Abstract
OBJECTIVE
Despite a substantial increase in the use of MRI for pretreatment evaluation of prostate cancer, its prognostic value in patients undergoing radiation therapy (RT) is not well known. Therefore, the purpose of this study was to systematically review the literature and perform a meta-analysis on the prognostic value of pretreatment MRI in patients with prostate cancer who underwent external beam radiation therapy (EBRT) or brachytherapy.
MATERIALS AND METHODS
PubMed and Embase databases were searched for studies published on or before March 13, 2019. We included studies that evaluated pretreatment MRI as a prognostic factor in prostate cancer regarding biochemical recurrence (BCR), metastatic failure, and overall or cancer-specific mortality. Effect sizes were measured in terms of the hazard ratio (HR) and were meta-analytically pooled using the random-effects model. The quality of the studies was independently evaluated using the Quality in Prognostic Studies tool.
RESULTS
Twelve studies (2205 patients) were included. All studies assessed BCR; metastasis was evaluated in three studies, and mortality was evaluated in one study. Extraprostatic extension (EPE), seminal vesicle invasion (SVI), large tumor size or volume, number of sextants involved, and tumor involvement of prostatic apex were significant prognostic factors of BCR (pooled HRs = 1.50–4.47). EPE, larger tumor size, greater tumor volume, presence of metastatic pelvic lymph nodes (LNs), and presence of SVI were significant risk factors for metastasis (pooled HRs = 1.12–11.96). Pelvic LN metastasis was significantly predictive of cancer-specific mortality (HR = 4.45 [95% CI, 1.30–15.23]).
CONCLUSION
Several pretreatment MRI findings were significant prognostic factors in patients with prostate cancer who underwent RT.
Keywords: meta-analysis, MRI, prognosis, radiotherapy, systematic review
Prostate cancer is highly prevalent in men across the world, particularly in the Western hemisphere. Outcomes are widely variable and are dependent on multiple factors, including patient and tumor characteristics. Management strategies are increasingly tailored to individual risk, the likelihood of adverse oncologic outcomes, and impact on quality of life. Commonly used risk stratification systems are based on clinical variables such as prostate-specific antigen (PSA) level, biopsy Gleason scores, and clinical stage from digital rectal examination, but there is significant variability in these assessments [1–3]. For instance, interobserver agreement for clinical staging is strikingly low, and Gleason scores are upgraded from biopsy to radical prostatectomy specimens in approximately one-third of patients [4, 5]. Therefore, there is a clinically unmet need to improve risk stratification of prostate cancer.
During recent years, there has been a substantial increase in the use of MRI in the pretreatment evaluation of prostate cancer. Advances in MRI technology and the resultant detailed anatomic and functional information obtained from multiparametric MRI protocols have led to more sensitive detection of clinically significant prostate cancer, more accurate staging, and potential for improved prognostic assessment [6–8]. However, most of the studies in the literature have been based on surgicopathologic specimens as the reference standard. This reference standard induces significant selection bias for patients who undergo radical prostatectomy and lacks generalizability to the population receiving radiation therapy (RT) [9, 10]. In fact, although risk stratification is important for all patients, it is even more crucial for those for whom there will be no whole-organ specimen for detailed pathologic analyses (i.e., pathologic extraprostatic extension [EPE], lymph node [LN] metastasis), such as patients triaged for active surveillance or those who undergo RT. MRI, because of its ability to depict and localize the dominant tumor, is being increasingly used in the pretreatment evaluation and planning of treatment in patients with prostate cancer in recent years; however, few investigators have evaluated the prognostic value of pretreatment MRI before RT, and there is no clear consensus regarding the utility of MRI for patients undergoing RT for prostate cancer.
Therefore, the purpose of this study was to systematically review the literature and perform a meta-analysis on the prognostic value of pretreatment MRI in patients with prostate cancer who underwent external beam radiation therapy (EBRT) or brachytherapy.
Materials and Methods
This systematic review and meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Literature Search
PubMed and Embase databases were systematically searched for studies published on or before March 13, 2019. The following search query was formulated using keywords and related descriptors: ((prostate OR prostatic) AND (radiotherapy OR “radiation therapy” OR “radiation treatment” OR RT OR brachytherapy OR “seed implant” OR “seed implantation”)) AND (“MR” OR MRI OR MR) AND (biochemical OR outcome OR failure OR recur* OR survival OR mortality OR death OR metastas*) AND (“hazard ratio” OR HR OR Cox OR Kaplan OR KM). The reference lists of the identified studies were also checked to find additional articles relevant to our research question.
Study Selection
Studies were included if they satisfied the following patient, index test, comparator, outcome, and study design criteria [11]: patients, patients with newly diagnosed prostate cancer; index test, pretreatment MRI; comparator, no comparator relevant to this meta-analysis; outcome, biochemical recurrence (BCR), metastatic failure, and overall or cancer-specific mortality; and study design, original research articles. Exclusion criteria were a study population of fewer than 10 patients, other publication types (e.g., conference abstracts and review articles), studies dealing with a different topic (e.g., salvage RT for postradical prostatectomy recurrence or MRI for detection of recurrent prostate cancer after primary RT), studies with insufficient survival data for meta-analysis pooling, and overlap in patient population with patient population of another study. When population overlap was present, the study providing more comprehensive information (e.g., evaluated more MRI findings) was included. Two reviewers (reviewers 1 and 2) performed the study selection process. When disagreement was present, a consensus was reached after discussion with a third reviewer (reviewer 3).
Data Extraction and Quality Assessment
Data were extracted from the included studies. The following patient and tumor characteristics were extracted: number of tumors, patient age, PSA levels, clinical stage of prostate cancer, biopsy Gleason scores of prostate cancer, and risk stratification. RT characteristics were extracted: the type of RT (EBRT or brachytherapy), radiation dose, and whether androgen deprivation treatment (ADT) was given. The following characteristics of the study were extracted: origin of study (authors, institution, and patient enrollment period), year of article publication, study design (prospective vs retrospective, multi- vs single-center), definition used for outcomes (i.e., Phoenix criteria or American Society for Radiation Oncology [ASTRO] definition for biochemical recurrence). MRI characteristics were also extracted: vendor and model of scanner, magnetic field strength, use of endorectal coils, MRI sequences used, and number of readers and their level of experience.
The quality of the studies was independently evaluated by two reviewers (reviewers 1 and 4) with the Quality in Prognostic Studies tool [12]. A third reviewer (reviewer 2) was consulted in case of discrepancy to reach a consensus.
Data Synthesis and Analysis
The outcomes were BCR, metastatic failure, and overall or cancer-specific mortality. The effect sizes of the prognostic value of each MRI finding were measured in terms of hazard ratios (HRs), where an HR of greater than 1 indicated a higher risk of having one of the outcomes when the MRI findings were present [13]. We obtained the HRs and corresponding standard errors for each predictor directly from the published data when it was feasible to do so. If that was not possible, we extracted survival data from Kaplan-Meier curves using Engauge Digitizer (version 3.0, Mark Mitchell, Baurzhan Muftakhidinov, Tobias Winchen, et al.) and indirectly calculated the HRs and standard errors using the method described by Tierney et al. [14]. We planned to use funnel plots and the Egger test to evaluate publication bias if more than 10 studies were included [15].
HRs were pooled using the random-effects model (DerSimonian and Laird) with the Meta package in R software (version 3.5.1, R Foundation for Statistical Computing) [16]. Higgins I2 was used to assess heterogeneity between studies [17]; p values of < 0.05 were considered to indicate statistical significance.
Results
Literature Search
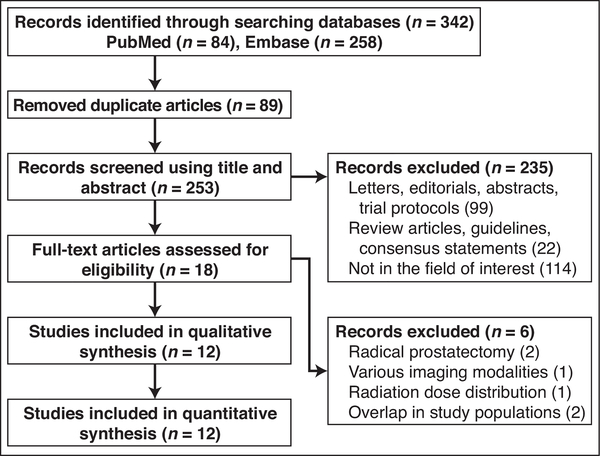
Three hundred forty-two studies were initially retrieved from the systematic search of PubMed and Embase. After 89 duplicate studies were excluded, 253 were screened using titles and abstracts. The full texts of the 18 potentially eligible studies were evaluated. Six studies were excluded for the following reasons: all or most patients underwent radical prostatectomy (n = 2) [18, 19], significant overlap in patient population (n = 2) [20, 21], various imaging modalities were used for pretreatment assessment [22], and the study assessed dose distribution of RT (n = 1) [23]. Ultimately, 12 studies assessing the prognostic value of pretreatment MRI in 2205 patients with prostate cancer before RT were included in this meta-analysis [24–35]. Figure 1 shows the flowchart for the study selection process based on PRISMA guidelines.
Fig. 1—
Flowchart shows study selection process based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Characteristics of Included Studies
The characteristics of the included studies are shown in Tables I and 2. In brief, the number of patients with prostate cancer in the studies ranged from 37 to 390. The population was based on only high-risk prostate cancer in two studies [28, 29], either intermediate or high risk in four [26, 27, 32, 34], a mixed population of low to high risk in four [30, 31, 33, 35], and unknown risk in two [24, 25]. Patients received EBRT in seven studies [25, 26, 28, 30, 31, 33, 35]; EBRT and brachytherapy in three [27, 29, 34]; either brachytherapy or both EBRT and brachytherapy in one [24]; and EBRT, brachytherapy, or both in one [32]. All or some of the men received ADT in all studies except one [33]. Only one study was prospective [27], and two were performed at multiple institutions [28, 33]. All 12 studies reported BCR, eight using the Phoenix criteria [25–29, 32, 34, 35] and four using ASTRO definition [24, 30, 31, 33]. Three studies assessed metastatic failure [27, 28, 31], and one study evaluated overall and cancer-specific mortality [28]. Three- or 1.5-T MRI scanners were used in all studies except one in which this information was not provided [24]. Endorectal coils were used in six studies [24, 25, 29, 31, 33, 34].
TABLE 1:
Patient, Tumor, and Study Characteristics
| First Author [Reference No.] | Year Study Was Published | Institution Where Study Was Performed | Enrollment Period | Prospective Design | BCR |
No. of Patients | Median Age (y) | Median PSA (ng/mL) | Clinical T Category | Median Gleason Score | RiskGroup | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definitiona | Median Follow-Up (mo) | |||||||||||
| Clarke [24] | 2002 | Inova Alexandria Cancer Center | 1994–1998 | No | ASTRO | 39 (3–72) | 390 | NR(NR) | NR (< 4 to > 30) | T1c-T3c | 6 (≥ 4–10) | NR |
| Fuchsjäger [25] | 2010 | MSKCC | 2000–2002 | No | Phoenix | 67 | 224 | 69 (45–82) | NR (< 5 to > 20) | T1c–T3b | 7(5–10) | NR |
| Gnep [26] | 2017 | Université de Rennes | 2009–2012 | No | Phoenix | 47(19–65) | 74 | 68(50–82) | 9.8(3.4–47.0) | T1c–T3 | 7 (6–9) | Intermediate or high |
| Gomez-Iturriaga [27] | 2018 | Hospital Universitario Cruces | 2010–2015 | Yes | Phoenix | 46(16–70) | 185 | 71 (56–82) | NR (< 10 to > 20) | T1–T3b | 7(6–10) | Intermediate or high |
| Goupy [28] | 2019 | 6 French institutionsb | 2008–2017 | No | Phoenix | 36c (33–39) | 276 | 69c (42–86) | 27b(2–217) | T1–T3 | 7(6–10) | High |
| Hegde [29] | 2017 | UCLA | 2010–2015 | No | Phoenix | 30.6(7.2–62.8) | 37 | 68(51–83) | 9.3(1.6–99.7) | T1c–T3b | 9(7–9) | High |
| Jackson [30] | 2005 | Royal Marsden Hospital | 1988–1999 | No | ASTRO | 46 | 199 | 69(52–79) | 14.5(1–373) | T1–T4 | 6(2–10) | Lowto high |
| Joseph [31] | 2009 | UCSF | 1998–2003 | No | ASTRO | 44(3–96) | 67 | 67c (49–78) | 9.3(1.7–36.8) | T1–T3 | 7(2–9) | Lowto high |
| Kauffmann [32] | 2018 | University of Chicago | 2006–2013 | No | Phoenix | 50 | 123 | 67 (47–88) | 9.2(1.9–109.1) | T1c–T3 | 7 (6–9) | Intermediate or high |
| Nguyen [33] | 2004 | 3 U.S. institutionsd | 1992–2001 | No | ASTRO | 28(4–103) | 250 | 72 (NR) | NR (< 4 to > 20) | T1c–T2c | ≤6 (2–10) | Lowto high |
| Riaz [34] | 2012 | MSKCC | 2000–2008 | No | Phoenix | 49(12–123) | 279 | NR | NR (≤ 10 to > 20) | T1 to ≥T3a | 7(6–10) | Intermediate or high |
| Yamaguchi [35] | 2016 | Osaka University Graduate School of Medicine | 2008–2014 | No | Phoenix | 29(11–83) | 101 | 71 (57–85) | 9.24(1.2–171.3) | T1c–T4 | 7(6–10) | Lowto high |
Note—Values in parenthesesare ranges. BCR = biochemical recurrence, PSA = prostate-specific antigen, ASTRO = American Society for Radiation Oncology, NR = not reported, MSKCC = Memorial Sloan Kettering Cancer Center, UCLA = University of California, Los Angeles, UCSF = University of California, San Francisco.
ASTRO definition = three consecutive increases in PSA above nadir, Phoenix criteria definition = posttreatment PSA nadir plus an increase of 2 ng/mL.
Centres de Lutte Contre le Cancer (CLCC) Eugène Marquis, CLCC René-Gauducheau, CLCC Oscar Lambret, Clinigue Pasteur, University Hospital La Cavale Bianche, and Clinique Saint-Yves.
Mean value.
St. Anne’s Hospital, Brigham and Women’s Hospital, and Hospital of the University of Pennsylvania.
TABLE 2:
Radiation Therapy (RT) and MRI Characteristics
| First Author [Reference No.] | RT |
MRI Acquisition |
MRI Interpretaron |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose (Gy) | ADT, % (No./Total No.) | Vendor | Model | Magnet Strength (T) | Endorectal Coil | Sequences | No. of Readers | Consensus Reading | Reader Experience (y) | |
| Clarke [24] | E and B, B | E and B, 45–50.4; B, 90–115 | 37(144/390) | NR | NR | NR | Yes | T2-weighted imaging | 3 | No | NR |
| Fuchsjäger [25] | E | 81–86.4 | 54(121/224) | GE | Signa Horizon | 1.5 | Yes | T2-weighted imaging | 2 | Yes | 5,1.5 |
| Gnep [26] | E | 74–80 | 57(42/74) | Philips | Achieva | 3 | No | T2-weighted imaging, DWI, DCE-MRI | 2 | Yes | 5,30 |
| Gomez-Iturriaga [27] | E and B | E, 37.5; B, 15 | All | Philips | Achieva | 1.5 | No | T2-weighted imaging, DWI, DCE-MRI | 2 | Yes | Specialist |
| Goupy [28] | E | 70–80 | All | NR | NR | 1.5 | No | T2-weighted imaging, DWI, DCE-MRI | 2 | No | Expert |
| Hegde [29] | E and B | E, 39.6–50.4; B, 15–24 | 81(30/37) | NR | NR | 3 | Yes for some patients | T2-weighted imaging, DWI, DCE-MRI | NR | NR | NR |
| Jackson [30] | E | 60–74 | All | Siemens | GBS1, Vision | 1.5 | No | T2-weighted imaging | 1 | NA | Consultant |
| Joseph [31] | E | 68–76 | 58(39/67) | Siemens | Signa | 1.5 | Yes | T2-weighted imaging, MRS | 1 | NA | >10 |
| Kauffmann [32] | E or B or both E and B | E, 75.6–79.2; B, 144–145 | 52(64/123) | Philips | Achieva | 1.5,3 | No | T2-weighted imaging | 2 | NR | Body imaging |
| Nguyen [33] | E | 70–72 | None | GE | NR | 1.5 | Yes | T2-weighted imaging | 3 | No | >4 |
| Riaz [34] | E and B | E, 50.4; B, LDR of 90–110 or HDRof 16.5–22.5 | 36(100/279) | GE | Signa Horizon | 1.5,3 | Yes | T2-weighted imaging | 2 | Yes | GU radiologist |
| Yamaguchi [35] | E | 70–78 | 76(77/101) | NR | NR | 1.5,3 | NR | T2-weighted imaging, DWI | 2 | Yes | 3,20 |
Note—ADT = androgen deprivation treatment, E = external beam RT, B = brachytherapy, NR = not reported, DCE-MRI = dynamic contrast-enhanced MRI, NA = not applicale, MRS = MR spectroscopy, LDR = low-dose rate, HDR = high-dose rate, GU = genitourinary.
Quality Assessment Using Quality in Prognostic Studies Tool
For the study participation domain, six studies showed a moderate risk because they used pretreatment MRI in RT planning, which may have introduced selection bias [24, 27–29, 32, 35]. Low risk of bias was assigned in all studies for the study attrition domain. In the prognostic factor measurement domain, one study was considered to be at high risk for bias because it analyzed outcome not by the performance of MRI findings but by the performance of MRI staging [24]. Moderate risk was assigned to three studies with unclear explanations of whether MRI analyses were performed in a blinded manner [26–28]. In the outcome measurement domain, one study showed a moderate risk of bias because it did not specify how the presence of metastasis was established [28]. In terms of study confounding, two studies that did not perform multivariate analysis showed a high risk of bias [24, 29]. For statistical analysis and reporting domain, all studies showed a low risk of bias.
Biochemical Recurrence
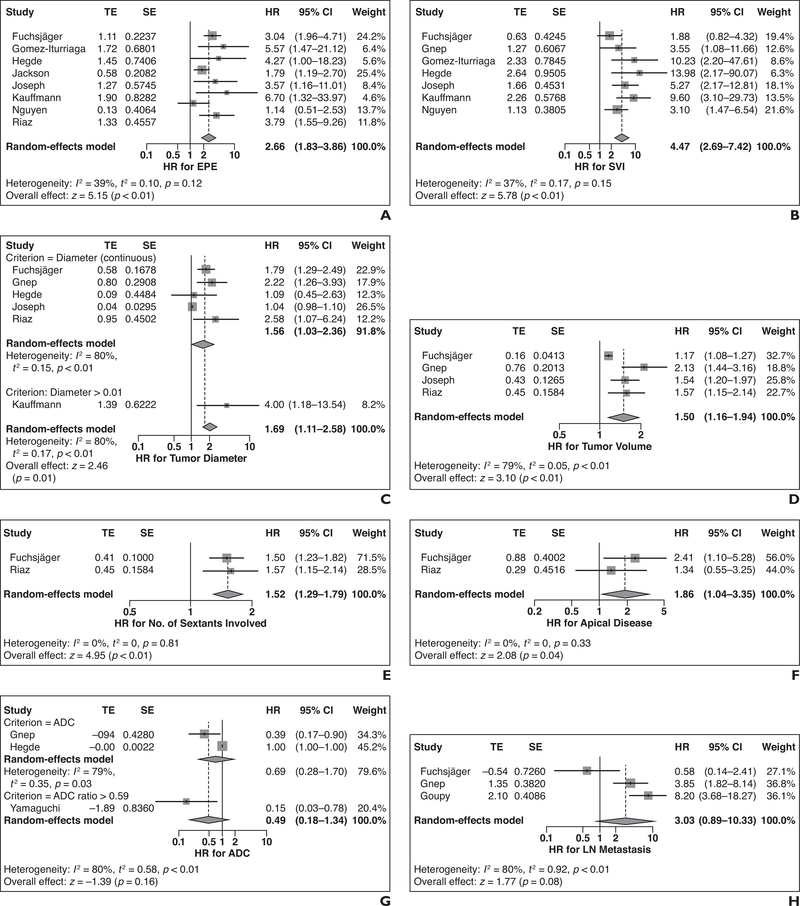
Forest plots for the predictive value of each MRI finding for BCR are shown in Figure 2. The use of pretreatment MRI was associated with a lower risk of BCR (n = 1 study; HR = 0.17 [95% CI, 0.06–0.47]; p = 0.0008) [24]. Both EPE (n = 8 studies; HR = 2.66 [95% CI, 1.83–3.86]) and SVI (n = 7 studies; HR = 4.47 [95% CI, 2.69–7.42]) on MRI were significant prognostic factors of BCR. Larger tumor size was a significant risk factor for BCR (n = 6 studies; HR = 1.69 [95% CI, 1.11–2.58]), both as a continuous variable (n = 5 studies; HR = 1.56 [95% CI, 1.03–2.36]) and when using a size cutoff of larger than 15 mm (n = 1 study; HR = 4.00 [95% CI, 1.18–13.54]). Both tumor volume on MRI (n = 4 studies; HR = 1.50 [95% CI, 1.16–1.94]) and number of sextants involved on MRI (n = 2 studies; HR = 1.52 [95% CI, 1.29–1.79]) were significant risk factors of BCR. Lesions involving the prostatic apex on MRI were associated with a higher risk of BCR (n = 2 studies; HR = 1.86 [95% CI, 1.04–3.35); however, lesions involving the midgland (n = 1 study; HR = 1.96 [95% CI, 0.69–5.52]) or lesions involving the base (n = 1 study; HR = 1.60 [95% CI, 0.84–3.04) were not associated with a higher risk of BCR. Apparent diffusion coefficient (ADC) values were not significant factors associated with BCR (n = 3; HR = 0.49 [95% CI, 0.18–1.34]), either measuring ADC as a continuous variable (n = 2 studies) or using an ADC ratio of greater than 0.59 (n = 1 study). In the latter study [35], ADC ratio was defined as the ADC of the tumor divided by that of biopsy-proven benign tissue. The presence of pelvic LN metastasis also showed a borderline trend for a higher risk of BCR (n = 3 studies; HR = 3.03 [95% CI, 0.89–10.33]). Heterogeneity of HRs among the included studies was considered low for the number of sextants involved, apical sextant involved, EPE, and SVI (I2 = 0–39%), whereas it was considered substantial for the other MRI findings (I2 = 79–80%). Publication bias was not assessed because there were fewer than 10 studies for all MRI findings.
Fig. 2—
Forest plots for predictive value of pretreatment MRI findings for biochemical recurrence (BCR) in patients with prostate cancer undergoing radiation therapy. Solid vertical lines show hazard ratio (HR) of 1, dotted vertical lines show pooled means, and diamonds show pooled indexes. TE = treatment estimate; SE = standard error. A–H, Forest plots show results for pretreatment MRI findings: extraprostatic extension (EPE) (A), seminal vesical invasion (SVI) (B), tumor diameter (C), tumor volume (D), number of involved sextants (E), apical disease (F), apparent diffusion coefficient (ADC) (G), and lymph node (LN) metastasis (H). HR of greater than 1 indicates higher risk of BCR when MRI findings were present. In G, ADC ratio was defined as ADC of tumor divided by that of biopsy-proven benign tissue.
Metastatic Failure, Overall Survival, and Cancer-Specific Mortality
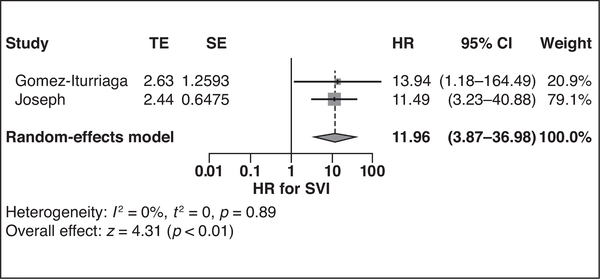
Three studies evaluated metastasis [27, 28, 31], and one study reported overall and cancer-specific survival [28]. SVI was a significant risk factor for developing metastasis in two studies (HR = 11.96 [95% CI, 3.87–36.98]) with no substantial heterogeneity (I2 = 0%), as shown in Figure 3. EPE, larger tumor size, greater tumor volume, presence of metastatic pelvic LNs, and three or more metastatic pelvic LNs were observed to be risk factors for metastasis in one study each (HR = 11.51 [95% CI, 1.36–97.29], 1.12 [95% CI, 1.02–1.20], 1.53 [95% CI, 1.08–2.16], 4.70 [95% CI, 1.95–11.36], and 8.20 [95% CI, 3.68–18.26], respectively). Pelvic LN metastasis was significantly predictive of cancer-specific mortality (HR = 4.45 [95% CI, 1.30–15.23]) but not overall mortality (p = 0.39). However, three or more metastatic pelvic LNs were significantly predictive of both cancer-specific mortality (HR = 7.60 [95% CI, 2.22–26.03]) and overall mortality (HR = 2.78 [95% CI, 1.06–7.29]).
Fig. 3—
Forest plot shows predictive value of pretreatment MRI finding of seminal vesical invasion (SVI) for metastatic failure. Solid vertical line shows hazard ratio (HR) of 1, dotted vertical line shows pooled means, and diamond shows pooled indexes. TE = treatment estimate, SE = standard error.
Discussion
We performed a meta-analysis on the prognostic value of prostate MRI before primary RT in patients with newly diagnosed prostate cancer and found that several MRI findings (EPE, SVI, tumor diameter, tumor volume, number of involved sextants, presence of apical disease) were significant risk factors for BCR and that SVI was associated with a higher risk of metastatic failure. RT is an important treatment modality for patients with prostate cancer, yielding oncologic outcomes comparable to surgery, especially in those with localized prostate cancer [36]. However, conventional risk stratification based on clinical variables is imperfect, and locally recurrent or metastatic disease after primary RT is not rare. Most recurrent tumors arise at the site of the primary tumor, and therefore accurate identification of the primary tumor before RT planning is crucial [37]. Several recent trials explored simultaneous integrated boost (SIB) to the dominant lesion visualized on MRI and have reported promising results of feasibility and improved genitourinary or rectal toxicity profiles [38, 39]. Treatment planning and tailoring (i.e., SIB) with consideration of the dominant lesion require identification and evaluation of the characteristics and extent of the dominant lesion, which can be provided by pretreatment multiparametric MRI [40]. In addition, incorporation of MRI findings into clinical nomograms has been shown to significantly improve prediction of biochemical failure after EBRT [21]. On the basis of the results of our study, MRI appears to provide incremental value in the pretreatment evaluation of prostate cancer before RT.
EPE and SVI were the two MRI findings that were most frequently evaluated as potential prognostic factors of BCR in these studies and also showed consistent results across studies. EPE and SVI yielded pooled HRs of 2.66 (95% CI, 1.83–3.86) and 4.47 (95% CI, 2.69–7.42) in eight and seven studies, respectively, with no substantial heterogeneity (I2 = 39% and 37%, respectively). These results can be intuitively understood because pathologically confirmed EPE and SVI are well-recognized risk factors of poorer oncologic outcomes, and both of these features are included in the TNM staging system [41–43]. Despite overlap in the 95% CIs, it was noteworthy that SVI showed a higher degree of risk of BCR than EPE (HR = 4.47 vs 2.66, respectively). These results are in line with the higher assigned stage of T3b for SVI compared with T3a for EPE and with the fact that SVI indicates a higher risk for occult LN metastasis than EPE [44]. Furthermore, the seminal vesicles are not always included in the RT field because of balancing between optimal tumor coverage, possibility of SVI, and rectal toxicity [45]. MRI has incremental value to conventional risk stratification systems in terms of assessment of EPE and SVI and therefore could potentially aid in RT treatment planning and estimation of prognosis.
MRI findings reflective of tumor burden—including tumor diameter, tumor volume, and number of sextants involved—were significantly predictive of BCR (HR = 1.50–1.69). It is well known that greater tumor volume on radical prostatectomy specimens leads to elevated risk for BCR, LN involvement, distant metastasis, and cancer-specific or overall mortality [46, 47]. Therefore, it is intuitive that greater tumor burden would lead to worse oncologic outcomes in patients undergoing RT. Although tumor volume measurement on MRI has shown promising results as a surrogate of pathologic tumor volume, with relatively high correlation and low interobserver variability in some studies, other studies have reported poor correlation, especially when using T2-weighted imaging alone [48–50]. Although further refinement of measuring tumor volume on MRI such as adding functional sequences (e.g., DWI) to T2-weighted imaging is needed, MRI is the best modality at the moment (compared with CT) to visualize the tumor and its burden; therefore, MRI findings reflective of tumor burden. should be obtained and used during RT planning [50].
With regard to the location of suspicious prostate cancer lesions on MRI, apical involvement was a risk factor for BCR, with an HR of 1.86 (95% CI, 1.04–3.35), but involvement of the prostatic base or midgland was not a risk factor for BCR. These results may be attributed to the fact that the prostatic apex is more difficult to localize on RT planning CT, with high variability between observers regarding delineation [51, 52]. Furthermore, there is evidence that the apex is one of the most common locations where tumors recur after primary RT, possibly because of undertreatment to avoid potential urethral strictures [53–55]. MRI has better soft-tissue resolution than CT and can better define the prostate apex and possible involvement of tumor, potentially improving RT planning outcomes.
LN metastasis suspicious on MRI and lower ADC were not significantly predictive of BCR. However, a borderline trend for elevated risk of BCR was recognized for LN metastasis (HR = 3.03 [95% CI, 0.89–10.33]; p = 0.08). Furthermore, pelvic LN metastasis was associated with a higher risk of metastasis, overall mortality, and cancer-specific mortality in one study [28]. However, strong conclusions cannot be drawn because few studies investigated these MRI findings (n = 3 studies for both). This paucity of studies could be related to the fact that patients with more extensive LN metastasis are more likely to have undergone further imaging workup and then systemic treatment after identification of distant metastasis. On the other hand, there is clinical background supporting the potential prognostic value of these two imaging findings. First, the presence of metastatic LNs at pathology after radical prostatectomy is known to be a risk factor for cancer-specific mortality [56], and it can be speculated that similar prognostic value may be extended to the population undergoing RT. Trials have shown that RT coverage of sentinel nodes is feasible [57, 58]. In addition, MRI has high specificity for detecting metastatic LNs and in turn may aid in selection and planning of RT coverage to suspicious LNs [59]. Nevertheless, the sensitivity of MRI, especially using size criteria (i.e., > 8 mm), is still low, and further improvement is needed using promising techniques such as DWI and ultrasmall paramagnetic iron oxide contrast agents [40]. ADC has been consistently observed as a functional MRI parameter associated with prostate cancer aggressiveness such as greater tumor volume, EPE, and higher Gleason scores [60–62]. Finally, the use of pretreatment MRI was a factor that was protective against BCR, possibly because of better RT planning with consideration of MRI findings.
There are limitations in this study. First, only 12 studies were included because most studies in the literature included patients who underwent radical prostatectomy, supporting the need to identify the prognostic value of pretreatment MRI before primary RT. Second, several older publications were included. Specifically, one-third of the studies included patients treated before the year 2000 [24, 30, 31, 33]. Because there have been technologic advances in MRI and RT, there may be concern for the application of the results derived from this meta-analysis; however, all but one study used 1.5- or 3-T scanners and the main MRI sequence used in the studies was T2-weighted imaging, which has seen relatively minor changes over the years compared with other functional sequences such as DWI. Nevertheless, we speculate that recent advances could actually potentiate the prognostic value of pretreatment MRI. For instance, multiparametric protocols including DWI and dynamic contrast-enhanced MRI could be used to enhance detection of the index lesion and improve assessment of local extent and estimation of tumor volume [40, 48, 50]. In addition, cutting-edge technology enables delivery of higher RT doses to areas of interest while avoiding unnecessary irradiation of surrounding structures [63]. Third, most of the included studies focused on BCR as the outcome, and there were only three studies that investigated metastatic failure, overall mortality, or cancer-specific mortality as the outcome. BCR does not necessarily lead to metastasis or mortality; therefore, future studies evaluating these outcomes are needed. Fourth, substantial heterogeneity was seen in tumor diameter, tumor volume, ADC, and presence of LN metastasis. Although neither meta-regression nor subgroup analyses were feasible because of the small number of included studies, this heterogeneity may be because of differences in the patient, tumor, MRI, and RT characteristics among the included studies [64]. Still, several MRI findings (EPE, SVI, apical disease, and number of sextants involved) did not show significant heterogeneity, strengthening their generalizability and applicability. Fifth, characteristics of studies assessing ADC limit drawing conclusions for several reasons: Only one study calculated ADC ratio [35], one study obtained ADCs from MRI reports [29], and another study did not use a uniform b value combination for performing DWI [26]. Further studies are warranted evaluating the prognostic value of ADC.
Conclusion
Several pretreatment MRI findings were significant prognostic factors with regard to BCR in patients with prostate cancer who underwent RT. Because of the paucity of studies assessing metastasis and overall or cancer-specific mortality, future studies are needed.
Acknowledgments
This study was funded by the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of interest/Disclosures: Since May 2017, Dr. Hricak has served on the Board of Directors of Ion Beam Applications (IBA), a publicly traded company, and she receives annual compensation for her service. Furthermore, Dr. Hricak is a member of the External Advisory Board of the University of Michigan Comprehensive Cancer Center, the International Advisory Board of the University of Vienna, Austria, and the Scientific Committee of the DKFZ (German Cancer Research Center), Germany; she does not receive financial compensation for any of these roles. The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
References
- 1.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280:969–974 [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1. Screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71:618–629 [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw 2016; 14:19–30 [DOI] [PubMed] [Google Scholar]
- 4.Angulo JC, Montie JE, Bukowsky T, et al. Interobserver consistency of digital rectal examination in clinical staging of localized prostatic carcinoma. Urol Oncol 1995; 1:199–205 [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012; 61:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 2017; 72:177–188 [DOI] [PubMed] [Google Scholar]
- 7.de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol 2016; 70:233–245 [DOI] [PubMed] [Google Scholar]
- 8.Woo S, Cho JY, Ku JH, Kim SY, Kim SH. Prostate cancer-specific mortality after radical prostatectomy: value of preoperative MRI. Acta Radiol 2016; 57:1006–1013 [DOI] [PubMed] [Google Scholar]
- 9.Boccon-Gibod L, Bertaccini A, Bono AV, et al. Management of locally advanced prostate cancer: a European consensus. Int J Clin Pract 2003; 57:187–194 [PubMed] [Google Scholar]
- 10.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997; 337:295–300 [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 13.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834 [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JG. Random-effects (DerSimonian and Laird) method for meta-analysis. handbook-5-1.cochrane.org/chapter_9/9_4_3_1_random_effects_dersimonian_and_laird_method_for.htm Updated March 2011. Accessed February 19, 2019
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwan GA, Ghai S, Margel D, et al. Magnetic resonance imaging detected prostate evasive anterior tumours: further insights. Can Urol Assoc J 2015; 9:E267–E272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng JR, Yang LY, Lin YC, et al. Metabolic volumetric parameters in 11C-choline PET/MR are superior PET imaging biomarkers for primary high-risk prostate cancer. Contrast Media Mol Imaging 2018; 2018:8945130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna DA, Coakley FV, Westphalen AC, et al. Prostate cancer: role of pretreatment MR in predicting outcome after external-beam radiation therapy—initial experience. Radiology 2008; 247:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westphalen AC, Koff WJ, Coakley FV, et al. Prostate cancer: prediction of biochemical failure after external-beam radiation therapy—Kattan nomogram and endorectal MR imaging estimation of tumor volume. Radiology 2011; 261:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinover WH, Hanlon A, Lee WR, Kaplan EJ, Hanks GE. Prostate carcinoma patients upstaged by imaging and treated with irradiation: an outcome-based analysis. Cancer 1996; 77:1334–1341 [DOI] [PubMed] [Google Scholar]
- 23.Zamboglou C, Klein CM, Thomann B, et al. The dose distribution in dominant intraprostatic tumour lesions defined by multiparametric MRI and PSMA PET/CT correlates with the outcome in patients treated with primary radiation therapy for prostate cancer. Radiat Oncol 2018; 13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke DH, Banks SJ, Wiederhorn AR, et al. The role of endorectal coil MRI in patient selection and treatment planning for prostate seed implants. Int J Radiat Oncol Biol Phys 2002; 52:903–910 [DOI] [PubMed] [Google Scholar]
- 25.Fuchsjäger MH, Pucar D, Zelefsky MJ, et al. Predicting post-external beam radiation therapy PSA relapse of prostate cancer using pretreatment MRI. Int J Radiat Oncol Biol Phys 2010; 78:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnep K, Fargeas A, Gutiérrez-Carvajal RE, et al. Haralick textural features on T2-weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer. J Magn Reson Imaging 2017; 45:103–117 [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Iturriaga A, Casquero F, Pijoan JI, et al. Pretreatment multiparametric magnetic resonance imaging findings are more accurate independent predictors of outcome than clinical variables in localized prostate cancer. Int J Radiat Oncol Biol Phys 2018; 101:1172–1178 [DOI] [PubMed] [Google Scholar]
- 28.Goupy F, Supiot S, Pasquier D, et al. Intensity-modulated radiotherapy for prostate cancer with seminal vesicle involvement (T3b): a multicentric retrospective analysis. PLoS One 2019; 14:e0210514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde JV, Demanes DJ, Veruttipong D, et al. Pretreatment 3T multiparametric MRI staging predicts for biochemical failure in high-risk prostate cancer treated with combination high-dose-rate brachytherapy and external beam radiotherapy. Brachytherapy 2017; 16:1106–1112 [DOI] [PubMed] [Google Scholar]
- 30.Jackson AS, Parker CC, Norman AR, et al. Tumour staging using magnetic resonance imaging in clinically localised prostate cancer: relationship to biochemical outcome after neo-adjuvant androgen deprivation and radical radiotherapy. Clin Oncol (R Coll Radiol) 2005; 17:167–171 [DOI] [PubMed] [Google Scholar]
- 31.Joseph T, McKenna DA, Westphalen AC, et al. Pretreatment endorectal magnetic resonance imaging and magnetic resonance spectroscopic imaging features of prostate cancer as predictors of response to external beam radiotherapy. Int J Radiat Oncol Biol Phys 2009; 73:665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauffmann G, Arif F, Patel P, Oto A, Liauw SL. Pretreatment multiparametric MRI is independently associated with biochemical outcome in men treated with radiation therapy for prostate cancer. Urol Oncol 2018; 36:471.e11–471.e18 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen PL, Whittington R, Koo S, et al. Quantifying the impact of seminal vesicle invasion identified using endorectal magnetic resonance imaging on PSA outcome after radiation therapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 59:400–405 [DOI] [PubMed] [Google Scholar]
- 34.Riaz N, Afaq A, Akin O, et al. Pretreatment endorectal coil magnetic resonance imaging findings predict biochemical tumor control in prostate cancer patients treated with combination brachytherapy and external-beam radiotherapy. Int J Radiat Oncol Biol Phys 2012; 84:707–711 [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Hori M, Suzuki O, et al. Clinical significance of the apparent diffusion coefficient ratio in prostate cancer treatment with intensity-modulated radiotherapy. Anticancer Res 2016; 36:6551–6556 [DOI] [PubMed] [Google Scholar]
- 36.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375:1415–1424 [DOI] [PubMed] [Google Scholar]
- 37.Arrayeh E, Westphalen AC, Kurhanewicz J, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys 2012; 82:e787–e793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundahl N, De Meerleer G, Villeirs G, et al. Combining high dose external beam radiotherapy with a simultaneous integrated boost to the dominant intraprostatic lesion: analysis of genitourinary and rectal toxicity. Radiother Oncol 2016; 119:398–404 [DOI] [PubMed] [Google Scholar]
- 39.Feng Y, Welsh D, McDonald K, et al. Identifying the dominant prostate cancer focal lesion using image analysis and planning of a simultaneous integrated stereotactic boost. Acta Oncol 2015; 54:1543–1550 [DOI] [PubMed] [Google Scholar]
- 40.Woo S, Ghafoor S, Vargas HA. Contribution of radiology to staging of prostate cancer. Semin Nucl Med 2019; 49:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buyyounouski MK, Choyke PL, McKenney JK, et al. Prostate cancer: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danneman D, Wiklund F, Wiklund NP, Egevad L. Prognostic significance of histopathological features of extraprostatic extension of prostate cancer. Histopathology 2013; 63:580–589 [DOI] [PubMed] [Google Scholar]
- 43.Potter SR, Epstein JI, Partin AW. Seminal vesicle invasion by prostate cancer: prognostic significance and therapeutic implications. Rev Urol 2000; 2:190–195 [PMC free article] [PubMed] [Google Scholar]
- 44.Pagliarulo V, Hawes D, Brands FH, et al. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol 2006; 24:2735–2742 [DOI] [PubMed] [Google Scholar]
- 45.Kestin L, Goldstein N, Vicini F, Yan D, Korman H, Martinez A. Treatment of prostate cancer with radiotherapy: should the entire seminal vesicles be included in the clinical target volume? Int J Radiat Oncol Biol Phys 2002; 54:686–697 [DOI] [PubMed] [Google Scholar]
- 46.Knoedler JJ, Karnes RJ, Thompson RH, Rangel LJ, Bergstralh EJ, Boorjian SA. The association of tumor volume with mortality following radical prostatectomy. Prostate Cancer Prostatic Dis 2014; 17:144–148 [DOI] [PubMed] [Google Scholar]
- 47.Hong MK, Namdarian B, Corcoran NM, et al. Prostate tumour volume is an independent predictor of early biochemical recurrence in a high risk radical prostatectomy subgroup. Pathology 2011; 43:138–142 [DOI] [PubMed] [Google Scholar]
- 48.Sun C, Chatterjee A, Yousuf A, et al. Comparison of T2-weighted imaging, DWI, and dynamic contrast-enhanced MRI for calculation of prostate cancer index lesion volume: correlation with whole-mount pathology. AJR 2019; 212:351–356 [DOI] [PubMed] [Google Scholar]
- 49.Harvey H, Orton MR, Morgan VA, et al. Volumetry of the dominant intraprostatic tumour lesion: intersequence and interobserver differences on multiparametric MRI. Br J Radiol 2017; 90:20160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazaheri Y, Hricak H, Fine SW, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology 2009; 252:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaughlin PW, Evans C, Feng M, Narayana V. Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate contouring accuracy. Int J Radiat Oncol Biol Phys 2010; 76:369–378 [DOI] [PubMed] [Google Scholar]
- 52.De Brabandere M, Hoskin P, Haustermans K, Van den Heuvel F, Siebert FA. Prostate post-implant dosimetry: interobserver variability in seed localisation, contouring and fusion. Radiother Oncol 2012; 104:192–198 [DOI] [PubMed] [Google Scholar]
- 53.Milosevic M, Voruganti S, Blend R, et al. Magnetic resonance imaging (MRI) for localization of the prostatic apex: comparison to computed tomography (CT) and urethrography. Radiother Oncol 1998; 47:277–284 [DOI] [PubMed] [Google Scholar]
- 54.Takeda T, Tin AL, Corradi RB, et al. Topography of prostate cancer recurrence after radiation therapy: a detailed mapping study of salvage radical prostatectomy specimens. Eur Urol 2018; 73:488–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merrick GS, Butler WM, Wallner KE, et al. Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol 2006; 175:1376–1380; discussion, 1381 [DOI] [PubMed] [Google Scholar]
- 56.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011; 185:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Müller AC, Eckert F, Paulsen F, et al. Nodal clearance rate and long-term efficacy of individualized sentinel node-based pelvic intensity modulated radiation therapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2016; 94:263–271 [DOI] [PubMed] [Google Scholar]
- 58.Ganswindt U, Paulsen F, Corvin S, et al. Optimized coverage of high-risk adjuvant lymph node areas in prostate cancer using a sentinel node-based, intensity-modulated radiation therapy technique. Int J Radiat Oncol Biol Phys 2007; 67:347–355 [DOI] [PubMed] [Google Scholar]
- 59.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. The diagnostic performance of MRI for detection of lymph node metastasis in bladder and prostate cancer: an updated systematic review and diagnostic meta-analysis. AJR 2018; 210:[web]W95–W109 [DOI] [PubMed] [Google Scholar]
- 60.Donati OF, Afaq A, Vargas HA, et al. Prostate MRI: evaluating tumor volume and apparent diffusion coefficient as surrogate biomarkers for predicting tumor Gleason score. Clin Cancer Res 2014; 20:3705–3711 [DOI] [PubMed] [Google Scholar]
- 61.Woo S, Cho JY, Kim SY, Kim SH. Extracapsular extension in prostate cancer: added value of diffusion-weighted MRI in patients with equivocal findings on T2-weighted imaging. AJR 2015; 204:[web]W168–W175 [DOI] [PubMed] [Google Scholar]
- 62.Donati OF, Mazaheri Y, Afaq A, et al. Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 2014; 271:143–152 [DOI] [PubMed] [Google Scholar]
- 63.Podder TK, Fredman ET, Ellis RJ. Advances in radiotherapy for prostate cancer treatment. Adv Exp Med Biol 2018; 1096:31–47 [DOI] [PubMed] [Google Scholar]
- 64.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011; 64:1187–1197 [DOI] [PubMed] [Google Scholar]