Abstract
Nerve tissue regeneration continues to represent an intractable obstacle to realizing the promise of tissue engineering. Although neurobiology works to shed light on the mechanisms governing neuronal growth and repair, considerable technical gaps remain that hinder progress. Chief among these is the absence of an appropriate culture environment to faithfully reproduce the neuronal niche ex vivo. We propose that the various multipotent cells found in the oral cavity may represent an important yet underutilized resource for preparing such neurogenic microenvironments. Similar to those of nerve tissue, these cell populations are of ectodermal origin and have clinically demonstrated neurogenic potential. Although there is a lack of consensus on whether putative types of oral and craniofacial stem cells constitute distinct populations, their contribution to neural tissue engineering may be twofold: as a cellular feedstock for neoneurogenesis and for the production of specialized in vitro environments for neurogenic differentiation, phenotype maintenance, and use in therapeutic applications.
Impact statement
We propose that addressing gaps in understanding the neurogenic role of dental stem cells and their microenvironment may yield efficient and reliable strategies for long-term neuronal cell culture and open new avenues for neural regeneration in both dental, nerve, and other tissues.
Keywords: mesenchymal stem cells, dental stem cells, neuronal niche, neurogenesis, regenerative medicine
In 2019, we attended the annual meetings of the International Society for Stem Cell Research (ISSCR) and the Tissue Engineering Regenerative Medicine International Society (TERMIS). At these meetings, neural diseases and regeneration attracted considerable attention. Attendees had ample opportunity to learn about advances in neuroscience ranging from developmental biology to neuropathology, and approaches for achieving reinnervation, highlighting the immense challenges and opportunities that these areas continue to represent for regenerative medicine. Although significant advances have been made toward understanding the biology of the nervous system, and advanced culture methodologies, such as neurospheres, have opened up new avenues for modeling the neuronal niche ex vivo, numerous scientific and technical obstacles remain before neoneurogenesis or neural repair become a clinical reality.
Given the immense interest in neural tissue engineering at these meetings, the abundance of presentations describing difficulties associated with the generation of functional neurons from transdifferentiated multipotent stromal cells was to be expected. However, we did not identify a single research poster or lecture at the ISSCR meeting addressing the variety of ectoderm-derived multipotent stem cells found in the oral cavity. This was surprising given the significant potential that these cells represent for both studying neural disease and regenerating nervous tissues. When oral cavity stem cells were discussed at TERMIS, they were frequently characterized within the context of mesenchymal stem/stromal cells (MSCs). However, rather than being mesenchymal in origin, oral cavity stem cells derive from the neural crest, a transient ectodermal structure. These misunderstandings about the origin and nature of oral cavity stem cells obscure their potential.
During development, the neural crest originates from the ectoderm at the margins of the neural tube and contributes to a variety of neural and non-neural tissues, including most of the peripheral nervous system. The cells that comprise the neural crest disperse laterally between the developing epidermis and neural tube, differentiating into a multitude of tissue and cell types, including Schwann cells, spinal ganglia, pigmented cells, as well as the ectomesenchyme of the head and neck.1
These migrating cranial neural crest cells interact at sites along the dental epithelium and in doing so induce the initial folding and thickening that mark sites of dental placodes or tooth development.2 In this way, in mammals teeth are considered an ectodermal tissue and quantitatively, most of the remaining cell types in adults are neural crest derived.2 Neural crest-derived tissue includes the pulp, periodontal ligament, and tissues supporting the root and its apex, including the following cell types: odontoblasts, periodontal ligament cells, glial cells, and dental pulp stem cells (DPSCs).2 In contrast, the remaining dental epithelium-derived tissue is the acellular enamel and a few cell rests of Malassez along the periodontal ligament; contributing mesoderm comprises the vascular and immune component to the pulp.2
Oral cavity-derived stem cells share this neural crest history and interestingly have been demonstrated to differentiate into neuronal cell types both in vitro and in vivo.3 Whether or not direct differentiation of oral cavity stem cells into functional neurons is a practical approach for tissue engineering, the common origin of oral cavity stem cells and various neural cell types make them an intriguing source of cells for studying neural physiology and regeneration. In this study, we offer that neoneurogenesis in adult teeth after complete pulpectomy may offer important clues for overcoming obstacles to neuronal repair. Furthermore, we propose that stem cell biologists and tissue engineers alike would benefit from more careful consideration of the multitude of stem cell populations found within the oral cavity and their unique properties.
Nerve regeneration is particularly challenging for a number of reasons. Relative to many other tissues, fewer precursor cells capable of regenerating nervous tissue are retained after development and the neurogenic potential of these populations wanes with age.3 Complicating this, methods for the in vitro maintenance of neural cell types are inadequate, hampering investigations into the mechanisms of initiation and progression of neurogenesis.4,5 Although some progress has been made in producing cells resembling neuronal progenitors from human-induced pluripotent stem cells, they are difficult to maintain and expand for more than several days, or reliably differentiate to mature neural cells.5,6
Another important factor inhibiting progress in studying neurogenesis ex vivo is the absence of a unique culture environment or neuronal niche reproducing appropriate physical and biochemical cues.7–10 Finally, a genuine need remains for an effective and scalable method of sourcing, expanding, and studying neural cell types. The only successful reinnervation procedure currently in clinical practice may point toward a path forward.
The oral cavity is an often-overlooked structure in medicine, yet dentistry has achieved impressive progress toward identifying and adopting successful regenerative strategies. Regenerative endodontic procedures are a group of developing techniques aimed at regenerating the dental pulp and restoring the function of the pulp-dentin complex.11 In the past, dental infections that reached the depth of the dental pulp were treated with nonsurgical root canal therapy in which a pulpectomy (the complete removal of all soft tissue within a tooth, including local nerve tissue) was performed, followed by filling of the remaining space with biocompatible materials.
This procedure removes the infection and eliminates the patient's pain by excising the nerve tissue.12 Although this procedure represents the standard of care, the end result is a functionally compromised devitalized tooth that is more prone to fracture and lacks sensation.11 In contrast, regenerative endodontic procedures have demonstrated restoration of nociception in teeth that had previously undergone pulpectomy.12 These techniques would ideally replace traditional root canal therapy for most cases. Currently, most studies have been performed in immature adult or deciduous (baby) teeth; however, in studies performed in adults with permanent dentition, successful resolution of apical periodontitis, with narrowing of the canal space and closure of the apex, have indicated healing of these tissues.11,13
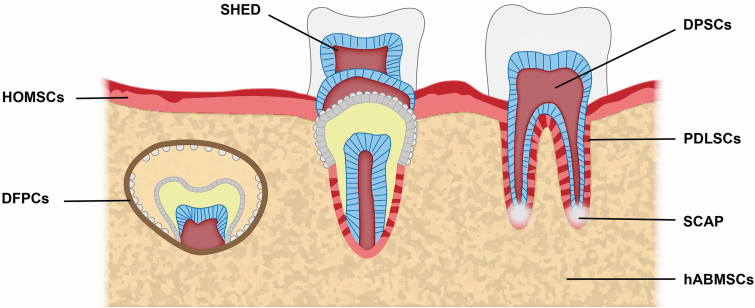
Interestingly, in many of these cases the patients regain sensation in their teeth, providing evidence of successful reinnervation after pulpectomy.13 The successful neuroregenerative outcomes achieved by endodontics are not accompanied by corresponding strategies in other tissues, suggesting special properties of the stem cells residing in the dental periapical niche. To this point, several groups have demonstrated the capability of different stem cell populations derived from dental pulp to promote reinnervation and restoration of movement in paraplegic rodent models, highlighting the novel regenerative capacity of these cells outside of oral medicine.3 Figure 1 shows the anatomical location of previously reported oral and craniofacial stem cells.14–21
FIG. 1.
Sources of putative oral and craniofacial stem cells. DFPCs, dental follicle precursor cells; DPSCs, dental pulp stem cells; hABMSCs, human alveolar bone-derived mesenchymal stem cells; HOMSCs, human oral mucosa stem cell; PDLSCs, periodontal ligament stem cells; SCAP, stem cells of the apical papilla; SHED, stem cells of human exfoliated deciduous teeth.
Successful endodontic neurogenesis is attributable to the unique cellular composition of the periapical tissues. In these procedures, stem cell recruitment is initiated by bleeding beyond the apex of the tooth, allowing the canal spaces to be infiltrated. This blood is rich with growth factors, extracellular matrix (ECM) proteins, and a heterogenous population of cells.13 However, despite promising clinical results, much progress remains to be achieved in isolating and defining the various types of oral and craniofacial stem cells present and determining their individual potential for neural regeneration.15–18 Recently, various groups have described a number of stem and progenitor cell populations in dental pulp tissue and defined obstacles to their utilization, including standardization of definitions, understanding cell potency, determining the relationship between phenotype and isolation/culture conditions, and assessment of “quality” metrics.15–22
The sheer diversity of putative stem cell types, based on anatomical and developmental origin (Table 1), is a source of confusion in the field. The absence of clear phenotypic distinctions between cell types also results in a lack of clarity around clonal heterogeneity and prohibits the field from reaching a consensus regarding which of these cell types are “real” versus experimental artifacts. Continued confusion over semantics and the absence of consensus regarding definitions of discrete cell types limits the ability of researchers to draw useful conclusions from existing literature and make effective comparisons across studies.23
Table 1.
Description of Selected Dental Stem Cell Types
| Cell type | Location | Properties | Markers |
|---|---|---|---|
| DPSCs | Dental pulp | First dental SC identified. Share commonalities with neural stem cells. Indicate neurogenic and vascular endothelial differentiation potential.11,12 | STRO-1, CD146, OCT4 |
| SHED | Exfoliated deciduous teeth | Faster proliferation, more immature DPSC, high CFUs. Suggested to be incapable of osteoblast or osteocyte differentiation.13,14 | STRO-1, CD146, OCT4 |
| SCAP | Apical papilla | Differentiable to odontoblast-like cell type. | STRO-1, CD24, CD29 |
| Comparable osteogenic and dentinogenic potential to DPSCs, less adipogenic potential.15 | |||
| PDLSCs | Periodontal ligament | Differentiate into cementoblast-like cells. Potential regeneration of periodontal structures.16 | STRO-1, CD146, tendon-specific TF |
| HOMSCs | Oral mucosa | Isolated from oral mucosa and gingiva; may include multiple cell types. Demonstrate MSC-like tri-lineage differentiation.17 | CD13, CD29, CD44, CD73, CD90, CD105, CD146, CD166, STRO-1, SSEA-4 |
| DFPCs | Dental follicle | Heterogeneous population of mixed cell phenotypes. Demonstrate osteogenic potential.18,19 | CD13, CD29, CD72, CD90, CD105, CD106, CD166, Notch1, STRO-1, Nestin |
| hABMSCs | Alveolar bone | Demonstrate significant bone-forming capacity and MSC-like tri-lineage differentiation.20 | CD73, CD90, CD105, CD146, CD166, STRO-1 |
CFU, colony forming unit; DFPCs, dental follicle precursor cells; DPSCs, dental pulp stem cells; hABMSCs, human alveolar bone-derived mesenchymal stem cells; HOMSCs, human oral mucosa stem cells; MSC, mesenchymal stem cell; PDLSCs, periodontal ligament stem cells; SC, stem cell; SCAP, stem cells of the apical papilla; SHED, stem cells of human exfoliated deciduous teeth.
Owing to a number of related morphological properties and similar differentiation potentials, the proposed stem cell populations derived from dental and periapical tissues are often compared with MSCs from ordinary sources such as bone marrow, adipose tissue, and umbilical cord tissue.19–21 It is convenient to conceptualize bones and teeth as analogous tissues due to their calcified structures, but they completely differ in their development and final compositions.
Although the various dental stem cell populations are ectodermal in origin (neural crest), cells that give rise to bones and marrow are largely mesodermal. These differences manifest themselves in distinct functional phenotypes.13–15 For example, although bone marrow-derived MSCs and DPSCs share fibroblast-like morphology and readily differentiate to osteogenic, adipogenic, and chondrogenic lineages, they reside within dramatically different microenvironments (niches) in vivo, which have considerable differences in structure, vascularity, mechanical properties, and resident cell types.14–16,21 False equivalency between dental and MSCs may obscure their distinct advantages within specific regenerative applications. Accordingly, there is an emerging consensus that MSC transdifferentiation to nonmesodermal lineages such as neuronal cell types is unlikely to represent an effective regenerative strategy or translatable process for nerve tissue engineering.24 In contrast, dental stem cells and neurons share an ectodermal origin. As such, dental stem cells may represent a more rational cell population to serve as a feedstock for nerve tissue engineering.
Given the evidence that dental stem cells support nerve regeneration either directly (through expansion and differentiation) or indirectly (through secretion of matrix components and soluble factors), this is a promising direction for future investigation.13 Elucidating the role of dental stem cells and their niche in reinnervation would yield critical insights into mechanisms of neoneurogenesis. One potential approach is employing methods used to produce and study other tissue-specific microenvironments in vitro.10 For example, various types of dental stem cells may be used to produce ECMs ex vivo, which can then be characterized for their biochemical, structural, and mechanical properties, as well as their ability to support expansion, phenotype maintenance, and/or differentiation of either dental stem cells or neural progenitors.
In addition to clinical applications, the ability of these cell-produced microenvironments to support neurogenesis would enable novel directions in basic research. It is also possible that dental stem cell-derived ECMs, or cocultures with dental stem cells, may support long-term culture of neurons. Moreover, this study may yield important insights into biomaterials design and the properties of scaffolds necessary for supporting expansion, phenotype retention, and directed differentiation of neural progenitors. If this line of study shows promise, translational applications may prove much broader than neurogenesis in the oral cavity.
Development-inspired scaffolds, possibly in combination with oral-derived stem cells or their secreted neurotrophic factors, for example, brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, nerve growth factor, may be applied for nerve tissue engineering for spinal cord damage or repair of the peripheral nervous system. As a whole, dental stem cell populations continue to harbor a compelling, if underexploited, potential to address some of the most critical unmet needs in neural tissue engineering.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by a VA Merit Review (1I01BX002145-01) to Dr. Chen and a National Institutes of Health/NIDCR grant (DE025286) to Dr. Yeh.
References
- 1. Kulesa P., Bailey C., Kasemeier-Kulesa J., and McLennan R.. Cranial neural crest migration: new rules for an old road. Dev Biol 344, 543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krivanek J., Adameyko I., and Fried K.. Heterogeneity and developmental connections between cell types inhabiting teeth. Front Physiol 8, 376, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luo L., He Y., Wang X., et al. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int 2018, 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reekmans K., Praet J., Daans J., et al. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev Rep 8, 262, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Chen K., Mallon B., McKay R., and Robey P.. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 14, 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon J., Amini S., and White M.. General overview of neuronal cell culture. Methods Mol Biol 1078, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai Y., Sun Y., Skinner C., et al. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev 19, 1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ninkovic J., and Götz M.. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol 17, 338, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Illes S., Theiss S., Hartung H.P., Siebler M., and Dihné M.. Niche-dependent development of functional neuronal networks from embryonic stem cell-derived neural populations. BMC Neurosci 10, 93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marinkovic M., Block T., Rakian R., et al. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol 52, 426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diogenes A., Henry M., Teixeira F., and Hargreaves K.. An update on clinical regenerative endodontics. Endod Topics 28, 2, 2013 [Google Scholar]
- 12. Murray P., Garcia-Godoy F., and Hargreaves K.. Regenerative endodontics: a review of current status and a call for action. J Endod 33, 377, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Lovelace T., Henry M., Hargreaves K, and Diogenes A.. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37, 133, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Gronthos S., Mankani M., Brahim J., Robey P., and Shi S.. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97, 13625, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miura M., Gronthos S., Zhao M., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100, 5807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chalisserry E., Nam S., Park S., and Anil S.. Therapeutic potential of dental stem cells. J Tissue Eng 8, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonoyama W., Liu Y., Yamaza T., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34, 166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo B., Miura M., Gronthos S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q., Nguyen A., Yu W., and Le A.. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. Dent Res J 91, 1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honda M., Imaizumi M., Suzuki H., Ohshima S., Tsuchiya S., and Satomura K.. Stem cells isolated from human dental follicles have osteogenic potential. Oral Surg Oral Med Oral Pathol Oral Radiol 111, 700, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Liu J., Yu F., Sun Y., et al. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 3, 627, 2015 [DOI] [PubMed] [Google Scholar]
- 22. Mason S., Tarle S.A., Osibin W., Kinfu Y., and Kaigler D.. Standardization and safety of Alveolar bone–derived stem cell isolation. Dent Res J 93, 55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marinkovic M., Block T., and Kondraske G.. Toward a unified “quality” framework for cell-based therapies. Cytotherapy 20, 1220, 2018 [DOI] [PubMed] [Google Scholar]
- 24. Langrzyk A., Nowak W.N., Stępniewski J., et al. Critical view on mesenchymal stromal cells in regenerative medicine. Antioxid Redox Signal 29, 169, 2018 [DOI] [PubMed] [Google Scholar]