Abstract
Background
There are only scarce data regarding the presentation, incidence, severity and outcomes of coronavirus disease 2019 (COVID-19) in patients undergoing long-term haemodialysis (HD). A prospective observational study was conducted in eight HD facilities in Alsace, France, to identify clinical characteristics of HD patients with COVID-19 and to assess the determinants of the risk of death.
Methods
All HD patients tested positive for COVID-19 from 5 March to 28 April 2020 were included. Collected data included patient characteristics, clinical features at diagnosis, laboratory data, treatments and outcomes.
Results
Among 1346 HD patients, 123 tested positive for COVID-19. Patients had a median age of 77 years (interquartile range 66–83), with a high number of comorbidities (3.2 ± 1.6 per patient). Symptoms were compatible in 63% of patients. Asthenia (77%), diarrhoea (34%) and anorexia (32%) were frequent at diagnosis. The delay between the onset of symptoms and diagnosis, death or complete recovery was 2 (0–5), 7 (4–11) and 32 (26.5–35) days, respectively. Treatment, including lopinavir/ritonavir, hydroxychloroquine and corticosteroids, was administered in 23% of patients. The median C-reactive protein (CRP) and lymphocyte count at diagnosis was 55 mg/L (IQR 25–106) and 690 Ly/µL (IQR 450–960), respectively. The case fatality rate was 24% and determinants associated with the risk of death were body temperature {hazard ratio [HR] 1.96 [95% confidence interval (CI) 1.11–3.44]; P = 0.02} and CRP at diagnosis [HR 1.01 (95% CI 1.005–1.017); P < 0.0001].
Conclusions
HD patients were found to be at high risk of developing COVID-19 and exhibited a high rate of mortality. While patients presented severe forms of the disease, they often displayed atypical symptoms, with the CRP level being highly associated with the risk of death.
Keywords: COVID-19, epidemiology, haemodialysis, mortality
KEY LEARNING POINTS
What is already known on this topic?
as of 28 April 2020, there were 129 859 cases of confirmed COVID-19 and 23 660 deaths in France.
there are only scarce data regarding the presentation, incidence, severity and outcomes of COVID-19 in patients undergoing long-term haemodialysis (HD).
What this study adds?
HD patients often displayed an atypical presentation, such as gastrointestinal involvement, less hyperthermia, a much more severe form of COVID-19 and delayed viral clearance.
the incidence and mortality rate of COVID-19 were much higher in HD patients than in the general population.
C-reactive protein and body temperature at admission were predictive of the risk of death.
What impact may this have on practice or policy?
screening indications should be comprehensive in this population to ensure adequate isolation and repeated reverse transcription–polymerase chain reaction testing would be advisable before ending isolation in COVID-19 HD patients.
we suggest strict implementation of ‘barrier gestures’ in these patients in whom strict containment cannot be secured due to the regular need for dialysis.
because of the substantial vulnerability of our patients, the prioritized distribution of personal protective equipment in a period of relative shortage or more extensive indications for hospitalization should be considered.
INTRODUCTION
Since late December 2019, an outbreak of a novel coronavirus, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan, China. The first case of coronavirus disease 2019 (COVID-19) in France was diagnosed on 24 January in Paris. A cluster was identified on 3 March, following an evangelist gathering between 17 and 21 February in Alsace, France. Since then, the northeastern regions of France have been severely struck by the pandemic (1178 in-hospital deaths in Alsace as of 28 April among 14 810 total in-hospital deaths in France and 1.89 million inhabitants in Alsace).
Early reports have suggested that patients with chronic kidney disease could be more susceptible to a severe form of COVID-19 {odds ratio [OR] 3.03 [95% confidence interval (CI) 1.09–8.47]} [1, 2]. However, there are only scarce data regarding the presentation, incidence, severity and outcomes of COVID-19 in HD patients. Currently, available information is based on case reports or small case series [3–5]. Given the rapid pace of the pandemic, we were compelled to gather an epidemiologic overview of COVID-19 HD patients and their specificities to optimize medical care.
Herein we report the results of an observational study in a cohort of HD patients from eight HD facilities in Alsace, France. This study provides an overview of the clinical presentation and outcomes of COVID-19 in HD patients in centres on the front line of COVID-19 during the first French wave of the epidemic.
MATERIALS AND METHODS
Data were collected from 5 March, the first confirmed dialysis case of COVID-19, to 28 April 2020 from adult (≥18 years old) patients undergoing long-term HD and testing positive for COVID-19. A positive test for COVID-19 was defined by either a positive reverse transcription–polymerase chain reaction (RT-PCR) test on a nasopharyngeal swab or compatible radiologic findings on a low-dose computed tomography (LD-CT) scan. Eight HD facilities in three cities participated in the study: Strasbourg [Clinique St Anne, Association pour l'utilisation du rein artificiel en Alsace (AURAL) Bergson, Hôpitaux Universitaires de Strasbourg], Colmar (AURAL Colmar, Hôpitaux Civils de Colmar) and Mulhouse (Hôpital Emile Muller, Diaverum private centre, AURAL Mulhouse). These eight facilities provided HD treatment for 1346 patients in Alsace.
The collected data were as follows:
General characteristics and demographics.
Comorbidities, including age >75 years, functional disabilities, obesity (body mass index >30 kg/m2), history of cancer (<5 years), current immunosuppression (history of transplantation, autoimmune disease, chemotherapy <5 years), chronic respiratory disease (e.g. chronic obstructive pulmonary disease, sleep apnoea treated with continuous positive airway pressure, need for long-term oxygen therapy), stroke, peripheral arterial disease, ischaemic heart disease and diabetes. A histroy of high blood pressure was not taken into account, due to its nearly ubiquitous prevalence in this population.
Diagnostic methods: RT-PCR on a nasopharyngeal swab, LD-CT scan indicating the extent of the impairment (minimal if <10% of the pulmonary parenchyma, mild if 10–25%, mild–severe if 25–50%, severe if 50–75%, critical if >75%).
Clinical presentation at diagnosis: date of onset of symptoms, date of diagnosis and dat of hospitalization, if needed. Clinical features and the severity of the infection at the time of diagnosis were documented [asymptomatic, moderate if not requiring oxygen supply or mild symptoms either with ambulatory care or hospitalization, severe if admission required for oxygen supply or severe symptoms, critical if hospitalization in an intensive care unit (ICU) or oxygen supply >10 L/min].
Laboratory characteristics at diagnosis: C-reactive protein (CRP), procalcitonin (PCT), lymphocyte count, serum albumin, electrolyte disorders (either plasma potassium <3.5 mmol/L or serum calcium <2.20 mmol/L or serum magnesium <0.70 mmol/L).
Treatment: oxygen therapy (none or need for a nasal cannula or simple face mask with flow rate in liters per minute), non-invasive ventilation or assisted ventilation, antibiotic and specific treatments (none, lopinavir/ritonavir combination, hydroxychloroquine, corticosteroids). The delay between initiation of therapy and the date of diagnosis and the onset of symptoms was also documented.
Laboratory evolution: at Day 7 and Day 14 (CRP, PCT, lymphocyte count, serum albumin, lactate dehydrogenase and ferritin).
Clinical outcomes: hospitalization, length of hospital stay, hospitalization in the ICU, evolution at Days 3, 7, 10, 14 and 21 after diagnosis (stability, improvement, deterioration or deceased) and weight loss. The patient’s condition on 28 April was also noted: discharged from hospital care, still hospitalized, transferred to a rehabilitation centre, ambulatory care or deceased. Recovery status and recovery time were reported. Complete recovery was defined as two consecutive negative RT-PCRs on nasopharyngeal swabs after a minimum time of 24 days after the onset of symptoms and with at least 48 h without symptoms.
Statistical analysis
Quantitative data are described as median and interquartile range (IQR) or mean and standard deviation (SD) according to the normality of the distribution. Distribution normality was tested graphically and with the Shapiro–Wilk test. Comparisons were performed using the Student’s t-test or the Mann–Whitney test, as appropriate. Qualitative data are described according to the frequency for each modality and compared with Fisher’s exact test.
Risk factors for death were analysed with a multivariate Cox model including variables with a P-value <0.2 in the univariate comparison between deceased and surviving patients. Statistical significance was defined as P < 0.05. All statistical analyses were performed with STATA/MP 13.1 (StataCorp, College Station, TX, USA).
RESULTS
Between 5 March and 28 April, 123 patients undergoing long-term haemodialysis (HD) were infected with COVID-19 (Mulhouse, n = 64; Strasbourg, n = 45; Colmar, n = 14), representing 9.1% of all patients undergoing long-term HD in our centres (n = 1346). The cumulative and incident cases in included HD facilities are presented in Supplementary data, Appendix 1.
Patient characteristics, demographics and comorbidities at the time of diagnosis are summarized in Table 1. Of note, a history of high blood pressure was found in 97.5% of patients.
Table 1.
Patient characteristics
| Characteristics | Results | Available/total, n/N |
|---|---|---|
| Demographics at the time of diagnosis | ||
| Age (years), median (IQR) | 77 (68–83) | 123/123 |
| Age >75 years, n (%) | 71 (58) | 123/123 |
| Gender (male), n (%) | 70 (57) | 122/123 |
| Functional disability, n (%) | 109/123 | |
| Total autonomy | 37 (34) | |
| Partial autonomy | 56 (51) | |
| None | 16 (15) | |
| Kidney disease, n (%) | 117/123 | |
| Diabetic | 42 (36) | |
| Glomerulonephritis | 17 (14.5) | |
| CIN/uropathy | 18 (15.5) | |
| Vascular | 14 (12) | |
| APKD | 10 (8.5) | |
| Unknown | 16 (13.5) | |
| Comorbidity at the time of diagnosis, n (%) | ||
| Presence of comorbidity | 118 (97.5) | 121/123 |
| Number of comorbidities, mean ± SD | 3.2 ± 1.6 | 118/123 |
| Cancer | 24 (20) | |
| Immunosuppression | 18 (15) | |
| Stroke | 26 (22) | |
| Peripheral arterial disease | 41 (35) | |
| Ischaemic heart disease | 54 (46) | |
| Diabetes | 63 (53) | 119/123 |
| Chronic respiratory disease | 40 (34) | 119/123 |
| Obesity | 40 (36) | 112/123 |
| Clinical features at the time of diagnosis, n (%) | ||
| Fever | 61 (57) | 107/123 |
| Body temperature (°C), mean ± SD | 37.8 ± 1 | 100/123 |
| Cough | 77 (69) | 112/123 |
| Dyspnoea | 55 (51) | 107/123 |
| SpO2 (%), median (IQR) | 95 (90–98) | 95/123 |
| SpO2 <93% | 42 (44) | |
| Asthenia | 82 (77) | 106/123 |
| Diarrhoea | 35 (34) | 102/123 |
| Anorexia | 33 (32) | 103/123 |
| Myalgia | 20 (20) | 102/123 |
| Anosmia | 6 (6) | 103/123 |
| Other ENT symptoms | 14 (14) | 103/123 |
| Headache | 11 (11) | 103/123 |
| Severity of the disease, n (%) | 120/123 | |
| Asymptomatic | 4 (3) | |
| Moderate | 62 (52) | |
| Severe | 43 (36) | |
| Critical | 11 (9) | |
CIN, chronic interstitial nephropathy; BMI, body mass index; SpO2: pulsatile saturation in oxygen; ENT, ear, nose and throat.
Diagnosis
The median time between first symptoms and diagnosis was 2 days (IQR 0–5). The origin of the contamination was unknown in the majority of cases (62.5%). Otherwise, the contamination mostly occurred in patients living in institutions (40%) or intrafamilial settings (29%). Of note, 21% of infections were due to nosocomial contamination (already in a hospital unit or rehabilitation unit for at least 7 days for another reason before the onset of symptoms) and 10% were related to an evangelist gathering cluster in Mulhouse. There was no evidence of either vertical or horizontal transmission within the HD units.
The clinical features and severity of the disease at the time of diagnosis are presented in Table 1. Symptoms were found to be compatible with COVID-19 infection (a combination of at least two symptoms among fever, cough and dyspnoea) in 63% of patients.
The primary method of diagnosis was RT-PCR of a nasopharyngeal swab for 112 patients, which was contributive in 88%. LD-CT scan was performed on 64 patients and was contributive in 88% and confirmed the diagnosis in 13 patients that had an initially negative RT-PCR. The extent of the impairment (available for 49/64 patients) on LD-CT scan was minimal in 13 (26.5%), mild in 21 (43%), mild–severe in 6 (12%), severe in 7 (14.5%) and critical in 2 (4%) patients.
Laboratory findings at the time of diagnosis are provided in Table 2. Of note, the median maximal CRP level was 112 mg/L (IQR 56–203) and was reached after a median delay of 7 days (IQR 4–12) after the onset of symptoms.
Table 2.
Laboratory characteristics of HD patients with COVID-19
| Characteristics | At diagnosis (N = 123) | Day 7 post-diagnosis (N = 102) | Day 14 post-diagnosis (N = 83) | Normal range |
|---|---|---|---|---|
| CRP (mg/L), median (IQR) | 55 (25–106), | 55 (15–113), | 19 (7–58), | 0–4 |
| n = 113 | n = 81 | n = 58 | ||
| Procalcitonin (µg/L), median (IQR) | 0.805 (0.475–2.115), | 1 (0.5–3.54), | 0.42 (0.2–2.03), | 0–0.5 |
| n = 32 | n = 31 | n = 14 | ||
| Lymphocyte count (Ly/µL), median (IQR) | 690 (450–960), | 635 (425–1010), | 870 (600–1250), | 1.000–4.000 |
| n = 109 | n = 76 | n = 57 | ||
| Serum albumin (g/L), mean ± SD | 35 ± 6, | NC | 32 ± 7, | 35–50 |
| n = 70 | n = 39 | |||
| Ferritin (µg/L), median (IQR) | NA | 1188 (840–2060), | NA | 23–322 |
| n = 41 | ||||
| LDH (U/L), median (IQR) | NA | 280 (215–346), | NA | 120–246 |
| n = 31 |
n, Number of available data/number of patients; LDH, lactate dehydrogenase; NA, not available.
Treatment
Sixty per cent of the patients required oxygen support therapy at the time of diagnosis. When oxygen therapy was needed, the median time between the onset of symptoms or time of diagnosis and maximal oxygen therapy was 8 days (IQR 3–11) and 2 days (IQR 0–8), respectively (data available in 29 patients).
Antibiotic therapy was initiated in 77% of patients after a median delay of 0 days (IQR −1–1) and 3 days (IQR 1–5) after diagnosis and symptoms onset, respectively. A putative antiviral therapy was initiated at the discretion of the physician in 23% of patients after a median delay of 2 days (IQR 1.5–3.5) and 4.5 days (IQR 3–9.5) after diagnosis and symptoms onset, respectively. This treatment was discontinued in 4/23 patients due to intolerance or medical contraindication. The main reasons for non-prescription of a specific therapy were the clinician’s choice and the absence of guidelines in HD patients (65%).
An overview of treatments is provided in Table 3.
Table 3.
Treatment in HD patients with COVID-19
| Treatment | Results | Available/total, n/N |
|---|---|---|
| Antibiotic treatment, n (%) | 76 (77) | 99/123 |
| Cephalosporin | 56 (78) | |
| Macrolide | 24 (33) | |
| Specific treatment, n (%) | 23 (23) | 100/123 |
| Lopinavir/ritonavir | 10 (38.5) | |
| Hydroxychloroquine | 12 (46) | |
| Corticosteroid | 5 (19) | |
| Oxygen therapy, n (%) | 68 (60) | 114/123 |
| Artificial ventilation | 5 (4) | |
| Initial oxygen therapy (L/min), median (IQR) | 0 (0–2) | 109/123 |
| Maximal oxygen therapy (L/min), median (IQR) | 2 (0–6) | 111/123 |
Outcomes at the time of writing
Hospitalization was required in 71% of patients after a median time of 2 days (IQR 0–5) after symptoms onset, seven of whom were transferred to the ICU. The median hospital stay was 9 days (IQR 4–14).
At the time of writing, follow-up data on 117/123 patients were available: 39 (33%) were discharged from the hospital, 11 (9%) remained hospitalized, 27 (23%) had only ambulatory care, 11 (9%) were transferred to a rehabilitation centre and 29 (24%) had died. Complete recovery was observed in 30 (24%) patients after a median interval of 32 days (IQR 26.5–35) after the onset of symptoms. In survivors at Day 21 (n = 63), 52% had a sustained positive RT-PCR on a nasopharyngeal swab or had no control exam until this time. Supplementary data, Appendix 2 depicts the time interval until complete recovery, death or the end of the observation time. Of note, a mean weight loss of −2.4 ± 2.7 kg (SD −13–1.5) was observed.
Deceased patients
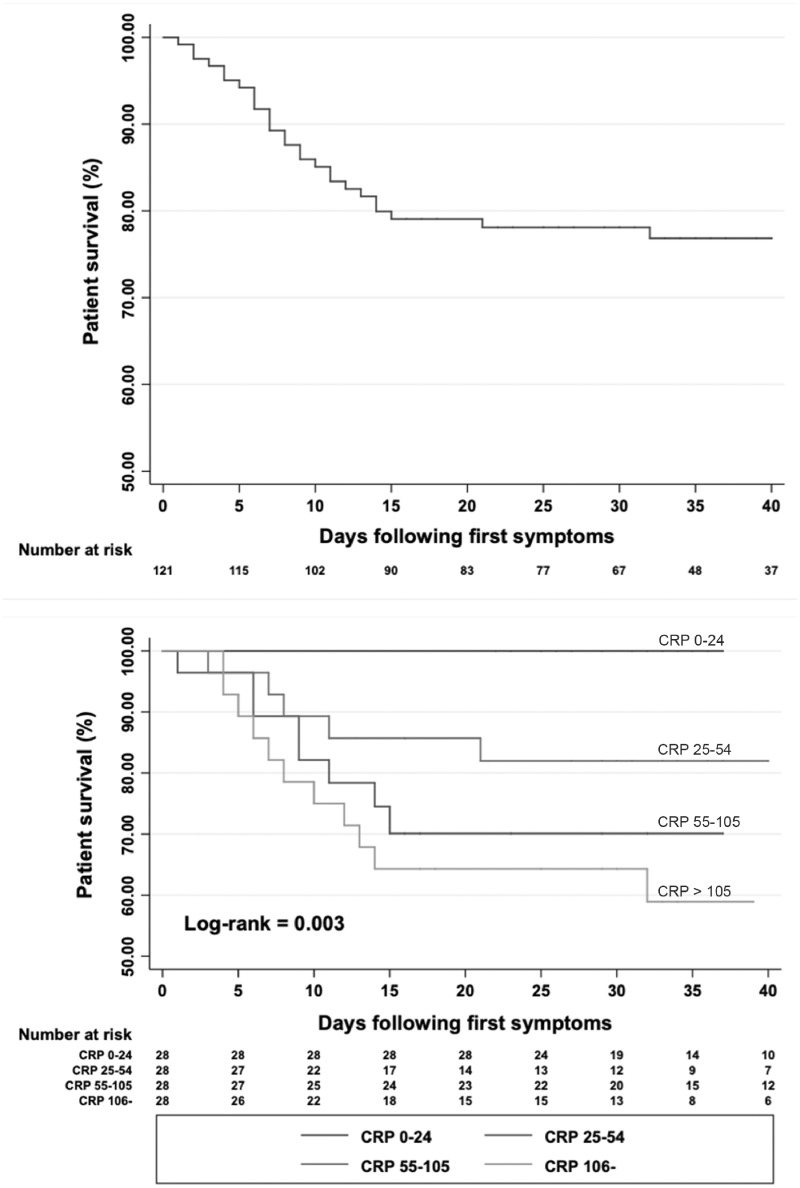
Twenty-nine (24%) patients died from COVID-19, three of whom died in the ICU. The median time between symptoms onset and death was 7 days (IQR 4–11). The Kaplan–Meier survival curve is presented in Figure 1 . A comparison of deceased and surviving patients is shown in Table 4.
FIGURE 1.
Kaplan–Meier survival curves in HD patients after onset of symptoms and according to CRP quartile.
Table 4.
Comparison of deceased and surviving patients
| Characteristics | Deceased patients (n = 29) |
Surviving patients (n = 94) |
P-value |
Available in each group/total, n/N | |||
|---|---|---|---|---|---|---|---|
| Demographics at the time of diagnosis | |||||||
| Age (years), median (IQR) | 80 (72–88) | 75.5 (64–83) | 0.04 | (29/94)/123 | |||
| Age >75 years, n (%) | 19 (66) | 52 (55) | 0.39 | 123/123 | |||
| Gender (male), n (%) | 18 (62) | 52 (56) | 0.67 | 122/123 | |||
| Functional disability, n (%) | 0.13 | 109/123 | |||||
| Total autonomy | 5 (20) | 32 (38) | |||||
| Partial autonomy | 14 (56) | 42 (50) | |||||
| None | 6 (24) | 10 (12) | |||||
| Comorbidity, n (%) | 28 (100) | 90 (97) | 1 | 121/123 | |||
| Number of comorbidities, mean ± SD | 3.4 ± 1.3 | 3.1 ± 1.6 | 0.28 | (28/90)/123 | |||
| Obesity, n (%) | 8 (33) | 32 (36) | 1.0 | 112/123 | |||
| Severity of the disease at diagnosis, n (%) | <0.001 | 120/123 | |||||
| Asymptomatic | 0 (0) | 4 (4) | |||||
| Moderate | 9 (31) | 53 (58) | |||||
| Severe | 11 (38) | 32 (35) | |||||
| Critical | 9 (31) | 2 (2) | |||||
| Clinical features at the time of diagnosis | |||||||
| Body temperature, mean ± SD | 38.2 ± 1 | 37.7 ± 1 | 0.056 | (24/76)/123 | |||
| SpO2, median (IQR) | 92 (85–97) | 96 (90–98) | 0.07 | (22/73)/123 | |||
| Laboratory and LD-CT scan characteristics, median (IQR) | |||||||
| CRP at diagnosis (mg/L) | 95 (49–192) | 44.5 (19–92) | 0.0003 | (25/88)/123 | |||
| CRP peak (mg/L) | 220 (117–272) | 98.5 (44–147) | 0.0002 | (23/70)/123 | |||
| Procalcitonin at diagnosis (ng/mL) | 1.7 (0.83–5.7) | 0.8 (0.34–1.7) | 0.12 | (5/27)/123 | |||
| Lymphocyte count at diagnosis (Lym/µL) | 530 (420–910) | 720 (461–995) | 0.36 | (23/86)/123 | |||
| CRP at D7 (mg/L) | 157 (72–222) | 43 (14–94) | 0.0004 | (11/70)/102 | |||
| Lymphocyte count at Day 7 (WBC/µL) | 410 (300–550) | 670 (450–1040) | 0.017 | (10/66)/102 | |||
| Extent of impairment on the initial LD-CT scan, n (%) | 0.54 | 48/62 | |||||
| Minimal | 2 (22) | 11 (28) | |||||
| Mild | 5 (56) | 16 (41) | |||||
| Mild to severe | 0 (0) | 6 (15) | |||||
| Severe | 1 (11) | 5 (13) | |||||
| Critical | 1 (11) | 1 (3) | |||||
| Therapy during period of care, n (%) | |||||||
| Antibiotic treatment | 19 (78) | 57 (71) | 0.56 | 99/123 | |||
| Specific treatment | 8 (28) | 15 (21) | 0.55 | 94/123 | |||
| Oxygen therapy | 24 (92) | 44 (50) | <0.005 | 114/123 | |||
| Artificial ventilation | 4 (15) | 1 (1) | 0.009 | 114/123 | |||
| Initial oxygen therapy (mL/min), median (IQR) | 2 (0–4) | 0 (0–2) | 0.0028 | (26/83)/123 | |||
| Maximal oxygen therapy (mL/min), median (IQR) | 15 (4–15) | 0 (0–3) | <0.0005 | (25/86)/123 | |||
SpO2, pulsatile saturation in oxygen.
At diagnosis, deceased patients presented a significantly older age, a more severe presentation, a higher CRP level, a higher body temperature and more often required oxygen therapy and at a higher flow. Patients who died more frequently had a typical presentation of the disease at diagnosis compared with survivors (85% versus 57%; P = 0.011), including dyspnoea (70% versus 45%; P = 0.027). Clinical features at diagnosis were critical in 31% (compared with 2% in survivors) and moderate in 31% (compared with 58% in survivors). Of note, compared with survivors, deceased patients more frequently had diabetic kidney disease (48% versus 32%) or an autosomal polycystic kidney disease (APKD) (15% versus 7%). None of the specific comorbidities was associated with the risk of death, while only a history of ischaemic heart disease tended to be more frequent in deceased patients (61% versus 41%; P = 0.084).
A Cox model was subsequently constructed for multivariate analysis. Included in this model were age, autonomy (total autonomy or not), pulsatile saturation in oxygen (SpO2) and oxygen therapy at diagnosis, body temperature and CRP at diagnosis. PCT was not included due to the amount of missing data. When taking into account all of the clinical symptoms, only body temperature at diagnosis was associated with the risk of death {hazard ratio [HR] 1.96 [95% confidence interval (CI) 1.11–3.44]; P = 0.02}. CRP at diagnosis was also associated with the risk of death [HR 1.01 (95% CI 1.005–1.017); P < 0.0001].
The results of the multivariate analysis are presented in Table 5, while the Kaplan–Meier survival curves according to CRP quartile are shown in Figure 1.
Table 5.
Multivariate analyses of factors associated with the risk of death
| Factors | HR | P-value | IQR |
|---|---|---|---|
| Age | 1.03 | 0.18 | 0.987–1.072 |
| Partial or no autonomy | 0.66 | 0.59 | 0.14–3.05 |
| Oxygen therapy | 1.13 × 1016 | 1.0 | |
| SpO2 at diagnosis | 0.97 | 0.31 | 0.90–1.03 |
| Body temperature at diagnosis (per 1°C) | 1.96 | 0.02 | 1.11–3.44 |
| CRP at diagnosis (per 1 mg/dL) | 1.01 | <0.0001 | 1.005–1.017 |
Multivariate analysis performed on only 83 patients due to missing data.
SpO2, pulsatile saturation in oxygen.
DISCUSSION
The rapid outbreak of the COVID-19 pandemic represented an unexpected challenge for the medical community. Although the epidemic curve is descending in many countries, the imminent end of containment measures raises the risk of a second wave [6, 7], and any new disease-related information would be useful to improve care. Given the lack of knowledge relative to specific and vulnerable patients, we report herein on a series of patients undergoing long-term HD in eight facilities in Alsace, France, where the first cluster of COVID-19 occurred.
The incidence of COVID-19 was much higher in our HD patients than in the background population (9.1% versus 0.16%). There are various putative explanations. First, our HD population carried comorbidities at risk of COVID-19 infection. Second, physical distancing is challenging during HD sessions, in the waiting room, during transport to HD facilities and in living facilites (many patients live in nursing homes). Finally, these patients are in contact with healthcare providers three times a week, such that screening was likely more thorough than in the general population. There was no evidence for transmission within our HD units, as the first cases occurred in patienta treated in separate rooms and containment measures were immediately applied for all suspected patients. Indeed, each patient presenting to the HD unit with any symptom suggestive of COVID-19 was admitted to a dedicated isolated room until the RT-PCR result was available. All patients with proven COVID-19 remained thereafter in an isolated room to avoid nosocomial transmission.
In the first reports in the Chinese population [2, 8], only 25–50% of patients had at least one comorbidity, which is far below our findings. Compared with hospitalized US patients who had more similar clinical characteristics and a significant number of comorbidities (88% with at least one comorbidity) [9], our patients were older and slightly less obese. Notwithstanding, our patients more frequently had diabetes, chronic respiratory disease and a history of cancer. The question of whether end-stage kidney disease (ESKD) per se or the comorbidities associated with ESKD worsens the outcome remains to be determined.
Compared with HD patients of the nationwide French Renal Epidemiology and Information Network (REIN) registry [10], patients in our cohort were older (74 ± 15 versus 69 ± 15 years) and were more likely to have APKD (8.5% versus 5.8%), obesity (36% versus 29%), chronic respiratory disease (34% versus 19%), cancer (20% versus 12%), ischaemic heart disease (45.6% versus 29%), stroke (22% versus 13%) and peripheral arterial disease (34.7% versus 21%). These observations highlight the substantial vulnerability of our cohort, i.e. the ‘frails upon the frails’. Moreover, the higher proportion of patients with partial (51.4% versus 8.5%) or an absence of autonomy (15% versus 8.5%) would explain both the higher vulnerability of these patients and the frequent need for external or institutional help, which could be a vector of contamination. This suggests that ‘barrier gestures’ should be strictly implemented in these patients in whom strict containment cannot be secured due to the need for dialysis. Screening indications should be comprehensive in this population to ensure adequate isolation when needed. Also, the favoured distribution of personal protective equipment in a period of relative shortage or wider indications for hospitalization should be considered.
Typical symptoms of COVID-19 infection have been initially described as fever, cough and dyspnoea [11]. Fever was inconsistent in our cohort compared with previous reports (57% versus 98%) [8, 11]. A lack of fever is frequent in infected HD patients, who tend to be slightly hypothermic in baseline conditions [12]. A considerable proportion of our patients presented with less typical symptoms, mostly asthenia, diarrhoea and anorexia. The latter has been previously described in the general COVID-19 population [8, 13–15] as well as in a case report of HD patients [16] and kidney transplants [17]. For example, diarrhoea, initially described as an uncommon symptom [8], was found in 34% of our patients. The extensive screening in our patients likely enabled the detection of atypical forms, although more frequent gastrointestinal involvement in patients with ESKD cannot be excluded.
Lymphopaenia was frequent in our cohort, as also previously described in the general COVID-19 population [8, 9, 11, 15], and associated with the severity of the disease [18]. This was attributed not only to a chronic endothelial dysfunction in ageing patients with chronic disease, but also to a direct cytotoxic action of the virus [15, 19]. Lymphopaenia should be considered both as part of the diagnosis and as a marker of the risk level of the disease [20]. In this study, CRP was higher than in previous studies conducted in the general COVID-19 population, even those patients with severe disease [8, 9, 11]. Of particular note, CRP was predictive of the risk of death in our cohort of COVID-19 HD patients. Such an association between CRP and disease severity was previously reported and associated with the extent of impairment on LD-CT scan (correlation coefficient 0.873, 0.734; P < 0.001) [21, 22]. Some authors have suggested the use of a predictive score based on CRP and lung volume involvement on LD-CT scan at the time of diagnosis [21, 22]. High PCT (>0.5 ng/mL), an uncommon finding in the general COVID-19 population [8, 9, 11], was more frequent in our cohort.
The mortality rate in our cohort was much higher than in the general COVID-19 population: 24% compared with the 1–5% mortality in the general COVID-19 population [23] and 8–15% mortality among patients >70 years old [9, 23]. An abysmal prognosis was previously described in a small cohort of kidney transplant recipients in the USA [17], with an early mortality rate of 28% at 3 weeks. A similar mortality rate of 26% was found in Italian [24] patients admitted to the ICU who had fewer comorbidities and were younger [median age 63 years (IQR 56–70)]. These findings thus emphasize the detrimental effect of ESKD and comorbidity burden over that of age. We did not find any association between age and risk of death in our cohort, in contrast to the general population. This discrepancy could be explained by the more advanced age of our cohort, as we compared old versus very old patients, which might have attenuated the differential risk. However, one should bear in mind that our HD population had a much higher mortality rate (16%) than that found in the general COVID-19 population (0.91%), with a steeper effect of age [10, 25]. Our results suggest an estimated mortality rate of 26.7%, but the exact case fatality rate (CFR) should take into account the asymptomatic patients, whose exact percentage was unknown in our population.
A noteworthy observation was the poor and prolonged viral clearance in our patients. Only 24% of our patients had viral clearance after a median of 32 days after onset of symptoms. while viral clearance was initially reported after a median time of 17–20 days in the general COVID-19 population [26, 27]. However, a recent study [28] found a viral clearance after a median of 24 days, with a maximum duration of 42 days, which is more in line with our population. Age and ESKD are well-identified causes of immune dysfunction, which could delay viral clearance [29]. In addition, the higher prevalence of a severe form of the disease could be associated with prolonged viral shedding [27]. Currently French guidelines [30] recommend the end of isolation 24 days after the onset of symptoms in immunocompromised patients, without control RT-PCR testing. Given the present data, it would seem cautious to perform another RT-PCR test before ending isolation. In our opinion, and considering the false negative rate of ∼30% [31, 32], a minimum of two consecutive negative swabs would appear to be a sound precaution.
Our study has certain limitations. The cohort was modestly sized and we could not perform a proper comparison due to the lack of other reported cohorts in HD patients. The number of missing data also represents a drawback. Finally, the absence of systematic screening in both the general and specific COVID-19 populations such as ours may limit our conclusions, especially regarding the CFR. Further larger studies are necessary to estimate the risk factors in the HD population. The high mortality rate in our patients also points to the need for randomized controlled studies on antiviral therapy.
CONCLUSION
In the present report, we describe a cohort of 123 HD patients presenting with symptomatic COVID-19 together with their clinical profiles and outcomes. HD patients were found to be at high risk of developing COVID-19. Several clinical characteristics, including atypical presentation, the predictive value of elevated CRP and delayed viral clearance, were worth noting and could be specific for this population. The outcomes were abysmal in this particularly vulnerable population, with a mortality rate of 24%.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We express our thanks to Nadia Honoré for compiling the REIN regional data, to the Clinical Research Assistant of AURAL for gathering clinical data and all the Alsace nephrologists on the frontline. This article is dedicated to the memory of our nephrologist colleague, Abdelmajid Ben Aicha, deceased of COVID-19. This observational study was nested in the French REIN registry, which obtained an agreement from the Commission nationale de l'informatique et des libertés for the use of personal data.
AUTHORS’ CONTRIBUTIONS
N.K., F.C., T.K., D.B.-K., T.H. and M.I. designed the study. N.K., F.C., C.M., A.L.F., T.N., M.I., P.P. and S.B. collected the study data. N.K., T.K., D.B.-K., T.H. and F.C. performed the statistical analyses and drafted the manuscript. All authors participated in the critical revision and approval of the article.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Henry BM, Lippi G.. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020; 52: 1193–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W, Liang W, Zhao Y. et al. Comorbidity and its impact on 1,590 patients with COVID-19 in China: a nationwide analysis. Respir Med 2020; 55: 2000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scarpioni R, Manini A, Valsania T. et al. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol 2020; 37: 2020-vol2. Doi: 10.1126/science.abc3517 [PubMed] [Google Scholar]
- 4. Wang R, Liao C, He H. et al. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis 2020; 76: 141–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberici F, Delbarba E, Manenti C. et al. Management of patients on dialysis and with kidney transplant during SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep 2020; 5: 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salje H, Kiem CT, Lefrancq N. et al. Estimating the burden of SARS-CoV-2 in France. Science2020; 369: 208–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung K, Wu JT, Liu D. et al. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet 2020; 395: 1382–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge H, Wang X, Yuan X. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020; 39: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lassalle M, Monnet E, Ayav C. et al. 2017 annual report of the Renal Epidemiology Information Network (REIN) registry. Transpl Int 2019; 32: 892–902 [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Usvyat LA, Raimann JG, Carter M. et al. Relation between trends in body temperature and outcome in incident hemodialysis patients. Nephrol Dial Transplant 2012; 27: 3255–3263 [DOI] [PubMed] [Google Scholar]
- 13. Holshue ML, DeBolt C, Lindquist S. et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan L, Mu M, Yang P. et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020; 115: 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J-J, Dong X, Cao Y-Y. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75: 1730–1741 [DOI] [PubMed] [Google Scholar]
- 16. Ferrey AJ, Choi G, Hanna RM. et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol 2020; 51: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akalin E, Azzi Y, Bartash R. et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382: 2475–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen T, Dai Z, Mo P. et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci 2020; doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bermejo-Martin J, Almansa R, Menedez R. et al. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J Infect 2020; 80: e23–e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan L, Wang Q, Zhang D. et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Sig Transduct Target Ther 2020; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L. C- reactive protein levels in the early stage of COVID-19. Med Mal Infect 2020; 50: 332–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng Z, Qin L, Cao Q. et al. Quantitative computed tomography of the coronavirus disease 2019 (COVID-19) pneumonia. Radiol Infect Dis 2020; doi: 10.1016/j.jrid.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239. [DOI] [PubMed] [Google Scholar]
- 24. Grasselli G, Zangrillo A, Zanella A. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nombre de décès quotidiens par département | Insee. https://www.insee.fr/fr/information/4470857 (3 May 2020, date last accessed)
- 26. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu K, Chen Y, Yuan J. et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao AT, Tong YX, Zhang S.. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson DW, Fleming SJ.. The use of vaccines in renal failure. Clin Pharmacokinet 1992; 22: 434–446 [DOI] [PubMed] [Google Scholar]
- 30.SARS coronavirus-2: clinical criteria for safely ending isolation of infected patients. https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=800 (3 May 2020, date last accessed)
- 31. Wang X, Tan L, Wang X. et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 2020; 94: 107–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lan L, Xu D, Ye G. et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020; 323: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.