Abstract
Background
Emerging data suggests PM2.5 (particulate matter with aerodynamic diameter <2.5 μm) may be associated with both earlier declines in episodic memory (EM) and increased depressive symptoms in older adults. Although late-life depressive symptoms are associated with EM, no longitudinal studies have examined the inter-relationship among PM2.5, depressive symptoms and EM.
Methods
Older women (n = 2,202; aged 67–83 in 1999) enrolled in the Women’s Health Initiative Study of Cognitive Aging completed up to eight annual assessments of depressive symptoms (15-item Geriatric Depression Scale) and EM (California Verbal Learning Test). A nationwide spatiotemporal model (1999–2010) was used to estimate ambient PM2.5 exposure at residential locations. Univariate and bivariate structural equation models (SEMs) for latent-change scores were used to examine how 3-year average PM2.5 preceding each assessment affects the temporal dynamics and bidirectional relations of annual changes in depressive symptoms and EM.
Results
In univariate SEMs, one inter-quartile (4.04 μg/m3) increment of 3-year PM2.5 was significantly (p<0.05) associated with accelerated declines in verbal learning (List A trials 1–3: β=−1.48) and free-recall memory (short-delay: β=−1.43; long-delay: β=−1.11), but not with change in depressive symptoms (β=0.12; p=0.71). In bivariate SEMs, significant associations were observed between PM2.5 and accelerated declines in EM measures (β=−1.44 to −0.99; p<0.05) and between EM performance and changes in depressive symptoms (β=−0.08 to −0.05; p<0.05), with significant indirect PM2.5 effects on changes in depressive symptoms (β=0.08 to 0.10; p<0.05). These findings were robust with adjustment for multiple demographic, lifestyle, and clinical factors, and remained after excluding subjects with dementia or mild cognitive impairment. No associations were found between PM2.5 and change in depressive symptoms or depressive symptoms and subsequent EM decline.
Conclusions
Findings suggest that PM2.5 neurotoxicity may damage brain areas implicated in EM, followed by manifestation of depressive symptoms. Our data did not support depressive symptoms as the neuropsychological mediator of accelerated brain aging associated with PM2.5 exposure.
Keywords: Particulate Matter, Depressive Symptoms, Episodic Memory, Structural Equation Modeling
1. Introduction
Long-term exposure to particulate air pollutants represents a novel environmental risk factor of accelerated brain aging (The Lancet Neurology 2018). An increasing number of epidemiologic studies have reported associations between late-life exposure to ambient PM2.5 (particulate matter with aerodynamic diameter <2.5 μm) and increased risks for cognitive decline (Cacciottolo et al. 2017; Tonne et al. 2014; Weuve et al. 2012) and dementia (Cacciottolo et al. 2017; Carey et al. 2018; Chen et al. 2017a; Chen et al. 2017b; Jung et al. 2015; Oudin et al. 2018). Recent longitudinal data also suggest PM2.5 exposure may increase the risk of depression in adults (Kim et al. 2016). Biologically plausible mechanisms underlying these neurotoxic effects on brain aging may include particle-induced neuroinflammation, oxidative stress, cerebral vascular damage, and neurodegeneration via direct or indirect pathways. For instance, air pollution exposure can trigger systemic or peripheral inflammation that affects the central nervous system and neurobiology in the brain. Experimental data also showed that airborne engineered particles may translocate to the brain, possibly resulting in direct damage whereby astroglia, brain capillaries, and microglia respond with chronic activation, inflammation, and oxidative stress (Béjot et al. 2018; Block and Calderón-Garcidueñas 2009).
A large body of literature has documented the close link between depressive symptoms, accelerated brain aging, and dementia (Byers and Yaffe 2011). Earlier prospective cohort studies found an increased risk for cognitive impairment or dementia associated with prior depression or depressive symptoms in late-life (Barnes et al. 2006; Barnes et al. 2012; Ownby et al. 2006; Saczynski et al. 2010; Wilson et al. 2002; Yaffe et al. 1999). More recent longitudinal analyses showed that late-life depressive symptoms are more likely to occur as the prodromal neuropsychiatric manifestation of Alzheimer’s disease and related dementias (ADRD; Steffens 2017). Even after accounting for neuropathological measures of ADRD (Wilson et al. 2014), depressive symptoms are still associated with cognitive decline in the elderly, although the exact directionality of this interrelation remains unclear. The same neural mechanisms may underlie both depressive symptoms and memory impairment (Disner et al. 2011). Depressive symptoms may lead to declines in episodic memory (Zahodne et al. 2014), which tends to decline with normal aging and represent one of the cognitive domains with early decline detectable in preclinical Alzheimer’s disease (Gallagher and Koh 2011). Neuroimaging studies have shown that hippocampal atrophy is elevated in individuals with untreated depression (Sheline et al. 2003), and the hippocampal networks play a key role in episodic memory. In contrast, other studies also found that poor episodic memory may actually lead to increased depressive symptoms over time (Jajodia and Borders 2011; Vinkers et al. 2004), lending support for the opposite direction of coupling effect. An individual’s self-awareness of their episodic memory impairment could lead to a psychological response of increased depression because they know their recollection of particular life experiences may fade or activities they used to enjoy (e.g., reading) become difficult (Ganguli 2009). They also may experience increased concern about the future and developing dementia.
Extant knowledge of cognitive neurosciences of brain aging therefore raises at least two possibilities about the longitudinal associations linking air pollution exposure with these two phenotypes of brain aging. First, depressive symptoms in late life, if directly affected by air pollution (e.g., PM2.5), may act as a neuropsychological mediator of exposure-associated cognitive impairment. Second, air pollution may indirectly lead to increased depressive symptoms via declines in episodic memory associated with exposure. To the best of our knowledge, no studies have examined whether and how air pollution exposure affects the temporal dynamics between depressive symptoms and episodic memory in late life. Understanding these complex associations is important from the public health perspectives, because each of the suggested pathways, if substantiated by empirical data, may point to different targets for primary prevention versus secondary interventions towards improving brain health of older people. Long-term studies with multiple assessments of these two phenotypes are needed to address whether air pollution exposures relate to the bidirectional changes in these two phenotypes of brain aging. In this longitudinal study we examined how exposure to PM2.5 affects the temporal dynamics and possibly bidirectional relation between episodic memory and depressive symptoms over time in a community-dwelling cohort of older women assessed annually from 1999 to 2010.
2. Materials and Methods
2.1. Study Population
This longitudinal cohort study included 2,202 community-dwelling older women (baseline age 66–83 years old) without dementia in 1999 when enrolled in the Women’s Health Initiative Study of Cognitive Aging (WHISCA; Resnick et al. 2004), an ancillary study to the Women’s Health Initiative Memory Study (WHIMS; Shumaker et al. 1998). The WHIMS (N=7,479) began in 1996 and was an ancillary study to the Women’s Health Initiative (WHI) Clinical Trial of Hormone Therapy (The Women’s Health Initiative Study Group 1998). Between 1999 and 2010, WHISCA participants (n=2304) completed annual neuropsychological assessments, including measures of depressive symptoms and episodic memory. Excluded from the present study were 102 women with missing data on relevant covariates, resulting in a final sample of 2,202.
2.2. Assessment of depressive symptoms
Depressive symptoms were assessed at baseline and at each annual follow-up (up to 8 assessments) using the 15-item Geriatric Depression Scale (GDS-15) (Yesavage and Sheikh 1986). The GDS-15 is a reliable and valid instrument, commonly used to assess depressive symptoms in older adults (Mitchell et al. 2010). Scores were standardized on a T-score metric (Mean = 50; SD = 10), based on the baseline GDS-15 mean and standard deviation. Higher scores reflect greater depression symptoms.
2.3. Assessment of verbal episodic memory
Verbal episodic memory was assessed using a modified version of the California Verbal Learning Test (CVLT) (Delis et al. 1987). Participants were read a list of 16-words and instructed to repeat as many of the words from the list as they could. This procedure was repeated two more times. Only three learning trials were administered in WHISCA instead of the standard five trials. Learning/immediate recall ability was measured by the total number of words correctly recalled over the three learning trials (trials 1–3). The participant was then asked to freely recall all of the words that they could from the first list (short-delay free recall). Approximately 20-minutes after the short-delay free recall trial, the participants were asked again to freely recall as many words from the initial list of words (long-delay free recall). Performance on each measure was also standardized on a T-score metric based on the baseline mean and standard deviation.
2.4. Assessment of ambient PM2.5
In this study, we focused on PM2.5, because its associations with cognitive deficits in both human studies (Clifford et al. 2016) and animal models (Fonken et al. 2011; Ku et al. 2017) were more established than the other regional air pollutants. Briefly, participants residential addresses were prospectively collected at each annual WHISCA assessment and geocoded using standardized procedures (Whitsel et al. 2004). Using the Bayesian Maximum Entropy (BME) method (Christakos 2000; Christakos et al. 2012), we constructed spatiotemporal models that are a function of space and time to generate individual-level, residence-specific PM2.5 estimates. The BME integrates daily observed PM2.5 data obtained from nationwide monitoring system of the U.S. Environmental Protection Agency Air Quality System, along with the output of chemical transport models that fully characterize the local emission sources, meteorology, chemicals transformations and transport of pollutants (Reyes et al. 2017). Estimates of daily PM2.5 exposures were statistically cross-validated with a 10-fold estimations analysis. The results showed that the BME estimates of PM2.5 exposure correlated well with EPA recorded concentrations (average Pearson’s R2 = .70). The resulting exposure estimates were then aggregated to represent the average PM2.5 exposure 3-years preceding each WHISCA assessment. In all analyses PM2.5 exposure was scaled to the interquartile range estimated for the baseline WHISCA assessment (4.04 μg/m3).
2.5. Classification of Mild Cognitive Impairment and dementia
Mild cognitive impairment (MCI) was classified using the Peterson’s criteria (Petersen et al. 1994); and all-cause dementia was defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-4) (American Psychiatric Association 1994) criteria. From 1999–2008 annual screenings were conducted in 49 WHIMS sites (including satellite clinics) by centrally-trained and regularly-certified interviewers administering the Modified Mini-Mental State (3MS) (Teng and Chui 1987). Women who screened positive according to age-/education-adjusted 3MS were administered extensive neuropsychological testing (Morris et al. 1989) and behavioral symptoms/function assessment. Beginning in 2008, a validated battery of tests was administered to participants annually by telephone using the Telephone Interview for Cognitive Status-modified (TICSm) (Rapp et al. 2012). For women who screened positive during telephone interviews (i.e., TICSm<31), the standardized Dementia Questionnaire (Kawas et al. 1994) was administered by telephone to knowledgeable informants to assess the participant’s dementia-related cognitive and behavioral changes and relevant medical history. All relevant assessments and information were submitted to a central adjudication committee for final classification, based on the DSM-IV. Data on MCI and dementia status were available up to December 2015.
2.6. Relevant Covariate Data
A structured questionnaire was administered at WHIMS baseline to gather information on the time independent covariates of demographics (age, race/ethnicity), geographic region of residence (Northeast, South, Midwest, and West), socioeconomic status (education; family income), lifestyle factors (smoking; alcohol use; physical activities), and clinical characteristics, including self-reported postmenopausal hormone treatment ever, history of cardiovascular disease (including previous coronary heart, stroke, or transient ischemic attack), hypertension (defined as elevated blood pressure or use of antihypertensive medication), and diabetes mellitus (defined as physician diagnosis plus oral medications, or insulin therapy). Good reliability and validity of the self-reported medical histories and the physical measures have been previously documented (Heckbert et al. 2004).
2.7. Statistical Analysis
Structural equation models (SEMs) for latent change scores (LCSs) (McArdle 2001) were constructed to examine the complex associations between PM2.5 exposure and temporal changes in the two inter-related neuropsychological processes (episodic memory; depressive symptoms) over the WHISCA study period. The SEMs with LCS are advantageous because they allows for the modeling of dynamic change between two variables from one time point to the next. The LCS approach estimates dynamic annual change by combining features of latent growth curve models, which determine the systematic change over time (Meredith and Tisak 1990), and autoregressive cross-lagged regressions, which estimate the proportional change over time (Selig and Little 2012). Because women were assessed annually, we examined change over one-year intervals with the WHISCA inception time denoted as the baseline. The supplemental methods section provides a more detailed description of our analytic approaches.
2.7.2. Univariate latent change score models.
To examine the association between PM2.5 exposure and annual change in each neuropsychological process we first constructed univariate LCS models separately for episodic memory and depressive symptoms (See Figure 1 for a depiction of the full univariate model). For episodic memory, individual-specific performance at baseline (intem,i) was estimated along with between-individual variability in initial performance (σ2intem). The equation to estimate annual individual-specific change in episodic memory for individual i at timepoint t was written as:
| (Equation 1), |
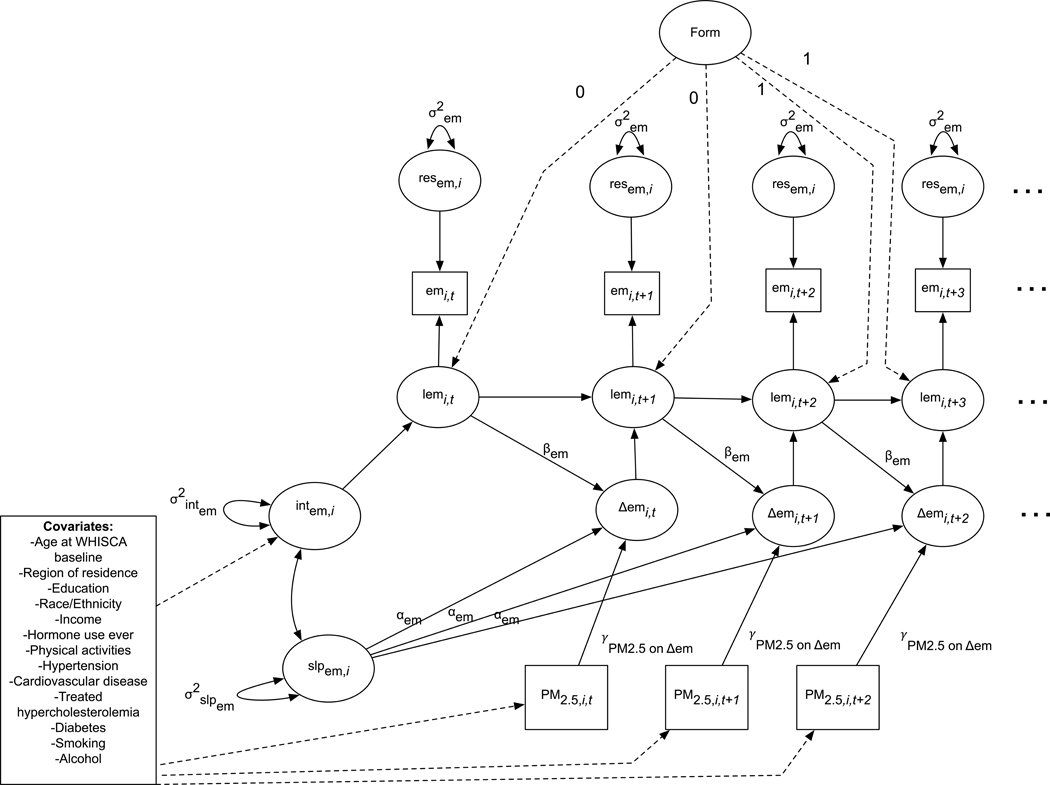
Fig. 1.
Simplified depiction of the univariate latent change score structural equation model estimating the effect of preceding 3-year average PM2.5 exposure on change in episodic memory performance. Intem,i = estimate of episodic memory (em) as measured by CVLT performance at WHISCA baseline for individual i. slpem,i = estimate of systematic linear change in episodic memory (em) for individual I as measured by CVLT performance. PM2.5,i,t = estimate of average daily particulate matter exposure for individual i, for the three-years prior to WHISCA assessment at time t. lemi,t = estimate of latent episodic memory (em) for individual i at time t as measured by CVLT performance. Δemi,t = estimate of latent change in episodic memory (em) for individual i at time t as measured by CVLT performance. Form = estimate of latent effect of the CVLT form. αem = the path coefficient from estimate of systematic linear change to change in episodic memory latent variable. This coefficient was constrained to equal 1.0 in all models. βem = the effect of proportional change in episodic memory. γPM2.5 on Δem = the effect of PM2.5 exposure on change in episodic memory. σ2int em = the variance in individual specific estimates of CVLT performance at the WHISCA baseline. σ2slpem = the variance in individual specific estimates of linear change in CVLT performance. resem,i = the residual of CVLT performance for individual i. σ2em = the unexplained residual variance in CVLT performance. Bolded pathways represent the indirect effects estimated in each model. Only the baseline assessment (lemi,t=0) is regressed onto the intercept factor (INTem,i).
where Δemi,t denotes the estimated individual-specific annual change in episodic memory. Individual-specific estimate of systematic linear change is represented by the slpem,I parameter. The effect estimate, denoted by αem, linking the latent slope factor (slpem,i) to annual change in episodic memory (Δemi,t), was fixed to equal 1.0. Proportional change, denoted by βem, is a fixed-effect estimate that quantifies the extent to which change from one time to the next is dependent on episodic memory performance at the previous year (denoted by lemi,t). The term γPM2.5 on Δem denotes the effect of time-varying PM2.5 exposure (PM2.5,i,t) on change in episodic memory performance. Error variance in CVLT performance (resem,i) was constrained to be equal across the study period. To account for the form effect due to the use of an alternative CVLT form at the second and third follow-up assessment (Resnick et al. 2009), we added a latent factor indicating the form effect, with the corresponding path set to 0 for the standard CVLT or 1 for the alternative form. To estimate the PM2.5 exposure effect on each measure of episodic memory in univariate SEM, we adjusted for the following covariates: age at the WHISCA baseline, race/ethnicity, geographic region of residence, education, household income, lifestyle factors (smoking; alcohol use; physical activities), and clinical characteristics (any prior hormone use ever, hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease). Each measure of the CVLT (trials 1–3, short-delay free recall, long-delay free recall) was modeled separately. Analogous equations can be written for univariate SEM to estimate the PM2.5 exposure effect on change of depressive symptoms. All covariates were assessed at the WHIMS baseline with the exception of age which was the baseline WHISCA age.
2.7.3. Bivariate latent change score models.
Bivariate LCS models allow us to examine how the level of one variable was associated with subsequent changes in a second variable. We employed bivariate LCS models to address two questions: (1) whether there was an indirect association between PM2.5 exposure and changes in depressive symptoms that may be mediated by episodic memory decline; or (2) whether there was an indirect PM2.5 exposure effect on declines in episodic memory mediated through increases in depressive symptoms associated with exposure.
Figure 2 depicts a simplified depiction of the two bivariate hypotheses (Figures S1 and S2 in Supplemental Materials present the full model estimated). These bivariate SEMs followed similar modeling structures as used in our previous work studying the directionality of the association between symptoms of anxiety and depression with cognitive performance (Petkus et al. 2019). In the first bivariate model the equation to estimate individual-specific change in depressive symptoms was written as:
| (Equation 2). |
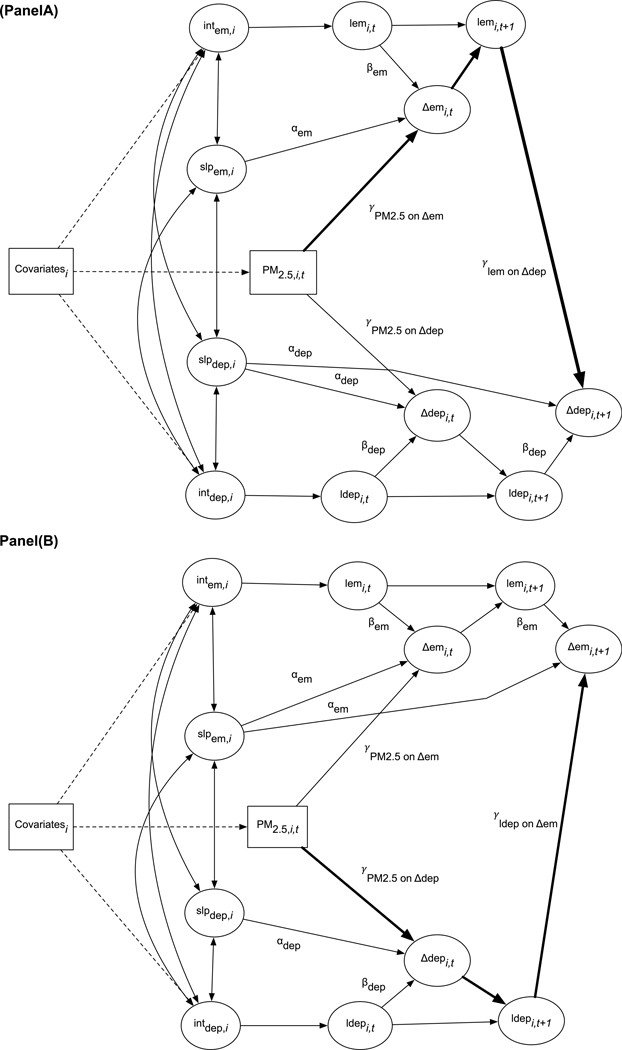
Fig. 2.
(A). Simplified depiction of the bivariate latent score structural equation model estimating the indirect effect of PM2.5 exposure on depressive symptoms. (B). Simplified depiction of the bivariate latent change score structural equation model estimating the indirect effect of PM2.5 exposure on change in episodic memory performance. Intem,i = estimate of episodic memory (em) at WHISCA baseline for individual i as measured by CVLT performance. slpem,i = estimate of systematic linear change in episodic memory (em) for individual i as measured by CVLT performance. PM2.5,i,t = estimate of average daily particulate matter exposure for individual i, for the three-years prior to WHISCA assessment at time t. lemi,t = estimate of latent episodic memory performance for individual i at time t as measured by CVLT performance. ldepi,t = estimate of latent depressive symptoms for individual i at time t as measured by the GDS-15. Δemi,t = estimate of latent change in episodic memory (em) for individual i at time t as measured by CVLT performance. Δdepi,t = estimate of latent change in depressive symptoms for individual i at time t as measured by the GDS-15. Bolded pathways represent the indirect effects estimated in each model. Note: The full diagram is presented in Supplemental Materials, Figs. S2 and S3. Raw scores, residual variances, change parameter labels were omitted to simplify the diagram. One sided arrows without labels in the diagram are fixed to equal 1.0. Covariates include the following variables: age at WHISCA baseline, region of residence, education, race/ethnicity, income, hormone use ever, high cholesterol, diabetes, smoking, alcohol use. Only the baseline assessments (lemi,t=0 or ldepi,t=0) are regressed onto the intercept factors (INTem,I or INTdep,i,t).
In bivariate models, the change in depressive symptoms (Δdepi,t) was a function of linear systematic change (slpdep,i), proportional change (βdep), the effect of time-varying PM2.5 exposure (γPM2.5 on Δdem), and the effect of episodic memory performance on subsequent changes in depressive symptoms (γlem on Δdep).
Following SEM path tracing conventions, the specific indirect effect of PM2.5 exposure on changes in depressive symptoms was estimated by multiplying the two estimated coupling parameters as depicted in the following equation:
| (Equation 3). |
The significance of the indirect effect was estimated by bootstrap calculation of asymmetric confidence intervals (MacKinnon et al. 2002). The significant indirect effect was supported if the confidence interval did not include zero. All bivariate LCS models were adjusted for the same set of covariates as described in the univariate LCS models. Analogous equations can be written to examine whether there was an indirect effect of PM2.5 exposure on changes in episodic memory that was mediated by changes in depressive symptoms.
We carried out additional analyses to evaluate the robustness of our findings. To explore whether any observed associations with PM2.5 could be explained by the underlying risk for clinically significant neurocognitive disorders, we repeated the analyses after excluding individuals who developed incident dementia or mild cognitive impairment by 2015. When examining the indirect effect of PM2.5 exposure on changes in CVLT Trials 1–3 performance, the SEMs would not converge after excluding individuals with either incident dementia or mild cognitive impairment by 2015. For this model we only examined individuals who had not developed dementia or mild cognitive impairment by the end of WHISCA in 2010. All LCS models were conducted using the SEM program MPLUS version 8 (Muthén and Muthén 1998–2018) which was run via the MPLUS Automation package (Hallquist and Wiley 2018) in R (Team 2018).
3. Results
On average, participants completed near six (mean±S.D.=5.68±2.02) assessments of episodic memory and depressive symptoms. Descriptive statistics for CVLT measures, GDS-15, and PM2.5 exposure are presented in supplemental Tables S1- S5. Bivariate correlations between respective CVLT measure, GDS-15, and PM2.5 exposure are presented in supplemental Figures S3-S5. Table 1 compares the distribution of the 3-year average PM2.5 exposure prior to the WHISCA assessment by population characteristics. Participants with higher levels of PM2.5 exposure estimates averaged over follow-up were more likely to be racial/ethnic minorities (African-American or Hispanic White), residing in the Midwest, a non-drinker or past-drinker, participating in some physical activity or 2–4 episodes/week, and reporting higher household incomes (≥$75,000).
Table 1.
Comparison of estimated PM2.5 exposure by baseline cohort characteristics (N = 2,202)
| Distribution of time-varying 3-year averagePM2.5a | |||||||
|---|---|---|---|---|---|---|---|
| Population Characteristics | N | Mean | SD | 25th | Median | 75th | pb |
| Overall | 2202 | 12.63 | 2.60 | 10.74 | 12.07 | 14.23 | |
| Region of Residence | <.001 | ||||||
| Northeast | 462 | 12.25 | 1.36 | 11.14 | 12.20 | 13.27 | |
| South | 319 | 12.23 | 1.77 | 10.56 | 11.87 | 14.04 | |
| Midwest | 856 | 12.91 | 2.44 | 10.68 | 12.38 | 15.39 | |
| West | 565 | 12.75 | 3.70 | 10.46 | 11.70 | 14.32 | |
| Race/Ethnicity | <.001 | ||||||
| African-American | 136 | 15.05 | 2.38 | 13.58 | 14.69 | 16.38 | |
| Hispanic White | 26 | 13.29 | 2.89 | 11.11 | 12.29 | 14.36 | |
| Non-Hispanic White | 1983 | 12.43 | 2.49 | 10.66 | 11.87 | 13.94 | |
| Other or Missing | 57 | 13.56 | 3.28 | 11.38 | 12.84 | 15.52 | |
| Education | .027 | ||||||
| Less than high school | 107 | 12.67 | 2.58 | 10.89 | 11.96 | 14.31 | |
| High school | 469 | 12.34 | 2.28 | 10.63 | 11.75 | 13.63 | |
| More than high school | 1626 | 12.71 | 2.68 | 10.76 | 12.12 | 14.32 | |
| Income (in USD) | .004 | ||||||
| < 9,999 | 483 | 12.38 | 2.56 | 10.61 | 11.73 | 14.21 | |
| 10,000–34,999 | 678 | 12.57 | 2.54 | 10.67 | 12.09 | 14.18 | |
| 35,000–49,999 | 469 | 12.59 | 2.56 | 10.68 | 11.96 | 14.01 | |
| 50,000–74,999 | 308 | 12.88 | 2.69 | 11.05 | 12.28 | 14.20 | |
| 75,000 or more | 194 | 13.21 | 2.79 | 10.96 | 12.61 | 15.11 | |
| Don’t know | 70 | 12.53 | 2.47 | 10.75 | 12.20 | 13.87 | |
| Lifestyle | |||||||
| Smoking status | .276 | ||||||
| Never smoked | 1214 | 12.66 | 2.62 | 10.73 | 12.04 | 14.31 | |
| Past smoker | 862 | 12.65 | 2.55 | 10.82 | 12.16 | 14.15 | |
| Current Smoker | 126 | 12.27 | 2.70 | 10.46 | 11.51 | 14.09 | |
| Alcohol use | .031 | ||||||
| Non-drinker | 271 | 13.07 | 2.75 | 10.66 | 12.50 | 15.07 | |
| Past drinker | 400 | 12.61 | 2.56 | 10.73 | 12.03 | 14.38 | |
| Less than 1 drink per day | 1263 | 12.55 | 2.56 | 10.73 | 12.00 | 14.01 | |
| More than 1 drink per day | 268 | 12.61 | 2.64 | 10.89 | 11.97 | 14.15 | |
| Moderate or strenuous activities ≥ 20 minutes | .294 | ||||||
| No activity | 1260 | 12.67 | 2.66 | 10.73 | 12.15 | 14.39 | |
| Some activity | 116 | 12.77 | 2.43 | 10.83 | 12.07 | 14.61 | |
| 2–4 episodes/week | 446 | 12.68 | 2.53 | 10.82 | 12.04 | 14.23 | |
| ≥4 episodes/week | 380 | 12.40 | 2.52 | 10.66 | 11.83 | 13.66 | |
| Physical Health | |||||||
| Hypertension | .321 | ||||||
| No | 1385 | 12.59 | 2.59 | 10.69 | 12.01 | 14.22 | |
| Yes | 817 | 12.70 | 2.60 | 10.78 | 12.16 | 14.26 | |
| Treated hypercholesterolemia | .267 | ||||||
| No | 1817 | 12.60 | 2.60 | 10.73 | 12.06 | 14.22 | |
| Yes | 385 | 12.76 | 2.57 | 10.82 | 12.16 | 14.27 | |
| Diabetes Mellitus | .593 | ||||||
| No | 2078 | 12.64 | 2.61 | 10.73 | 12.07 | 14.26 | |
| Yes | 124 | 12.51 | 2.44 | 10.87 | 12.12 | 13.60 | |
| Cardiovascular disease | .166 | ||||||
| No | 1847 | 12.60 | 2.59 | 10.73 | 12.03 | 14.19 | |
| Yes | 355 | 12.81 | 2.66 | 10.80 | 12.33 | 14.75 | |
| Prior hormone therapy | .500 | ||||||
| No | 1189 | 12.66 | 2.52 | 10.73 | 12.13 | 14.20 | |
| Yes | 1013 | 12.59 | 2.68 | 10.76 | 12.00 | 14.28 | |
PM2.5 represents the distribution of the individual-level summary of all time-varying 3-year exposures aggregated from the daily exposure levels estimated at each residential location using the spatiotemporal model.
p values estimated from ANOVA F-tests or t-tests
All univariate LCS models fit data acceptably, with Root Mean Square Residual Approximations (RMSEA) meeting suggested cutoffs (Byrne 2005) for a very close model fit (RMSEA’s ranged from 0.048 to 0.055). See supplemental Table S6 for all model fit indices and supplemental Table S7 for growth parameter estimates from the univariate models. Supplemental figure S6 presents the estimated mean score on each outcome with 20 randomly selected individual trajectories to demonstrate variability around the average trajectory. 3-year average PM2.5 exposure was negatively associated with change in all three CVLT measures, indicating the episodic memory declines were accelerated by increased exposures before each assessment (see Table 2 for parameter estimates). Although PM2.5 exposure was associated with increasing depressive symptoms, this association was not statistically significant.
Table 2.
Univariate structural equation models examining the associations between 3-year average PM2.5 exposure and change in verbal episodic memory and depressive symptoms (N = 2,202).
| Univariate Model 1 Estimatesa of PM2.5 effect on change | ||
|---|---|---|
| Outcome | γPM2.5 on Δem or γPM2.5 on Δdep | 95% Confidence Interval |
| CVLT measures | ||
| Trials 1–3 | −1.48 | (−2.10, −0.85) |
| Short-delay free recall | −1.43 | (−2.12, −0.73) |
| Long-delay free recall | −1.11 | (−1.79, −0.42) |
| Depressive symptoms | ||
| GDS-15 | 0.12 | (−0.51, 0.74) |
Abbreviations: CVLT = California Verbal Learning Test; GDS-15 = 15 item Geriatric Depression Scale
Estimates bolded if statistically significant at p<0.05
All estimates derived from the latent change score structural equation model (SEM) as depicted in figure 1 b, with PM2.5 scaled by interquartile range (4.04 μg/m3). In all models, the effect of time-varying PM2.5 exposure on initial CVLT performance, and on initial GDS-15 were adjusted for age at WHISCA baseline, race/ethnicity, geographic region of residence, education, household income, lifestyle factors (smoking, alcohol use, physical activities) and clinical characteristics (use of hormone treatment; hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease)
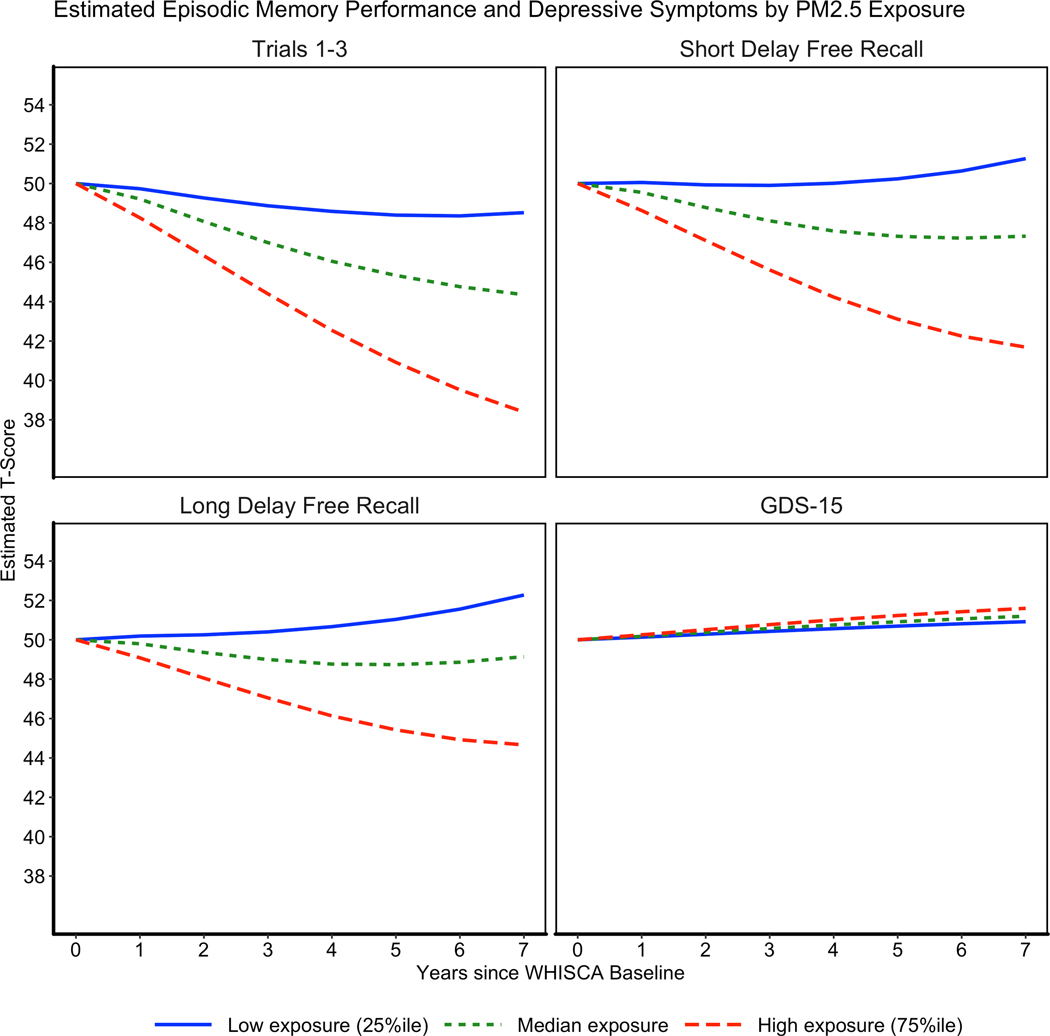
Figure 3 depicts estimated trajectories of episodic memory or depressive symptoms associated with either relatively low (25th percentile), average (median), or relatively high (75th percentile) ambient PM2.5 exposure for each WHISCA assessment, among women with the average levels of episodic memory performance or depressive symptoms at WHISCA baseline.
Fig. 3.
Graphs of the estimated performance on the California Verbal Learning Test measures and depressive symptoms by low (25th percentile), median, and high (75th percentile) average daily PM2.5 exposure.
The results of bivariate LCS models examining the indirect effect of PM2.5 on changes in depressive symptoms are presented in Table 3. All models exhibited good model fit (RMSEA < 0.05). Consistent with univariate models, increased PM2.5 was associated with greater declines across all three CVLT measures. Women with worse performance on all three CVLT measures tended to have increasing depressive symptoms over the subsequent year. Significant indirect effects of PM2.5 on increasing depressive symptoms were present across all three CVLT measures. These indirect effects suggest that PM2.5 exposure was associated with greater declines in episodic memory which were then associated with increasing depressive symptoms over time. The direct effect of PM2.5 exposure on changes in depressive symptoms was not significant.
Table 3.
Bivariate latent change score structural equation models examining the direct effect of PM2.5 exposure on changes in depressive symptoms and the indirect effects mediated by episodic memory declines (N = 2,202).
| CVLT Measures | |||
|---|---|---|---|
| Trials 1–3 | Short Delay Free Recall | Long Delay Free Recall | |
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Estimatesa of Direct Effect | |||
| Effect of PM2.5 on annual change in depressive (γPM2.5 on Δdep) | 0.07 (−0.53, 0.67) | 0.07 (−0.53, 0.67) | 0.01 (−0.58, 0.61) |
| Estimatesa of Indirect Effect | |||
| Effects of PM2.5 on annual changes in CVLT variable (γPM2.5 on Δem) | −1.44 (−2.08, −0.81) | −1.42 (−2.11, −0.72) | −0.99 (−1.68, −0.29) |
| Effects of CVLT performance on annual change in depressive symptoms (γLem on Δdep) | −0.05 (−0.10, −0.01) | −0.07 (−0.11, −0.02) | −0.08 (−0.13, −0.03) |
| Indirect effect of PM2.5 on annual change in depressive symptoms | 0.08 (0.00, 0.19) | 0.10 (0.03, 0.17) | 0.08 (0.01, 0.15) |
Abbreviations: CVLT = California Verbal Learning Test
Estimates bolded if statistically significant at p<0.05
All estimates derived from the bivariate structural equation models (SEM) as depicted in figure 2 panel B, with PM2.5 scaled by baseline interquartile range (4.04 μg/m3). In all models, the initial level of PM2.5, CVLT performance, and GDS-15 were adjusted for initial age, race/ethnicity, geographic region of residence, education, household income, lifestyle factors (smoking, alcohol use, physical activities), clinical characteristics (use of hormone treatment; hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease)
Results of bivariate LCS models examining the indirect effect of PM2.5 exposure on changes in episodic memory are presented in Table 4. All models exhibited acceptable model fit (RMSEA < 0.05). There were statistically significant direct effects of PM2.5 exposure on declines in episodic memory, but no evidence supporting indirect effects of PM2.5 exposure. PM2.5 exposure was not directly associated with change in depressive symptoms, and depressive symptoms were not associated with subsequent changes in episodic memory.
Table 4.
Bivariate latent change score structural equation models examining the direct effect of PM2.5 exposure on changes in episodic memory and the indirect effect mediated by depressive symptoms (N = 2,202).
| CVLT Measures | |||
|---|---|---|---|
| Trials 1–3 | Short Delay Free Recall | Long Delay Free Recall | |
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Estimatesa of Direct Effect | |||
| Effect of PM2.5 on annual change in episodic memory (γPM2.5 on Δem) | −1.26 (−1.90, −0.63) | −1.45 (−2.45, −0.75) | −1.00 (−1.70, −0.31) |
| Estimatesa of Indirect Effect | |||
| Effects of PM2.5 on annual changes in GDS-15 (γPM2.5 on Δdep) | 0.13 (−0.48, 0.64) | 0.09 (−0.52, 0.70) | 0.11 (−0.51, 0.72) |
| Effects of GDS-15 performance on annual change in episodic memory (γLdep on Δem) | −0.01 (−0.07, 0.05) | 0.04 (−0.01, 0.08) | 0.02 (−0.02, 0.06) |
| Indirect effect of PM2.5 on annual change in CVLT | −<0.01 (−0.01, 0.01) | <0.01 (−0.02, 0.03) | <0.01 (−0.01, 0.02) |
Abbreviations: CVLT = California Verbal Learning Test
Estimates bolded if statistically significant at p<0.05
All estimates derived from the bivariate structural equation models (SEM) as depicted in figure 2 panel B, with PM2.5 scaled by baseline interquartile range (4.04 μg/m3). In all models, the effect of time-varying PM2.5 exposure on initial CVLT performance, and on initial GDS-15 were adjusted for initial age, race/ethnicity, geographic region of residence, education, household income, lifestyle factors (smoking, alcohol use, physical activities), clinical characteristics (use of hormone treatment; hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease)
Results from additional analyses excluding women with incident dementia by 2015 are similar (Supplemental Materials, Tables S8-S10). Although removing the incident dementia cases attenuated the parameter estimates of the PM2.5 effects on changes in depressive symptoms, both the estimates of total effect (Table S8) and direct effect (Table S9) remained statistically non-significant. Additional analyses restricted to women without mild cognitive impairment or dementia by 2015 also revealed very similar results as the sensitivity analyses excluding women with incident dementia by 2015 (Supplemental Materials, Tables S11-S13).
4. Discussion
This is the first study to examine whether exposure to ambient air pollutants in late life affects the temporal dynamics and bidirectional relation of changes in depressive symptoms and episodic memory. In a geographically-diverse cohort of older women, long-term exposure to ambient PM2.5 estimated at the residential locations was associated with accelerated declines in episodic memory over the 8-year study period. We did not find any significant direct association between PM2.5 exposure and annual change in depressive symptoms. However, in bivariate models, we observed a significant indirect effect of PM2.5 exposure on increasing depressive symptoms via declines in episodic memory. Our data did not support depressive symptoms as a neuropsychological mediator of brain aging associated with PM2.5 exposure. These same associations were observed in older women who remained cognitively-intact during the follow-up, suggesting that neurocognitive disorders including dementia and its underlying neuropathological processes could not fully explain our findings. Taken together these results suggest that PM2.5 exposure may exert a neurotoxic effect on brain areas implicated in episodic memory followed by a neuropsychological manifestation of depressive symptoms.
Our study demonstrates supporting evidence for accelerated decline in episodic memory associated with long-term PM2.5 exposure. In univariate SEMs, higher PM2.5 exposure was associated with greater annual declines in verbal learning as well as in short- and long-delay free recalls. This observation expanded the earlier report of PM2.5-associated memory decline in a 5-year follow-up study which included only two repeated measures of verbal learning without assessing long-delay recall (Tonne et al. 2014). In our study, the putative adverse PM2.5 effect sustained in the bivariate SEMs (Table 3), and the observed declines in episodic memory were further associated with subsequent increases in depressive symptoms, resulting in statistically significant indirect effects of PM2.5 on increasing depressive symptoms (Table 3). It is noteworthy that the observed indirect association between PM2.5 exposure and change in depressive symptoms was only modestly diminished after excluding incident cases of dementia or mild cognitive impairment. These findings lead to two possible interpretations. First, long-term air pollution exposure may accelerate declines in episodic memory, while increased depressive symptoms may be indicative of emotional reaction to self-awareness of cognitive declines (Ganguli 2009) in the affected individuals or the psychological consequence, such as social and behavioral changes (e.g., changes in friendships and family relationships, ability to cope with stress, or engagement in positively reinforcing activities) associated with cognitive deficits. Second, the observed indirect effect on depressive symptoms suggests that PM2.5 neurotoxicity may perpetuate some underlying brain aging processes, causing damage to brain regions and neural networks essential to maintain episodic memory and emotional health in late life.
Based on the bivariate LCS models, we found no statistically significant indirect effect of PM2.5 on episodic memory decline mediated by changes in depressive symptoms (Table 4), while the direct association between exposure and episodic memory declines remained. These findings did not support the hypothesis that late-life depressive symptoms act as a neuropsychological mediator linking PM2.5 exposure with accelerated cognitive decline. To the best of our knowledge, only one study (Tallon et al. 2017) attempted to test this hypothesis and suggested the possible mediation role of depressive symptoms. However, limited by only two repeated measures, Tallon et al., were unable to examine the change in either the hypothesized mediator or the cognitive outcome, and the direct exposure effect defined in their SEM was cross-sectional in nature. In the present study, we found no statistically significant evidence that the annual change in GDS-15 in older women was directly affected by PM2.5 exposure, as shown in both univariate (Table 2) and bivariate SEMs (Table 4) adjusting for multiple potential confounders. Two previous studies, one conducted on community dwelling populations residing in Boston (aged ≥65 years) (Wang et al. 2014) and the other across the U.S. (aged 57–85 years) (Pun et al. 2017), reported null associations between long-term PM2.5 exposure and change in depressive symptoms across two assessments. Collectively, these epidemiological data suggest that the neurotoxic effects of late-life PM2.5 exposure on brain aging may not be primarily operated by aggravating the longitudinal change in depressive symptoms.
Our study results, as well as the growing literature on air pollution neurotoxicology, point to several important directions for future research in environmental neurosciences of brain aging associated with exposure to ambient air particles. Early decline of episodic memory is detectable in preclinical Alzheimer’s disease. Episodic memory also declines with normal aging, related to volumetric reductions of the hippocampus and other medial temporal lobe structures (Dickerson and Eichenbaum 2010). The indirect effects on increased depressive symptoms imply that part of the observed PM2.5 neurotoxicity on episodic memory decline may also confer neural dysfunction in the fronto-striatal and limbic systems that is well-documented in late-life depression (Alexopoulos 2002). Although animal studies (Fonken et al. 2011; Liu et al. 2018) suggested PM2.5 exposure may alter brain structures including hippocampal subfields, extant cross-sectional data with regional brain MRI measures (Chen et al. 2015; Power et al. 2018; Wilker et al. 2015) did not show associations between PM2.5 and hippocampal volumes. Longitudinal brain MRI studies are needed to examine whether air pollution neurotoxicity contributes to brain atrophy in hippocampus and other medial temporal lobe structures. In a whole-brain MRI analysis of using voxel-based morphometry of a subset of WHIMS participants, higher PM2.5 exposure was associated with smaller volumes of prefrontal cortex, but not with hippocampal volumes (Casanova et al. 2016). Interestingly, we also found older women with elevated depressive symptoms had smaller gray matter volumes in frontal lobe subregions, but not in hippocampus or other medial temporal lobe structures (Goveas et al. 2011). These observations indicate the need to further examine the role of prefrontal cortex and related networks in mediating the episodic memory decline associated with PM2.5 exposure. Also, an increasing number of studies suggest that white matter architecture may represent a novel target of airborne particle-induced neurotoxicity in laboratory animals (Allen et al. 2014; Woodward et al. 2017) and humans (Chenet al. 2015; Peterson et al. 2015). White matter abnormalities play an important role in late-life depression even in the absence of changes in gray matter (Sexton et al. 2012). In the above-mentioned whole-brain MRI analysis (Casanova et al. 2016), increased PM2.5 exposure was also associated with smaller volumes of subcortical white matter including areas involved in salience network, and aberrant processing of this network has been linked to cortical dysfunction and apathy commonly seen in late-life depression (Uddin 2015). Future research with diffusion tensor imaging can help elucidate whether PM2.5 exposures disrupt white matter tracts in the fronto-striatal-limbic circuitry. Future studies also need to examine whether neurotoxic effects of ambient air particles compromise the functional connectivity, including the possible changes in resting-state (Fjell et al. 2015; Fjell et al. 2016), in the neural networks that modulate positive emotions and reward responses in late life. The inter-relation between memory decline and depressive symptoms has been overlooked in air pollution neurotoxicology. Carefully-designed experiments with late-life inhalation exposure and repeated multimodal behavioral assessments are much needed to clarify the temporal dynamics as well as the inter-relation of memory loss and depressive-like behaviors. Such animal models can also shed important lights on underlying mechanisms, no matter through common pathways or sequential neuropathological events that are driving these different phenotypes of brain aging in response to air pollution.
We recognize several limitations of our study. First, although the PM2.5 spatiotemporal model was statistically cross-validated (average Pearson’s R2=0.70), (Cacciottolo et al. 2017; Reyes et al. 2017) the resulting exposure estimates were still subject to measurement errors. However, such estimation errors are likely non-differential and tend to attenuate the observed associations. Second, the present study focused on regional PM2.5 only, so we did not investigate its chemical constituencies (e.g., black carbon; inorganic secondary aerosols), other exposure sources (e.g., from near-roadways), or possible interactions with other pollutant mixtures. Third, we examined only the inter-relation of neuropsychological processes related to emotion health and brain aging. Although our data did not support the hypothesis that depressive symptoms are a neuropsychological mediator of brain aging associated with PM2.5 exposure, we could not rule out the possibility that increased PM2.5 exposure may interfere with the regulation of emotions (e.g., emotional arousal) (Dolcos et al. 2014) and decline in other cognitive domains (e.g., working memory) in vulnerable populations. Fourth, although our analyses showed that the PM2.5-associated episodic memory primarily resulted from the direct exposure effects (Tables 3 and 4), data on late-life depression were only collected on symptoms in this community-based sample. Therefore, we could not rule out the possibility that late-onset major depression, if affected by air pollution exposure and sustained over time, may still contribute to the progression of brain aging or ADRD with accelerated decline in episodic memory. Fifth, our modeling approach was based on the assumption that each neuropsychological process is homogeneous, which disregards the potential heterogeneities present in the longitudinal trajectories of brain aging phenotypes. Sixth, the bivariate SEM for LCS is not equipped to examine the possible exposure effects on concurrent neuropsychological processes of brain aging, including episodic memory decline and depressive symptoms that correlated with each other. However, the observed lack of direct exposure effect on change in depressive symptoms does not provide a strong support for this alternative hypothesis. Lastly, our findings may not be generalizable to men or younger women.
Our study has several strengths. First, women were prospectively followed over a long period of time (8 years), with annual assessments of both their EM and depressive symptoms, allowing us to closely examine the temporal dynamics. Second, the use of sophisticated SEMs for latent change scores allowed us to examine the complex associations between PM2.5 exposure and temporal changes in the two inter-related neuropsychological processes of EM and depressive symptoms. Third, the comprehensive data in the WHIMS cohort allowed us to account for a number of important covariates and reduce potential sources of biases.
5. Conclusions
Our study substantiates the epidemiologic evidence that long-term PM2.5 exposure in late life may accelerate declines in episodic memory. Exposure was indirectly associated with increases in depressive symptoms through declines in episodic memory. Our data did not support depressive symptoms as the neuropsychological mediator of accelerated brain aging associated with PM2.5 exposure, but suggested changes in depressive symptoms may result indirectly from episodic memory decline associated with exposure. These findings suggest that PM2.5 neurotoxicity may damage brain areas implicated in episodic memory, possibly involving networks critical to emotion regulation in late life.
Supplementary Material
6. Acknowledgments
The WHIMS was funded by Wyeth Pharmaceuticals, St Davids, PA, USA, and Wake Forest University. This study is supported R01AG033078 and R01ES025888. Petkus and Chen are supported in part by the RF1AG054068. The Women’s Health Initiative Study of Cognitive Aging was supported by the Department of Health and Human Services and the National Institute on Aging (N01-AG-1–2106), The research was also supported by the Alzheimer’s Disease Research Center at USC (P50A05142) and by the Southern California Environmental Health Sciences Center (5P30ES007048).
The WHI program is funded by the National Heart, Lung, and Blood Institute (NIH) through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C.
Abbreviations:
- PM2.5
particulate matter with aerodynamic diameter <2.5 μm
- ADRD
Alzheimer’s disease and related dementias
- WHISCA
Women’s Health Initiative Study of Cognitive Aging
- WHIMS
Women’s Health Initiative Memory Study
- WHI
Women’s Health Initiative
- GDS-15
15-item Geriatric Depression Scale
- CVLT
California Verbal Learning Test
- BME
Bayesian Maximum Entropy
- MCI
Mild Cognitive Impairment
- DSM-4
Diagnostic and Statistical Manual of Mental Disorders – 4th edition
- 3MS
Modified Mini-Mental State Exam
- TICSm
Telephone Interview for Cognitive Status-modified
- SEM
Structural Equation Modeling
- LCS
latent change score
- em
episodic memory
- dep
depressive symptoms as measured by the 15-item Geriatric Depression Scale
- lem
latent episodic memory score
- ldep
latent depressive symptoms
- resem
error variance of episodic memory
- resdep
error variance of depressive symptoms
- RMSEA
root mean square error of approximation
Footnotes
Declarations of interest: none
7. References
- Alexopoulos GS Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry 2002;10:687–695 [PubMed] [Google Scholar]
- Allen JL; Liu X; Weston D; Prince L; Oberdörster G; Finkelstein JN; Johnston CJ; Cory-Slechta DA Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci 2014;140:160–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed) edêds. Arlington, VA, US: American Psychiatric Publishing, Inc.; 1994 [Google Scholar]
- Barnes DE; Alexopoulos GS; Lopez OL; Williamson JD; Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006;63:273–279 [DOI] [PubMed] [Google Scholar]
- Barnes DE; Yaffe K; Byers AL; McCormick M; Schaefer C; Whitmer RA Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjot Y; Reis J; Giroud M; Feigin V. A review of epidemiological research on stroke and dementia and exposure to air pollution. Int J Stroke 2018;13:687–695 [DOI] [PubMed] [Google Scholar]
- Block ML; Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 2009;32:506–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL; Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol 2011;7:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BM Factor analytic models: viewing the structure of an assessment instrument from three perspectives. J Pers Assess 2005;85:17–32 [DOI] [PubMed] [Google Scholar]
- Cacciottolo M; Wang X; Driscoll I; Woodward N; Saffari A; Reyes J; Serre ML; Vizuete W; Sioutas C; Morgan TE; Gatz M; Chui HC; Shumaker SA; Resnick SM; Espeland MA; Finch CE; Chen JC Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 2017;7:e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM; Anderson HR; Atkinson RW; Beevers SD; Cook DG; Strachan DP; Dajnak D; Gulliver J; Kelly FJ Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018;8:e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova R; Wang X; Reyes J; Akita Y; Serre ML; Vizuete W; Chui HC; Driscoll I; Resnick SM; Espeland MA; Chen JC A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front Hum Neurosci 2016;10:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H; Kwong JC; Copes R; Hystad P; van Donkelaar A; Tu K; Brook JR; Goldberg MS; Martin RV; Murray BJ; Wilton AS; Kopp A; Burnett RT Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ Int 2017a;108:271–277 [DOI] [PubMed] [Google Scholar]
- Chen H; Kwong JC; Copes R; Tu K; Villeneuve PJ; van Donkelaar A; Hystad P; Martin RV; Murray BJ; Jessiman B; Wilton AS; Kopp A; Burnett RT Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 2017b;389:718–726 [DOI] [PubMed] [Google Scholar]
- Chen JC; Wang X; Wellenius GA; Serre ML; Driscoll I; Casanova R; McArdle JJ; Manson JE; Chui HC; Espeland MA Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann Neurol 2015;78:466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos G. Modern spatiotemporal geostatistics edêds: Oxford University Press; 2000 [Google Scholar]
- Christakos G; Bogaert P; Serre M. Temporal GIS: advanced functions for field-based applications edêds: Springer Science & Business Media; 2012 [Google Scholar]
- Clifford A; Lang L; Chen R; Anstey KJ; Seaton A. Exposure to air pollution and cognitive functioning across the life course--A systematic literature review. Environ Res 2016;147:383–398 [DOI] [PubMed] [Google Scholar]
- Delis DC; Kramer JH; Kaplan E; Ober BA California verbal learning test research edition manual. San Antonio: The Psychological Corporation; 1987; [Google Scholar]
- Dickerson BC; Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 2010;35:86–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG; Beevers CG; Haigh EA; Beck AT Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011;12:467–477 [DOI] [PubMed] [Google Scholar]
- Dolcos S; Katsumi Y; Dixon RA The role of arousal in the spontaneous regulation of emotions in healthy aging: a fMRI investigation. Front Psychol 2014;5:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM; Sneve MH; Grydeland H; Storsve AB; de Lange AG; Amlien IK; Røgeberg OJ; Walhovd KB Functional connectivity change across multiple cortical networks relates to episodic memory changes in aging. Neurobiol Aging 2015;36:3255–3268 [DOI] [PubMed] [Google Scholar]
- Fjell AM; Sneve MH; Storsve AB; Grydeland H; Yendiki A; Walhovd KB Brain Events Underlying Episodic Memory Changes in Aging: A Longitudinal Investigation of Structural and Functional Connectivity. Cereb Cortex 2016;26:1272–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK; Xu X; Weil ZM; Chen G; Sun Q; Rajagopalan S; Nelson RJ Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry 2011;16:987–995, 973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M; Koh MT Episodic memory on the path to Alzheimer’s disease. Curr Opin Neurobiol 2011;21:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M. Depression, cognitive impairment and dementia: Why should clinicians care about the web of causation? Indian J Psychiatry 2009;51 Suppl 1:S29–34 [PMC free article] [PubMed] [Google Scholar]
- Goveas JS; Espeland MA; Hogan P; Dotson V; Tarima S; Coker LH; Ockene J; Brunner R; Woods NF; Wassertheil-Smoller S; Kotchen JM; Resnick S. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women’s Health Initiative MRI Study. J Affect Disord 2011;132:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN; Wiley JF MplusAutomation: An R Package for Facilitating Large-Scale Latent Variable Analyses in Mplus. Structural Equation Modeling: A Multidisciplinary Journal 2018;25:621–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckbert SR; Kooperberg C; Safford MM; Psaty BM; Hsia J; McTiernan A; Gaziano JM; Frishman WH; Curb JD Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol 2004;160:1152–1158 [DOI] [PubMed] [Google Scholar]
- Jajodia A; Borders A. Memory predicts changes in depressive symptoms in older adults: a bidirectional longitudinal analysis. J Gerontol B Psychol Sci Soc Sci 2011;66:571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR; Lin YT; Hwang BF Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis 2015;44:573–584 [DOI] [PubMed] [Google Scholar]
- Kawas C; Segal J; Stewart WF; Corrada M; Thal LJ A validation study of the Dementia Questionnaire. Arch Neurol 1994;51:901–906 [DOI] [PubMed] [Google Scholar]
- Kim KN; Lim YH; Bae HJ; Kim M; Jung K; Hong YC Long-Term Fine Particulate Matter Exposure and Major Depressive Disorder in a Community-Based Urban Cohort. Environ Health Perspect 2016;124:1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku T; Li B; Gao R; Zhang Y; Yan W; Ji X; Li G; Sang N. NF-κB-regulated microRNA-574–5p underlies synaptic and cognitive impairment in response to atmospheric PM. Part Fibre Toxicol 2017;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X; Qian X; Xing J; Wang J; Sun Y; Wang Q; Li H. Particulate Matter Triggers Depressive-Like Response Associated With Modulation of Inflammatory Cytokine Homeostasis and Brain-Derived Neurotrophic Factor Signaling Pathway in Mice. Toxicol Sci 2018;164:278–288 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP; Lockwood CM; Hoffman JM; West SG; Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 2002;7:83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ A latent difference score approach to longitudinal dynamic structural analyses. Structural equation modeling: Present and future 2001:342–380 [Google Scholar]
- Meredith W; Tisak J. Latent curve analysis. Psychometrika 1990;55:107–122 [Google Scholar]
- Mitchell AJ; Bird V; Rizzo M; Meader N. Diagnostic validity and added value of the Geriatric Depression Scale for depression in primary care: a meta-analysis of GDS30 and GDS15. J Affect Disord 2010;125:10–17 [DOI] [PubMed] [Google Scholar]
- Morris JC; Heyman A; Mohs RC; Hughes JP; van Belle G; Fillenbaum G; Mellits ED; Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology 1989;39:1159-1159 [DOI] [PubMed] [Google Scholar]
- Muthén LK; Muthén BO Mplus User’s Guide. Eighth Edition Los Angeles, CA: Muthén & Muthén; 1998–2018 [Google Scholar]
- Oudin A; Segersson D; Adolfsson R; Forsberg B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS One 2018;13:e0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL; Crocco E; Acevedo A; John V; Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006;63:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC; Smith GE; Ivnik RJ; Kokmen E; Tangalos EG Memory function in very early Alzheimer’s disease. Neurology 1994;44:867–867 [DOI] [PubMed] [Google Scholar]
- Peterson BS; Rauh VA; Bansal R; Hao X; Toth Z; Nati G; Walsh K; Miller RL; Arias F; Semanek D; Perera F. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015;72:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ; Filoteo JV; Schiehser DM; Gomez ME; Petzinger G. Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: A bidirectional analysis. Neuropsychology 2019;33:35–46 [DOI] [PubMed] [Google Scholar]
- Power MC; Lamichhane AP; Liao D; Xu X; Jack CR; Gottesman RF; Mosley T; Stewart JD; Yanosky JD; Whitsel EA The Association of Long-Term Exposure to Particulate Matter Air Pollution with Brain MRI Findings: The ARIC Study. Environ Health Perspect 2018;126:027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC; Manjourides J; Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ Health Perspect 2017;125:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR; Legault C; Espeland MA; Resnick SM; Hogan PE; Coker LH; Dailey M; Shumaker SA; Group CS Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc 2012;60:1616–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM; Coker LH; Maki PM; Rapp SR; Espeland MA; Shumaker SA The Women’s Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials 2004;1:440–450 [DOI] [PubMed] [Google Scholar]
- Resnick SM; Espeland MA; An Y; Maki PM; Coker LH; Jackson R; Stefanick ML; Wallace R; Rapp SR; Investigators W s.H.I.S.o.C.A. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab 2009;94:4152–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JM; Xu Y; Vizuete W; Serre ML Regionalized PM2.5 Community Multiscale Air Quality model performance evaluation across a continuous spatiotemporal domain. Atmos Environ (1994) 2017;148:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski JS; Beiser A; Seshadri S; Auerbach S; Wolf PA; Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 2010;75:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP; Little TD Autoregressive and cross-lagged panel analysis for longitudinal data. 2012; [Google Scholar]
- Sexton CE; Allan CL; Le Masurier M; McDermott LM; Kalu UG; Herrmann LL; Mäurer M; Bradley KM; Mackay CE; Ebmeier KP Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry 2012;69:680–689 [DOI] [PubMed] [Google Scholar]
- Sheline YI; Gado MH; Kraemer HC Untreated depression and hippocampal volume loss. Am J Psychiatry 2003;160:1516–1518 [DOI] [PubMed] [Google Scholar]
- Shumaker SA; Reboussin BA; Espeland MA; Rapp SR; McBee WL; Dailey M; Bowen D; Terrell T; Jones BN The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 1998;19:604–621 [DOI] [PubMed] [Google Scholar]
- Steffens DC Late-Life Depression and the Prodromes of Dementia. JAMA Psychiatry 2017;74:673–674 [DOI] [PubMed] [Google Scholar]
- Tallon LA; Manjourides J; Pun VC; Salhi C; Suh H. Cognitive impacts of ambient air pollution in the National Social Health and Aging Project (NSHAP) cohort. Environ Int 2017;104:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RCR: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018 [Google Scholar]
- Teng EL; Chui HC The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry 1987; [PubMed] [Google Scholar]
- The Lancet Neurology. Air pollution and brain health: an emerging issue. Lancet Neurol 2018;17:103. [DOI] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- Tonne C; Elbaz A; Beevers S; Singh-Manoux A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 2014;25:674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015;16:55–61 [DOI] [PubMed] [Google Scholar]
- Vinkers DJ; Gussekloo J; Stek ML; Westendorp RG; van der Mast RC Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004;329:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y; Eliot MN; Koutrakis P; Gryparis A; Schwartz JD; Coull BA; Mittleman MA; Milberg WP; Lipsitz LA; Wellenius GA Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect 2014;122:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J; Puett RC; Schwartz J; Yanosky JD; Laden F; Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 2012;172:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel EA; Rose KM; Wood JL; Henley AC; Liao D; Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol 2004;160:1023–1029 [DOI] [PubMed] [Google Scholar]
- Wilker EH; Preis SR; Beiser AS; Wolf PA; Au R; Kloog I; Li W; Schwartz J; Koutrakis P; DeCarli C; Seshadri S; Mittleman MA Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 2015;46:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS; Barnes LL; Mendes de Leon CF; Aggarwal NT; Schneider JS; Bach J; Pilat J; Beckett LA; Arnold SE; Evans DA; Bennett DA Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002;59:364–370 [DOI] [PubMed] [Google Scholar]
- Wilson RS; Capuano AW; Boyle PA; Hoganson GM; Hizel LP; Shah RC; Nag S; Schneider JA; Arnold SE; Bennett DA Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 2014;83:702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC; Pakbin P; Saffari A; Shirmohammadi F; Haghani A; Sioutas C; Cacciottolo M; Morgan TE; Finch CE Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging 2017;53:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K; Blackwell T; Gore R; Sands L; Reus V; Browner WS Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 1999;56:425–430 [DOI] [PubMed] [Google Scholar]
- Yesavage JA; Sheikh JI 9/Geriatric Depression Scale (GDS). Clinical Gerontologist 1986;5:165–173 [Google Scholar]
- Zahodne LB; Stern Y; Manly JJ Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. J Am Geriatr Soc 2014;62:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.