Abstract
Objective
The objective of this study is to systematically synthesize the currently available literature on various modes of transmission (congenital, intrapartum, and postpartum), clinical features and outcomes of SARS-CoV-2 infection in neonates.
Methods
We conducted a comprehensive literature search using PubMed, EMBASE, and Web of Science until 9 June 2020. A combination of keywords and MeSH terms, such as COVID-19, coronavirus, SARS-CoV-2, 2019-nCoV, severe acute respiratory syndrome coronavirus 2, neonates, newborn, infant, pregnancy, obstetrics, vertical transmission, maternal–foetal transmission and intrauterine transmission, were used in the search strategy. We included studies reporting neonatal outcomes of SARS-CoV-2 proven pregnancies or neonatal cases diagnosed with SARS-CoV-2 infection.
Results
Eighty-six publications (45 case series and 41 case reports) were included in this review. Forty-five case series reported 1992 pregnant women, of which 1125 (56.5%) gave birth to 1141 neonates. A total of 281 (25%) neonates were preterm, and caesarean section (66%) was the preferred mode of delivery. Forty-one case reports describe 43 mother-baby dyads of which 16 were preterm, 9 were low birth weight and 27 were born by caesarean section. Overall, 58 neonates were reported with SARS-CoV-2 infection (4 had a congenital infection), of which 29 (50%) were symptomatic (23 required ICU) with respiratory symptoms being the predominant manifestation (70%). No mortality was reported in SARS-CoV-2-positive neonates.
Conclusion
The limited low-quality evidence suggests that the risk of SARS-CoV-2 infections in neonates is extremely low. Unlike children, most COVID-positive neonates were symptomatic and required intensive care. Postpartum acquisition was the commonest mode of infection in neonates, although a few cases of congenital infection have also been reported.
Keywords: breast milk, congenital infection, COVID-19, neonates, pregnancy
INTRODUCTION
Novel coronavirus infection (later termed as COVID-19) was declared a global pandemic on 11 March 2020 and as of 12 June 2020, the number of confirmed cases has reached 7 410 510 and 418 294 (5.6%) deaths have been reported worldwide [1]. A significant number of pregnant females are also affected, as they are equally susceptible to SARS-CoV-2 infection [2]. Neonatal SARS-CoV-2 infections are rare, and till now a handful of cases are reported. Although the newborns are considered at risk for vertical and postpartum horizontal transmission, there is a dearth of data on the clinical features, outcome, mode of transmission and mode of delivery for neonates. Also, there is uncertainty about the transmission of the SARS-CoV-2 virus through the placenta and breast milk [3–9].
Therefore, we performed this systematic review to synthesize the currently available literature on various modes of transmission (congenital, intrapartum and postpartum), clinical features and outcomes of SARS-CoV-2 infection in neonates.
MATERIALS AND METHODS
Search strategy
This study was conducted following the Meta-analysis Of Observational Studies in Epidemiology guidelines [10]. A predefined search strategy was developed, and three investigators (S.K.D., J.M., and J.K.) independently performed a literature search in MEDLINE, EMBASE and Web of Science for the original articles published between 1 December 2019 and 9 June 2020. Terms used for literature search were COVID-19, coronavirus, SARS-CoV-2, 2019-nCoV, severe acute respiratory syndrome coronavirus 2, neonates, newborn, infant, pregnancy, obstetrics, vertical transmission, maternal–foetal transmission, and intrauterine transmission. Specific search strategies were created for each electronic database separately, by using the MeSH terms, Emtree terms and terms described above (Supplementary Table S1). The electronic search was also supplemented by a hand search of bibliography of the included studies and relevant review articles. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines [11]. No language restrictions were used.
Study selection
A predefined set of criteria was used for the assessment of the eligibility of the studies for this systematic review. Studies enrolling neonates and/or pregnant mothers and reporting data on COVID-19 testing of the neonates were considered eligible for the review. Initially, two researchers (J.M. and S.K.D.) independently screened the title and abstract for the eligibility. Later three authors (S.K.D., J.M. and J.K.) examined the full-text articles for inclusion and exclusion criteria. Studies were included if they met the following criteria: (i) studies reporting the neonatal outcome of pregnancy with RT-PCR proven SARS-CoV-2 infection, (ii) studies reporting clinical manifestations, disease severity, laboratory investigations and outcome of RT-PCR proven SARS-CoV-2 infection in neonates (postnatal age < 29 days for the term and postmenstrual age up to 44 weeks for preterm neonates), (iii) all types of study designs: cohort, cross-sectional studies, case–control studies, case series and case reports. Correspondences or letters fulfilling the above criteria were also included. We excluded: (i) studies with term neonates aged more than 28 days and preterm neonates with postmenstrual age more than 44 weeks, (ii) studies reporting COVID-19-positive pregnancy without any neonatal outcomes, (iii) studies not reporting the neonatal COVID-19 status, (iv) studies reporting about other serotypes of coronavirus or testing methods other than RT-PCR, (v) narrative or systematic review, (vi) conference proceedings and (vii) editorial, perspective, etc. not meeting the inclusion criteria.
Data extraction and quality assessment
A structured performa was used for the data extraction. Two investigators independently extracted the desired data from the full-text of the eligible studies. The details of extracted data parameters are given in Supplementary AppendixTable S2. The studies published in Chinese language were first translated to English language using Google translation and then the desired data were extracted. Any disagreement between two investigators was resolved through discussion with the third investigator (J.K.). A researcher (J.K.) independently rechecked the extracted data for its accuracy and completeness. The quality of the included studies in this systematic review was assessed using the Newcastle Ottawa scale [12]. Two investigators (S.K.D. and J.K.) independently assigned an overall risk of bias to each eligible study, and if they disagreed, another researcher (J.M.) was involved to resolve the discrepancy.
Data synthesis and statistical analysis
We summarized the relevant clinical details of the neonates and pregnant mothers described in the included studies. Clinical details, demographics, the time of doing RT-PCR for SRS-CoV-2 infection in neonates and outcomes of the SARS-CoV-2-positive neonates were summarized separately. Mother was considered to have SARS-CoV-2 (COVID-19) infection only if the RT-PCR from nasopharyngeal/oropharyngeal swab was positive [13]. Neonate was considered to have COVID-19 infection if the RT-PCR from nasopharyngeal/oropharyngeal swab from infant or blood from neonate/umbilical cord or amniotic fluid or tissue sample from the foetal side of the placenta was positive for SARS-CoV-2 [13]. The neonates with SARS-CoV-2 infection were further classified to characterize the mode of transmission (congenital, acquired intrapartum and acquired postpartum) [13]. Percentages and mean/median values were calculated to describe categorical and continuous variables, respectively. SPSS v23 was used for statistical analysis.
RESULTS
Study selection and characteristics
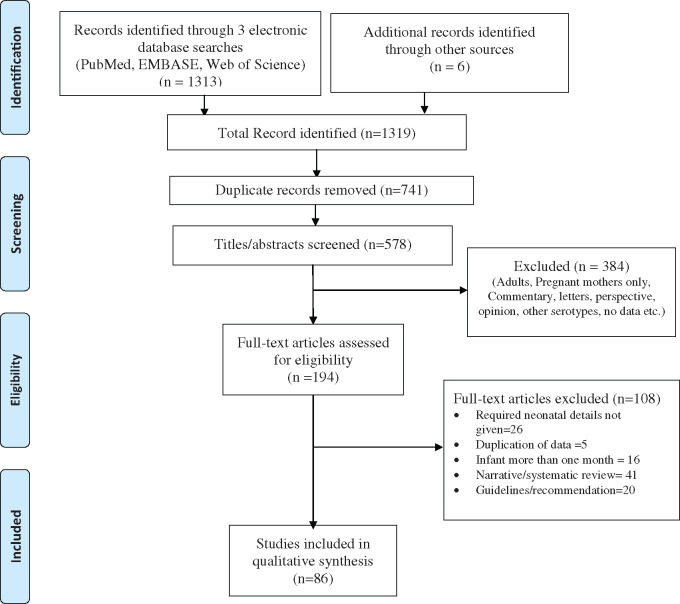
We found a total of 1313 records. The detailed process of selection of final included studies for this systematic review is described in Fig. 1. After removing 741 duplicates, 578 articles were screened for eligibility through titles and abstracts. A total of 384 articles were excluded, and 194 articles were retrieved for full-text assessment. After a thorough screening of full-text articles, 85 (45 case series/cohort and 41 case reports) publications were included for the qualitative synthesis. Quality assessment was done for 45 studies, of which 9 were rated as good, 21 as fair, and 15 of poor quality by the Newcastle Ottawa scale [12].
Fig. 1.
PRISMA flow diagram.
Clinical details
Forty-five studies [3, 5, 7, 14–55] described 1992 pregnant women with gestation ranging from 5 to 41 weeks. Birth was reported amongst 1125 (56.5%) of these. The mode of delivery was available for 1114 pregnancies, and caesarean section (65%) was more frequent than vaginal delivery. A total of 1141 neonates were born of which, 281 (25%) were preterm (<37 weeks). SARS-CoV-2 testing was done for 1005 (88%) neonates and 39 (3.9%) turned out to be positive on RT-PCR (Table 1). Forty-one case reports [4, 6, 8, 56–93] described 43 mother-baby dyads, of which 16 (37.2%) were preterm (<37 weeks), 9 (21%) were low birth weight (<2500 g) and 27 (62.8%) were born by caesarean section (Table 2). All 43 were tested for SARS-CoV-2 infection using nasopharyngeal or oropharyngeal specimen and 19 neonates (44.2%) have positive RT-PCR for SARS-CoV-2.
Table 1.
Details of included studies (case series and cohort)
| Author | Date of publication | Country | Study design | Study quality | Pregnant women (n) | Gestational age (weeks) | Vaginal delivery (total delivered) | Live births (n) | Preterm, n (%) | Birth weight (g) | Neonates tested(n) | COVID- positive neonates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breslin et al. [14] | 9 April 2020 | USA | Retrospective | Fair | 43 | 37 (32–38)a | 10 (18) | 18 | 1 (5) | – | 18 | 0 |
| Buonsenso et al. [15] | 21 April 2020 | Italy | Observational | Poor | 7 | 8–37b | 0 (2) | 2 | 1 (50) | 2300, 3390 | 2 | 2 |
| Campbell et al. [46] | 26 May 2020 | USA | Case series | Poor | 30 | Term | 20 (30) | 30 | 0 | 3370 (621)c | 30 | 0 |
| Cao et al. [16] | 10 April 2020 | China | Retrospective | Fair | 10 | 33–40b | 2 (10) | 11 | 4 (36) | 2050–3800b | 4 | 0 |
| Chen et al. [3] | 12 February 2020 | China | Retrospective | Fair | 9 | 36–39b | 0 (9) | 9 | 4 (44) | 1880–3730b | 6 | 0 |
| Chen et al. [17] | 16 March 2020 | China | Case series | Poor | 4 | 37–39b | 1 (4) | 4 | 0 | 3050–3800b | 3 | 0 |
| Chen et al. [18] | 28 March 2020 | China | Observational | Fair | 5 | 38–41b | 3 (5) | 5 | 0 | 3235–4050b | 5 | 0 |
| Chen et al. [19] | 17 April 2020 | China | Retrospective | Poor | 118 | – | 5 (68) | 68 | 14 (21) | – | 8 | 0 |
| Chen et al. [20] | 10 March 2020 | China | Case series | Poor | 17 | – | 0 (17) | 17 | 3 (18) | – | 0 | 0 |
| Chen et al. [21] | 08 May 2020 | China | Case series | Poor | 3 | – | 0 (2) | 2 | 1 (33) | – | 2 | 0 |
| Fan et al. [22] | 17 March 2020 | China | Case series | Fair | 2 | 36–37b | 0 (2) | 2 | 0 | 2890, 3400 | 2 | 0 |
| Ferrazzi et al. [23] | 07 April 2020 | Italy | Retrospective | Fair | 42 | – | 24 (42) | 42 | 11 (26) | 840–4040b | 42 | 3 |
| Govind et al. [24] | 07 May 2020 | England | Observational | Fair | 9 | 36.8 (27–39)a | 1 (9) | 9 | 7 (78) | – | 9 | 1 |
| Hernández et al. [47] | 05 June 2020 | Spain | Case series | Good | 3 | 39 | – (3) | 3 | 1 (33) | 1135–3700b | 3 | 3 |
| Hantoushzadeh et al. [25] | 24 April 2020 | Iran | Case series | Fair | 9 | 32.7 (28–38)a | 0 (5) | 6 | 5 (83) | 1180–3200b | 6 | 1 |
| Hirshberg et al. [26] | 01 May 2020 | USA | Case series | Fair | 5 | 25–31b | 0 (3) | 3 | 3 (100) | 1500–2110 | 3 | 0 |
| Huang et al. [27] | 08 May 2020 | China | Retrospective | Poor | 8 | 28–39b | 1 (6) | 6 | 3 (50) | 1520–4200b | 6 | 0 |
| Kayem et al. [44] | 31 May 2020 | France | Case series | Poor | 617 | 22–37b | 94 (181) | 190 | 50 (28) | – | 190 | 2 |
| Khan et al. [28] | 19 March 2020 | China | Case series | Fair | 3 | 34–39b | 3 (3) | 3 | 1 (33) | 2890–3730b | 3 | 0 |
| Khan et al. [48] | 27 March 2020 | China | Case series | Fair | 17 | 35–41b | 0 (17) | 17 | 3 (18) | 2300–3750b | 17 | 0 |
| Knight et al. [49] | 08 June 2020 | UK | Case series | Fair | 427 | 38 (36–40)a | 106 (253) | 259 | 63 (24) | – | 259 | 12 |
| Li et al. [45] | 30 March 2020 | China | Case–control | Good | 16 | 38 (0.2)a | 3 (16) | 17 | 4 (23) | 3066 (560)c | 3 | 0 |
| Liao et al. [29] | 29 April 2020 | China | Retrospective | Good | 10 | 38 (1.43)a | 10 (10) | 10 | 1 (10) | 3283 (449)c | 7 | 0 |
| Liu et al. [30] | 27 February 2020 | China | Retrospective | Fair | 13 | 32–38b | 0 (10) | 10 | 7 (70) | – | 9 | 0 |
| Liu et al. [31] | 17 March 2020 | China | Observational | Fair | 10 | 38 (1.5)c | 1 (10) | 10 | 2 (20) | 3293 (425)c | 10 | 0 |
| Liu et al. [32] | 07 March 2020 | China | Retrospective | Poor | 15 | 37 (1)c | 1 (11) | 11 | – | – | 11 | 0 |
| Martinez-Perez et al. [50] | 08 June 2020 | Spain | Case series | Poor | 82 | – | 41 (82) | 82 | 25 (30) | 910–4750b | 82 | 5 |
| Ochiai et al. [51] | 04 June 2020 | Japan | Case series | Poor | 52 | 38 | 0 (2) | 2 | 0 | 3715, 2805 | 2 | 0 |
| Pereira et al. [5] | 22 May 2020 | Spain | Observational | Good | 60 | 32 (5–41)a | 18 (23) | 23 | 2 (9) | – | 23 | 0 |
| Pierce-Williams et al. [33] | 04 May 2020 | USA | Cohort | Good | 64 | 34 (4.2)c | 8 (32) | 32 | 19 (59) | 2403 (858)c | 33 | 1 |
| Qadri and Mariona [52] | 20 May 2020 | USA | Case series | Poor | 16 | 22–40b | 8 (12) | 12 | 1 (8) | 2830–4215b | 12 | 0 |
| Qiancheng et al. [34] | 22 April 2020 | China | Retrospective | Fair | 28 | 38 (36–39)a | 5 (22) | 23 | 1 (4) | 2915–3390b | 22 | 0 |
| Salvatori et al. [35] | 15 May 2020 | Italy | Case series | Fair | 2 | 39–41b | – (2) | 2 | 0 | 3120, 4440 | 2 | 2 |
| Sun et al. [36] | 19 April 2020 | China | Case series | Fair | 3 | 31–37b | 0 (3) | 3 | 2 (67) | – | 3 | 1 |
| White et al. [53] | 04 June 2020 | USA | Case series | Good | 3 | 39 | 2 (3) | 3 | 0 | – | 2 | 2 |
| Wu et al. [37] | 05 May 2020 | China | Case series | Fair | 13 | 5–38b | 1 (5) | 5 | 2 (40) | 2300–3910b | 5 | 0 |
| Xu et al. [38] | 28 April 2020 | China | Retrospective | Fair | 5 | 34–39b | 4 (5) | 5 | 2 (40) | 2450–3760b | 5 | 0 |
| Yan et al [39] | 17 April 2020 | China | Retrospective | Good | 116 | 38.4 (37–39)a | 14 (100) | 100 | 21 (21) | 3108 (526)c | 86 | 0 |
| Yang et al [40] | 5 April 2020 | China | Prospective | Good | 7 | 36–38b | 0 (7) | 7 | 4 (57) | 2096 (660)c | 6 | 0 |
| Yu et al. [41] | 24 March 2020 | China | Retrospective | Fair | 9 | 39 (37–41)a | 0 (7) | 7 | 0 | 3000–3500b | 3 | 1 |
| Zeng et al. [7] | 26 March 2020 | China | Prospective | Poor | 6 | – | – (6) | 6 | – | – | 6 | 0 |
| Zeng et al. [42] | 26 March 2020 | China | Cohort | Fair | 33 | – | 0(33) | 33 | 4(12) | – | 19 | 3 |
| Zeng et al. [54] | 21 May 2020 | China | Case series | Poor | 16 | 37 (34–41)a | 4 (16) | 12 | 3 (25) | 3175 (478)c | 16 | 0 |
| Zhang et al [55] | 25 March 2020 | China | Case series | Good | 16 | 29 (2.9)c | 0 (10) | 10 | 1 (10) | – | 10 | 0 |
| Zhu et al. [43] | 10 February 2020 | China | Case series | Poor | 9 | 31–39b | 2 (9) | 10 | 5 (50) | 1720–3800b | 10 | 0 |
Median (IQR).
Range.
Mean (SD).
Table 2.
Details of included studies (case reports)
| Author | Date of publication | Country | No. of COVID+ mothers | COVID+ neonates (n) | Gestation (weeks) | Birth weight (g) | Mode of delivery | Apgar (1/5 min) | Symptoms |
|---|---|---|---|---|---|---|---|---|---|
| Aghdam et al. [56] | 1 April 2020 | Iran | 1 | 1 | – | 3460 | CS | – | Fever, lethargy, mottling, tachypnoea, RD |
| Aguilar et al. [57] | 27 April 2020 | Spain | 1 | 1 | – | – | – | – | Seizures, Hypertonia, Fever, Watery stools |
| Alzamora et al. [58] | 18 April 2020 | Peru | 1 | 1 | 33 | 2970 | CS | 6/8 | RD, cough |
| Blauvelt et al. [59] | 8 May 2020 | USA | 1 | 0 | 28 | 1880 | CS | 4/8 | HMD, leukopenia, mild acidosis |
| Carosso et al. [60] | 14 April 2020 | Italy | 1 | 1 | 37 | 3120 | VD | 9/10 | Asymptomatic |
| Cook et al. [86] | 19 May 2020 | UK | – | 1 | 27 | – | – | – | Poor feeding, dyspnoea, respiratory failure, shock |
| De Socio et al. [61] | 1 May 2020 | Italy | 1 | 0 | 40 | – | VD | 10/10 | Asymptomatic |
| Fontanella et al. [92] | 29 May 2020 | Netherlands | 1 | 0 | 40 | – | CS | 9/9 | Asymptomatic |
| Groß et al. [8] | 21 May 2020 | Germany | 2 | 2 | – | – | – | – | Breathing difficulty |
| Han et al. [62] | 16 April 2020 | Korea | 1 | 1 | 38 | 3730 | VD | – | Fever, nasal blockage, tachycardia, cough |
| Iqbal et al. [63] | 1 April 2020 | USA | 1 | 0 | 39 | – | VD | 8/9 | Asymptomatic |
| Jain et al. [93] | 5 June 2020 | India | 2 | 0 | Term | 2865/– | CS | –/– | Asymptomatic/second-asphyxia, shock, ventilated |
| Kirtsman et al. [64] | 14 May 2020 | Canada | 1 | 1 | 40 | 2930 | CS | 9/9 | Hypothermia, feeding difficulty, hypoglycaemia |
| Kuhrt et al. [65] | 8 May 2020 | England | 1 | 0 | 32 | 2190 | CS | 8/9 | Ventilated |
| Lang et al. [6] | 8 May 2020 | China | 1 | 0 | 35 | – | CS | 9/10 | Asymptomatic |
| Lee et al. [66] | 31 March 2020 | Korea | 1 | 0 | 37 | 3130 | CS | 9/10 | Asymptomatic |
| Li et al. [67] | 5 March 2020 | China | 1 | 0 | 35 | – | CS | – | Asymptomatic |
| Li et al. [68] | 5 May 2020 | China | 1 | 0 | 35 | 2700 | CS | 1/1 | Birth asphyxia, died |
| Li et al. [69] | 2020 | China | 1 | 1 | 38 | – | CS | – | – |
| Liao et al. [70] | 26 March 2020 | China | 1 | 0 | 35 | – | CS | – | – |
| Lorenz et al. [71] | 12 May 2020 | Germany | 1 | 1 | 40 | – | VD | 9/9 | Fever, encephalitis like symptoms, cough |
| Lowe et al. [72] | 15 April 2020 | Australia | 1 | 0 | 40 | – | VD | 9/9 | Asymptomatic |
| Lu et al. [73] | 23 April 2020 | China | 1 | 0 | 38 | 3470 | CS | 9/9 | Asymptomatic |
| Lyra et al. [74] | 20 April 2020 | Portugal | 1 | 0 | 39 | 3110 | CS | 8/9 | Asymptomatic |
| Mehta et al. [87] | 16 May 2020 | USA | 1 | 1 | 28 | 925 | CS | 5/6 | Asymptomatic |
| Munoz et al. [75] | 22 April 2020 | USA | 1 | 1 | 36 | – | – | – | Hypotension, hypothermia, tachypnoea |
| Peng et al. [4] | 6 April 20 | China | 1 | 0 | 35 | 2600 | CS | 9/10 | HMD, tachypnoea, apnoea |
| Perrone et al. [88] | 21 May 2020 | Italy | 1 | 0 | 32 | – | VD | – | Asymptomatic |
| Piersigilli et al. [76] | 7 May 2020 | Belgium | 1 | 1 | 26 | 960 | CS | 5/8 | HMD, PDA, pneumothorax |
| Salik and Mehta [89] | 25 May 2020 | USA | 1 | 1 | 37 | 1900 | – | – | Tetralogy of Fallot |
| Sharma et al. [77] | 20 April 2020 | India | 1 | 0 | 38 | – | CS | – | Asymptomatic |
| Sinelli et al. [78] | 1 May 2020 | Italy | 1 | 1 | – | – | VD | 9/10 | RD |
| Wang et al. [85] | 28 February 2020 | China | 1 | 0 | 30 | 1830 | CS | 9/10 | Asymptomatic |
| Wang et al. [79] | 12 March 2020 | China | 1 | 1 | 40 | 3205 | CS | 8/9 | Vomiting, lymphopenia, deranged LFT |
| Wang et al. [90] | 22 March 2020 | China | 1 | 1 | 38 | 3030 | VD | – | Vomiting |
| Xia et al. [80] | 17 March 2020 | China | 1 | 0 | 37 | 3100 | CS | 9/10 | – |
| Xiong et al. [81] | 7 April 2020 | China | 1 | 0 | 38 | 3070 | VD | 9/10 | Asymptomatic |
| Yilmaz et al. [91] | 17 May 2020 | Turkey | 1 | 0 | 38 | 2900 | CS | 9/10 | Asymptomatic |
| Zamaniyan et al.[82] | 17 April 2020 | Iran | 1 | 1 | 32 | 2350 | CS | 8/9 | Fever |
| Zambrano et al. [83] | 25 March 2020 | Honduras | 1 | 0 | 32 | 1500 | VD | – | – |
| Zhou et al. [84] | 28 April 2020 | China | 1 | 0 | 37 | – | CS | – | – |
CS, caesarean section; HMD hyaline membrane disease; LFT, liver function tests RD, respiratory distress; VD, vaginal delivery.
We identified a total of 58 SARS-CoV-2 RT-PCR-positive neonates. The clinical details, demographics and outcome of these neonates are described in Table 3. Of these 58 neonates, maternal COVID testing details were available for 53 and all were positive for SARS-CoV-2 infection. The perinatal characteristics and clinical features of SARS-CoV-2-positive neonates are summarized in Table 4. Most of the neonates became symptomatic beyond 24 h of birth. Among term neonates, 10 had onset of symptoms in first week (7 on Day 2 of life itself), 3 each in second and third weeks and 4 in fourth week of life. Except one (Meconium aspiration syndrome), none of these term infants had any other neonatal illness to explain the symptoms. In preterm, only one had symptoms on first day, one on second day and rest five had at or beyond Day 7 of life. Among all COVID-positive neonates, 22 (38%) required ICU admission and 10 (17%) were ventilated (invasive and non-invasive). Separate details for invasive and non-invasive ventilation were not available as most except one [71] did not report it clearly.
Table 3.
Clinical details, mode of transmission and outcome of COVID-positive neonates (n=58)
| Author | Neonates (n) | Mother COVID + | MOD | GA (weeks)/ weight (g) | NP swab positive (DOL) | NP swab negative (DOL) | Direct breast feeding | Clinical features | ICU stay | MV | Final outcome | Mode of transmission |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aghdam et al. [56] | 1 | – | CS | Term/3460 | 15 | – | – | Fever, mottling, respiratory distress | Yes | No | Discharged | Postpartum acquired |
| Aguilar et al. [57] | 1 | – | – | – | 26 | – | Yes | Seizures, fever, irritability, watery stools | Yes | No | Discharged | Postpartum acquired |
| Alzamora et al. [58] | 1 | Yes | CS | 33/2970 | 1 | – | No | Respiratory distress | Yes | Yes | – | Not assigneda |
| Buonsenso et al. [15] | 2 | Yes | CS | 38/3390 | 15 | – | Yes | Asymptomatic | No | No | Discharged | Postpartum acquired |
| 35/2300 | – | 1 | No | Asymptomatic | No | No | Discharged | Congenital (confirmed)b | ||||
| Carosso et al. [60] | 1 | Yes | VD | 37/3120 | At birth | 3 | – | Asymptomatic | No | No | Discharged | Congenitalc |
| Cook et al. [86] | 1 | – | – | 27/– | 56 | – | – | Poor feeding, dyspnoea, respiratory failure, shock | Yes | Yes | Admitted | Postpartum acquired |
| Ferrazzi et al. [23] | 3 | Yes | VD | – | 1 | – | Yes | Asymptomatic | – | – | – | Not assigneda |
| CS | – | 3 | – | Yes | Asymptomatic | – | – | – | Postpartum acquired | |||
| VD | Term/– | 3 | – | No | GI and respiratory | Yes | Yes | – | Postpartum acquired | |||
| Govind et al. [24] | 1 | Yes | CS | 38/4165 | – | – | No | Desaturation, fever | Yes | Yes | – | Postpartum acquired |
| Hernández et al. [47] | 3 | Yes | VD | Term/3700 | 2 | 10 | – | MAS | Yes | Yes | Discharged | Postpartum acquired |
| Yes | VD | Preterm/1135 | 78 | 84 | – | – | Yes | Yes | Discharged | Postpartum acquired | ||
| Yes | VD | Term/3550 | 6 | 13 | – | Asymptomatic | No | No | Discharged | Postpartum acquired | ||
| Groß et al. [8] | 2 | Yes | – | – | 4 | – | Yes | Respiratory distress | No | No | Discharged | Postpartum acquired |
| 11 | 24 | Yes | Respiratory distress, hypoxia | – | No | Discharged | Postpartum acquired | |||||
| Han et al. [62] | 1 | Yes | VD | 38/3730 | 27 | – | Yes | Fever, cough, vomiting | Yes | No | Discharged | Postpartum acquired |
| Hantoushzadeh et al. [25] | 1 | Yes | CS | 30/– | 7 | – | No | Pneumonia | Yes | – | Admitted | Postpartum acquired |
| Kayem et al. [44] | 2 | Yes | – | – | – | – | – | Asymptomatic/hypoxia | – | – | – | Details not available |
| Kirtsman et al. [64] | 1 | Yes | CS | 35/2930 | At birth | 7 | Yes | Asymptomatic | No | No | Discharged | Congenital (probable)d |
| Knight et al. [49] (individual patient details not available) | 6 | Yes | 2 VD, 4 CS | 3 preterm | <12 h | – | – | – | – | – | – | Not assigneda |
| 3 Term | ||||||||||||
| 6 | Yes | 2 VD, 4 CS | 4 preterm | >12 h | – | – | – | 1 | – | – | Postpartum acquired | |
| 2 Term | ||||||||||||
| Li et al. [69] | 1 | Yes | CS | 38/– | 3 | – | – | Asymptomatic | No | No | Discharged | Postpartum acquired |
| Lorenz et al. [71] | 1 | Yes | VD | 40/– | 2 | – | – | Lethargy, pneumonia, encephalitis syndrome (fever, seizures, altered sensorium) | Yes | CPAP | Discharged | Postpartum acquired |
| Martinez-Perez et al. [50] | 5 | Yes | CS | Term/– | 10 | – | Yes | COVID symptoms | – | – | – | Postpartum acquired |
| Yes | CS | Term/– | 10 | – | Yes | COVID symptoms | – | – | – | Postpartum acquired | ||
| Yes | VD | Preterm/– | 1 | 2 | – | Asymptomatic | No | No | – | Not assigneda | ||
| Yes | VD | Preterm/– | 1 | 2 | – | Asymptomatic | No | No | – | Not assigneda | ||
| Yes | CS | – | 1 | 2 | – | Asymptomatic | No | No | – | Not assigneda | ||
| Mehta et al. [87] | 1 | Yes | CS | 28/925 | 3 | – | No | Asymptomatic | Yes | No | Admitted | Postpartum acquired |
| Munoz et al. [75] | 1 | – | – | 36/– | 17 | – | – | Hypotension, tachycardia, hypothermia, tachypnoea | Yes | Yes | Discharged | Postpartum acquired |
| Piersigilli et al. [76] | 1 | Yes | CS | 26/960 | 7 | 21 | No | HMD, pneumothorax | Yes | Yes | Admitted | Postpartum acquired |
| Pierce-Williams et al. [33] | 1 | Yes | – | – | 2 | – | – | Asymptomatic | – | – | Discharged | Postpartum acquired |
| Salik and Mehta [89] | 1 | Yes | – | 37/1.9 | 7 | 13 | – | Tet spells, tachypnoea, pneumonia | Yes | Yes | – | Postpartum acquired |
| Salvatori et al. [35] | 2 | Yes | – | 41/4440 | 18 | – | Yes | Asymptomatic | No | No | Discharged | Postpartum acquired |
| 39/3120 | 10 | – | Yes | Cough, diarrhoea, poor feeding | No | No | Discharged | Postpartum acquired | ||||
| Sinelli et al. [78] | 1 | Yes | VD | Term/– | 2 | – | No | Hypoxia, cyanosis, poor sucking | Yes | No | Discharged | Postpartum acquired |
| Sun et al. [36] | 1 | Yes | VD | 37/– | 6 | – | No | Asymptomatic | No | – | – | Postpartum acquired |
| Wang et al. [90] | 1 | – | VD | 38/3030 | 23 | – | Yes | Vomiting | – | No | Discharged | Postpartum acquired |
| White et al. [53] | 2 | Yes | VD | 39 | 17 | – | Yes | Fever, shock, rhinorrhoea, hypoxia | Yes | No | Discharged | Postpartum acquired |
| Yes | CS | 39 | 25 | – | Yes | Fever, rhinorrhoea, desaturation | Yes | No | Discharged | Postpartum acquired | ||
| Wang et al. [79] | 1 | Yes | CS | 40/3205 | 2 | 17 | Yes | Vomiting, deranged LFT, pneumonia | No | No | Discharged | Postpartum acquired |
| Yu et al. [41] | 1 | Yes | CS | 39/3250 | 2 | 17 | – | Respiratory distress | Yes | No | Discharged | Postpartum acquired |
| Zamaniyan et al. [82] | 1 | Yes | CS | 32/2350 | 1 | – | No | Fever | No | No | – | Congenital (confirmed)e |
| Zeng et al. [42] | 3 | Yes | CS | 40/3250 | 2 | 6 | – | Lethargy, fever | Yes | No | Discharged | Postpartum acquired |
| 40/3360 | 2 | 6 | – | Lethargy, vomiting | Yes | No | Discharged | Postpartum acquired | ||||
| 31/1580 | 2 | 7 | – | HMD, sepsis | Yes | Yes | Admitted | Postpartum acquired |
BPD, bronchopulmonary dysplasia; CS, caesarean section; DOL, day of life; GA, gestational age; ICU, intensive care unit; MAS, meconium Aspiration syndrome; MOD, mode of delivery; NP, nasopharyngeal swab; VD, vaginal delivery.
NP swab positive on Day 1 but other tests not done, so difficult to tell whether it was congenital/intrapartum or postpartum.
Placenta, umbilical cord blood and breast milk positive, baby’s NP swab-negative.
Same NP sample negative at 37 h. Placenta-negative, SARS-CoV-2 IgG antibodies positive.
NP swab taken at birth and placental swab (foetal side) positive.
Amniotic fluid PCR positive.
Table 4.
Summary of perinatal characteristics and clinical symptoms of COVID-positive neonates (n=58)
| Parameters | Number (%) |
|---|---|
| Gestational age | |
| Term (≥37 weeks) | 29 (50) |
| Preterm (<37 weeks) | 20 (34.4) |
| ≥28 weeks | 3 (5.2) |
| 29–33 weeks | 4 (6.9) |
| 34–36 weeks | 3 (5.2) |
| Exact gestation not given | 10 (17.2) |
| Details not available | 9 (15.5) |
| Mode of delivery | |
| Vaginal | 18 (31) |
| Caesarean | 29 (50) |
| Details not available | 11 (19) |
| Mode of transmission | |
| Congenital | 4 (6.9) |
| Postpartum acquired | 41 (70.7) |
| Intrapartum acquired | 0 (0) |
| Could not assigned | 13 (22.4) |
| Clinical features | |
| Asymptomatic | 13 (22.4) |
| Fever | 9 (15.5) |
| Respiratory symptoms (respiratory distress/hypoxia/ desaturation/cough, etc.) | 24 (41.4) |
| Gastrointestinal symptoms | 5 (vomiting—4 and diarrhoea—1) (8.6) |
| Lethargy | 3 (5.2) |
| Poor feeding | 3 (5.2) |
| Details not available | 13 (22.4) |
The outcome (discharge/death) has been reported for 31 neonates of which 26 have been discharged to home and 5 were still admitted. No mortality has been reported in SARS-CoV-2-infected neonates.
Mode of transmission
To understand the mode of transmission as well as to maintain the uniformity in reporting we classified the SARS-CoV-2-infected neonates into various categories [13]. Of these 58 live-born SARS-CoV-2 cases, 4 (7%) were congenital in origin (2 confirmed, 1 probable and 1 not sure), 41 were acquired in the postpartum period and the remaining 13 neonates could not be classified due to non-availability of complete details.
SARS-CoV-2 secretion in breast milk
A few studies tested breast milk for the SARS-CoV-2 virus and have reported conflicting results [3, 6, 8, 19, 37, 64]. Initial studies did not find any SARS-CoV-2 RNA in breast milk [3, 6, 19, 22, 67, 69]. However, recently few authors reported detection of SARS-CoV-2 in breast milk [8, 37, 64].
DISCUSSION
This review summarizes the perinatal characteristics, clinical features and outcome of RT-PCR proven SARS-CoV-2 infection in neonates. As described previously, the total number of reported paediatric cases is quite less than the adults of which the neonates are just a handful. Most of the reported neonatal infections are acquired in the postpartum period, and the overall prognosis is excellent.
Unlike older children and adults in which most of the infections are clinically asymptomatic, two-thirds of the neonatal cases were symptomatic [94]. As given in results, most of the neonates were clinically well before appearance of symptoms, suggesting that these symptoms are unlikely to be due to the prematurity or other non-COVID illness. Similar to the children and adults, respiratory symptoms were the predominant manifestations in neonates too, however, unlike them, fever was seen in one-fifth of the cases only [94–96]. Like older children, about 10% of the neonates had gastrointestinal manifestations, and the overall prognosis of SARS-CoV-2 infection in neonates is better than adults [94–97]. Although the frequency of SARS-CoV-2-positive neonates is extremely low, a significant proportion of the affected neonates requiring intensive care and mechanical ventilation suggests that the disease in neonates is more severe than older children [96–98].
Although the protective effect of caesarean section against SARS-CoV-2 transmission to neonate lacks evidence and most of the guidelines advise to reserve a caesarean section for obstetric indication only, the proportion of caesarean section was much more than vaginal deliveries [99, 100]. Higher rates for caesarean delivery may be due to either clinician preference or maternal sickness or comorbidities. Due to population-based differences in mode of delivery like Chinese having preference for caesarean section even in non-COVID pregnancies, we analysed the data as per country of origin of the study [101]. Caesarean section rate for COVID-19 pregnancies in China was found to be as high as 86% when compared with 53% in other countries. Also, data suggest an overall preference for caesarean delivery worldwide.
Many studies have highlighted the association of COVID-19 and increased preterm deliveries [5, 23]. In our review also about one-fourth of the neonates were born premature, which is much more than overall global (10.6%) and China’s (6.9%) preterm birth rate [102]. The exact reason for the higher preterm birth rate could not be delineated from this review.
Since the beginning of the pandemic, there is a debate about vertical transmission of SARS-CoV-2 infection. Early reports from China suggested that the intrauterine vertical transmission is unlikely [3–6, 9]. However, the detection of antibodies in cord blood and neonate raised concerns [7]. Ideally, to prove a vertical transmission, testing of placental tissue, amniotic fluid before rupture of membranes, umbilical cord blood, neonatal blood in the first 12 h and neonatal throat/nasopharyngeal swab for RT-PCR in the immediate postpartum period are recommended [13]. Initial studies that tested all these specimens did not find any evidence to suggest vertical transmission [22, 41, 85]. However, recently published reports suggest otherwise [15, 60, 64, 82]. Lack of intrapartum transmission in this review suggests that vaginal delivery may not be a risk factor for COVID-19 transmission to the neonate and it has been supported by many studies documenting the absence of SARS-CoV-2 in vaginal secretions [37, 103]. However, the intrapartum transmission cannot be ruled out with certainty as its diagnosis requires neonate’s nasopharyngeal swab testing immediately after birth (after cleaning the baby) and at 24–48 h age. However, in most reports, neonates were first tested beyond 24–48 h after birth. Also, recently SARS-CoV-2 has been documented in vaginal secretions too [64]. Overall, evidence suggests that congenital infection is possible but the incidence is extremely low and most of the cases are acquired in the postpartum period only.
Although the separation of COVID-19-positive mother from the infant might decrease the risk of postpartum transmission, it deprives the neonate of the benefits of breastfeeding. Although earlier studies advocated the safety of breast milk, detection of SARS-CoV-2 RNA from breast milk in recent studies is of concern [3, 6, 8, 19, 22, 37, 64, 67, 79, 104]. Further exploration of the safety of breast milk feeding is warranted [8, 37, 64]. As of now, considering the huge survival benefits of breast milk feeding against unknown potential threat associated with SARS-CoV-2 transmission, breast milk feeding (direct or expressed) should be given to the infants as and when the clinical condition of mother and baby permits. Mother should take adequate respiratory and hand hygiene precautions.
A number of organizations have established registries for a better understanding of COVID-19 in pregnancy and the neonatal period; however, generally their data are not available in the public domain. Data summary from the National registry for surveillance and epidemiology of perinatal COVID-19 infection (NPC-19 registry) maintained by the neonatal group of the American Academy of Pediatrics (AAP) is open to public [105]. Until 13 June 2020, there were 176 participating centres from all across the world and they enrolled 747 COVID-19-positive mothers. COVID testing was done for 624 only, of which 25 (4%) were positive. They did not provide separate data on the clinical course and outcome of COVID-positive neonates. This registry includes published and unpublished cases from various countries.
We used an extensive search strategy without any language restrictions in order to capture a global picture of COVID-19. When compared with previous reviews in which almost all the studies were from China, this review contains studies from many other countries [2, 106, 107]. Therefore, the results are likely to be representative of larger population. We included the neonatal age group only because the detailed information on clinical features, mode of transmission and outcome in this age group were lacking. To ensure uniformity, we included studies reporting RT-PCR-based diagnosis of COVID-19 only and classified cases using an explicit standard classification system [13]. This review also has several limitations too. The main limitation arises from the nature of the included studies. One-third of the neonatal data is from the case reports which are expected to have high publication bias and are not suitable for inferential statistics. Also, the included case series lack internal controls and represent low-quality evidence. Furthermore, the information on indications for preterm birth and caesarean section was not reported. There is a paucity of data on mode of transmission as only a few studies tested all the required maternal and foetal samples to ascertain the mode of transmission. Although we used structured exhaustive criteria to assign the mode of transmission, but due to limited numbers, inadequate testing of required specimens and lack of standard criteria for classifying the mode of transmission, it is difficult to assign the mode of transmission with certainty. Although we followed an extensive process to exclude duplicates, the possibility of a case report later published as a part of a larger retrospective cohort cannot be ruled out with certainty.
However, given the urgency of the situation and lack of large prospective cohort studies, it would still be valuable to synthesize and critically analyse these data for future case management as well as in the planning of further studies. Also, substantial data from various registries are expected in the future which may guide us better in understanding the disease and its management.
CONCLUSIONS
The limited low-quality evidence suggests an extremely low risk of SARS-CoV-2 infections in neonates. Unlike children most of the neonates with proven SARS-CoV-2 infection were symptomatic, and a significant proportion of them required intensive care. Postpartum infection is the commonest mode of acquisition in neonates, although a few cases of congenitally acquired infection are also reported.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Tropical Pediatrics online.
Conflict of interest: All authors declare no competing interests.
Supplementary Material
REFERENCES
- 1.World Health Organization. Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (13 June 2020, date last accessed).
- 2. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynecol Obstet 2020;150:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Guo J, Wang C, et al. Clinical characteristics, and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peng Z, Wang J, Mo Y, et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health 2020;13:818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira A, Cruz‐Melguizo S, Adrien M, et al. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand 2020;99:839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang G, Zhao H.. Can SARS-CoV-2-infected women breastfeed after viral clearance? J Zhejiang Univ Sci B 2020;21:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020;323:1848–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groß R, Conzelmann C, Müller JA, et al. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020;395:1757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 2020;144:799–805. [DOI] [PubMed] [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. ; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (31 May 2020, date last accessed).
- 13. Shah PS, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand 2020;99:565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol 2020;2:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buonsenso D, Costa S, Sanguinetti M, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol 2020;37:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao D, Yin H, Chen J, et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. Int J Infect Dis 2020;95:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Peng H, Wang L, et al. Infants born to mothers with a new coronavirus (COVID-19). Front Pediatr 2020;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen S, Liao E, Cao D, et al. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 2020. (Epub ahead of print). doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020;382:e100. NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen R, Zhang Y, Huang L, et al. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing cesarean delivery: a case series of 17 patients. Can J Anesth 2020;67:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen S, Huang B, Luo DJ, et al. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020;49:418–23. [DOI] [PubMed] [Google Scholar]
- 22. Fan C, Lei D, Fang C, et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG 2020. (Epub ahead of print). doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Govind A, Essien S, Karthikeyan A, et al. Re: novel Coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol 2020; 251:272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19. Am J Obstet Gynecol 2020; 223;109.e1–109.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirshberg A, Kern-Goldberger AR, Levine LD, et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol 2020; S0002-9378:30515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang W, Zhao Z, He Z, et al. Unfavorable outcomes in pregnant patients with COVID-19. J Infect 2020; S0163445320302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan S, Peng L, Siddique R, et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol 2020;41:748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao J, He X, Gong Q, et al. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID‐19 pandemic. Int J Gynecol Obstet 2020;150:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect 2020; S0163445320301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu W, Wang J, Li W, et al. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med 2020;14:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am J Roentgenol 2020;215:127–132. [DOI] [PubMed] [Google Scholar]
- 33. Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol 2020;100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiancheng X, Jian S, Lingling P, et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis 2020;95:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salvatori G, De Rose DU, Concato C, et al. Managing COVID-19-positive maternal–infant dyads: an Italian experience. Breastfeed Med 2020;15:347–8. [DOI] [PubMed] [Google Scholar]
- 36. Sun M, Xu G, Yang Y, et al. Evidence of mother-to-newborn infection with COVID-19. Br J Anaesth 2020;125:e245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Y, Liu C, Dong L, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG 2020;127:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu L, Yang Q, Shi H, et al. Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull (Beijing). 2020;doi: 10.1016/j.scib.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223:111.e1-111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang P, Wang X, Liu P, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol 2020;127:104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 2020;20:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 2020;174:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020;9:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kayem G, Alessandrini V, Azria E, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020;101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in Southern Connecticut. JAMA 2020;323:2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gregorio-Hernández R, Escobar-Izquierdo AB, Cobas-Pazos J, et al. Point-of-care lung ultrasound in three neonates with COVID-19. Eur J Pediatr 2020;179:1279–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khan S, Jun L, Nawsherwan, et al. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect 2020;26:788–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knight M, Bunch K, Vousden N, et al. ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martínez-Perez O, Vouga M, Cruz Melguizo S, et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA 2020. (Epub ahead of print). doi: 10.1001/jama.2020.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ochiai D, Kasuga Y, Iida M, et al. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet. 2020;150:268–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qadri F, Mariona F.. Pregnancy affected by SARS-CoV-2 infection: a flash report from Michigan. J Matern Fetal Neonatal Med 2020;1–3. [DOI] [PubMed] [Google Scholar]
- 53. White A, Mukherjee P, Stremming J, et al. Neonates hospitalized with community-acquired SARS-CoV-2 in a Colorado neonatal intensive care unit. Neonatology 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zeng Y, Lin L, Yan Q, et al. Update on clinical outcomes of women with COVID-19 during pregnancy. Int J Gynaecol Obstet 2020;150:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020;55:166–71. [DOI] [PubMed] [Google Scholar]
- 56. Kamali Aghdam M, Jafari N, Eftekhari K.. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020;52:427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chacón-Aguilar R, Osorio-Cámara JM, Sanjurjo-Jimenez I, et al. COVID-19: fever syndrome and neurological symptoms in a neonate. An Pediatr (Engl Ed) 2020;92:373–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alzamora MC, Paredes T, Caceres D, et al. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol 2020;37:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blauvelt CA, Chiu C, Donovan AL, et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020;136:46–51 . [DOI] [PubMed] [Google Scholar]
- 60. Carosso A, Cosma S, Borella F, et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol 2020;249:98–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Socio GV, Malincarne L, Arena S, et al. Delivery in asymptomatic Italian woman with SARS-CoV-2 infection. Mediterr J Hematol Infect Dis 2020;12:e2020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han MS, Seong MW, Heo EY, et al. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iqbal SN, Overcash R, Mokhtari N, et al. An uncomplicated delivery in a patient with COVID-19 in the United States. N Engl J Med 2020;382:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020;192:E647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuhrt K, McMicking J, Nanda S, et al. Placental abruption in a twin pregnancy at 32 weeks' gestation complicated by COVID-19, without vertical transmission to the babies. Am J Obstet Gynecol 2020;100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee DH, Lee J, Kim E, et al. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confirmed patient. Korean J Anesthesiol 2020. (Epub ahead of print). doi: 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis 2020;26:1335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li J, Wang Y, Zeng Y, et al. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynecol Obstet 2020;150:126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li M, Xu M, Zhan W, et al. Report of the first cases of mother and infant infections with 2019 novel coronavirus in Xinyang City Henan Province. Chin J Infect Dis 2020. https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-2207 (9 June 2020, date last accessed). [Google Scholar]
- 70. Liao X, Yang H, Kong J, et al. Chest CT findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Med J 2020;37:226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lorenz N, Treptow A, Schmidt S, et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J 2020;39:e212. [DOI] [PubMed] [Google Scholar]
- 72. Lowe B, Bopp B.. COVID‐19 vaginal delivery—a case report. Aust N Z J Obstet Gynaecol 2020;60:465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lu D, Sang L, Du S, et al. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol 2020. (Epub ahead of print). doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lyra J, Valente R, Rosário M, et al. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port 2020;33:429–31. [DOI] [PubMed] [Google Scholar]
- 75. Coronado Munoz A, Nawaratne U, McMann D, et al. Late-onset neonatal sepsis in a patient with COVID-19. N Engl J Med 2020;382:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Piersigilli F, Carkeek K, Hocq C, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health 2020;4:476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sharma KA, Kumari R, Kachhawa G, et al. Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int J Gynecol Obstet 2020;150:116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sinelli M, Paterlini G, Citterio M, et al. Early neonatal SARS-CoV-2 infection manifesting with hypoxemia requiring respiratory support. Pediatrics 2020;146:e20201121. [DOI] [PubMed] [Google Scholar]
- 79. Wang S, Guo L, Chen L, et al. A case report of neonatal COVID-19 infection in China. Clin Infect Dis 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xia H, Zhao S, Wu Z, et al. Emergency caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth 2020;124:e216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xiong X, Wei H, Zhang Z, et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J Med Virol 2020. (Epub ahead of print). doi: 10.1002/jmv.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zamaniyan M, Ebadi A, Aghajanpoor Mir S, et al. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn 2020. (Epub ahead of print). doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zambrano LI, Fuentes-Barahona IC, Bejarano-Torres DA, et al. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis 2020;101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhour R, Chen Y, Lin C, et al. A case of asymptomatic pregnant woman with new coronavirus infection in lung imaging. Chin J Perinat Med 2020;23:E006. [Google Scholar]
- 85. Wang X, Zhou Z, Zhang J, et al. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cook J, Harman K, Zoica B, et al. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc Health 2020;S2352-4642:30166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mehta H, Ivanovic S, Cronin A, et al. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep Womens Health 2020;27:e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Perrone S, Giordano M, Meoli A, et al. Lack of viral transmission to preterm newborn from a COVID-19 positive breastfeeding mother at 11 days postpartum. J Med Virol 2020. (Epub ahead of print). doi: 10.1002/jmv.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salik I, Mehta B.. Tetralogy of Fallot palliation in a COVID-19 positive neonate. J Clin Anesth 2020;66:109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang J, Wang D, Chen GC, et al. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yilmaz R, Kiliç F, Arican Ş, et al. Anesthetic management for cesarean birth in pregnancy with the novel coronavirus (COVID-19). J Clin Anesth 2020;66:109921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fontanella F, Hannes S, Keating N, et al. COVID-19 infection during the third trimester of pregnancy: current clinical dilemmas. Eur J Obstet Gynecol Reprod Biol 2020;;251:268–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jain P Thakur A Kler N,et al. . Manifestations in Neonates Born to COVID-19 Positive Mothers. Indian J Pediatr 2020;87:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020. (Epub ahead of print). doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 95. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Meena J, Yadav J, Saini L, et al. Clinical features and outcome of SARS-CoV-2 infection in children: a systematic review and meta-analysis. Indian Pediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020. doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
- 99. Chawla D, Chirla D, Dalwai S, et al. ; Federation of Obstetric and Gynaecological Societies of India (FOGSI). Perinatal-neonatal management of COVID-19 infection—guidelines of the Federation of Obstetric and Gynecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr 2020;57:536–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Boelig RC, Manuck T, Oliver EA, et al. Labor and delivery guidance for COVID-19. Am J Obstet Gynecol 2020;2:100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lumbiganon P, Laopaiboon M, Gülmezoglu AM, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08. Lancet 2010;376:1902. [DOI] [PubMed] [Google Scholar]
- 102. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martins-Filho PR, Santos VS, Santos HP Jr.. To breastfeed or not to breastfeed? Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19. Rev Panam Salud Publica 2020;44:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.NPC-19 Registry. https://my.visme.co/view/ojq9qq8e-npc-19-registry (13 June 2020, date last accessed).
- 106. Smith V, Seo D, Warty R, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One 2020;15:e0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Juan J, Gil MM, Rong Z, et al. Effects of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol 2020;56:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.