Abstract
Background
Emerging evidence suggests that black and Hispanic communities in the United States are disproportionately affected by coronavirus disease 2019 (COVID-19). A complex interplay of socioeconomic and healthcare disparities likely contribute to disproportionate COVID-19 risk.
Methods
We conducted a geospatial analysis to determine whether individual- and neighborhood-level attributes predict local odds of testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We analyzed 29 138 SARS-CoV-2 tests within the 6-county catchment area for Duke University Health System from March to June 2020. We used generalized additive models to analyze the spatial distribution of SARS-CoV-2 positivity. Adjusted models included individual-level age, gender, and race, as well as neighborhood-level Area Deprivation Index, population density, demographic composition, and household size.
Results
Our dataset included 27 099 negative and 2039 positive unique SARS-CoV-2 tests. The odds of a positive SARS-CoV-2 test were higher for males (odds ratio [OR], 1.43; 95% credible interval [CI], 1.30–1.58), blacks (OR, 1.47; 95% CI, 1.27–1.70), and Hispanics (OR, 4.25; 955 CI, 3.55–5.12). Among neighborhood-level predictors, percentage of black population (OR, 1.14; 95% CI, 1.05–1.25), and percentage Hispanic population (OR, 1.23; 95% CI, 1.07–1.41) also influenced the odds of a positive SARS-CoV-2 test. Population density, average household size, and Area Deprivation Index were not associated with SARS-CoV-2 test results after adjusting for race.
Conclusions
The odds of testing positive for SARS-CoV-2 were higher for both black and Hispanic individuals, as well as within neighborhoods with a higher proportion of black or Hispanic residents—confirming that black and Hispanic communities are disproportionately affected by SARS-CoV-2.
Keywords: Bayesian statistics, COVID-19, disparities, geographic information systems, SARS-CoV-2
Coronavirus disease 2019 (COVID-19) was first reported in the United States in January 2020. In less than 1 month, cases had been confirmed in all 50 states [1]. As of June 30, 2020, 2 545 250 cases and 126 369 deaths had been reported [2]. Emerging data suggest that particular racial and ethnic groups in the United States population may be disproportionately affected by the pandemic. For example, surveys of hospitalization data gathered by the Centers for Disease Control and Prevention found that black patients comprised 33% of COVID-19-related hospitalizations despite representing just 18% of the catchment population [3]. Similarly, a recent report from the Baltimore-Washington DC region found that >40% of Hispanics were positive [4].
Geographic, racial, and socioeconomic disparities in disease risk have implications for pandemic mitigation, suppression, and surveillance strategies. Disproportionate comorbidity burdens may increase the risk of disease or adverse outcomes among the most vulnerable. Access to medical evaluation may be hindered by proximity to healthcare facilities, access to reliable transportation, and differences in insurance. Financial strain may hinder the ability of individuals to adhere to social distancing and stay-at-home orders, and fear of exposure may inhibit sick individuals from seeking timely medical care.
To investigate the potential influence of geographic and racial disparities on the likelihood of COVID-19 disease, we conducted a geospatial analysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test results using clinical testing data from the Duke University Health System (DUHS). We hypothesized that the spatial distribution for the probability of having a positive test result would be heterogenous with test positivity being more likely among residents living in urban, low-income, and minority communities.
METHODS
Ethical Approval
This study was determined exempt by the DUHS Institutional Review Board. Waivers of informed consent and Health Insurance Portability and Accountability Act (HIPAA) were granted.
Data Preparation
Novel coronavirus nucleic acid amplification testing data were obtained from the electronic health records of patients within the DUHS. The DUHS clinical sites include 3 inpatient hospitals and many outpatient facilities. We queried all patients whose record included a test whose name included the terms “COVID” or “SARS” from March 11, 2020 (the date of the first test performed) through June 26, 2020. Our unit of analysis was by unique individual test. Tests were deduplicated according to the following rules. For multiply tested individuals, we only included 1 test every 14 days counting sequentially from their first test. For any individual who tested positive, we included their first positive test in our analysis, but we did not include subsequent test results after this first positive subsequent tests.
Of the subjects identified, we obtained the test result, date of the test, date of birth, gender, self-reported race, self-reported ethnicity, and the longitude and latitude coordinates of their residential address. Ethnicity was consolidated into the categories “Hispanic,” “Not Hispanic,” and “Unavailable.” Individual race was grouped into the categories “Black,” “White,” “Asian,” “Multiracial,” “Other,” and “Unavailable.” The category “Other” included individuals who had self-reported their race as “Other” as well as a small number of individuals who identified as “Native American,” “Alaska Native,” “Pacific Islanders,” and “Native Hawaiians.” We excluded a small number of records that were missing gender or date of birth. The SARS-CoV-2 test results were dichotomized as Positive or Negative. We also excluded tests that were either not performed or that were indeterminate. Age at the time of testing was computed in decimal years. The date of testing was expressed as the day of the year.
To evaluate neighborhood demographic characteristics, we obtained census block group-level population density, average household size, percentage of black population, and percentage of Hispanic population from the American Community Survey 2014–2018 5-year estimates. We also obtained the Area Deprivation Index, which is a composite index of socioeconomic disadvantage, using 17 variables from the Census and the American Community Survey [5–7]. The raw Area Deprivation Index values were converted to percentiles for the entirety of North Carolina. Our previous work has shown that the distribution of Area Deprivation Index values and percentile for the Raleigh-Durham metropolitan area is similar to the statewide values [8].
For ease of computation and interpretability, numeric variables (household size, population density, age, and day) were centered on 0 by subtracting their mean, then scaled by dividing by their standard deviation. Variables that were already on a percent scale (percentage of black population and percentage of Hispanic population) or a percentile scale (Area Deprivation Index) were centered on 0 by subtracting 0.5.
We chose to limit our analysis to those subjects whose address fell in 1 of 6 counties in North Carolina: Durham, Chatham, Orange, Person, Granville, or Wake. The DUHS is the major health system within Durham County, and the DUHS catchment extends into the city of Raleigh. Within the city of Raleigh as well as in the southwestern extent of our study area, the DUHS patient catchment overlaps with other health systems from which we did not have access to patient records. Thus, the density of patient address locations declined with increasing distance from Durham. To maximize data density and to rationally exclude spatial outliers, we used ArcGIS to perform a 2 standard deviational ellipse. This method draws the smallest possible ellipse that contains 95% of all data points. Thus, our analysis was limited to just those subjects whose address fell both within the 6-county study region and within this ellipse.
Analysis
Our primary model was a logistic generalized additive model (GAM). Generalized additive models are regression models that use nonparametric polynomial functions to model nonlinear relationships between independent variables and an outcome variable of interest [9]. We used the statistical programming language R (www.r-project.org) and the brms and mgcv packages [9–11]. mgcv is a comprehensive package for the specification of GAMs. brms, through its dependency on mgcv, allows the construction of Bayesian GAMs that are sent to the program Stan (www.mc-stan.org) for sampling of the posterior probability distribution. The response variable in our models was the binary result of COVID testing (negative vs positive); our individual-level linear predictors were gender, race, ethnicity, and test date (expressed as day of the year); and our neighborhood-level linear predictors were average household size, population density, percentage black, percentage Hispanic, and Area Deprivation Index percentile.
We used a tensor product thin plate spline of longitude and latitude to model geographic heterogeneity of COVID testing results in 2-dimensional geographic space. Tensor product splines allow for different degrees of smoothness or wiggliness in the x (longitude) and y (latitude) dimensions. We also chose to use thin plate splines for patient age and for day, with the foreknowledge that availability of testing and in particular for testing for pediatric subjects varied temporally. Thus, age and day represented a varying testing landscape and not merely a reflection of SARS-CoV-2 epidemiology. For this stage of model selection, we used mgcv, which uses maximum likelihood estimation and which estimates models very quickly compared with its Bayesian counterpart, brms. A key parameter for GAMs is the number of “knots,” or junctions between smoothing polynomial segments. We selected the number of knots through a trial and error process, incrementally increasing the number of knots and comparing models using analysis of deviance. The number of knots for our final model was ultimately chosen once model performance no longer improved with increasing knot numbers.
For our final model, which was estimated using brms, we chose loosely regularizing priors for our fixed parameters, selecting normal distributions with mean 0 and standard deviation 1. Default priors were accepted for smoothed terms, which were a minimally informative Student t distribution. We ran 2 models: (1) a partially adjusted model that included only geographic coordinates, subject age, and date of test and (2) a fully adjusted model that also included individual gender, race, and ethnicity, the block group-level predictors, a nested random intercept term for tract and block group. Among 417 duplicate tests from 265 unique subjects, only their first positive test was included in our analysis. There were 4569 tests among 3122 subjects who only had negative tests; analysis of these test results was limited to 1 test every 14 days, counting in intervals from their first test.
To evaluate spatial statistical trends, we predicted our fit models onto a longitude-latitude grid covering the entire geographic area of interest. Census data were matched by block group. We expressed our results as a local odds ratio (OR), which was computed by dividing local odds by the average odds for the entire study area. We defined a local OR as “significant” where there was at least a 95% probability that the local odds differed from the mean odds for the entire study area. We used contours to circumscribe these areas, using red and blue to denote areas with significantly higher or lower OR, respectively.
RESULTS
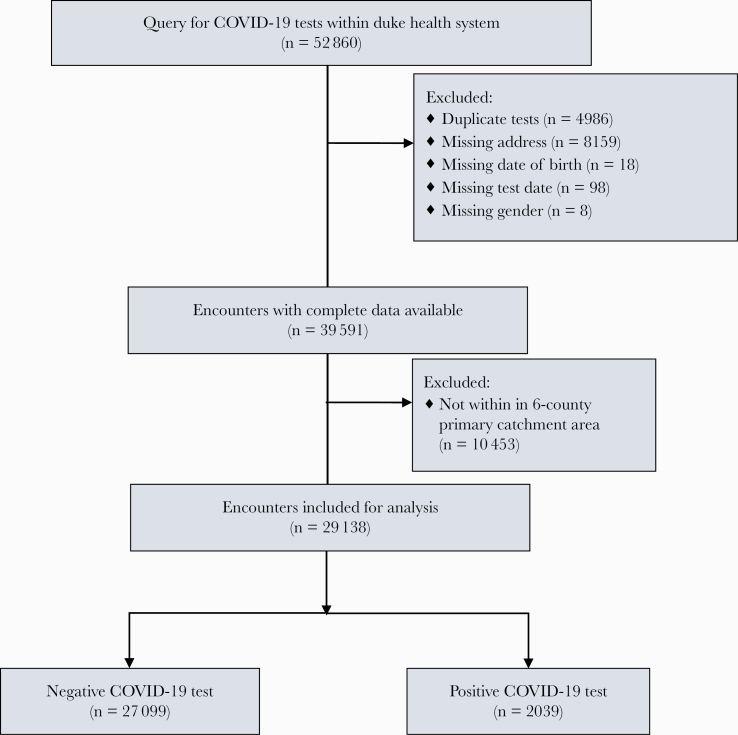
Figure 1 summarizes cohort selection. Our initial query yielded 52 860 total SARS-CoV-2 tests run through the DUHS. A total of 4986 duplicate tests were excluded, 8159 records were excluded due to missing address information, and 124 records were excluded due to missing information (either sex, date of birth, or test date). After limiting to DUHS’s main local catchment area (Durham and its surrounding 5 counties), we were left with 29 138 SARS-CoV-2 tests from 26 732 unique subjects (Figure 1). Results were analyzed by unique test result. There were 2039 positive (7.0%) and 27 099 negative tests (93.0%). Patient demographic characteristics are presented in Table 1. Block group-matched census traits stratified by race are presented in Supplemental Table S1.
Figure 1.
Cohort selection. COVID-19, coronavirus disease 2019.
Table 1.
Demographic Characteristics of the Study Population
| Characteristics | Cohort n (%) | |||
|---|---|---|---|---|
| Overall n = 29 138 | COVID-19 Negative n = 27 099 (93.0) | COVID-19 Positive n = 2039 (7.0) | %COVID Positive, by Covariate | |
| Race | ||||
| White | 15 824 (54.3) | 15 288 (56.4) | 536 (26.3) | 3.4 |
| Black | 8393 (28.8) | 7887 (29.1) | 506 (24.8) | 6.0 |
| Asian | 993 (3.4) | 947 (3.5) | 46 (2.3) | 4.6 |
| Native American | 78 (0.3) | 74 (0.3) | 4 (0.2) | 5.1 |
| Multiracial | 1546 (5.3) | 1187 (4.4) | 359 (17.6) | 23.2 |
| Unavailable | 1048 (3.6) | 822 (3.0) | 226 (11.1) | 21.6 |
| Other | 1239 (4.3) | 879 (3.2) | 360 (17.7) | 29.1 |
| Ethnicity | ||||
| Non-Hispanic | 25 172 (86.5) | 24 120 (89.1) | 1052 (51.7) | 4.2 |
| Hispanic | 2958 (10.2) | 2075 (7.7) | 883 (43.4) | 29.9 |
| Unavailable | 984 (3.4) | 883 (3.3) | 101 (5.0) | 10.3 |
| Gender | ||||
| Female | 17 510 (60.1) | 16 448 (60.7) | 1062 (52.1) | 6.1 |
| Male | 11 628 (39.9) | 10 651 (39.3) | 977 (47.9) | 8.4 |
| Age Group | ||||
| 0–18 years | 2148 (7.4) | 1847 (6.8) | 301 (14.8) | 14.0 |
| 19–24 years | 1608 (5.5) | 1431 (5.3) | 177 (8.7) | 11.0 |
| 25–50 years | 11 809 (40.5) | 10 832 (40.0) | 977 (47.9) | 8.3 |
| >50 years | 13 571 (46.6) | 12 987 (47.9) | 584 (28.6) | 4.3 |
Abbreviations: COVID, coronavirus disease; COVID-19, coronavirus disease 2019.
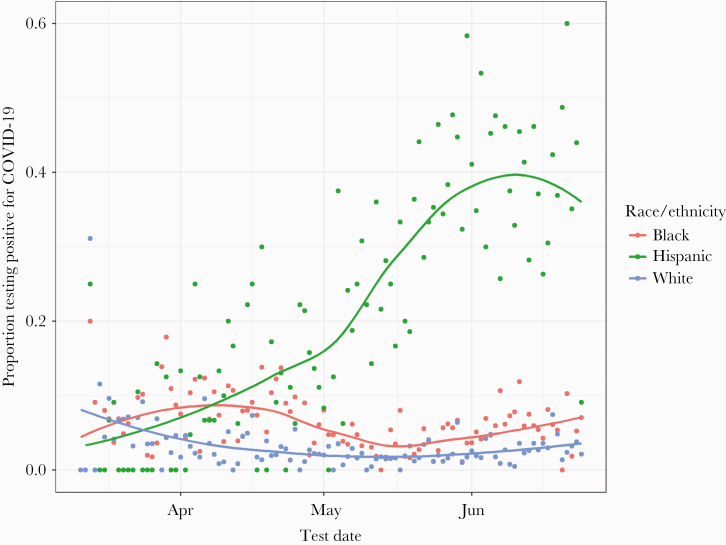
The temporal trends in SARS-CoV-2 positivity were fairly constant over time among most racial and ethnic groups. However, we observed a dramatic increase in the Hispanic population between May and June 2020 (Figure 2).
Figure 2.
Temporal trends in coronavirus disease 2019 (COVID-19) positivity by race/ethnicity. Proportion of positive COVID-19 tests over time, stratified by race/ethnicity. For ease of visualization, data are shown only for black, white, and Hispanic groups. Fitted lines represent a locally weighted scatter-plot smoother (LOESS) regression.
Hispanic individuals were slightly overrepresented among those with missing data, representing 13.8% of those with missing data versus 8.7% of those with complete data. The COVID-19 positivity rate was also slightly higher among subjects with missing address or demographic data relative to subjects with complete data (9.3% vs 6.0%). On inspection for temporal trends in SARS-CoV-2 positivity stratified by race/ethnicity, we observed a dramatic increase in proportion testing positive among Hispanic individuals, which was particularly pronounced between May and June 2020 (Figure 2).
The impact of individual- and neighborhood-level covariates on SARS-CoV-2 testing results is presented in Table 2. Gender, race, ethnicity, and age were associated with the probability of a positive SARS-CoV-2 test. The odds of a positive SARS-CoV-2 test were higher for males (OR, 1.43; 95% credible interval [CI], 1.30–1.58), blacks (OR, 1.47; 95% CI, 1.27–1.70), and Hispanics (OR, 4.25; 95% CI, 3.55–5.12). Some neighborhood marginal effects were associated with the OR of a positive SARS-CoV-2 test, including percentage black population (OR, 1.14; 95% CI, 1.05–1.25) and percentage Hispanic population (OR, 1.23; 95% CI, 1.07–1.41). Population density, average household size, and Area Deprivation Index, on the other hand, were not associated with SARS-CoV-2 testing results. The variance ratio of the fitted model (equivalent to the intraclass correlation coefficient) was 0.07 (95% CI, 0.00–0.13), indicating that the odds of a positive SARS-CoV-2 test is sensitive to a significant amount of unmeasured neighborhood variance.
Table 2.
Association of Individual and Neighborhood Variables With COVID-19 Testing Resulta
| Variable | OR | 95% CI | P|>|0 |
|---|---|---|---|
| Sex (Male) | 1.43 | 1.30–1.58 | 1.00 |
| Race (Asian) | 1.35 | 0.97–1.83 | .96 |
| Race (Black) | 1.47 | 1.27–1.70 | 1.00 |
| Race (Multiracial) | 2.23 | 1.81–2.73 | 1.00 |
| Race (Native American) | 0.91 | 0.33–2.16 | .44 |
| Race (Other) | 2.21 | 1.78–2.74 | 1.00 |
| Race (Unavailable) | 2.68 | 2.13–3.36 | 1.00 |
| Ethnicity (Hispanic) | 4.25 | 3.55–5.12 | 1.00 |
| Ethnicity (Unavailable) | 1.59 | 1.23–2.06 | 1.00 |
| Area Deprivation Index | 1.05 | 0.96–1.05 | .86 |
| Average Household Size | 0.97 | 0.90–1.05 | .27 |
| Percent Black Population | 1.14 | 1.05–1.25 | 1.00 |
| Percent Hispanic Population | 1.23 | 1.07–1.41 | 1.00 |
| Population Density | 1.03 | 0.93–1.13 | .71 |
Abbreviations: CI, credible interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
aFor this bayesian model, 95% CI represents the 95% posterior credible interval, and P|>|0 is the probability that a given independent variable will have a nonzero influence on the OR of a positive COVID-19 test result.
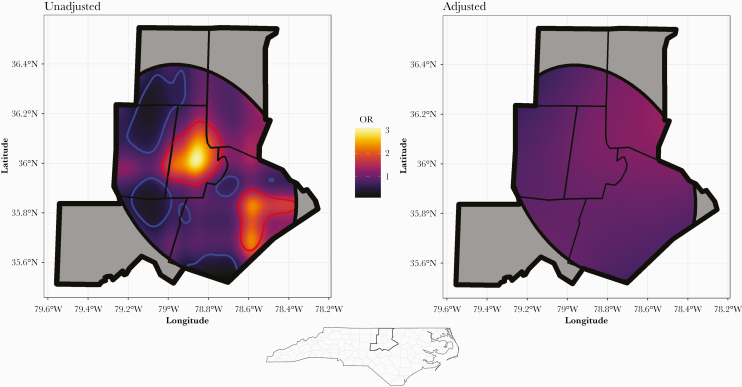
The odds that a SARS-CoV-2 test would be positive were spatially heterogeneous, with the local OR of a positive test ranging from 0.17 to 3.03 (Figure 3). In the cities of Durham and Raleigh, there were areas with a significantly high OR of a positive test. We identified several smaller areas in Person, Orange, Chatham, and Wake Counties where the OR of a positive test was significantly low. Adjustment for both individual and areal variables blunted the overall OR range to 0.64 to 1.34 and abrogated the high and low OR clusters seen in our unadjusted model.
Figure 3.
Spatial distribution of coronavirus disease 2019 testing results. The study area depicted is a 6-county area around Durham, NC. The elliptical shape that intersects the study area was a 2-standard deviational ellipse, the smallest possible ellipse containing 95% of the subject locations. The odds of a positive test were modeled using the home address coordinate locations of individual subjects as a smoothed, 2-dimensional independent variable. These models were then predicted on a dense grid of coordinate pairs covering the study area. The local odds ratio (OR), depicted in the color background, was computed by dividing the odds at each coordinate pair in the prediction grid by the average odds. Areas circumscribed by high (red) or low (blue) contours are those in which the local OR has at least a 95% probability of differing from the average. Areas with the highest OR in our unadjusted model included the cities of Durham and Raleigh. Adjusting for individual and neighborhood variables eliminated much of the geographic heterogeneity in OR.
DISCUSSION
Although early reporting suggests the potential for racial disparities in COVID-19 disease burden, published data and formal epidemiologic studies are limited to date. Most published geospatial analyses have been conducted at larger spatial scales, and they have analyzed data aggregated at the county or state level. In this study, we examined the association between both individual and geographic predictors of a positive SARS-CoV-2 test. It is widely recognized that the COVID-19 pandemic has disproportionately affected racial and ethnic minorities, and this is documented in an emerging body of literature. Our study is unique in its use of individual location data to evaluate not only individual variables, but also the effect of neighborhood variables and location itself. The OR of testing positive was increased across a range of minority groups—most notably blacks, Hispanics, and those reporting a multiracial background. Neighborhood-level variables representing racial and ethnic composition were also associated with a greater OR of a positive SARS-CoV-2 test. The spatial distribution of testing results revealed a higher OR of a positive test in the urban centers of Durham and Raleigh. This corresponds closely to racial and ethnic segregation within these communities, and it accounts for why the effect of location was blunted by adjustment for individual race and ethnicity. Household size, Area Deprivation Index, and population density were not clearly associated with individual SARS-CoV-2 testing results, but our models indicate that there remains substantial unmeasured variance at the neighborhood level. It is likely that many exposures, including nutrition, number of people in the home, housing quality, wealth, education, and healthcare access, produce an environment of disparate health risk in segregated neighborhoods.
Our findings are consistent with other early reports noting an increased burden of COVID-19 disease among blacks and Hispanics [4, 12–18]. In particular, a similar sharp increase within Hispanic communities was recently described in the Baltimore-Washington DC area, slightly preceding the time period examined here [4]. A complex interplay of socioeconomic factors and structural disparities across multiple levels (environment, occupation, housing, multigeneration living arrangements, education, transportation) likely contribute to increased risk [19]. The COVID-19 pandemic exhibits a disparity among minorities that is well documented with numerous other health conditions, including diabetes mellitus, hypertension, and cardiovascular disease [20, 21]. Other recent county-level geospatial analyses have found correlations between higher rates of air pollution, unemployment, and uninsured status among the minority communities most affected by COVID-19 [22]. It is well documented that healthcare service access is patterned by race and socioeconomic status, and these inequities further influence access to testing and clinical outcomes [23]. Of particular relevance with COVID-19, minorities may be disproportionately represented in service industries considered essential during the pandemic—placing them at elevated risk of exposure to SARS-CoV-2. Still more troubling are the potential implications for the immigrant communities where fear of deportation may further hinder access to testing and appropriate healthcare, household occupancy is often higher, and the pressure to continue working even more severe [24].
The delayed but dramatic increase in SARS-CoV-2 test positivity rates among Hispanic individuals was not associated with any specific geographic or occupational setting, and we are left to speculate on how the explosive emergence of COVID-19 in this population came about. It is most likely that COVID-19 cases among Hispanic individuals increased simultaneously in geographically discontinuous areas. This could be understood by socially segregated networking within the Hispanic community, for instance among geographically separated family members or shared meeting spaces such as churches and workplaces that draw from several discontinuous neighborhoods. Although outbreaks have previously been reported within churches, nursing facilities, congregate living settings, and prisons, the emergence we observed in the local Hispanic population seems unlikely to be related to any of these [25–27]. Lack of close geographic case clustering argues against a typical point source (as might be seen with a church, prison or congregate setting), and the majority of Hispanic individuals testing positive for SARS-CoV-2 appear to be young (median age, 33.5; interquartile range, 21.5–46.5), community-dwelling individuals. Similar community outbreaks affecting young healthy individuals have been reported among workers in essential industries where social distancing might not be feasible (eg, meat packing workers or warehouse workers) or where exposure is an occupational hazard (eg, healthcare workers) [28].
Our study does carry several limitations. Our geospatial patient locations were extracted from electronic medical records, and we could not verify our subjects’ addresses. A patient’s residential address is usually not their sole location, and they cannot account for their exposures away from the home. Our use of neighborhood-level risk factors is limited block group-level resolution; this is the smallest level census unit in which robust demographic data are made public, but block groups do not tend to correspond to real-world neighborhood definitions and are variable in shape, area, and their relationship with neighboring block groups. Perhaps most importantly, many of the same limitations to healthcare access among marginalized and minority populations might also limit our assessment of these communities in particular; in other words, the highest risk communities may be undertested compared with more affluent areas. Thus, it could be that our work “underestimates” the abundance of positive SARS-CoV-2 tests within minority communities that still lack access to testing.
CONCLUSIONS
Factors contributing to COVID-19 risk are complex, but emerging data suggest that black and Hispanic populations are at elevated risk. Further research with more detailed, prospective collection of subject-specific, clinical and socioeconomic data will be needed to dissect out the drivers of increased COVID-19 risk among minorities. Although ongoing research will take time, urgent action is needed on the part of healthcare providers, public health officials, and government leaders to assure the protection of the most vulnerable populations amid this rapidly evolving pandemic. Moreover, enhanced risk awareness in vulnerable communities may increase demand for testing and improve the palatability of risk mitigation strategies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table S1. Block group-level variables stratified by subject race/ethnicity.
Acknowledgments
Author contributions. P. M. L., W. P., and N. A. T. contributed to data acquisition and statistical analysis. C. W. W., P. M. L., W. P., N. A. T., V. S. M.-B., A. M. P., and G. M. M. P. contributed to writing, editing, and manuscript preparation. N. A. T. and P. M. L. had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence–United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) cases in the U.S. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 28 October 2020..
- 3. Centers for Disease Control (CDC). Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019–COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez DA, Hinson JS, Klein EY, et al. SARS-CoV-2 positivity rate for latinos in the Baltimore-Washington, DC region. JAMA 2020; 324:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. HIPxChange. Area deprivation index databases. Available at: https://www.hipxchange.org/ADI. Accessed 28 October 2020.
- 6. Knighton AJ, Savitz L, Belnap T, et al. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS (Wash DC) 2016; 4:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Program UHI. Health innovation program. Area deprivation index. UW health innovation program; 2014. Available at: http://www.hipxchange.org/ADI2014. Accessed 28 October 2020.
- 8. Lantos PM, Hoffman K, Permar SR, et al. Neighborhood disadvantage is associated with high cytomegalovirus seroprevalence in pregnancy. J Racial Ethn Health Disparities 2018; 5:782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood SN. Generalized Additive Models: An Introduction with R. 2nd ed. Boca Raton: CRC Press/Taylor & Francis Group; 2017. [Google Scholar]
- 10. Wood S Package “mgcv”: mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation Available at: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed 28 October 2020.
- 11. Bürkner P-C brms: an R package for Bayesian multilevel models using stan. J Stat Softw 2017; 80:28. [Google Scholar]
- 12. Yancy CW COVID-19 and African Americans. JAMA 2020; 323:1891–2. [DOI] [PubMed] [Google Scholar]
- 13. Fouad MN, Ruffin J, Vickers SM. COVID-19 is out of proportion in African Americans. This will come as no surprise. Am J Med 2020; 133:e544–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SJ, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav 2020; doi: 10.1177/1090198120929677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahajan UV, Larkins-Pettigrew M. Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health (Oxf) 2020; 42:445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okoh AK, Sossou C, Dangayach NS, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health 2020; 19:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khose S, Moore JX, Wang HE. Epidemiology of the 2020 pandemic of COVID-19 in the state of Texas: the first month of community spread. J Community Health 2020; 45:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020; doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 19. Schulz AJ, Mehdipanah R, Chatters LM, et al. Moving health education and behavior upstream: lessons from COVID-19 for addressing structural drivers of health inequities. Health Educ Behav 2020; doi: 10.1177/1090198120929985. [DOI] [PubMed] [Google Scholar]
- 20. Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA 2019; 322:2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas SJ, Booth JN 3rd, Dai C, et al. Cumulative incidence of hypertension by 55 years of age in blacks and whites: the CARDIA study. J Am Heart Assoc 2018; 7:e007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol 2020; 47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev 2000; 57:108–45. [DOI] [PubMed] [Google Scholar]
- 24. Dyer O Covid-19: black people and other minorities are hardest hit in US. BMJ 2020; 369:m1483. [DOI] [PubMed] [Google Scholar]
- 25. Wallace M, Hagan L, Curran KG, et al. COVID-19 in correctional and detention facilities–United States, February-April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:587–90. [DOI] [PubMed] [Google Scholar]
- 26. Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice–Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:606–10. [DOI] [PubMed] [Google Scholar]
- 27. Mosites E, Parker EM, Clarke KEN, et al. ; COVID-19 Homelessness Team Assessment of SARS-CoV-2 infection prevalence in homeless shelters–four U.S. Cities, March 27-April 15, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dyal JW, Grant MP, Broadwater K, et al. COVID-19 among workers in meat and poultry processing facilities–19 states, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.