Abstract
This study explored the diagnostic and therapeutic significance of vitamin D binding protein (VDBP) and miR-155-5p for diabetic nephropathy and the correlation with urinary microalbumin. A total of 145 patients with type 2 diabetes who attended the Hwamei hospital were selected as research objects and assigned to diabetic nephropathy group (DN group) and diabetes group according to whether they suffered from diabetic nephropathy (DN). The expression levels of urine VDBP and serum miR-155-5p in the two groups were detected, and the correlation between urinary microalbumin (mAlb), serum cystatin C (Cys C) and 24-h urinary protein was analyzed. The predictive value of single and joint detection of urinary VDBP and serum miR-155-5p for DN onset and poor prognosis was analyzed. In DN patients, urine VDBP and serum miR-155-5p were highly expressed, and urine VDBP, serum miR-155-5p and mAlb, Cys C and 24-h urine protein were positively correlated (P<0.05). Moreover, the joint detection of urine VDBP and serum miR-155-5p was more valuable in diagnosis and poor prognosis prediction of DN patients than its single detection. Urine VDBP and serum miR-155-5p have good diagnostic value for DN, but their joint diagnostic value is higher, and their expression levels are all related to mAlb of DN patients, which may be used as new biological indicators for diagnosis and disease assessment.
Keywords: VDBP, miR-155-5p, diabetic nephropathy, diagnostic value, urinary microalbumin, correlation
Introduction
Diabetic nephropathy (DN) is one of the main complications of diabetic microangiopathy and one of the main causes of uremia. The morbidity and mortality of DN in diabetic patients are both high, which is a serious threat to their quality of life and health (1,2). Clinically, the early symptoms of DN onset are not obvious, and the indicator changes are usually not obvious in routine detection, therefore difficult to detect. However, in the late stage, the disease progresses very quickly, and the liver and kidney functions also decline rapidly, which will also lead to poor prognosis due to lack of timely treatment (3,4). Therefore, for DN patients, early diagnosis and timely treatment have important clinical significance.
Vitamin D binding protein (VDBP) belongs to the binding protein albumin family. It is mainly synthesized by liver and then reabsorbed by proximal tubular epithelial cells after glomerular filtration (5). Previous studies confirmed that urine VDBP was a new biomarker of tubulointerstitial injury independent of proteinuria, and it mainly appeared in the early stage of inflammatory reaction and tubulointerstitial fibrosis and had high sensitivity to renal inflammation (6). Inflammation played a vital role in the pathogenesis of DN (7). In addition, previous studies found that abundant miRNA expression could be seen in kidney tissues of DN patients, and reported that its expression could reflect the condition of patients to a certain extent, and that miRNA and DN were closely related (8). miR-155-5p is a miRNA that plays an important regulatory role in the development and progression of tumors, and its diagnostic and prognostic value in tumors has also been confirmed (9). Previous study also discovered that miR-155-5p was highly expressed in DN, and it was considered that its expression was tied to the severity of DN patients (10).
However, there is relatively scarce research on the diagnostic value of VDBP and miR-155-5p in DN at present, so we explored the diagnostic value of VDBP and miR-155-5p in DN, and analyzed the correlation between VDBP and urinary microalbumin (mAlb) (11), which is the main basis indicator for DN diagnosis to provide more reference schemes.
Patients and methods
General information
From April 2015 to January 2017, 145 patients with type 2 diabetes were selected as research targets, including 75 male patients and 70 female patients. Their average age was (68.02±5.33) years, including 71 DN patients (DN group) and 74 non-DN patients (diabetes group). Inclusion criteria were as follows: Patients who met the diagnostic criteria for type 2 diabetes of the American Diabetes Association (12). Patients diagnosed as DN by pathological (biopsy) diagnosis were included in DN group. Exclusion criteria were as follows: patients with primary renal diseases, other diabetic complications, ketoacidosis or other metabolic diseases; patients who had used immunosuppressive agents or nephrotoxic drugs in the recent past; patients with malignant tumors, severe infectious diseases, other severe diabetic complications; patients who refused to participate in the study.
The study was approved by the Ethics Committee of HwaMei Hospital (Ningbo, China). All patients and their families agreed to participate in the study and signed informed consents were obtained from the patients and/or their guardians.
Indicator detection RT-PCR detection of miR-155-5p expression
Venous blood (3 ml) of all patients was drawn on an empty stomach, and then centrifuged for 10 min at 8,000 x g at 4˚C. Serum was taken for detection, and total RNA in serum was extracted with TRIzol reagent (15596018; Thermo Fisher Scientific). The purity and concentration of RNA were detected with ultraviolet spectrophotometer, and then 5 µg of total RNA was taken, respectively, and reverse transcription cDNA was performed following the instructions of the reverse transcription kit (AQ202-01; TransGen Biotech). The reaction parameters were as follows: 37˚C for 10 min, 45˚C for 30 min, 72˚C for 4 min. miR-155-5p amplification system (PCR kit; AQ201-01; TransGen Biotech): cDNA 1 µl, upstream and downstream primers 0.4 µl each, 2X TransTaq® Tip Green qPCR SuperMix 10 µl, Passive Reference Dye (50X) 0.4 µl, ddH2O supplemented to 20 µl. Amplification conditions: PCR reaction conditions were as follows: Pre-denaturation at 94˚C for 45 sec, denaturation at 94˚C for 10 sec, annealing extension at 60˚C for 45 sec, a total of 40 cycles. Each sample was provided with 3 repeated wells, and the experiment was carried out 3 times. U6 was used as the internal reference and 2-ΔΔct was used to analyze the data.
Detection of VDBP, mAlb and other biochemical indicators
Urine VDBP (ml023696; Shanghai Enzyme Linked Biotechnology Co., Ltd.) was detected by ELISA and mAlb was detected by immunoturbidimetry. Fasting venous blood (5 ml) of patients was drawn, and then centrifuged for 10 min at 8,000 x g at 4˚C. Serum was taken and the serum glycosylated hemoglobin A1c (HbA1c), serum creatinine (SCr), blood urea (UREA) and cystatin C (Cys C) were detected by automatic biochemical analyzer, and 24-h urine protein of patients was detected.
Statistical analysis
In this study, the experimental data were statistically analyzed via SPSS 19.0 software, the counting data were checked through Chi-square test, and the measurement data were assessed via mean ± standard deviation. Moreover, t-test was employed for comparison between the two groups, GraphPad Prism 6 software was employed for drawing the experimental illustrations, and Pearson's was employed for correlation analysis. ROC results were analyzed via STATA software. SPSS was used to analyze data of the two groups, binary logistic analysis was used to calculate the predictive factors, and then ROC curve analysis was carried out to calculate the diagnostic sensitivity and specificity. Difference of P<0.05 was considered to be statistically significant.
Results
General data
There was no significant difference in sex, age and BMI between the two groups (P>0.05), as shown in Table I.
Table I.
General data.
| Factor | DN group (n=71) | Diabetes group (n=74) | t/χ2 value | P-value |
|---|---|---|---|---|
| Sex | 0.008 | 0.927 | ||
| Male | 37 (52.11) | 38 (51.35) | ||
| Female | 34 (47.89) | 36 (48.65) | ||
| Age, years) | 0.003 | 0.959 | ||
| ≤68 | 31 (43.66) | 32 (43.24) | ||
| >68 | 40 (56.34) | 42 (56.76) | ||
| BMI, kg/m2 | 0.007 | 0.932 | ||
| ≤23 | 36 (50.70) | 37 (50.00) | ||
| >23 | 35 (49.30) | 37 (50.00) | ||
| Educational level | 0.011 | 0.916 | ||
| Below junior high school | 32 (45.07) | 34 (45.95) | ||
| Junior high school and above | 39 (54.93) | 40 (54.05) | ||
| Place of residence | 0.000 | 0.987 | ||
| Countryside | 45 (63.38) | 47 (63.51) | ||
| City or town | 26 (36.62) | 27 (36.49) | ||
| Fasting blood glucose (mmol/l) | 8.35±1.58 | 8.47±1.53 | 0.465 | 0.643 |
| HbA1c (mmol/l) | 8.32±1.65 | 8.41±1.74 | 0.319 | 0.750 |
| SCr (µmol/l) | 93.21±21.66 | 48.26±9.52 | 16.29 | <0.001 |
| UREA (mmol/l) | 8.67±1.18 | 5.13±0.42 | 24.26 | <0.001 |
HbA1c, hemoglobin A1c; SCr, serum creatinine; UREA, blood urea; DN, diabetic nephropathy.
Expression levels of serum miR-155-5p and urine VDBP
The expression levels of serum miR-155-5p and urine VDBP in the DN group were significantly higher than those in the diabetes group, with statistically significant difference (P<0.05), as shown in Table II.
Table II.
Expression of serum miR-155-5p and urine VDBP.
| Factor | DN group (n=71) | Diabetes group (n=74) | t value | P-value |
|---|---|---|---|---|
| miR-155-5p | 3.25±1.53 | 1.82±0.93 | 6.832 | <0.001 |
| Urine VDBP (ng/ml) | 61.98±31.85 | 22.51±9.16 | 10.23 | <0.001 |
VDBP, vitamin D binding protein; DN, diabetic nephropathy.
Detection of other relevant indicators
In order to further compare differences between DN patients and non-DN patients, we further compared the expression levels of mAlb, Cys C and 24-h urine protein of patients in the two groups. The results showed that mAlb, Cys C and 24-h urine protein in the DN group were significantly higher than those in the diabetes group, with statistically significant differences (P<0.05), as shown in Fig. 1.
Figure 1.
Detection of other relevant indicators of DN. (A) Urine mAlb level of patients in the two groups. (B) Serum Cys C level of patients in the two groups. (C) 24-h urine protein level of patients in the two groups. *P<0.05. DN, diabetic nephropathy; mAlb, microalbumin; Cys C, cystatin C.
Correlation analysis of serum miR-155-5p, urine VDBP and mAlb, Cys C and 24-h urine protein in DN patients
Serum miR-155-5p and mAlb, Cys C and 24-h urine protein in DN patients were positively correlated (P<0.05), while urine VDBP and mAlb, Cys C and 24-h urine protein were positively correlated (P<0.05), as shown in Table III and Fig. 2.
Table III.
Correlation analysis of serum miR-155-5p, urine VDBP and mAlb, Cys C and 24-h urine protein in DN patients.
| Serum miR-155-5p | Urine VDBP | |||
|---|---|---|---|---|
| Indicators | r value | P-value | r value | P-value |
| mAlb | 0.676 | <0.001 | 0.807 | <0.001 |
| Cys C | 0.649 | <0.001 | 0.772 | <0.001 |
| 24-h urine protein | 0.700 | <0.001 | 0.823 | <0.001 |
VDBP, vitamin D binding protein; mAlb, microalbumin; Cys C, cystatin C; DN, diabetic nephropathy.
Figure 2.
Correlation analysis of serum miR-155-5p, urine VDBP, mAlb, Cys C and 24-h urine protein in DN patients. (A) miR-155-5p and mAlb were positively correlated. (B) miR-155-5p and Cys C were positively correlated. (C) miR-155-5p was positively correlated with 24-h urine protein. (D) Urine VDBP and mAlb were positively correlated. (E) Urine VDBP was positively correlated with Cys C. (F) There was a positive correlation between urine VDBP and 24-h urine protein. VDBP, vitamin D binding protein; mAlb, microalbumin; Cys C, cystatin C; DN, diabetic nephropathy.
Diagnostic value of single and joint detection of serum miR-155-5p and urine VDBP in DN
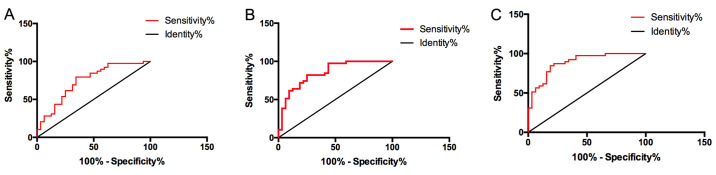
The diagnostic sensitivity of miR-155-5p to DN was 75.68%, specificity was 64.79%, AUC was 0.746, 95% CI was 0.664-0.828, and cut-off value was 2.6; the diagnostic sensitivity of urine VDBP to DN was 85.14%, specificity was 83.10%, AUC was 0.859, 95% CI was 0.787-0.931, and cut-off value was 31.24 ng/ml; the diagnostic sensitivity of joint diagnosis of serum miR-155-5p and urine VDBP was 93.24%, specificity was 76.06%, AUC was 0.904, and 95% CI was 0.851-0.958. Although serum miR-155-5p and urine VDBP had certain diagnostic value for DN, the sensitivity and AUC of joint diagnosis were higher than that of single diagnosis, and the former had higher diagnostic value, as shown in Fig. 3.
Figure 3.
Diagnostic value of single and joint detection of serum miR-155-5p and urine VDBP in DN. (A) The diagnostic sensitivity of serum miR-155-5p to DN was 75.68%, specificity was 64.79%, AUC was 0.746, 95% CI was 0.664-0.828, and cut-off value was 2.6. (B) The diagnostic sensitivity of urine VDBP to DN was 85.14%, specificity was 83.10%, AUC was 0.859, 95% CI was 0.787-0.931, and the cut-off value was 31.24 ng/ml. (C) The diagnostic sensitivity of joint diagnosis of serum miR-155-5p and urine VDBP for DN was 93.24%, specificity was 76.06%, AUC was 0.904, and 95% CI was 0.851-0.958. VDBP, vitamin D binding protein; DN, diabetic nephropathy.
Prognostic value of serum miR-155-5p and urine VDBP in poor prognosis of patients with DN
All DN patients were followed up for 2 years, among them 32 patients entered end-stage renal disease (ESRD) during the follow-up period. The serum miR-155-5p and urine VDBP of ESRD patients were significantly higher than those of ESRD patients without ESRD, with statistically significant difference (P<0.05). ROC curve showed that the sensitivity, specificity, AUC, 95% CI and cut-off value of serum miR-155-5p were 74.36, 65.63%, 0.744, 0.628-0.861, 3.409, 82.05 and 71.88%, respectively, for predicting poor prognosis of DN patients. AUC was 0.849, 95% CI was 0.759-0.939, and the cut-off value was 62.06 ng/ml. The sensitivity of joint detection of serum miR-155-5p and urine VDBP to predict poor prognosis of DN patients was 89.74%, specificity was 65.63%, AUC was 0.886, 95% CI was 0.810-0.963, and the joint detection had higher prediction value for poor prognosis of DN patients, as shown in Table IV and Fig. 4.
Table IV.
Expression of serum miR-155-5p and urine VDBP in DN patients with different prognosis.
| Factor | ESRD group (n=32) | Non-ESRD group (n=39) | t value | P-value |
|---|---|---|---|---|
| miR-155-5p | 4.06±1.09 | 2.82±0.88 | 5.305 | <0.001 |
| Urine VDBP (ng/ml) | 73.82±24.25 | 43.58±18.93 | 5.706 | <0.001 |
VDBP, vitamin D binding protein; DN, diabetic nephropathy; ESRD, end-stage renal disease.
Figure 4.
Predictive value of serum miR-155-5p and urine VDBP for poor prognosis of DN patients. (A) The predictive sensitivity of serum miR-155-5p to the occurrence of ESRD was 74.36%, specificity was 65.63%, AUC was 0.744, 95% CI was 0.628-0.861, and cut-off value was 3.409. (B) The predictive sensitivity of urine VDBP to the occurrence of ESRD was 782.05%, specificity was 71.88%, AUC was 0.849, 95% CI was 0.759-0.939, and the cut-off value was 62.06 ng/ml. (C) The predictive sensitivity of joint detection of serum miR-155-5p and urine VDBP for the occurrence of ESRD was 89.74%, specificity was 65.63%, AUC was 0.886, and 95% CI was 0.810-0.963. VDBP, vitamin D binding protein; DN, diabetic nephropathy; ESRD, end-stage renal disease.
Discussion
Clinically, pathogenesis of DN has a relatively complicated mechanism, and its occurrence is also a common cause of renal failure. However, if diagnosis and treatment can be carried out as soon as possible, the progression of patients can be delayed or even reversed (13,14). Therefore, it is of great clinical significance to find a diagnosis method with high sensitivity and specificity for DN patients.
In the present study, it was first found that urine VDBP and serum miR-155-5p in DN patients were higher than those in diabetic patients, which suggested that both urine VDBP and serum miR-155-5p might be tied to the pathogenesis of DN. As a glycosylated α-globulin, VDBP is mainly synthesized by liver and filtered by glomerulus. In addition to binding and transporting vitamin D and its metabolic products, it can also be converted into an activating factor of macrophages to promote polarization of macrophages (15,16). The increase of VDBP is bound with the injury degree of tubulointerstitium and inflammatory reaction (17). However, miR, as a non-coding microRNA, was revealed previously to exert a crucial influence on the development and progression of DN by regulating various mechanisms, which suggested that it might be a target for diagnosis and treatment of DN (18). These studies also confirmed our conclusion. Subsequently, in order to further explore the clinical value of urine VDBP and serum miR-155-5p on DN, other DN-related indicators were also detected, mAlb, Cys C and 24-h urine protein, and the correlation between them and urine VDBP as well as serum miR-155-5p were analyzed; the results showed that mAlb, Cys C and 24-h urine protein in DN patients were significantly higher than those in normal diabetic patients. mAlb is a sensitive and reliable indicator for DN kidney injury, and was proven previously to be a vital reference indicator for DN diagnosis (19). Cys C, as an endogenous indicator, can freely filter through glomerulus and be reabsorbed and degraded in proximal convoluted tubules. Cys C is very sensitive to the early function of glomerulus and is also a sensitive indicator reflecting glomerular filtration function (20). However, 24-h proteinuria is one of the important indicators for judging glomerular function. When 24-h proteinuria increases, it indicates further aggravation of metabolic disorder and kidney injury in vivo (21). Through correlation analysis of urine VDBP, serum miR-155-5p and the nephrotic sensitive indicators, it was discovered that urine VDBP, serum miR-155-5p and mAlb, Cys C and 24-h urine protein were positively correlated, which further indicated that the occurrence of urine VDBP, serum miR-155-5p and DN were relevant.
Subsequently, ROC analysis was carried out in order to study the diagnostic value of urine VDBP and serum miR-155-5p for DN. ROC is currently a common method for evaluating the medical diagnostic efficiency. And the results demonstrated that although urine VDBP and serum miR-155-5p had good diagnostic value, the AUC of their joint detection for DN diagnosis was as high as 0.904, which indicated that urine VDBP and serum miR-155-5p might be used as a new diagnostic mode for DN diagnosis. Previous studies (22) reported that miR-155-5p was highly expressed in renal tissues of DN patients, and its expression gradually increased with the progression of the disease, which revealed that it might also be tied to disease progression. Once entering stage ESRD, DN patients become more difficult to treat, and their condition will be almost impossibile to reverse, which also indicates poor prognosis (23). Therefore, patients were divided into ESRD group and non-ESRD group according to the follow-up situation, and the predictive value of urine VDBP and serum miR-155-5p was analyzed for poor prognosis of DN patients; the results showed that urine VDBP had higher sensitivity to poor prognosis of DN patients than serum miR-155-5p, but the sensitivity was the highest when joint detection was performed, and the diagnostic AUC could be as high as 0.886, which indicated that the joint detection of urine VDBP and serum miR-155-5p also had higher predictive value for their poor prognosis. This was also the first time that the joint detection of urine VDBP and serum miR-155-5p was found to have good predictive value for the onset and prognosis of DN.
In conclusion, urine VDBP and serum miR-155-5p have good diagnostic value for DN, but their joint diagnostic value is higher, and their expression levels are all linked to mAlb of DN patients, which may be used as new biological indicators for diagnosis and disease assessment. However, we did not further analyze the risk factors for poor prognosis of DN patients, nor did we conduct relevant basic experiments to clarify the effects of VDBP and miR-155-5p on the kidneys. Therefore, further study with expanded sample numbers is still required.
Acknowledgements
Not applicable.
Funding
The study was supported by the Nephrology Research Center of East Zhejiang (Zhejiang Health Commission 2015, no. 21) and the ‘Ningbo City Focuses on Fostering Disciplines’ project (no. 2016022).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XB and LG conceived and designed the study. XB, QL, KT and LG were responsible for the acquisition, analysis and interpretation of the data. XB drafted the manuscript. QL revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of HwaMei Hospital (Ningbo, China). Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhuang L, Jin G, Hu X, Yang Q, Shi Z. The inhibition of SGK1 suppresses epithelial-mesenchymal transition and promotes renal tubular epithelial cell autophagy in diabetic nephropathy. Am J Transl Res. 2019;11:4946–4956. [PMC free article] [PubMed] [Google Scholar]
- 2.Coskun ZM, Ersoz M, Adas M, Hancer VS, Boysan SN, Gonen MS, Acar A. Kruppel-like transcription factor-4 gene expression and DNA methylation status in type 2 diabetes and diabetic nephropathy patients. Arch Med Res. 2019;50:91–97. doi: 10.1016/j.arcmed.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Yu T, Zhang Z, Fang J, Wang Y, Yang Y, Liu L, Zou G, Gao H, Zhuo L, et al. Prognostic nomogram and score to predict renal survival of patients with biopsy-proven diabetic nephropathy. Diabetes Res Clin Pract. 2019;155(107809) doi: 10.1016/j.diabres.2019.107809. [DOI] [PubMed] [Google Scholar]
- 4.Grujicic M, Salapura A, Basta-Jovanovic G, Figurek A, Micic-Zrnic D, Grbic A. Non-diabetic kidney disease in patients with type 2 diabetes mellitus - 11-year experience from a single center. Med Arh. 2019;73:87–91. doi: 10.5455/medarh.2019.73.87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawzy MS, Beladi FI. Association of circulating vitamin D, VDBP, and vitamin D receptor expression with severity of diabetic nephropathy in a group of Saudi type 2 diabetes mellitus patients. Clin Lab. 2018;64:1623–1633. doi: 10.7754/Clin.Lab.2018.180401. [DOI] [PubMed] [Google Scholar]
- 6.Mirković K, Doorenbos CR, Dam WA, Lambers Heerspink HJ, Slagman MC, Nauta FL, Kramer AB, Gansevoort RT, van den Born J, Navis G, et al. Urinary vitamin D binding protein: A potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013;8(e55887) doi: 10.1371/journal.pone.0055887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu J, Huang W, Zhang W, Zhao T, Gao C, Gan W, Rao M, Chen Q, Guo M, Xu Y, et al. Sodium butyrate alleviates high-glucose -induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol. 2019;75(105832) doi: 10.1016/j.intimp.2019.105832. [DOI] [PubMed] [Google Scholar]
- 8.Ebadi Z, Moradi N, Kazemi Fard T, Balochnejadmojarrad T, Chamani E, Fadaei R, Fallah S. Captopril and spironolactone can attenuate diabetic nephropathy in Wistar rats by targeting microRNA-192 and microRNA-29a/b/c. DNA Cell Biol. 2019;38:1134–1142. doi: 10.1089/dna.2019.4732. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Zhang Y, Liu W, Xiao H, Qi Y, Li J, Luo B. Latent membrane protein 2A inhibits expression level of Smad2 through regulating miR-155-5p in EBV-associated gastric cancer cell lines. J Med Virol. 2020;92:96–106. doi: 10.1002/jmv.25579. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Wang G, Liang Y, Zhou X. Expression profiling and clinical significance of plasma microRNAs in diabetic nephropathy. J Diabetes Res. 2019;2019(5204394) doi: 10.1155/2019/5204394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Wang X, Zhang Y, Li H, Xu Y, Zheng D. The longitudinal effect of subclinical hypothyroidism on urine microalbumin-to-urine creatinine ratio in patients with type 2 diabetes mellitus. BMC Endocr Disord. 2019;19(84) doi: 10.1186/s12902-019-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krueger DA, Northrup H, Northrup H, Krueger DA, Roberds S, Smith K, Sampson J, Korf B, Kwiatkowski DJ, Mowat D, et al. International Tuberous Sclerosis Complex Consensus Group: Tuberous sclerosis complex surveillance and management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocak MZ, Aktas G, Erkus E, Duman TT, Atak BM, Savli H. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28:844–847. doi: 10.29271/jcpsp.2018.11.844. [DOI] [PubMed] [Google Scholar]
- 14.Prabu P, Rome S, Sathishkumar C, Gastebois C, Meugnier E, Mohan V, Balasubramanyam M. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the ‘Asian Indian phenotype’. Diabetes Metab. 2019;45:276–285. doi: 10.1016/j.diabet.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Fawzy MS, Abu AlSel BT. Assessment of vitamin D-binding protein and early prediction of nephropathy in type 2 Saudi diabetic patients. J Diabetes Res. 2018;2018(8517929) doi: 10.1155/2018/8517929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoukry A, Bdeer S-A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem. 2015;408:25–35. doi: 10.1007/s11010-015-2479-y. [DOI] [PubMed] [Google Scholar]
- 17.Tian XQ, Zhao LM, Ge JP, Zhang Y, Xu YC. Elevated urinary level of vitamin D-binding protein as a novel biomarker for diabetic nephropathy. Exp Ther Med. 2014;7:411–416. doi: 10.3892/etm.2013.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhbari M, Khalili M, Shahrabi-Farahani M, Biglari A, Bandarian F. Expression level of circulating cell free miR-155 gene in serum of patients with diabetic nephropathy. Clin Lab. 2019;65(65) doi: 10.7754/Clin.Lab.2019.190209. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Xu W, Zhou Z, Liu J, Li X, Chen L, Weng J, Yu Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes. 2015;123:360–367. doi: 10.1055/s-0035-1545345. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqi Z, Karoli R, Kaul A, Fatima J, Varshney S, Beg MS. Evaluation of neutrophil gelatinase-associated lipocalin and cystatin C as early markers of diabetic nephropathy. Ann Afr Med. 2017;16:101–106. doi: 10.4103/aam.aam_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Zhu H, Yang JH, Gao ZA, Yuan XX, Li XC, Wang JY, Chang BC. Can urine albumin/creatinine ratio replace 24 hs urinary albumin? Zhonghua Nei Ke Za Zhi. 2019;58:377–381. doi: 10.3760/cma.j.issn.0578-1426.2019.05.009. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, Yin Q, Chen L, Cui T, Zhang J, et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: Implications for glomerular endothelial injury. BMC Nephrol. 2014;15(142) doi: 10.1186/1471-2369-15-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Ouyang J, Li S, Wang H, Lian B, Liu Z, Xie L. The analysis of risk factors for diabetic nephropathy progression and the construction of a prognostic database for chronic kidney diseases. J Transl Med. 2019;17(264) doi: 10.1186/s12967-019-2016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.