Abstract
Introduction: Multiple sclerosis (MS) is a chronic disorder of the central nervous system with an untreatable late progressive phase. Molecular maps of different stages of brain lesion evolution in patients with progressive multiple sclerosis (PMS) are missing but critical for understanding disease development and to identify novel targets to halt progression.
Materials and Methods: The MS Atlas database comprises comprehensive high-quality transcriptomic profiles of 98 white matter (WM) brain samples of different lesion types (normal-appearing WM [NAWM], active, chronic active, inactive, remyelinating) from ten progressive MS patients and 25 WM areas from five non-neurological diseased cases.
Results: We introduce the first MS brain lesion atlas (msatlas.dk), developed to address the current challenges of understanding mechanisms driving the fate on a lesion basis. The MS Atlas gives means for testing research hypotheses, validating biomarkers and drug targets. It comes with a user-friendly web interface, and it fosters bioinformatic methods for de novo network enrichment to extract mechanistic markers for specific lesion types and pathway-based lesion type comparison. We describe examples of how the MS Atlas can be used to extract systems medicine signatures and demonstrate the interface of MS Atlas.
Conclusion: This compendium of mechanistic PMS WM lesion profiles is an invaluable resource to fuel future MS research and a new basis for treatment development.
Keywords: human brain lesions, lesion-specific heatmaps and networks, MS atlas, multiple sclerosis, natalizumab, transcriptome, VLA4
Introduction
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disorder of the central nervous system (CNS).1 It is one of the most common causes of neurological disability in young adults2–4 and the incidence is increasing.5,6 In about 50% of patients with relapsing multiple sclerosis (RMS), the disease evolves into a progressive phase. At this stage, progression is relentless, and treatments become ineffective. Lesions in the white matter (WM) characterize MS from the early phase. As the disease progresses, quantitative and qualitative changes in the WM can be observed. However, key aspects of progressive multiple sclerosis (PMS) pathogenesis are still unsolved, making it challenging to develop treatments. One reason is that direct studies on brain lesions of PMS patients are sparse and the evolution of acute lesion in the MS brain and their fate are not well characterized, mainly due to the limited access of brain tissue. Brain samples from MS patients can be obtained either by needle biopsy or by autopsy. However, needle biopsy is done in MS only if there is doubt about differentiation from other diseases, so usually biopsy samples do not represent typical MS. Needle biopsy does not allow either the examination of different lesion types, as only minuscule samples from the atypical brain lesion are obtained, and examination of the whole brain for different lesion types is not possible. In addition, knowledge from human MS brain lesions is mostly based on candidate gene approaches such as immunohistochemistry, microarrays, and quantitative polymerase chain reaction. In the past three decades, biobanking of fresh-frozen tissues and advanced technologies in transcriptomics and genomics contributed to more comprehensive studies on the brain material. Unfortunately, most of the generated gene lists do not overlap, which may be due to the use of targeted amplicon sequencing and microarrays, and lack of correction for multiple testing.7–19
To allow for identifying systems biology expression signatures that describe brain lesion type formation, evolution, and progression, we have assembled the first interactive MS lesion expression map (MS Atlas). At its core sits a database of preanalyzed whole-genome next-generation RNA sequencing-based transcriptomic profiles for stages of lesion formation gathered from 98 post mortem human brain samples of 10 patients with PMS and 5 non-neurological disease control cases, including normal appearing white matter (NAWM), active, inactive, chronic active, and remyelinating lesions. We applied strict preprocessing and conservative statistics and detected thousands of genes that are significantly differentially expressed during lesion evolution compared with control samples (Fig. 1). Our MS Atlas features an online web-based data analysis platform to identify and extract mechanistic pathways and gene sets that distinguish lesion types and are candidate drivers of different lesion type formation.
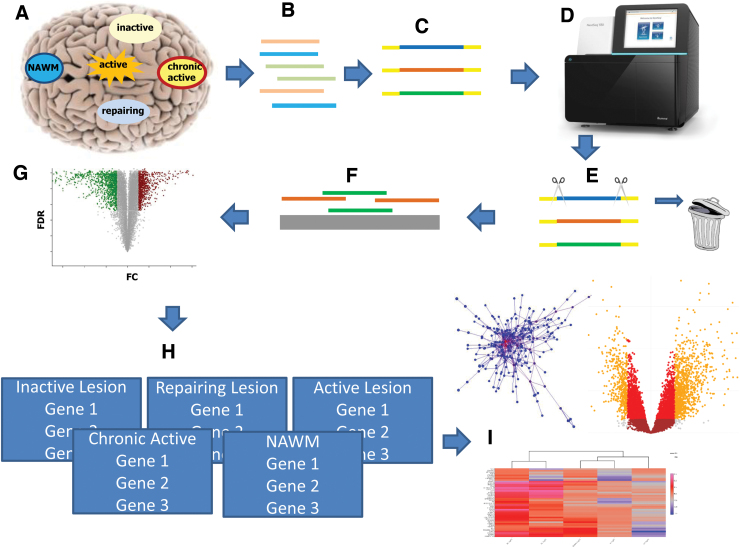
FIG. 1.
Graphical summary of the study. (A) With immunohistochemistry, we classified different lesion types (NAWM, active, inactive, chronic active, and remyelinating), and microdissected 98 of these brain areas from 10 progressive MS and 5 non-neurological disease brains. (B) Total RNA (microRNA, lncRNA, and protein coding) was extracted and (C) library was generated. (D) The templates were sequenced on a NextSeq550 using paired end, followed by (E) quality control, where all reads <Q20 were removed. (F) Remaining reads were aligned to the human genome and counted. (G) Statistical calculations were performed in R and the significant threshold was set to FDR <0.05. (H) MS Atlas was implemented based on RShiny offering three major visualizations (I): heatmaps, mechanistic candidate networks, and volcano plots. FDR, false discovery rate; logFC, log2-fold-changes; MS, multiple sclerosis; NAWM, normal appearing white matter.
The MS Atlas will fuel future research projects and significantly aid in advancing not only the MS field but also for research in other neurological diseases, as it allows researchers to (1) search for certain molecules of interest as drug targets or biomarkers of brain lesion genesis, (2) compare gene panels extracted from functional cell or animal studies, and (3) discover mechanistic markers using de novo network enrichment from genes of interest in different lesion types. We showed that the drug target known to be only effective in early disease stages is present in the active—but less in the chronic active lesion types characteristic of progressive MS. The MS Atlas is extendible and will continuously be updated with future transcriptome profiles. We also aim to integrate genome-wide methylome data from the same tissues, making it possible for the user to correlate the gene expression with methylation status, and to mine its mechanistic joint effects on lesion evolution.
Methods
Ethics Approval and Consent to Participate
MS and control tissue samples were supplied by the UKMSTB (UK Multicentre Research Ethics Committee, MREC/02/2/39), funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland (registered charity 207,495).
Human postmortem brain tissue
Seventy-five snap-frozen tissue blocks from 10 PMS patients and 25 blocks from five donors without neurological disease20 have been obtained from the UK Multiple Sclerosis Tissue Bank (UKMSTB) at Imperial College London (Table 1). All tissues were obtained within 30 h after death. The age of patients at death was 52.4±10.2 years, and the age of the controls was 56.4±14.1 years. We examined 4–10 brain areas/lesions from each brain: altogether 20 NAWM areas (7 patients), 17 active lesions (8 patients), 14 inactive lesions (5 patients), 6 remyelinating lesions (4 patients), 17 chronic active lesions (7 patients), and 25 control WM areas (5 controls).
Table 1.
Sample Information for All 10 Multiple Sclerosis and 5 Control Patients
| Case | Gender | Age | Control WM areas |
|---|---|---|---|
| C1 | Male | 35 | 5 |
| C2 | Male | 68 | 5 |
| C3 | Male | 68 | 5 |
| C4 | Female | 50 | 5 |
| C5 | Female | 61 | 5 |
| Lesion type |
|||||||
|---|---|---|---|---|---|---|---|
| AL | IL | CA | RL | NAWM | |||
| MS1 |
Male |
39 |
3 |
0 |
0 |
0 |
3 |
| MS2 |
Female |
54 |
1 |
5 |
0 |
0 |
0 |
| MS3 |
Female |
61/62 |
0 |
0 |
3 |
1 |
5 |
| MS4 |
Male |
51 |
2 |
1 |
5 |
0 |
2 |
| MS5 |
Female |
45 |
2 |
0 |
0 |
0 |
4 |
| MS6 |
Male |
42 |
4 |
1 |
2 |
2 |
2 |
| MS7 |
Male |
50 |
2 |
0 |
1 |
1 |
0 |
| MS8 |
Male |
75 |
0 |
0 |
5 |
0 |
4 |
| MS9 |
Female |
53 |
0 |
7 |
0 |
0 |
1 |
| MS10 | Female | 54 | 2 | 0 | 1 | 1 | 0 |
Lesion types are AL, IL, CL, RL, and NAWM.
AL, active; CL, chronic active; IL, inactive; MS, multiple sclerosis; NAWM, normal appearing white matter; RL, remyelinating/repairing; WM, white matter.
Immunohistochemistry and lesion classification
Snap-frozen tissue has been sectioned and stained for classification of NAWM, active, inactive, and remyelinating lesions based on antibodies against myelin oligodendrocyte glycoprotein to detect myelin integrity and human leukocyte antigen D related (HLA-DR+) to characterize the inflammatory state.21 For staining with very late antigen 4 (VLA-4)/integrin α-4 antibody (ab77528; abcam), we used tissue from one MS patient and control.
RNA extraction from specific histological brain areas
The brain fields of interest were microdissected in a cryostat (10–100 mg/sample). Total RNA has been isolated with miRNeasy Mini Kit from Qiagen, and DNAse I treatment (RNAse-Free DNAse Set; Qiagen) was applied to eliminate genomic DNA interference. RNA concentration and purity have been measured on a NanoDrop spectrophotometer ND-1000 (Thermo Scientific) and the integrity of RNA (RIN) was measured using the Bioanalyzer 2100 (Agilent Technologies). The fragmentation time and cleanup steps during library preparation have been adapted for each sample based on the RIN value.
RNA-seq
One microgram of RNA per sample was processed to remove ribosomal RNA followed by library preparation using TruSeq Stranded Total RNA Library Prep Kit with Ribo-Zero Human/Mouse/Rat Set (Illumina). The quality and fragmentation size of the libraries were estimated by High Sensitivity DNA chip on the Agilent 2100 Bioanalyzer and the concentration determined with Qubit dsDNA HS Assay (Life Technologies, Carlsbad, CA). Two pM pooled indexed libraries were loaded into flow cell followed by cluster generation and indexed paired-end sequencing (80+7 + 80 bp) on Illumina NextSeq500/550 [High Output v2 kit (150 cycles)].
Raw data analysis and quality control
The data were demultiplexed by the Illumina machine and exported in the FASTQ file format. Afterward, the read quality for the 100 samples was accessed through FastQC.22 Trimmomatic23 was used to trim the reads and remove any hypothetical adapter contamination. The software was provided with the correct Illumina adapter sequences and the quality cutoff for the leading/trailing bases as well as for the sliding windows was set to 20. The minimal length of the trimmed reads was set to 17 to include potentially present microRNA. In the next step, STAR aligner was utilized for read mapping against the human genome (hg38, downloaded May 8, 2017). The mapped reads were further processed using htseq-count24 in strict mode to access raw read counts for every gene.
Two samples have been excluded during quality control. Some of the remaining 98 samples had to be resequenced due to low read count. Throughout all sequencing and resequencing steps we ensured the minimization of batch effects by randomly distributing the samples over all 15 different flow cells. In total, we ended up with 73 cases and 25 controls.
Statistics
The unique Ensemble identifiers (IDs) were mapped to gene symbols using the R package “org.Hs.eg.db.”25 In case the package was missing a gene symbol the Ensemble ID was replaced with a unique number. During the mapping process, about 25% of the Ensemble IDs could not be mapped to a gene symbol. Note that the gene symbols are solely used for result visualization, whereas all analyzes have been performed based on the unambiguous Ensemble IDs. We used EdgeR26 to process the raw read counts and scan for significant genes for the different lesion types. All samples have been normalized for library sizes. Five generalized linear models were trained to reveal differentially expressed genes between control (WM) and each of the five lesions types (NAWM, active, chronic active, inactive, and remyelinating). All models were adjusted for age and gender. Our models additionally account for lesion distribution, since from every patient multiple samples of the same lesion types have been extracted and used. We obtain, for every lesion, a list of genes with the corresponding log2-fold-changes (logFC) and the p-value corrected for multiple testing using false discovery rate (FDR)-correction (Benjamini–Hochberg).27
The MS Atlas database and online analysis platform
The processed data were then integrated into a database, that we make publicly available to the research community together with a web-interface based on RShiny.28 Besides data download, the platform offers three major visualization tools to compare the different lesion types and extract markers at different levels in the system biology value chain. Heatmaps and volcano plots allow for the extraction of gene panels associated with lesion type. Network enrichment methodology (i.e., KeyPatwhayMiner29,30) enables the identification of mechanistic (i.e., subnetwork-based) markers. We integrated the human protein–protein interaction network from the Integrated Interactions Database31 filtered for only brain tissue-specific interactions with experimental evidence, orthologous mice genes, and computational prediction. The MS Atlas online platform produces visualizations on-the-fly using a variety of R packages (Table 2).
Table 2.
R-Packages
Code availability
The code used for the analysis of differential gene expression as well as the source code for the online tool are available as GitHub repository (https://github.com/frischt/msatlas). Used R packages with specified version can be found in Table 2.
Data Records
The raw data of all 98 samples can be downloaded from the gene expression omnibus (GEO) database (GSE138614) as FASTQ files. In addition, the raw read counts as well as the results of differential expression calling with edgeR are available as text files. The GEO data set is accompanied by the standard excel file giving detailed information about each sample, including patient ID and lesion type.
Technical validation: drug target expression during lesion genesis
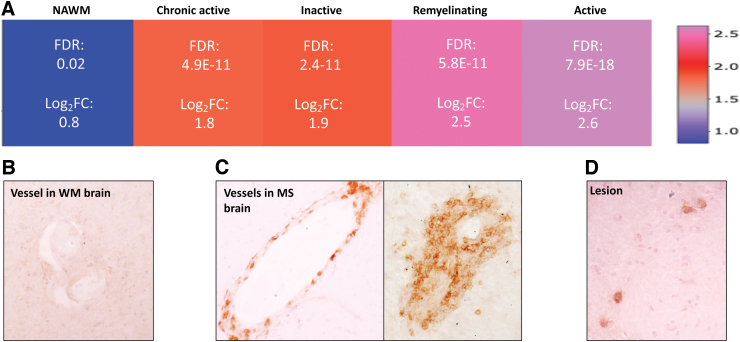
Natalizumab is a monoclonal antibody used in the treatment of RMS patients.32 It blocks the alpha4beta1 integrin (VLA-4)-mediated trafficking of pathogenic lymphocytes through the blood–brain barrier, and prevents inflammation in the CNS.33 Although natalizumab is one of the most effective treatments in RMS patients,34 its efficacy in PMS is limited.35 Our database and validation by immunohistochemistry indicate that VLA-4 is highly expressed in active lesions even in the PMS phase, but it is significantly upregulated in all lesion types compared with the NAWM (Fig. 2); the limited efficacy in the ASCEND clinical trial of PMS may be related to the increasing number of chronic active lesions in this phase of the disease with less expression of VLA-4 compared with the active lesions.36,37 Alternatively, additional mechanisms of inflammation that are unrelated to VLA-4 and/or increasing dominance of pathways independent of inflammation may contribute.38
FIG. 2.
Expression of drug target in the MS Atlas and evaluation on protein level. (A) Gene expression pattern of VLA-4 (drug target) in NAWM, active-, inactive-, remyelinating-, and chronic active lesion extracted from the MS Atlas. (B) Immunohistochemistry of VLA-4 in WM brain tissue, from non-neurological disease. (C) High protein expression of VLA-4 in vessels of MS brain tissue. (D) Detection of VLA-4 in lesions of PMS brain tissue. WM, white matter.
Usage Notes
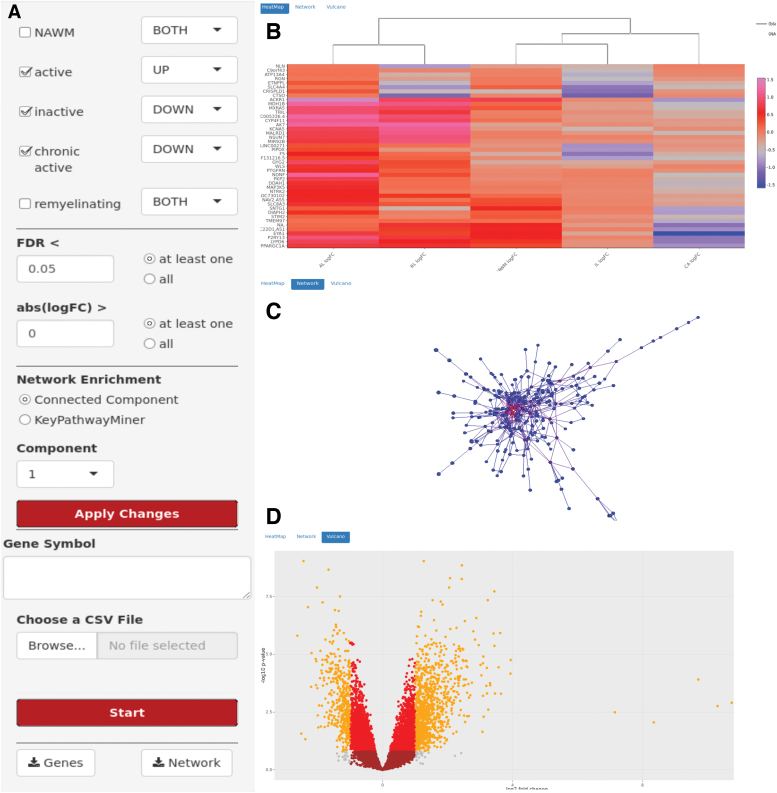
MS Atlas web interface offers three major visualization themes: heatmaps, mechanistic candidate networks, and volcano plots. The user can adjust several parameters (Fig. 3A) to choose statistical significance levels of the profiled genes. Initially, the user selects a (set of) lesion type(s) of interest. The user is further asked to choose whether the interest is in up-, down-, or overall deregulated genes. To study the evolution and development of lesion types, one might, for example, filter for genes downregulated in chronic but upregulated in inactive lesions. In the next step those genes can be sorted by FDR-corrected p-value and filtered for a minimal logFC value. The user may also directly search for a specific gene of interest and check for its expression changes across different lesion types. The MS Atlas platform will then visualize the gene's expression across the selected lesions in a heatmap (Fig. 3B). Genes and lesions are ordered based on a hierarchical clustering using the Euclidean distance metric. The color represents logFC (lesion vs. control). The individual logFC of a gene in a lesion is shown in a tool-tip when the mouse is hovering over the corresponding field. All data shown in the heatmap can be exported as CSV file or PNG image.
FIG. 3.
Web-interface of the MS Atlas. (A) The MS Atlas web interface offers adjustment for statistical parameters (e.g., lesion types, FDR, and logFC) and subnetwork extraction. Significant genes will be visualized in a (B) heatmap, (C) mechanistic candidate network, and (D) volcano plot.
Furthermore, to suggest potential mechanistic markers putatively driving lesion type evolution, selected genes can be projected onto the human protein–protein interaction network (Fig. 3C). Since the network contains >400,000 interactions and 13,000 genes, displaying the full network would not help extracting useful information. Instead, we integrated the de novo network enrichment method KeyPathwayMiner, which extracts subnetworks that distinguish, on a mechanistic level, between MS lesion types and, thus, provides first hints on how lesion evolution is driven and controlled on a systems biology level. We allow to specify a number of exception genes (k), which do not necessarily have to be significantly differentially expressed between lesion types (i.e., outliers) but still play a central role in the interaction network. A mouse click on a node/gene in the network reveals additional information. The key networks can be exported in SIF format for downstream analyses in Cytoscape39 or as PNG image file.
Finally, the platform allows for on-the-fly visualization of volcano plots for all genes of a selected lesion (Fig. 3D), where two thresholds have been chosen (FDR <0.05 and logFC >1.5) to color-code the genes accordingly.
Abbreviations Used
- CNS
central nervous system
- FDR
false discovery rate
- HLA-DR+
human leukocyte antigen D related
- logFC
log2-fold-changes
- MS
multiple sclerosis
- NAWM
normal appearing white matter
- PMS
progressive multiple sclerosis
- RMS
relapsing multiple sclerosis
- UKMSTB
UK Multiple Sclerosis Tissue Bank
- WM
white matter
Author Disclosure Statement
Z.I. reports personal fees from Biogen, Sanofi-Genzyme, Merck, and Novartis, outside the submitted study. The other authors declare that they have no competing interests.
Funding Information
Lundbeckfonden R118-A11472 (to Z.I.), Lundbeckfonden R260-2017-1247 and R296-2018-2502 (to M.L.E.), Scleroseforeningen R458-A31829-B15690 and R487-A33600-B15690 (to Z.I.), Region of Southern Denmark 14/24200 (to Z.I.), Jascha Fonden 5589 (to Z.I.), Direktør Ejnar Jonasson kaldet Johnsen og hustrus mindelegat 5609 (to Z.I.), Odense University Hospital 29A-1501 (to Z.I.), Sanofi-Genzyme REG-NOBA-COMPL-SD-017 (to Z.I.), and FIKP 2 theme 20765/3/2018/FEKUTSTRAT (to Z.I.). J.B. and T.F. are grateful for financial support from Braumbach's VILLUM Young Investigator grant no. 13154. J.B.'s study was also funded by H2020 project no. 777111 (REPOTRIAL).
Cite this article as: Frisch T, Elkjaer ML, Reynolds R, Michel TM, Kacprowski T, Burton M, Kruse TA, Thomassen M, Baumbach J, Illes Z (2020) Multiple sclerosis atlas: a molecular map of brain lesion stages in progressive multiple sclerosis, Network and Systems Medicine 3:1, 122–129, DOI: 10.1089/nsm.2020.0006.
References
- 1. Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson DW, Ellenberg JH, Leventhal CM, et al. Revised estimate of the prevalence of multiple sclerosis in the united states. Ann Neurol. 1992;31:333–336 [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez M, Noseworthy JH, Lucchinetti C, Weinshenker BG. Multiple sclerosis. N Engl J Med. 1992;343:938–952 [DOI] [PubMed] [Google Scholar]
- 4. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517 [DOI] [PubMed] [Google Scholar]
- 5. Alroughani R, Ahmed SF, Behbehani R, et al. Increasing prevalence and incidence rates of multiple sclerosis in Kuwait. Mult Scler J. 2014;20:543–547 [DOI] [PubMed] [Google Scholar]
- 6. Bentzen J, Flachs EM, Stenager E, Brønnum-Hansen H, Koch-Henriksen N. Prevalence of multiple sclerosis in Denmark 1950–2005. Mult Scler J. 2010;16:520–525 [DOI] [PubMed] [Google Scholar]
- 7. Becker KG, Mattson DH, Powers JM, et al. Analysis of a sequenced cDNA library from multiple sclerosis lesions. J Neuroimmunol. 1997;77:27–38 [DOI] [PubMed] [Google Scholar]
- 8. Whitney LW, Becker KG, Tresser NJ, et al. Analysis of gene expression in multiple sclerosis lesions using cDNA microarrays. Ann Neurol. 1999;46:425–428 [DOI] [PubMed] [Google Scholar]
- 9. Baranzini SE, Elfstrom C, Chang S-Y, et al. Transcriptional analysis of multiple sclerosis brain lesions reveals a complex pattern of cytokine expression. J Immunol. 2000;165:6576–6582 [DOI] [PubMed] [Google Scholar]
- 10. Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735 [DOI] [PubMed] [Google Scholar]
- 11. Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508 [DOI] [PubMed] [Google Scholar]
- 12. Mycko MP, Papoian R, Boschert U, et al. cDNA microarray analysis in multiple sclerosis lesions: detection of genes associated with disease activity. Brain. 2003;126:1048–1057 [DOI] [PubMed] [Google Scholar]
- 13. Mycko MP, Brosnan CF, Raine C, et al. Transcriptional profiling of microdissected areas of active multiple sclerosis lesions reveals activation of heat shock protein genes. J Neurosci Res. 2012;90:1941–1948 [DOI] [PubMed] [Google Scholar]
- 14. Tajouri L, Mellick A, Ashton K, et al. Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res Mol Brain Res. 2003;119:170–183 [DOI] [PubMed] [Google Scholar]
- 15. Lindberg R. Multiple sclerosis as a generalized CNS disease? Comparative microarray analysis of normal appearing white matter and lesions in secondary progressive MS. J Neuroimmunol. 2004;152:154–167 [DOI] [PubMed] [Google Scholar]
- 16. Zeis T, Graumann U, Reynolds R, Schaeren-Wiemers N. Normal-appearing white matter in multiple sclerosis is in a subtle balance between inflammation and neuroprotection. Brain. 2008;131:288–303 [DOI] [PubMed] [Google Scholar]
- 17. Cunnea P, McMahon J, O'Connell E, et al. Gene expression analysis of the microvascular compartment in multiple sclerosis using laser microdissected blood vessels. Acta Neuropathol. 2009;119:601–615 [DOI] [PubMed] [Google Scholar]
- 18. Han MH, Lundgren DH, Jaiswal S, et al. Janus-like opposing roles of cd47 in autoimmune brain inflammation in humans and mice. J Exp Med. 2012;209:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waller R, Woodroofe N, Wharton SB, et al. Gene expression profiling of the astrocyte transcriptome in multiple sclerosis normal appearing white matter reveals a neuroprotective role. J Neuroimmunol. 2016;299:139–146 [DOI] [PubMed] [Google Scholar]
- 20. Elkjær M, Frisch T, Reynolds R, et al. Molecular signature of different lesion types in the brain white matter of patients with progressive multiple sclerosis. Acta Neuropathol Commun. 2019;7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds R, Roncaroli F, Nicholas R, et al. The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol. 2011;122:155–170 [DOI] [PubMed] [Google Scholar]
- 22. Andrews S. FastQC: a quality control tool for high throughput sequence data, 2010. Available at www.bioinformatics.babraham.ac.uk/projects/fastqc
- 23. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anders S, Theodor Pyl P, Huber W. HTseq—a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carlson M. org.Hs.eg.db: Genome Wide Annotation for Human. R package version 3.5.0, 2018. Available at https://bioconductor.org/packages/org.Hs.eg.db
- 26. McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300 [Google Scholar]
- 28. Chang W, Cheng J, Allaire JJ, et al. shiny: Web Application Framework for R. R package version, 1(0), 2018. Available at https://shiny.rstudio.com
- 29. List M, Alcaraz N, Dissing-Hansen M, et al. Keypathwayminerweb: online multi-omics network enrichment. Nucleic Acids Res. 2016;44(W1):W98–W104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alcaraz N, Mcginty H, Weile J, et al. Keypathwayminer: detecting case-specific biological pathways using expression data. Internet Math. 2011;7:299–313 [Google Scholar]
- 31. Kotlyar M, Pastrello C, Sheahan N, Jurisica I. Integrated interactions database: tissue-specific view of the human and model organism interactomes. Nucleic Acids Res. 2015;44(D1):D536–D541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clerico M, Artusi C, Di Liberto A, et al. Long-term safety evaluation of natalizumab for the treatment of multiple sclerosis. Exp Opin Drug Safety. 2017;16:963–972 [DOI] [PubMed] [Google Scholar]
- 33. Engelhardt B, Kappos L. Natalizumab: targeting alpha-4-integrins in multiple sclerosis. Neurodegenerat Dis. 2008;5:16–22 [DOI] [PubMed] [Google Scholar]
- 34. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910 [DOI] [PubMed] [Google Scholar]
- 35. Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17:405–415 [DOI] [PubMed] [Google Scholar]
- 36. Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78:710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luchetti S, Fransen NL, van Eden CG, et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135:511–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2019;9:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wickham H. conflicted: An Alternative Conflict Resolution Strategy. R package version 0.1.0, 2018. Available at https://cran.r-project.org/web/packages/conflicted/index.html
- 41. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York, NY: 2016 [Google Scholar]
- 42. Sievert C, Parmer C, Hocking T, et al. plotly: Create Interactive Web Graphics via “plotly.js.” R package version 4.7.1, 2017. Available at https://plotly.com
- 43. Sali A. shinycssloaders: Add CSS Loading Animations to “shiny” Outputs. R package version 0.2.0, 2017. Available at https://cran.r-project.org/web/packages/shinycssloaders/index.html
- 44. Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40:1–29 [Google Scholar]
- 45. Galili T, O'Callaghan A, Sidi J, Sievert C. heatmaply: an R package for creating interactive cluster heatmaps for online publishing. Bioinformatics. 2018;34:1600–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almende BV, Thieurmel B, Robert T. visNetwork: Network Visualization Using “vis.js” Library. R package version 2.0.9, 2019. Available at https://datastorm-open.github.io/visNetwork/