently, late neurological findings suggesting an autoimmune mechanism, even in children having as an extreme example a GBS.
Encephalopathy of variable severity was found to be the predominant definite neurological complication, although some of the children with severe illness could have developed encephalopathy due to various reasons other than direct neurotrophic effects of the virus, such as hypoxia and septic shock. As compared to adults, neurological complications in children have been reported only in few cases and the illness appears to be less severe in children [6]. Various studies have described up to 24% of patients having some central nervous system (CNS) complaints and 8% having a peripheral nervous system-related complications in adults. In adults also the commonest neurological complication is meningoencephalitis-like presentation (including confusion, agitation, dysexecution syndrome). However, arterial ischemic stroke, acute necrotizing hemorrhagic encephalopathy of childhood, critical illness polyneuropathy and myopathy, Miller-Fisher syndrome, acute myelitis, ataxia, corticospinal tract signs and subarachnoid hemorrhage have not been reported till now in children [7, 38, 39]. Only one child with intracranial hemorrhage has been reported until now in those suffering from COVID-19. While some of the adult reports have speculated up to 10% of patients with severe COVID-19 illness have subclinical seizures, in children, however, it has not been explored. Up to 5% of adult patients have been speculated to be at risk for developing stroke, but it seems less likely for children, as the co-morbidities and risk factors in children are fewer [6, 7]. Another notable difference between adults and children with COVID-19 was found in a study comparing the clinical characteristics of 25 adults and 7 children with confirmed COVID-19 by Han, et al. In this study, none of the children had myalgia, while 13 adults had myalgia. Thus myalgia was disproportionally more common among adults, reaching 52%, although the children had elevated creatine kinase (CK) levels more frequently than adults (57.1% vs. 4%) [40].
The finding in this systematic review that encephalopathy and seizures were more common with severe COVID-19 illness and more so in those with the multisystem inflammatory response or Kawasaki-like presentation, has also been found in adults. Although the non-specific neurological symptoms appear to be clinically insignificant, often they might be the only symptom of COVID-19 [41]. As COVID-19 is predominantly considered as a respiratory illness, several adult cases with isolated neurological symptoms were misdiagnosed initially. Even some studies considered anorexia, dizziness, nausea/vomiting and dyspnea/breathlessness as neurological manifestations [6].
Recently studies have reported adult patients with COVID-19 have been behaving as patients with ‘happy hypoxia’, i.e. they deceptively did not have increased work of breathing, despite decreased oxygen saturation, until they end up in the advanced stage. The more promising explanation for that seems to be pulmonary microembolism, but the possibility of subclinical neurological involvement cannot be ruled out altogether. Another subset of severe patients described they required an active and conscious breath to maintain normal breathing rhythm [11, 12, 42]. As the angiotensin-converting enzyme-2 (ACE-2) receptor is expressed in the cardiorespiratory center in the medulla, so neuroinvasion might have impaired control of these vital functions in these patients. However, at the current stage, this is merely a speculation and further clinical and experimental studies are needed in this regard to delineating any role of the nervous system in respiratory failure [41].
In adults, at least six large case series of neurological involvement in COVID-19 have been reported, apart from several case reports. But in children, individual case series regarding this perspective is yet to be reported, but it seems to be underdiagnosed and underreported, as children are often unable to express symptoms and in a critically ill child neurological symptoms are often attributed to the severity of systemic illness by clinicians. This appears to be truer for anosmia and ageusia, which has been reported as an early sign of COVID-19 in 30–80% cases, but in children have been rarely reported. However, one study which describes the prevalence of anosmia/ageusia in 62% of children is consistent with adult data. The anosmia in COVID-19 has been attributed to direct invasion of the olfactory neuroepithelium, as it expresses the ACE-2 receptor and thereby causing inflammatory changes in bilateral olfactory clefts [5, 9, 41].
Aseptic neuroinflammation has been described as the underlying etiology behind encephalitis, GBS, myelitis and other complications. In consistent with the above facts, in most cases in adults and children with neurological complications, lymphocytic pleocytosis and elevated CSF protein have been demonstrated, but the virus could not be isolated in most cases. However, in one adult case who clinically presented with transient seizures and encephalopathy, SARS-CoV-2 could be detected in CSF by polymerase chain reaction (PCR) and neuroimaging also showed findings suggestive of meningoencephalitis, but PCR from the nasopharyngeal specimen was negative. Thus, direct neuroinvasion in COVID-19 although seems to be rare, but possible clinically. Moreover, the sensitivity of PCR, currently the method used to detect viruses might have low sensitivity in CSF. Thus, a negative CSF PCR report in these cases needs to be interpreted cautiously [43]. One interesting perspective regarding children is a febrile seizure in children suffering from COVID-19. It is well known that the etiopathogenesis behind febrile seizure is a lower seizure threshold in children and more common in respiratory illnesses than gastrointestinal illnesses. Neuroimaging and EEG findings are available only in few children with COVID-19 who had seizures or encephalopathy. While in children mostly the magnetic resonance imaging (MRI)/CT brain was normal, in adults sometimes enhancement of meninges, affected regions of the brain, spinal cord and nerve roots have been reported in patients with meningoencephalitis, myelitis and GBS. Apart from infarcts and definite abnormalities in cerebrovascular perfusion in adult COVID-19 patients with stroke, some cerebrovascular perfusion abnormalities could be demonstrated in severe COVID-19 cases without stroke. These findings suggest direct neuroinvasion at least in some cases, although disruption of blood–brain barrier and neuroinflammation might also lead to such radiological findings. Similarly, raised CSF protein, lymphocytic pleocytosis and elevated CSF immunoglobulin all favor neuroinflammation. But rarely even in adult cases, the virus was isolated from CSF by PCR, when it could not be isolated from respiratory specimens, suggesting direct neuroinvasion [5, 7].
In another adult patient with acute hemorrhagic necrotizing encephalopathy, also disruption of the blood–brain barrier was proposed as the pathogenesis behind hemorrhagic lesions in multiple brain regions [44]. EEG predominantly showed background abnormalities like diffuse slowing and occasionally focal epileptiform abnormalities (frontal/temporal) in children and adult patients [45]. One retrospective series in 28 adult severe COVID-19 patients with encephalopathy/seizure-like events has reported sporadic epileptiform abnormalities in 40.9% patients and frontal sharp wave transients also in a proportion of patients. In consistent with this finding, frontal sharp waves were seen in a pediatric case with seizure also. Nerve conduction study showed delayed conduction velocity, absent F-wave, and attenuated amplitude of action potentials in adult COVID-19 patients with GBS [6, 7, 41].
Two potential pathways including direct neural tissue invasion and maladaptive inflammatory response have been proposed to explain the impairment of the nervous system reported in some cases with COVID-19. SARS-CoV-2 could invade the CNS either by the neuronal retrograde or hematogenous route. ACE-2 receptors that help in viral entry into cells are specifically enriched in the vascular endothelium of the brain. By infecting endothelial cells of the blood–brain barrier and blood–CSF barrier SARS-CoV-2 can enter into CNS through the hematogenous route, as demonstrated by SARS-CoV-2 by Paniz-Mondolfi, et al. [45] in the postmortem brain tissue.
According to the other potential mechanism, the virus initially invades peripheral nerve terminal and subsequently transported into CNS by retrograde neuronal transport. Even disruption of nasal epithelium can facilitate neuronal dissemination as suggested by studies involving SARS-CoV and OC43-CoV in animals. To further support this hypothesis, entry genes required for SARS-CoV-2 including the ACE-2 gene are richly expressed in the human olfactory neuroepithelium, especially in sustentacular cells. But definite evidence to favor this transmission route is still lacking [5, 8, 45].
The other recently postulated more promising mechanism for neuronal and other tissue injury relies on maladaptive multisystem inflammatory response syndrome, a cytokine storm, resembling that seen in hemophagocytic lymphohistiocytic syndrome. Rapid viral replication leads to increased proinflammatory cytokine (IFN-γ, TNF-α, IL-1β, IL-4 and IL-10) secretion, which can disrupt BBB and cause neuroinflammation. In consistent with the above facts, in some adult patients with severe COVID-19 having neurological complications, elevated IgG and oligoclonal bands were detected in CSF. Even the neutralizing antibodies like anti-S protein IgG can alter the inflammatory response and cause severe tissue injury in various organs, acting as a double-edged sword. In an adult case of COVID-19 with acute hemorrhagic necrotizing encephalopathy also the authors attributed the cytokine storm and over-activated immune response for causing disruption of blood-brain barrier (BBB) and hemorrhagic necrosis of brain parenchyma [5, 45].
This systematic review has several limitations. None of the studies included in the review specifically intended to determine the prevalence of neurological complications in COVID-19 and all of them focused predominantly on respiratory symptoms. Thus, there is a high probability that they might have underreported the non-specific neurological features, although seizures and encephalopathy being contrasting features are more likely to be reported than missed. Most of the studies are retrospective case series and hence subjected to documentation error and other biases. As most of the studies included in the review are not of good quality, with high heterogeneity, which compromises the overall reliability of the results. Since seizures as such appeared to be extremely rare, thus we have included even case reports. However, we excluded them during combining study results to avoid affecting the overall frequency. Moreover, children suffering from COVID-19 are proposed to be at increased risk for neuropsychiatric complications [46]. Despite all these issues, this is the first systematic review regarding neurological complications of COVID-19 in children and hence is likely to be informative to pediatricians treating these children in providing a comprehensive overview. With the reporting of more children with various neurological complications, precise information regarding the treatment, semiology of seizure, EEG and MRI brain finding and long-term prognosis is likely to be revealed [47].
CONCLUSION
Neurological complications are rare in children suffering from COVID-19. Still, these children are at risk of developing seizures and encephalopathy, more in those suffering from severe illness. However, acute symptomatic seizures in children with COVID-19 can be managed successfully with antiepileptic drugs, with favorable short-term outcomes.
ACKNOWLEDGEMENT
PROSPERO ID (CRD42020197016).
REFERENCES
- 1. Balasubramanian S, Rao NM, Goenka A, et al. Coronavirus disease 2019 (COVID-19) in children—what we know so far and what we do not. Indian Pediatr 2020;57:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ebina-Shibuya R, Namkoong H, Shibuya Y, et al. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki Disease cases. Int J Infect Dis 2020;97:371–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennon TR, Penque MD, Abdul-Aziz R, et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol 2020; doi: 10.1016/j.ppedcard.2020.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAbee GN, Brosgol Y, Pavlakis S, et al. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol 2020;109:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020; doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci 2020;77:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg 2020;194:105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheraton M, Deo N, Kashyap R, et al. A review of neurological complications of COVID-19. Cureus 2020;12:e8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 2020;142:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Souza TH, Nadal JA, Nogueira RJN, et al. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol 2020; doi: 10.1002/ppul.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CDC COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill 2020;25:pii=2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, Chen K, Liu M, et al. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect 2020;81:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet Lond Engl 2020;395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Wang H, Wang F, et al. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis 2020;98:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun D, Chen X, Li H, et al. SARS-CoV-2 infection in infants under 1 year of age in Wuhan City, China. World J Pediatr 2020;16:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang B, Liu S, Zhang J, et al. Children hospitalized for coronavirus disease 2019 (COVID-19): a multicenter retrospective descriptive study. J Infect 2020;81:e74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Arch Pédiatr 2020;27:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parri N, Lenge M, Buonsenso D; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med 2020;383:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Ju XL, Xie F, et al. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China]. Zhonghua Er Ke Za Zhi Chin J Pediatr 2020;58:269–74. [DOI] [PubMed] [Google Scholar]
- 23. Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open 2020;3:e2010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai K, Liu W, Liu C, et al. Clinical analysis of 25 COVID-19 infections in children. Pediatr Infect Dis J 2020;39:e100–3. [DOI] [PubMed] [Google Scholar]
- 26. Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan Y, Tan B, Pan J, et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol 2020;127:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu C, Cui C, Hautefort C, et al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an International Multicenter Study. Otolaryngol Head Neck Surg 2020, doi: 10.1177/0194599820934376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun D, Li H, Lu X-X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr 2020;16:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma H, Hu J, Tian J, et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med 2020;18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dugue R, Cay-Martínez KC, Thakur KT, et al. Neurologic manifestations in an infant with COVID-19. Neurology 2020;94:1100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marhaeni W, Wijaya AB, Kusumaningtyas P, et al. Thalassemic child presenting with anosmia due to COVID-19. Indian J Pediatr 2020, doi: 10.1007/s12098-020-03370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parri N, Magistà AM, Marchetti F, et al. ; on behalf of the CONFIDENCE and COVID-19 Italian Pediatric Study Networks. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr 2020;179:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;e2010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khalifa M, Zakaria F, Ragab Y, et al. Guillain-Barre syndrome associated with SARS-CoV-2 detection and a COVID-19 infection in a child. J Pediatr Infect Dis Soc 2020; doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank CHM, Almeida TVR, Marques EA, et al. Guillain–Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr 2020; doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avula A, Nalleballe K, Narula N, et al. COVID-19 presenting as stroke. Brain Behav Immun 2020;87:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 2020;76:233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han Y-N, Feng Z-W, Sun L-N, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J Med Virol 2020; doi: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 41. Correia AO, Feitosa PWG, Moreira JLDS, et al. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res 2020;37:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020;296:E119–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galanopoulou AS, Ferastraoaru V, Correa DJ, et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open 2020;5:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 2020;92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panda PK, Sharawat IK. COVID-19 (SARS-CoV-2 infection) and children: pediatric neurologist’s perspective. Indian J Pediatr 2020;87:556–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panda PK, Dawman L, Panda P, et al. Feasibility and effectiveness of teleconsultation in children with epilepsy amidst the ongoing COVID-19 pandemic in a resource-limited country. Seizure 2020;81:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]