We are facing a full-blown pandemic, caused by a recently identified beta-coronavirus [1] now known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical disease, coined coronavirus disease 2019 (COVID-19), is quite heterogeneous. While most patients are asymptomatic or have minor symptoms, a minority of patients develop more serious and potentially life-threatening respiratory disease [2]. In this issue of Nephrology Dialysis Transplantation, Portolés and coworkers report on a large European cohort of patients with COVID-19. They explore kidney involvement as part of the clinical spectrum of SARS-CoV-2 infection [3].
For cell entry, the virus uses a spike protein to bind to an extracellular domain of the human angiotensin-converting enzyme 2 (ACE2) [4, 5]. ACE2 is a carboxypeptidase, hydrolysing single C-terminal amino acids from peptides, e.g. angiotensin I and angiotensin II [6]. High expression levels of ACE2 in the upper and lower airways explain the respiratory tropism of SARS-CoV-2.
The kidney is another organ with ACE2 expression, predominantly by proximal tubular cells and podocytes [7]. Until very recently, it was not known whether this would promote kidney tropism. The virus that caused the SARS epidemic in 2003 (SARS-CoV) equally uses ACE2 as a cellular receptor for cell entry. While a minority of SARS-CoV patients did develop acute kidney injury (AKI), this was attributed to critical illness with acute tubular necrosis in post-mortem kidney tissue. With electron microscopy, no viral particles could be detected in these specimens [8]. In kidney tissue obtained at autopsy of 26 critically ill patients with COVID-19, diffuse proximal tubule injury also was the main finding on light microscopy [9]. In a second series of three patients, of which kidney tissue was available in two (one native kidney and one transplanted kidney), clear endotheliitis was noted [10]. In these studies, virus-like particles were observed on electron microscopy in tubular epithelial cells, podocytes and endothelium. However, whether these structures actually are virions is disputed by others [11, 12]. Nevertheless, the presence of SARS-CoV-2 RNA and proteins has convincingly been demonstrated in glomerular epithelial, endothelial and tubular cells of patients who had died from COVID-19 [9, 13].
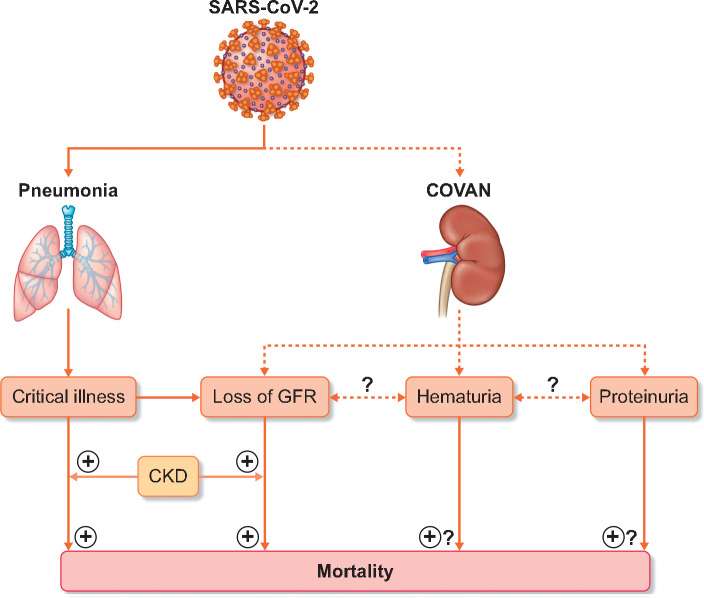
These findings indicate kidney tropism of SARS-CoV-2 and may promote direct damage to kidney tissue in addition to classical critical illness-associated acute kidney injury. This puts SARS-CoV-2 in an expanding list of other viruses with proven kidney tropism, including hantavirus [14], the Middle East respiratory syndrome coronavirus [15], polyomavirus (polyomavirus-associated nephropathy) [16] and the human immunodeficiency virus (HIV-associated nephropathy) [17]. In analogy, we propose to use the term coronavirus-associated nephropathy (COVAN; Figure 1).
FIGURE 1.
Schematic representation of the potential contribution of various renal manifestations of SARS-CoV-2 infection, including COVAN, to an increased risk of mortality. COVAN presents itself as loss of glomerular filtration rate, microscopic haematuria and/or proteinuria. These have all been associated with overall mortality, although a causal relationship has not been proven. In addition, pre-existent CKD is a modifier of the risk of COVID-19-associated mortality.
Our understanding of the clinical picture of COVAN remains patchy. Correlation between histology and functional findings is mostly lacking. In the abovementioned autopsy studies, urinalysis was available in only a minority of patients [9, 13]. Moreover, these biopsy data originate from a highly selected group of deceased patients, after a period of critical illness. To date, kidney histology of COVID-19 patients with a less severe clinical course has not been reported.
Clinical case series from different viral hotspots can partly fill this gap. Early reports, although of great importance to alert the world of trouble ahead, were hampered by a number of drawbacks, i.e. patient selection with overrepresentation of critically ill patients, as well as retrospective data collection [18, 19]. The latter is of importance, as urinalysis may not be routinely (nor be repeatedly) performed in patients with predominantly respiratory symptoms. Two reports from the same group (Tongji University, Wuhan) focused on markers of kidney function [20, 21]. They performed a prospective cohort study in 701 patients, admitted to a designated tertiary care hospital. Of these, 5.1% developed AKI. More than 40% of all patients had proteinuria and ∼25% had haematuria. Of note, despite the fact that this was a prospective study, missing data are non-negligible. Proteinuria and haematuria were measured in 442 of the 701 included patients (37% missing data) [21]. They also performed a retrospective study in 467 patients, of which 333 patients had dipstick analysis upon submission. Noteworthy, 254/333 (76%) patients were also included in the first (prospective) study cohort. In the retrospective cohort, 10.5% developed AKI, 66% had proteinuria and 42% had haematuria [20]. Of 5449 patients with COVID-19 admitted to 13 academic and community hospitals in metropolitan New York, 36.6% developed AKI, which was strongly associated with the need for mechanical ventilation. Urine dipstick studies were available in one-third of the patients with AKI and showed >1+ haematuria in 46% of patients and >1+ proteinuria in 26% [22]. Together, these data suggest that the clinical picture of COVID-19-related kidney disease is broader than an acute decrease in glomerular filtration rate due to acute tubular necrosis.
In this issue of Nephrology Dialysis Transplantation, data from a large European cohort study of patients with COVID-19 are reported [3]. The Puerta de Hierro Hospital is located in Madrid, one of the European regions most affected by COVID-19. Strengths of the cohort study are standardized data collection at admission and follow-up, inclusion of all patients (less focus on intensive care unit), knowledge of pre-existent chronic kidney disease (CKD) and availability of spot urine samples. A total of 1603 patients were included. About a fifth of hospitalized patients had elevated creatinine levels upon admission. Of these, ∼40% were known to have pre-existent CKD. Proteinuria was found in nearly 40%, and haematuria in slightly over half of the patients.
Differences in the composition of the cohorts, the different genetic backgrounds (Han Chinese versus multiracial versus Caucasian) and differences in the organization of healthcare, could all explain why the prevalence of AKI varied from 5.1 to 36.6%. Nevertheless, the prevalence of proteinuria and haematuria in a significant proportion of patients is quite a common finding, supporting the existence of COVAN as a separate disease entity. The challenge for the nephrological community is to characterize the renal phenotype of COVID-19 in more detail. Relevant questions are whether the haematuria is of glomerular or non-glomerular origin and whether the proteinuria is associated with haematuria. In addition, the type of proteins that are predominantly present in the urine should be assessed. Kidney biopsies could be helpful to correlate laboratory findings to histological changes as evaluated by light microscopy, immunofluorescence and electron microscopy.
A next important question is what COVAN portents in the short and the long term. Portolés et al. found a clear association between loss of kidney function and an increase in short-term mortality [3]. This is in line with the Wuhan and New York data [21, 22]. Such associations, however, are not specific for COVID-19. Critical illness is associated with both poor outcomes and risk of AKI, and it is near-impossible to disentangle cause and consequence. Of greater relevance is the observation that new-onset haematuria is associated with higher mortality. The Tongji data equally show that haematuria and proteinuria predict short-term mortality [21]. It is not yet clear how to understand this observation. The number of patients diagnosed with haematuria is higher than the number of patients experiencing loss of glomerular filtration rate. Is haematuria a marker of more severe COVID-19 (and need of urinary catheter insertion), or does this indicate a subgroup of patients in which kidney tropism, resulting in COVAN, contributes to worse outcome?
As the pandemic unfolds, our understanding of COVID-19 continues to grow. The Madrid dataset provides further support for COVAN as part of the clinical spectrum. What is not known at this time is whether this is a transient phenomenon without long-term consequences. Will kidney tropism of SARS-CoV-2 result in an increased risk for progressive CKD? Ideally, part of the Spanish cohort will be followed over time. Also, we do not know whether asymptomatic individuals infected by SARS-CoV-2, or those with COVID-19 and minor respiratory symptoms, will develop COVAN that is detectable via urinary indices. If this were to be the case, then unexplained haematuria and/or proteinuria should warrant testing for COVID-19. We believe the evidence is insufficient to draw this conclusion. At the same time, we believe the evidence is sufficient to consider COVAN as part of the clinical spectrum of COVID-19.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest related to the content of this manuscript.
(See related article by Portolés et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant 2020; 35: 1353–1361)
REFERENCES
- 1. Zhu N, Zhang D, Wang W. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Portolés J, Marques M, Lopez P. et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant 2020; 35: 1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan R, Zhang Y, Li Y. et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367: 1444–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine-Weber H, Schroeder S. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perico L, Benigni A, Remuzzi G.. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron 2020; 144: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan XW, Xu D, Zhang H. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 2020; 46: 1114–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varga Z, Flammer AJ, Steiger P. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldsmith CS, Miller SE, Martines RB. et al. Electron microscopy of SARS-CoV-2: a challenging task. Lancet 2020; 395: e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller SE, Brealey JK.. Visualization of putative coronavirus in kidney. Kidney Int 2020; 98: 231–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puelles VG, Lütgehetmann M, Lindenmeyer MT. et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; Doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hung T, Zhou JY, Tang YM. et al. Identification of Hantaan virus-related structures in kidneys of cadavers with haemorrhagic fever with renal syndrome. Arch Virol 1992; 122: 187–199 [DOI] [PubMed] [Google Scholar]
- 15. Alsaad KO, Hajeer AH, Al BM. et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology 2018; 72: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randhawa PS, Finkelstein S, Scantlebury V. et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 1999; 67: 103–109 [DOI] [PubMed] [Google Scholar]
- 17. Canaud G, Dejucq-Rainsford N, Avettand FÃ. et al. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol 2014; 25: 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu JT, Leung K, Leung GM.. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020; 395: 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 Pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]