Abstract
Background
Paroxysmal atrial fibrillation (pAF) recurrence after radiofrequency catheter ablation (RFCA) is linked to low-voltage zone (LVZ). This study explored whether serum soluble ST2 (sST2) levels can predict the size of LVZs in patients with pAF.
Material/Methods
A total of 177 patients with pAF treated with RFCA were consecutively enrolled in this study. One hundred twenty-five patients (70.6%) with <20% LVZ were assigned to Group A, and 52 patients (29.4%) with a LVZ >20% were assigned to Group B. Levels of soluble ST2 (sST2), growth and differentiation factor (GDF-15) and tissue inhibitor of MMP1 (TIMP-1) were measured.
Results
The sST2 levels were higher in Group B than in Group A (23.9±3.3 vs. 30.9±5.0 ng/mL, P<0.000). In multivariable logistic regression analysis, sST2 was the only independent parameter for predicting left atrial LVZ (odds ratio, 1.611 [1.379–1.882]; P<0.001). The cut-off value of sST2 obtained by receiver operating characteristic (ROC) analysis was 26.65 ng/mL for prediction of LVZ (sensitivity: 86.5%, specificity: 84.8%). The under-curve area was 0.895 (0.842–0.948) (P<0.001). At 12-month follow-up, patients with sST2 <26.65 ng/mL had more patients free from atrial arrhythmias compared to patients with sST2 >26.65 ng/mL (88.6% vs. 69.8%, P<0.01).
Conclusions
We demonstrated that sST2 levels are higher in pAF patients with LVZ >20% compared to those with a smaller LVZ. Also increased sST2 levels can serve as a novel predictor of AF recurrence rate in patients who have undergone RFCA.
MeSH Keywords: Atrial Fibrillation, Biological Markers, Catheter Ablation
Background
Radiofrequency catheter ablation (RFCA) has become first-line treatment for patients with paroxysmal atrial fibrillation (pAF) who are intolerant or refractory to antiarrhythmic medication [1]. AF recurrence is a major complication after RFCA.
AF is a critical marker for predicting circumferential pulmonary vein isolation (CPVI) outcome [2,3]. The DECAAF Study showed that the recurrence rate in patients with AF area >20% was approximately 47.1% [4]. Yamaguchi et al. also emphasized that the survival rate was significantly reduced in patients with low-voltage zone (LVZS) >20% [5].
Some studies have provided evidence that late gadolinium enhancement (LGE)-magnetic resonance imaging (MRI) can be used to evaluate left atrial (LA) fibrosis [6,7]. However, this method requires initial visualization using a volume-rendering tool in Corview (Marrek), which is crucial for assessment. Although MRI is an advanced technique, it is costly and not extensively practiced in the clinic. Bipolar voltage mapping with electrophysiology analysis is a good standard for accurate and reliable detection and quantification of AFs [8–10]. Stegmann et al. also used 20% as a boundary to evaluate the relationship between low-voltage area in LGE and LVZ in three-dimensional electroanatomic mapping [11]. However, measuring AF requires an invasive procedure. Thus, searching for a simplified clinical method is imperative. As a result, identifying new biomarkers has become an important goal.
Some studies have yielded meaningful results. A number of biomarkers have been implied to be associated with cardiac fibrosis. A study [12] evaluating the association between extracellular matrix regulatory factors and AF risk showed that increased tissue inhibitors of metalloproteinases (TIMP) levels were significantly associated with AF risk. Growth differentiation factor (GDF-15) is a tumor growth factor-β family member. Marin’s research concludes that GDF-15 is regarded as a useful biomarker for risk stratification in AF [13]. Serum soluble ST2 (sST2) is a family member of the interleukin-1 receptors. Ma et al. show that sST2 is probably an objective biomarker that can predict AF patients’ risk of emergency admission or heart failure (HF), and elevated sST2 concentration may be involved in progression of AF [14]. However, TIMP-1, GDF-15, and sST2 have not been investigated for evaluating LA LVZ in AF patients. In this study, we aimed to explore whether there is a positive relationship between LA LVZ and levels of TIMP-1, GDF-15, and sST2 in serum.
Material and Method
Study design and population
During the period between January 2018 and December 2018, a total of 183 consecutive patients with pAF who underwent RFCA in the Cardiology Department of Beijing Anzhen Hospital were enrolled in this trial (Figure 1).
Figure 1.
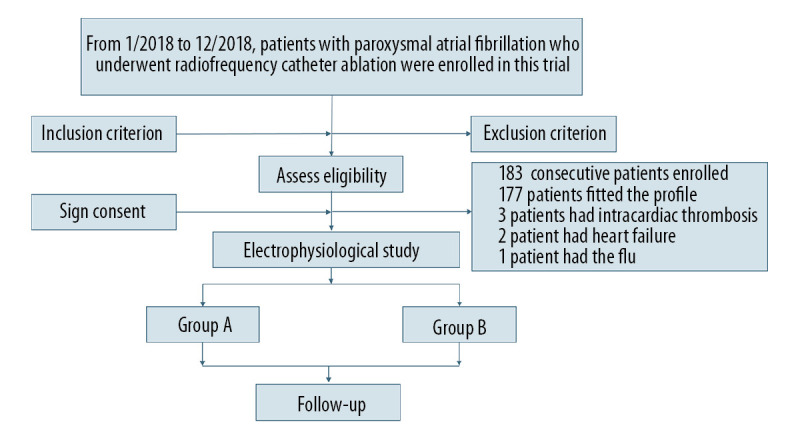
Flow diagram showing population selection and study design.
This study was approved by the Medical Ethics Committee of Beijing Anzhen Hospital. All participants evaluated in this trial signed written informed consent.
Inclusion and exclusion criteria
Patients aged 18 to 80 years with symptomatic pAF intolerant of or refractory to antiarrhythmic medication or who wanted to take RFCA were eligible. (Based on duration of AF, patients diagnosed with pAF were defined as having AF terminating spontaneously or onset lasting <7 days with intervention).
Patients who had any of the following were excluded: (a) LA appendage thrombosis; (b) abnormal cardiac structures disease (i.e. severe mitral, tricuspid, aortic malformation); (c) HF; (d) history of RFCA, cryoballoon ablation, and cardiac surgical procedures; (e) mental disease; (f) estimated glomerular filtration rate (eGFR) <30 mL/min; (g) septic shock; (h) advanced malignant tumor; (i) pregnancy; (j) cardiac tamponade or major hydropericardium; or (k) LA anteroposterior diameter >50 mm.
Data collection
Data on demographics including gender and age were collected from the hospital database. Blood samples for TIMP-1, GDF-15 and sST2 testing were collected from each participant in sodium citrate tubes 1 day prior to the operation when patients were in sinus rhythm, then centrifuged, and plasma was stored at −80°C. Baseline clinical characteristics of all participants, including body mass index (BMI); history of coronary heart disease (CHD); alanine transaminase, aspartate aminotransferase, eGFR, creatinin, uric acid, glucose (GLU), total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride levels; LA anteroposterior (LAAP) diameter; and ejection fraction (EF) were collected from medical records and confirmed by study physicians.
Voltage mapping and RFCA
Voltage mapping procedures were performed on patients under local anesthesia. Electrode placement, transseptal puncture and CPVI were performed for every participant according to standard protocols. Briefly, after performing bilateral femoral vein punctures to obtain access to the heart, two 6-F introducer sheathes (Input; Medtronic, Minneapolis, United States) were inserted into the left femoral vein and two guidewires for the long sheath were inserted into the right femoral vein. A 10-polar diagnostic catheter was then inserted into the coronary sinus (CS) using fluoroscopic guidance, and transseptal puncture was performed twice and two non-steerable long sheathes were placed into the LA. A mapping catheter (PentaRay®; Biosense Webster, Diamond Bar, California, United States) and an ST ablation catheter (Thermocool smarttouch®; Biosense Webster, Diamond Bar, California, United States) were inserted into the LA through non-steerable long sheathes, followed by voltage mapping conducted using PentaRay. Point-by-point CPVI was performed using irrigated ablation catheters (Thermocool Smarttouch; Biosense Webster, Diamond Bar, California, United States) in a power control mode at 35 W (irrigation flow 17 mL/min). RFCA was performed using Visitag (Carto, Biosense Webster) guidance with catheter stability (2.5 mm for 3 s) and contact force (CF; >3 g for 25% of time) settings. The ablation index (AI) had a target value of 500 for the anterior LA wall and 400 for the posterior wall. During the procedure, heparin was delivered to maintain an activated clotting time between 300 and 350 s.
Participant grouping
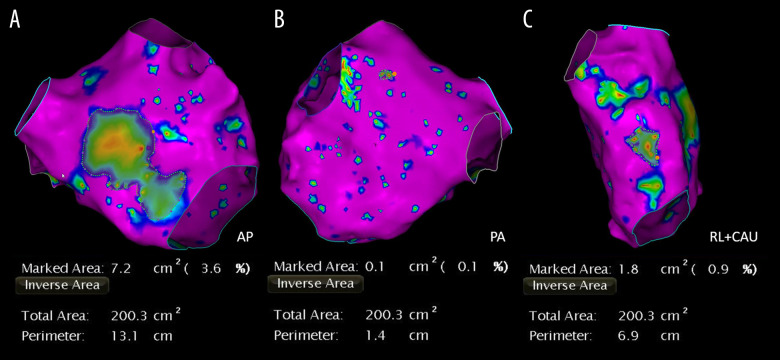
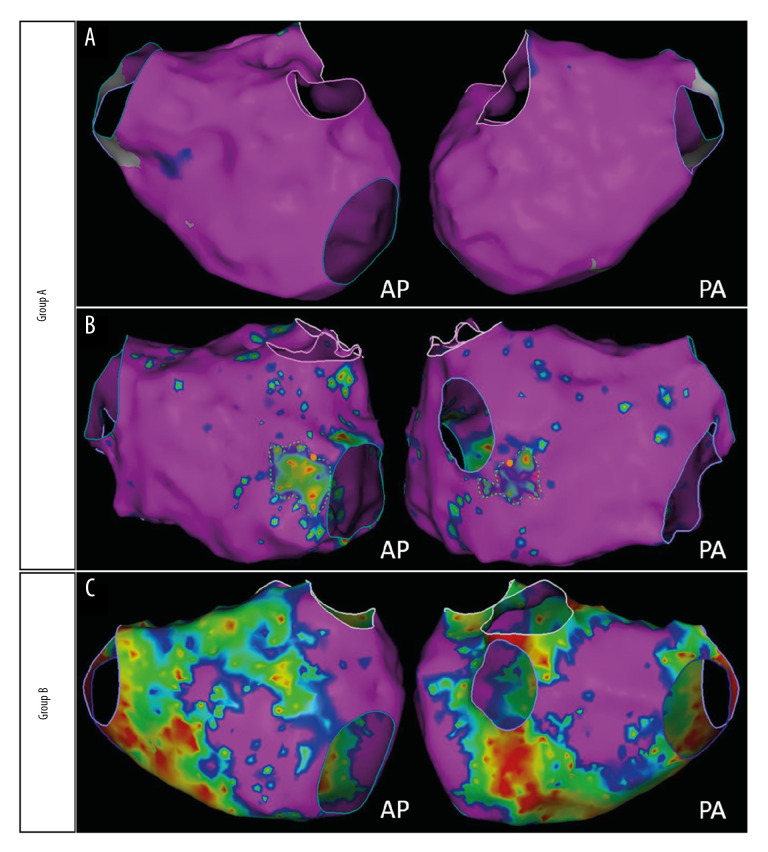
According to results from bipolar voltage mapping, areas were defined using different colors as follows: (1) normal substrate (purple): bipolar voltage >0.5 mV in contiguous areas; (2) LVZ (colorful): bipolar voltage <0.5 mV in contiguous areas (Figure 2). The area of LA LVZ was measured offline within each region separately using the Carto system and presented in cm2 (total area refers to the entire LA area except for the pulmonary veins and mitral valve) (Figure 2). Many studies show that after RFCA, the recurrence rate of patients with an LA LVZ >20% was approximately 47.1% [4,15,16]. Based on those findings, patients were divided into Group A (no LVZ or <20%) and Group B (LVZ >20%) after quantification of the LA LVZ was obtained using bipolar voltage mapping (Figure 3).
Figure 2.
Low-voltage zone measurement using bipolar voltage mapping. Purple areas indicate normal LA substrate and colorful areas indicate low voltage zone. Total area refers to the entire left atrial area except for the pulmonary veins and mitral valve. Areas are measured in the AP (A), PA (B), and RL+CAU (C) positions.
Figure 3.
Grouped according to the size of the LA LVZ. Group A: patients with few or even none LVZ (A) and LA LVZ less than 20% (B). Group B: patients with LVZ greater than 20% (C).
Follow-up and clinical outcomes
Outpatients were followed up through hospital visit at 3, 6, and 12 months postoperatively. During this follow-up period, patients’ vital signs were recorded and cardiac ultrasound and 24-hour Holter monitoring was performed. AF recurrence was defined as recorded episodes of AF, atrial flutter (AFL), or atrial tachycardia (AT) lasting for at least 30 s 3 months after CPVI.
Primary outcomes
The effectiveness of serum levels of TIMP-1, GDF-15 and sST2 in predicting LVZ served as the primary outcomes in this study. TIMP-1, GDF-15, and sST2 levels were measured using enzyme-linked immunosorbent assay kits (bsk11022, bsk11045 and bsk11100; Bioss antibodies). The limit of detection for sST2 was 15 pg/mL, with a mean inter-assay coefficient of variation <9.0%. Technicians were blind to the baseline patient characteristics.
Secondary outcome
Recurrence and composite outcome events served as the secondary outcome in this study. Recurrence was defined as the rate of pAF and composite outcome events at 12-month follow-up. Composite outcome events included: 1) major adverse events during the procedure (cardiac perforation, hydropericardium, malignant arrhythmias, sudden cardiac death, atrial-esophageal fistula, and acute myocardial infarction); and 2) rehospitalization rate (caused by AF, HF, and/or other heart-related complications).
Statistical analysis
Data were analyzed using SPSS statistical software (IBM, Version 23) and R (version 3.6.3). Continuous variables with normal distribution are expressed as mean ± standard deviation (SD), and non-uniformly distributed variables are expressed as median (Q1, Q3). Comparisons of means between groups were analyzed using the independent sample t-test for normally distributed data and the Mann-Whitney U-test for non-uniformly distributed data. The χ2 test was used to compare categorical variables. Results are presented as either the relative or absolute change in the marker with a 95% CI or P value. P<0.03 was considered statistically significant in the primary outcomes, and P<0.02 was considered statistically significant in the secondary outcome.
Factors associated with low-voltage zone were tested by univariate and binary logistic regression analyses. Variables with P<0.1 in the univariate analysis were tested in the multivariate model. Results were expressed as the P value and odds ratio (OR) in confidence interval (CI) of 95%. Receiver operating characteristic (ROC) analysis was done to determine the cut-off value of sST2 to predict LVZ. Hosmer-Lemeshow χ2 test was used to assess model goodness-of-fit.
Results
Baseline clinical materials
The study included 177 patients (male: 123, 69.5%) who underwent RFCA. According to reference standard of LA) LVZ, 125 patients were assigned to Group A (no LVZ or <20%), and 52 patients were assigned to Group B (LVZ >20%).
Patients with low-voltage area >20% were older (P<0.03) and had higher eGFR and EF (Table 1).
Table 1.
Comparison of baseline clinical materials.
| Group A (n=125) | Group B (n=52) | P valve | |
|---|---|---|---|
| Age, y | 56.4±9.4 | 62.4±9.3 | 0.002 |
| Male, n (%) | 87 (69.6%) | 36 (69.2%) | 0.961 |
| BMI, kg/m2 | 31.4±7.0 | 32.3±8.7 | 0.501 |
| AF duration, y | 24 (12, 48) | 24 (12, 48) | 0.789 |
| Hypertension, n (%) | 70 (56.0%) | 27 (51.9%) | 0.620 |
| Diabetes, n (%) | 24 (19.2%) | 8 (6.4%) | 0.548 |
| CHD, n (%) | 16 (12.8%) | 3 (5.8%) | 0.169 |
| ALT, U/L | 23.9±10.2 | 25.9±10.2 | 0.646 |
| AST, U/L | 24.1±8.7 | 22.7±5.8 | 0.284 |
| eGFR | 92.0±18.4 | 98.2±19.1 | 0.046 |
| CREA, mol/L | 72.0±16.8 | 69.1±14.8 | 0.272 |
| UA, mol/L | 343.4±100.9 | 340.7±92.0 | 0.865 |
| GLU, mmol/L | 5.9±1.6 | 5.2±0.8 | 0.093 |
| TCHO, mmol/L | 4.7±1.7 | 4.6±1.2 | 0.682 |
| LDL, mmol/L | 2.9±1.0 | 2.9±1.0 | 0.888 |
| HDL, mmol/L | 1.2±0.2 | 1.1±0.3 | 0.278 |
| TG, mmol/L | 1.8±1.1 | 1.7±0.9 | 0.494 |
| hs CRP, mg/L | 0.9 (0.6,2.1) | 1.0 (0.6,2.0) | 0.964 |
| LAAP-diameter, mm | 40.4±5.1 | 41.6±5.0 | 0.157 |
| EF,% | 61.6±6.0 | 60.3±5.5 | 0.078 |
Normally distributed continuous variables are expressed as mean±standard deviation; non-uniformly distributed data are expressed as median (Q1, Q3); numbers are presented as number of patients (%). BMI – body mass index; AF – atrial fibrillation; CHD – coronary heart disease; ALT – alanine transaminase; AST – aspartate aminotransferase; eGFR – estimated glomerular filtration rate; CREA – creatinine; UA – uric acid; GLU – glucose; TCHO – total cholesterol; LDL – low-density lipoprotein; HDL – high-density lipoprotein; TG – triglyceride; LAAP-diameter – left atrial anteroposterior diameter; EF – ejection fraction.
Primary outcomes
Serum sST2 concentration was significantly lower in Group A than in Group B (23.9±3.3 vs. 30.9±5.0, P<0.000) (Table 2). However, there was no meaningful difference in serum TIMP-1 and GDF-15 levels between the groups (82.2±24.1 vs. 84.4±27.9; 3.0±0.1 vs. 3.0±0.1, P>0.05) (Table 2).
Table 2.
Comparison of primary outcomes.
| Group A (n=125) | Group B (n=52) | P valve | |
|---|---|---|---|
| TIMP-1 | 82.2±24.1 | 84.4±27.9 | 0.612 |
| Log10(GDF-15), ng/ml | 3.0±0.1 | 3.0±0.1 | 0.215 |
| sST2, ng/ml | 23.9±3.3 | 30.9±5.0 | 0.000 |
Normally distributed continuous variables are expressed as mean ± standard deviation; non-uniformly distributed data are expressed as median (Q1, Q3). Hs CRP – high-sensitive C-reactive protein; sST2 – soluble ST2; GDF-15 – growth differentiation factor 15.
Multivariate relationships of LA LVZ
Age, eGFR, GLU, EF, and sST2 were related to LA LVZ. In multivariable logistic regression analysis, sST2 was the only independent parameter for predicting LA LVZ (1.611 [1.379–1.882]; P<0.001) (Table 3).
Table 3.
Multivariate relationships of LA LVZ.
| Variables | OR | β | P |
|---|---|---|---|
| Age (years) | 1.039 [0.984–1.097] | 0.039 | 0.163 |
| eGFR, mol/L | 1.021 [0.996–1.046] | 0.021 | 0.101 |
| GLU, mmol/L | 0.716 [0.442–1.158] | −0.335 | 0.173 |
| EF,% | 0.274 [0.000–685.477] | −1.294 | 0.746 |
| sST2, ng/ml | 1.611 [1.379–1.882] | 0.477 | <0.001 |
LA – left atrial; OR – odds ratio; sST2 – soluble ST2.
ROC curve analysis to determine predictive value of sST2 for LA LVZ
The cut-off value for sST2 obtained by ROC curve analysis was 26.65 ng/mL for prediction of LA LVZ (sensitivity: 86.5%, specificity: 84.8%). Area under the curve was 0.895 (0.842–0.948) (P<0.001, Figure 4). We also used Hosmer-Lemeshow χ2 test and confirmed that goodness-of-fit was acceptable (P=0.387).
Figure 4.
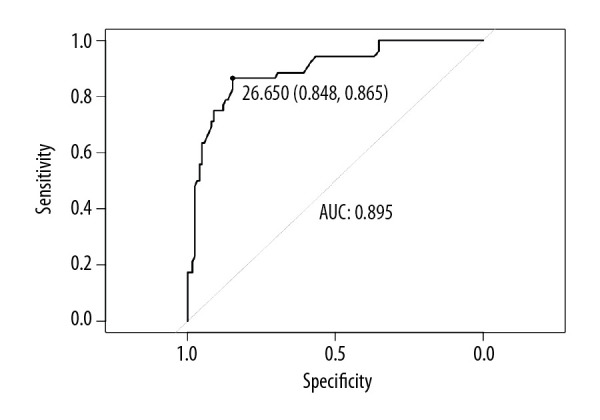
ROC curve analysis predictive value of sST2 of LA LVZ. sST2=26.65 ng/mL; sensitivity=86.5%; specificity=84.8%; AUC=0.895; 95% CI=0.842–0.948, P<0.001. AUC – area under the curve; CI – confidence interval; ROC – receiver operating characteristic; sST2 – soluble ST2.
Evaluating accuracy of sST2 for diagnosis LA LVZ
Table 4 shows sST2 level in relation to diagnosis of LA LVZ. The number of true positives, false positives, false negatives and true negatives can be easily identified.
Table 4.
Contingency table for evaluating the accuracy of sST2 for diagnosis LA LVZ.
| Voltage mapping | sST2 | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 44 | 8 | 52 |
| Negative | 19 | 106 | 125 |
| Total | 63 | 114 | 177 |
Comparison of secondary outcome between two groups
We also conducted a 12-month follow-up after the procedure to evaluate the prediction value of sST2 in secondary outcomes (recurrence and composite outcome events). Patients were divided into Group 1 (sST2 <26.65 ng/mL) and Group 2 (sST2 >26.65 ng/mL).
There were 101 patients (88.6%) in Group 1 and 44 patients (69.8%) in Group 2 that were free from atrial arrhythmias (P<0.010). In addition, six patients (5.3%) in Group 1 and five patients (7.9%) in Group 2 developed composite outcome events with no significant difference between them (P>0.010) (Table 5).
Table 5.
Comparison of secondary outcomes between sST2 levels.
| Group 1 (n=114) | Group 2 (n=63) | P valve | |
|---|---|---|---|
| No atrial fibrillation, % | 101 (88.6%) | 44 (69.8%) | 0.002 |
| Composite outcome events, % | 6 (5.3%) | 5 (7.9%) | 0.481 |
| Major adverse events, % | 0 | 0 | |
| Rehospitalization, % | 5 (4.0%) | 5 (7.9%) | |
| Atrial flutter, % | 5 (4.0%) | 4 (6.3%) | |
| Heart failure, % | 0 | 1 (1.6%) |
Discussion
In this study, sST2 was the only independent parameter for predicting LA LVZ (odds ratio, 1.611 [1.379–1.882]; P<0.001). The cut-off value of sST2 obtained by receiver operating characteristic curve analysis was 26.65 ng/mL for prediction of low voltage zone (sensitivity: 86.5%, specificity: 84.8%). The area under the curve was 0.895 (0.842–0.948) (P<0.001). At 12-month follow-up, patients with sST2 <26.65 ng/mL were more likely to be free from AF, AFL, and AT compared to patients with sST2 >26.65 ng/mL (88.6% vs. 69.8%, P<0.01).
Patients with severe atrial fibrosis who undergo RFCA have been reported to have a high AF recurrence rate. Based on standard criteria, atrial LVZ evaluated by voltage mapping mirrors the severity of atrial fibrosis. The DECAAF Study enrolled 260 patients who underwent CPVI. After full LA pathophysiological evaluation, participants were divided into four groups according to the area of atrial fibrosis. At 325-day follow-up, the recurrence rate in patients in the group with stage 3 (LA fibrosis area between 20–30%) and in patients in the group with stage 4 (LA fibrosis area >30%) was approximately 47.1% [4]. A LVZ of 20% could be used to predict the recurrence rate of RFCA [5,6,17]. Siebermair et al. used UTAH classification and also defined atrial LGE ≥20% as extensive [17].
Noninvasive examinations to diagnose atrial fibrosis remain under investigated. P wave peak time (PWPT) has been used to predict ischemic stroke in patients with pAF [18]. MRI-guided diagnosis is impractical due to its high cost, but advances in biochemistry and molecular biology have highlighted the significance of identifying novel biomarkers. Insults such as inflammation, oxidative stress, and atrial stretch can cause anisotropy and reentry by fibroblast proliferation and extracellular matrix deposition, which is reflected by changes in circulating levels of some specific molecules [19]. Compared with routine examinations, such as enhanced computed tomography scans and cardiac MRI, biomarkers with a high sensitivity and specificity have high health-economic value and have been the focus of studies in the field.
A growing body of evidence show that the sST2/IL-33 system plays an important role in hemodynamic stress in patients with non-ischemic cardiovascular disease. For instance, ST2L has been shown to interact with IL-33 to reduce fibrosis [15]. Hemodynamic disturbances in the atrial and ventricular wall stretches myocytes, which subsequently increases sST2 levels [20]. Through competitive binding with IL-33, the function of cell transmembrane receptor ST2L is downregulated. Therefore, sST2 has been used as a biomarker to screen patients with high risk of cardiovascular death and heart failure [16]. In contrast, sST2 levels have been shown to be increased in patients with AF [14]. In addition, a study has shown that sST2 levels correlate with AF recurrence in patients with pAF following cryoballoon ablation [21]. However, whether there is any correlation between the severity of atrial fibrosis and sST2 levels or whether sST2 can predict AF recurrence through assessing the degree of atrial fibrosis has not been well explored.
In this study, compared to the group with a LVZ <20% of total LA, the group with a larger LVZ had meaningful higher levels of sST2, indicating a correlation between the size of the LVZ and sST2 levels. Because the degree of atrial fibrosis was associated with the LA LVZ as revealed by intracardiac bipolar voltage mapping, we hypothesize that there is a correlation between sST2 levels and atrial fibrosis. Future studies should be designed to determine if there is a direct correlation between sST2 levels and atrial fibrosis. However, serum levels of GDF-15, a stress-responsive cytokine induced by oxidation and inflammation [22], and TIMP-1, a biomarker of atrial remodeling [23], were not markedly different between the two groups, despite some studies suggesting that GDF-15 levels were related with LA/LA appendage thrombus in patients with nonvalvular AF [24]. The discrepancy in these studies warrants further exploration.
We also compared the recurrence and composite outcome events rate at 12 months post-operatively among the two groups in our study. We found that Group A included more patients free from AF, AFL, or AT compared to Group B. However, there were no marked differences in the composite outcome events rate between them. This finding is consistent with recent studies and indicates that patients with a LVZ >20% have higher recurrence rates after RFCA compared to patients with no or <20% LVZ [6]. Given that LVZ is closely associated with atrial fibrosis and that sST2 levels are closely related with the degree of atrial fibrosis, we conclude that sST2 levels may serve as a novel biomarker to predict recurrence rates in patients with pAF after RFCA.
Limitations
This trial had a small sample size, and long-term AF recurrence rates were not analyzed, both of which compromise the strength of our findings. Atrial stunning usually occurs in patients with AF after cardioversion and the duration depends on the duration of AF, LAAP, and structural heart disease [25]. In this study, we enrolled pAF patients with LAAP <50 mm without structural heart disease. Atrial stunning may affect low-voltage area but we considered atrial stunning would not significantly affect it.
Conclusions
We demonstrated that sST2 levels are higher in patients with pAF with LVZ >20% compared to those with a smaller LVZ. We further demonstrated that increased sST2 levels can serve as a novel predictor of AF recurrence rate in patients who have undergone RFCA.
Footnotes
Source of support: Departmental sources
References
- 1.Okamatsu H, Okumura K. Strategy and outcome of catheter ablation for persistent atrial fibrillation-impact of progress in the mapping and ablation technologies. Circ J. 2017;82(1):2–9. doi: 10.1253/circj.CJ-17-1205. [DOI] [PubMed] [Google Scholar]
- 2.Moteleb A, Zarif JK, Ali AN. Incidence of atrial fibrosis in non-valvular atrial fibrillation patients and its impact on recurrence after pulmonary vein antral isolation. J Atr Fibrillation. 2018;11(1):1773. doi: 10.4022/jafib.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Niu XH, Yin X, et al. Elevated circulating fibrocytes is a marker of left atrial fibrosis and recurrence of persistent atrial fibrillation. J Am Heart Assoc. 2018;7(6):e008083. doi: 10.1161/JAHA.117.008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA. 2014;311(5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Tsuchiya T, Fukui A, et al. Impact of the extent of low-voltage zone on outcomes after voltage-based catheter ablation for persistent atrial fibrillation. J Cardiol. 2018;72(5):427–33. doi: 10.1016/j.jjcc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Chelu MG, King JB, Kholmovski EG, et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J Am Heart Assoc. 2018;7(23):e006313. doi: 10.1161/JAHA.117.006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kheirkhahan M, Baher A, Goldooz M, et al. Left atrial fibrosis progression detected by LGE-MRI after ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2020;43(4):402–11. doi: 10.1111/pace.13866. [DOI] [PubMed] [Google Scholar]
- 8.Seitz J, Bars C, Gitenay E, et al. What is the relevance of low-voltage maps to the underlying atrial scar? JACC Clin Electrophysiol. 2019;5(11):1278–79. doi: 10.1016/j.jacep.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Arentz T, Cochet H, et al. Extent and spatial distribution of left atrial arrhythmogenic sites, late gadolinium enhancement at magnetic resonance imaging, and low-voltage areas in patients with persistent atrial fibrillation: Comparison of imaging vs. electrical parameters of fibrosis and arrhythmogenesis. Europace. 2019;21(10):1484–93. doi: 10.1093/europace/euz159. [DOI] [PubMed] [Google Scholar]
- 10.Herczeg S, Walsh K, Keaney JJ, et al. Quantitative assessment of left atrial scar using high-density voltage mapping and a novel automated voltage analysis tool. J Interv Card Electrophysiol. 2019 doi: 10.1007/s10840-019-00570-7. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Stegmann C, Jahnke C, Paetsch I, et al. Association of left ventricular late gadolinium enhancement with left atrial low voltage areas in patients with atrial fibrillation. Europace. 2018;20(10):1606–11. doi: 10.1093/europace/euy013. [DOI] [PubMed] [Google Scholar]
- 12.Marin F, Roldan V. Biomarkers: GDF-15 and risk stratification in atrial fibrillation. Nat Rev Cardiol. 2015;12(1):8–9. doi: 10.1038/nrcardio.2014.190. [DOI] [PubMed] [Google Scholar]
- 13.Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: Importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22(9):987–93. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Yuan H, Luan HX, et al. Elevated soluble ST2 concentration may involve in the progression of atrial fibrillation. Clin Chim Acta. 2018;480:138–42. doi: 10.1016/j.cca.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Nortamo S, Ukkola O, Lepojarvi S, et al. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. Int J Cardiol. 2017;248:173–78. doi: 10.1016/j.ijcard.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Parikh RH, Seliger SL, Christenson R, et al. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc. 2016;5(8):e003188. doi: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siebermair J, Suksaranjit P, McGann CJ, et al. Atrial fibrosis in non-atrial fibrillation individuals and prediction of atrial fibrillation by use of late gadolinium enhancement magnetic resonance imaging. J Cardiovasc Electrophysiol. 2019;30(4):550–56. doi: 10.1111/jce.13846. [DOI] [PubMed] [Google Scholar]
- 18.Oz A, Cinar T, Kizilto Guler C, et al. Novel electrocardiography parameter for paroxysmal atrial fibrillation in acute ischaemic stroke patients: P wave peak time. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-137540. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci. 2019;4(5):640–54. doi: 10.1016/j.jacbts.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broch K, Andreassen AK, Ueland T, et al. Soluble ST2 reflects hemodynamic stress in non-ischemic heart failure. Int J Cardiol. 2015;179:378–84. doi: 10.1016/j.ijcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Okar S, Kaypakli O, Sahin DY, et al. Fibrosis marker soluble ST2 predicts atrial fibrillation recurrence after cryoballoon catheter ablation of nonvalvular paroxysmal atrial fibrillation. Korean Circ J. 2018;48(10):920–29. doi: 10.4070/kcj.2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94(21):11514–19. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanciu AE, Vatasescu RG, Stanciu MM, et al. The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine. 2018;103:63–68. doi: 10.1016/j.cyto.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Hu XJ, Zhan R, Xu S, et al. Growth differentiation factor 15 is associated with left atrial/left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Clin Cardiol. 2018;41(1):34–38. doi: 10.1002/clc.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan IA. Atrial stunning: basics and clinical considerations. Int J Cardiol. 2003;92(2–3):113–28. doi: 10.1016/s0167-5273(03)00107-4. [DOI] [PubMed] [Google Scholar]