Abstract
Background:
Many people living with HIV (PLWH) have comorbidities which are risk factors for severe coronavirus disease 2019 (COVID-19) or have exposures that may lead to acquisition of severe acute respiratory distress syndrome coronavirus 2. There are few studies, however, on the demographics, comorbidities, clinical presentation, or outcomes of COVID-19 in people with HIV.
Objective:
To evaluate risk factors, clinical manifestations, and outcomes in a large cohort of PLWH with COVID-19.
Methods:
We systematically identified all PLWH who were diagnosed with COVID-19 at a large hospital from 3 March to 26 April 2020 during an outbreak in Massachusetts. We analyzed each of the cases to extract information including demographics, medical comorbidities, clinical presentation, and illness course after COVID-19 diagnosis.
Results:
We describe a cohort of 36 PLWH with confirmed COVID-19 and another 11 patients with probable COVID-19. Almost 85% of PLWH with confirmed COVID-19 had a comorbidity associated with severe disease, including obesity, cardiovascular disease, or hypertension. Approximately 77% of PLWH with COVID-19 were non-Hispanic Black or Latinx whereas only 40% of the PLWH in our clinic were Black or Latinx. Nearly half of PLWH with COVID-19 had exposure to congregate settings. In addition to people with confirmed COVID-19, we identified another 11 individuals with probable COVID-19, almost all of whom had negative PCR testing.
Conclusion:
In the largest cohort to date of PLWH and confirmed COVID-19, almost all had a comorbidity associated with severe disease, highlighting the importance of non-HIV risk factors in this population. The racial disparities and frequent link to congregate settings in PLWH and COVID-19 need to be explored urgently.
Keywords: congregate setting, coronavirus disease 2019, HIV, pandemic, racial disparities, severe acute respiratory distress syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), has spread around the world, but little is known about how this disease affects people living with HIV (PLWH). The few reports to date on this subject have analyzed small numbers of PLWH and conclusions have been limited [1–3].
In the general population, major risk factors for severe COVID-19 include advanced age, hypertension, diabetes, obesity, and cardiovascular disease [4–7]. More than 40% of PLWH in the United States are now more than 50 years old and many have overlapping comorbidities associated with severe COVID-19 [8]. In addition, as people with HIV age, they may be living in congregate settings, including skilled nursing facilities, where rapid spread of SARS-CoV-2 infection has occurred [9,10]. There are no reports to date on the risk for COVID-19 among PLWH in congregate settings. Finally, there is little information available regarding the clinical manifestations and outcomes of COVID-19 in people with HIV.
Given the uncertainties regarding COVID-19 in people with HIV, we evaluated the demographics, risk factors, clinical presentation, and diagnosis of COVID-19 in this population. Here we report the characteristics and outcomes of the largest cohort to date of PLWH with confirmed COVID-19 (n = 36, Table 1 ) and a second cohort with probable COVID-19 (n = 11, Table 2).
Table 1.
Confirmed coronavirus disease 2019 cases among a cohort of people living with HIV.
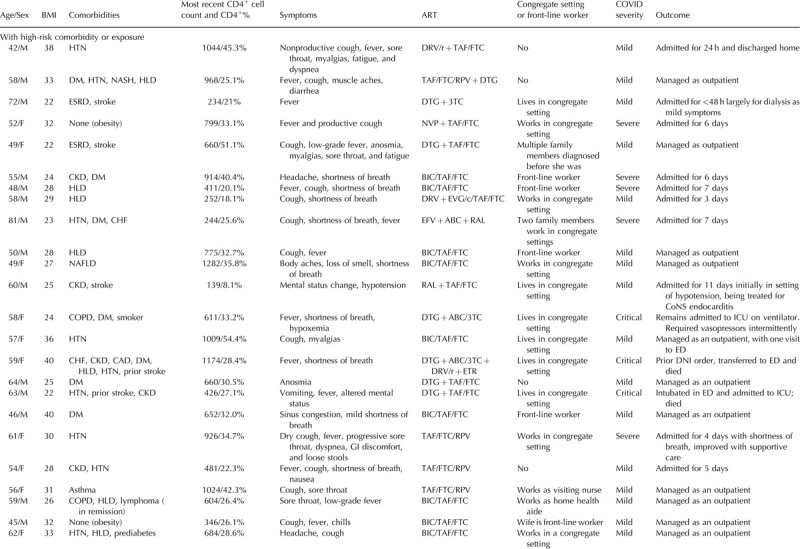
Table 1 (Continued).
Confirmed coronavirus disease 2019 cases among a cohort of people living with HIV.
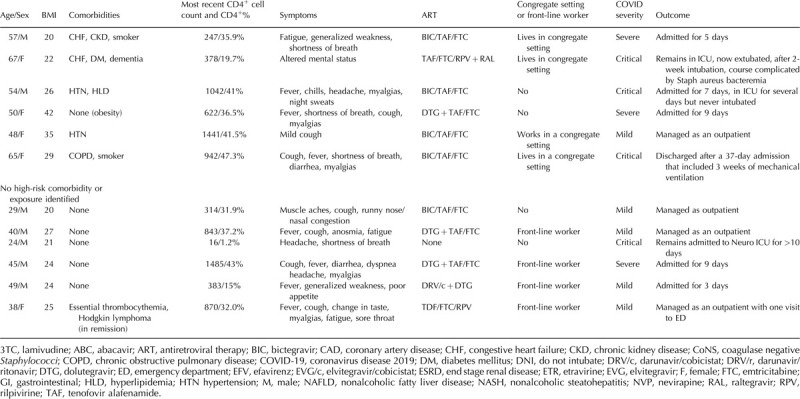
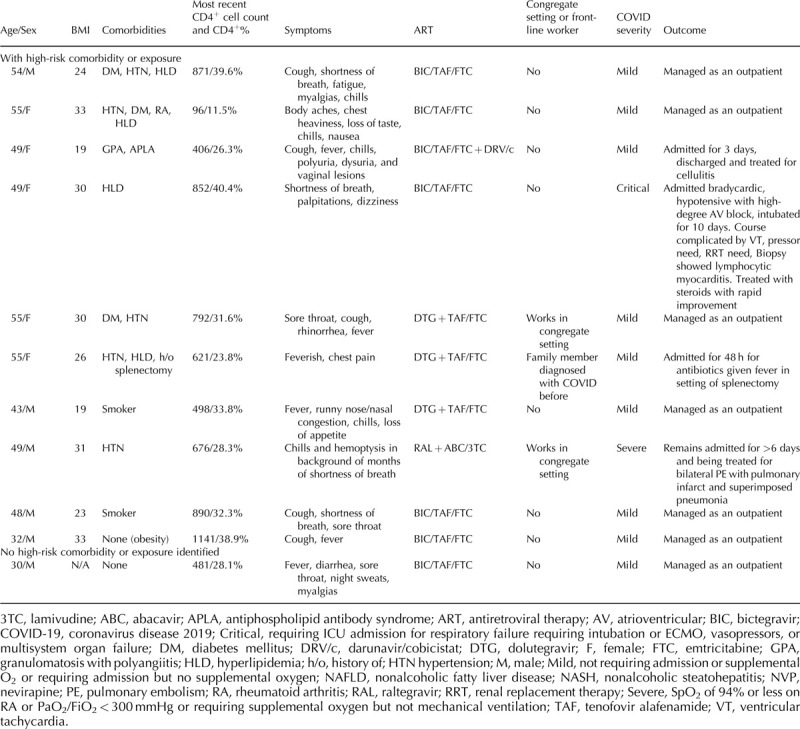
Table 2.
Probable coronavirus disease 2019 cases among a cohort of people living with HIV.
Methods
Using an electronic registry, all PLWH with COVID-19 cared for at our hospital from 3 March to 26 April 2020 were systematically identified. COVID-19 risk or diagnosis was captured by a flag in the electronic medical record. HIV diagnosis was identified based on International Classification of Diseases codes 042/B20, HIV RNA drawn or HIV Ag/Ab reactive; all HIV diagnoses were then confirmed by manual review. Suspected cases were PLWH cared for in our clinic who presented with typical symptoms including fever, chills, myalgias, and cough, without an alternative diagnosis during the study time period. The study was deemed exempt by our Institutional Review Board.
Data collected from manual chart reviews included presenting symptoms, duration of symptoms at time of presentation, race/ethnicity, comorbidities, and BMI. Each patient's current antiretroviral regimen and most recent CD4+ cell count were collected. Their type of work and place of residence were also recorded. Particular types of employment such as in grocery stores, convenience stores, or healthcare were considered ‘front-line work.’ Congregate settings included group homes, assisted living facilities, and skilled nursing facilities. COVID-19 illness severity was graded for each patient. Mild or moderate COVID-19 was defined as not requiring supplemental oxygen (either in an outpatient or inpatient setting). Severe COVID-19 was defined as hospitalized patients who required supplemental oxygen or had a peripheral capillary oxygen saturation of 94% or less on ambient air but did not require care in the ICU. Patients with critical disease required ICU-level care, often with mechanical ventilation.
Results
We identified 36 people with HIV who had confirmed cases of COVID-19 based on a positive nasopharyngeal swab PCR (tested a mean of 5.7 days after symptom onset, range 1–17). Another 11 PLWH had probable COVID-19 based on typical symptoms and lack of an alternative diagnosis during a period of low circulation of other respiratory viruses. Of the probable cases, 10 were PCR negative (tested a mean of 7.1 days after symptom onset, range 2–16) and one was not tested (he was diagnosed early in the outbreak when testing was not widely available).
Among those with confirmed COVID-19, almost two-thirds (21/36) required hospitalization, including eight with severe and seven with critical illness. Two patients (5.6%) died and four remain hospitalized, including two people in intensive care. At presentation, 21 (58.3%) reported fever, 20 (55.6%) reported cough, and 14 (38.9%) reported shortness of breath. Thirty-three (91.7%) reported at least one of these symptoms. Seven (19.4%) reported gastrointestinal symptoms including anorexia, nausea, vomiting or diarrhea, and five (13.8%) reported anosmia or dysgeusia.
The average age of the cohort was 53.4 years (range 24–81 years), with a higher age for those who required hospital admission compared with those who were managed as outpatients (55.9 versus 50 years, respectively). Thirty (83.3%) had comorbidities associated with severe COVID-19. The most common comorbidities were obesity defined as BMI more than 30 (12/36; 33.3%), hypertension (11/36; 30.6%), diabetes (8/36; 22.2%), hyperlipidemia (8/36; 22.2%), and chronic kidney disease (8/36; 22.2%). Of those who were hospitalized, 18/21 (85.7%) had a comorbidity associated with severe COVID-19; of those who were managed as outpatients, 12/15 (80%) had a comorbidity.
Regarding the demographics of the patients in this cohort, 16 (44.4%) were non-Hispanic Black and 12 (33.3%) were Hispanic/Latinx. For comparison, our clinic population includes just over 1300 PLWH, of whom around 50% are White, 30% Black, and 10% Hispanic/Latinx. Nearly half of the patients or their family members (16/36 or 44.4%) lived or worked in a congregate setting (group home, assisted living, or skilled nursing facility). Eleven others either worked in ‘front-line’ jobs including home healthcare and retail/grocery stores or had household members working in these positions.
Two patients had a CD4+ cell count less than 200 cells/μl, and one patient, who was simultaneously diagnosed with COVID-19, HIV/AIDS, and cryptococcal meningitis, was not on antiretroviral therapy (ART). Of those on ART, 29/35 were on an integrase strand transfer inhibitor, nine were on a nonnucleoside reverse transcriptase inhibitor, and four were on protease inhibitors. Thirty were on a tenofovir-containing regimen.
In the cohort of patients with probable COVID-19, 4/11 required hospital admission, including one with severe disease and one with critical illness who required mechanical ventilation in the setting of biopsy proven lymphocytic myocarditis. Nine (81.8%) had mild/moderate disease. There were no deaths in this group, and none remain in the hospital. All presented with typical symptoms including fever, cough, myalgias, anosmia, etc. The average age was 47.2 years (range 32–55 years). Ten (90.9%) had a comorbidity associated with severe COVID-19. Six (54.5%) were non-Hispanic Black, two (18.2%) were Hispanic/Latinx, and three (27.3%) were White. Two worked in congregate settings and one had a family member diagnosed with COVID-19 before she developed symptoms. One had a CD4+ cell count less than 200 cells/μl and all were on ART.
Discussion
In this study, we describe the largest cohort to date (n = 36) of PLWH with confirmed COVID-19. Most patients presented during their first week of symptoms, though some presented later. Although most patients had mild or moderate disease, 58.3% required hospitalization, and 5.6% died. The vast majority (91.7%) presented with typical symptoms of COVID-19. The average age of the cohort was 53.4 years with a higher age for those requiring admission (55.9 years) compared with those managed as outpatients (50 years), consistent with what has been reported in large series of people without HIV [6,11]. Risk factors for severe COVID-19 in PLWH also appear to be similar to those in people without HIV: of note, nearly 85% of those in our cohort had a well recognized risk factor or comorbidity, highlighting the importance of non-HIV risk factors in this population.
That almost 80% of people in this cohort compared with 40% of our HIV clinic population were Black or Hispanic/Latinx highlights the urgency of understanding and mitigating racial disparities in COVID-19. Racial disparities in incidence and outcomes for COVID-19 are being increasingly recognized [7,12,13]. Racial disparities in prevalence and outcomes for PLWH are also well described [14,15]. Some of the same structural forces that are associated with higher rates of HIV – such as poverty or unstable housing – also contribute to likelihood of SARS-CoV-2 infection. These ‘twin’ pandemics highlight the impact of social forces on disparate infectious diseases. And, because risk factors for COVID-19 and HIV may overlap, people with COVID-19 should be tested for HIV if not previously assessed.
The significant proportion (44% of people in our cohort) with exposure to long-term care facilities suggests clinicians should be attentive to the possibility of COVID-19 among PLWH in congregate settings. This may be particularly important as more PLWH move into congregate settings as the population ages. Given the multiple outbreaks described in skilled nursing facilities as well as in correctional facilities and other congregate settings, public health officials, and policy makers must work quickly to protect these highly vulnerable populations [9,16,17]. That some in our cohort had household members with exposure to congregate settings or front-line work underscores the importance of asking about these potential exposures for patients and all members of their household.
Finally, the identification of individuals with a compatible syndrome but negative PCR testing, emphasizes the importance of not discounting the possibility of COVID-19 in PLWH with negative PCR tests. Widespread access to testing, including PCR and serologic assays, will inform optimal diagnostic approaches, for PLWH and the general population.
Acknowledgements
We are grateful to our patients, colleagues, and all front-line workers involved in the fight against COVID-19.
Author contributions: E.A.M., A.Y.K., V.A.T, and R.T.G. designed the study. E.A.M., A.Y.K., K.L.A., N.B., J.T.C., R.M.H., C.K.L., W.H., T.M., S.N., B.O.O., G.R., S.S., V.A.T., K.Z., and R.T.G. wrote and reviewed the article.
Conflicts of interest
A.Y.K. has served on the scientific advisory board of Biomarin. N.B. serves on the Board of Directors of Allergan LLC. J.T.C. owns individual stocks for Johnson & Johnson and Pfizer. G.R. receives research support paid to the institution from Gilead, Citius Pharm, Emergent Biosolutions, Pfizer, and Leonard Meron Bioscience. R.T.G. has served on a scientific advisory board for Merck and Gilead. The other authors report no conflicts of interest.
References
- 1.Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, Miro JM. COVID-19 in HIV Investigators COVID-19 in patients with HIV: clinical case series. Lancet HIV 2020; 7:e314–e316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmen-Tuohy S, Carlucci PM, Zacharioudakis IM, Zervou FN, Rebick G, Klein E, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qingxian C, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen. Diabetes Care 2020; [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. The Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. The OpenSAFELY Collaborative OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020. [Google Scholar]
- 8.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000–2020. PLoS One 2018; 13:e0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–2090.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aidala AA, Wilson MG, Shubert V, Gogolishvili D, Globerman J, Rueda S, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health 2016; 106:e1–e23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Patel AP, Paranjpe MD, Kathiresan NP, Rivas MA, Khera AV. Race, socioeconomic deprivation, and hospitalization for covid-19 in english participants of a National Biobank. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross CP, Essien UR, Pasha S, Gross JR, Wang S-Y, Nunez-Smith M. Racial and ethnic disparities in population level Covid-19 mortality. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagwat P, Kapadia SN, Ribaudo HJ, Gulick RM, Currier JS. Racial disparities in virologic failure and tolerability during firstline HIV antiretroviral therapy. Open Forum Infect Dis 2019; 6:ofz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burt RD, Glick SN. Racial disparities in HIV. Lancet HIV 2017; 4:e281–e282.. [DOI] [PubMed] [Google Scholar]
- 16.McMichael TM, Clark S, Pogosjans S, Kay M, Lewis J, Baer A, et al. Public Health – Seattle & King County, EvergreenHealth, and CDC COVID-19 Investigation Team COVID-19 in a long-term care facility – King County, Washington, February 27–March 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:339–342.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace M, Hagan L, Curran KG, Williams SP, Handanagic S, Bjork A, et al. COVID-19 in correctional and detention facilities – United States, February–April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:587–590.. [DOI] [PubMed] [Google Scholar]


