Abstract
Purpose
To investigate whether AMP-activated protein kinase (AMPK) is required for the reduction of high mobility group box 1 (HMGB1) by exchange proteins activated by cAMP 1 (Epac1) in the retinal vasculature.
Methods
We measured AMPK phosphorylation in normal and diabetic Epac1 floxed and cdh5/Epac1 Cre mice. We also treated primary human retinal endothelial cells (RECs) in normal (5-mM) or high (25-mM) glucose with an Epac1 agonist and AMPK or insulin-like growth factor receptor binding protein 3 siRNA. We measured protein levels of AMPK, sirtuin 1 (SIRT1), and HMGB1.
Results
AMPK phosphorylation was reduced in cdh5/Epac1 Cre mice, suggesting that Epac1 regulated AMPK actions. High-glucose culturing conditions reduced AMPK levels in RECs, but the levels were increased by the Epac1 agonist, supporting the idea that Epac1 regulates AMPK. The Epac1 agonist was not able to reduce HMGB1 levels or increase SIRT1 when AMPK was blocked by AMPK siRNA, thus demonstrating that Epac1 requires AMPK to regulate SIRT1 and HMGB1.
Conclusions
Epac1 requires AMPK to increase SIRT1 and reduce HMGB1 in the diabetic retinal vasculature. This finding provides another pathway by which Epac1 may protect the retina during diabetes.
Keywords: Epac1, AMPK, inflammation, diabetic retinopathy, endothelial cells
Diabetic complication rates continue to climb, despite the best efforts to understand the pathology of the disease. In the past decade, the immune response has been recognized as a key player in diabetic retinopathy.1 High glucose has been shown to trigger a dangerous response that leads to increased high mobility group box 1 (HMGB1) activation.2,3 We recently reported that inhibition of HMGB1 by glycyrrhizin reduced retinal damage in response to high glucose.4 Additionally, exchange proteins activated by cAMP 1 (Epac1) can significantly reduce HMGB1 levels in ischemia–reperfusion and diabetic models, reducing retinal damage and inflammatory mediators.5,6 We have shown that Epac1 reduced a number of inflammatory pathways commonly associated with diabetic retinopathy.7 The mechanism by which Epac1 blocks HMGB1 actions in the retinal vasculature has yet to be determined.
We recently reported that Epac1 required sirtuin 1 (SIRT1) and insulin-like growth factor receptor binding protein 3 (IGFBP-3) to regulate HMGB1.8 Other studies have suggested that AMP-activated protein kinase (AMPK) is strongly linked to SIRT1 and may play a role in the diabetic retina,9 thus we wondered whether Epac1 could regulate AMPK. Others have reported that increasing AMPK and SIRT1 levels led to reduced inflammatory cytokines and apoptosis in a rat sepsis model.10 Similarly, the link between AMPK and SIRT1 was shown to be protective against diabetic nephropathy.11 Several different diabetic models have shown decreased AMPK levels, including neurons and muscle. These levels were improved by treatment with AMPK agonists.12 Additional work in streptozotocin and db/db mouse models showed decreased AMPK phosphorylation in dorsal root ganglion cells, leading to diabetic neuropathy.13 Because AMPK is often linked with SIRT1 actions, we questioned whether Epac1 required AMPK to regulate SIRT1 and reduce retinal inflammation via HMGB1 in the retinal vasculature.
Work on caloric restriction has shown that resveratrol actions on AMPK are Epac1 dependent,14 and other adipocyte studies have reported that increased levels of cAMP and Epac1 were associated with increased AMPK phosphorylation.15 Thus, we have focused our studies on the actions of Epac1, AMPK, and SIRT1. We hypothesize that Epac1 will increase IGFBP-3, leading to increased AMPK phosphorylation and SIRT1 activation in the retinal vasculature and in retinal endothelial cells (RECs) grown in high glucose, reducing HMGB1 actions.
Methods
Mice
All animal procedures complied with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research, were approved by the Institutional Animal Care and Use Committee of Wayne State University, and conformed to National Institutes of Health guidelines (Protocol 17-07-301). Epac1 floxed mice (B6;129S2-Rapgef3tm1Geno/J mice) and B6 FVB-Tg (cdh5-cre)7Mlia/J Cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). After two generations, Epac1 floxed mice were bred with cdh5-Cre mice to generate conditional knockout mice for Epac1, where Epac1 is eliminated in endothelial cells.5 Some mice were made diabetic with 60-mg/kg streptozotocin dissolved in citrate buffer, which was administered for 5 consecutive days, as we have done previously.5 Diabetes was accepted if glucose levels were greater than 250 mg/dL. Protein samples were taken from 3-month-old male and female Epac1 and cdh5/Epac1 Cre mice, and diabetic samples were collected after 2 months of diabetes in the mice. All mice were euthanized using a ketamine/xylazine overdose, followed by cervical dislocation.
Retinal Endothelial Cells
Primary human RECs were purchased from Cell Systems (Kirkland, WA, USA). Cells were grown in a Cell Systems medium (Complete Medium Formulated at Normal Blood Glucose Level, 5 mM) with microvascular growth supplement (MVGS), 10-µg/mL gentamycin, and 0.25-µg/mL amphotericin B (Thermo Fisher Scientific, Waltham, MA, USA). When cells reached confluence, some dishes were moved to a Cell Systems high-glucose medium (25-mM) for a minimum of 3 days prior to experiments. Only dishes prior to passage 6 were used. Cells were starved by incubation in high or normal glucose medium without the MVGS for 12 hours prior to treatments.
Cell Treatments
Some of the cells in normal or high glucose were treated with an Epac1 agonist (8-CPT-2′-O-Me-cAMP, 10 µM, 24 hours), as we have done previously.16 Additional cells in high glucose were transfected with IGFBP-3 siRNA (Dharmacon, Lafayette, CO, USA), AMPK siRNA (Dharmacon), or scrambled siRNA (Dharmacon) prior to treatment with the Epac1 agonist.
Western Blotting
Western blotting was carried out as previously described.7,8 Briefly, cell lysates or whole retina lysates were separated onto precast Invitrogen Tris-Glycine Gels (Thermo Fisher Scientific) and then blotted onto nitrocellulose membrane. After blocking in Tris-Buffered Saline Tween-20, membranes were treated with Epac1, phosphorylated AMPK (Tyr172), total AMPK, SIRT1, HMGB1 (Abcam, Cambridge, MA, USA), or beta-actin (Santa Cruz Biotechnology, Dallas, CA, USA) primary antibodies. Secondary antibodies labeled with horseradish peroxidase were also used. A chemiluminescence reagent kit (Thermo Fisher Scientific) was used to visualize antigen–antibody complexes. Images were acquired on an Azure C500 (Azure Biosystems, Dublin, CA, USA), and optical densities were determined using Image Studio Lite software (LI-COR Biosciences, Lincoln, NE, USA).
Statistics
An unpaired two-tailed t-test was used to obtain the statistics shown in Figure 1, and one-way ANOVA with Tukey's post hoc test was used for the statistical analyses shown in Figures 2 to 4. All analyses were carried out using Prism software (GraphPad, San Diego, CA, USA). Data are presented as mean ± SEM. P < 0.05 was taken as being statistically significant.
Figure 1.
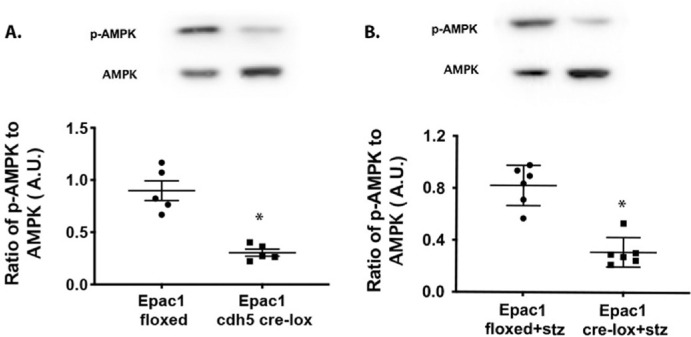
Epac1 regulates AMPK phosphorylation in the retina. Epac1 floxed and cdh5/Epac1 Cre mice (A) and diabetic Epac1 floxed and cdh5/Epac1 Cre mice (B) were used to determine if Epac1 regulates phosphorylation of AMPK in whole retinal lysates in normal and diabetic mice. *P < 0.05 versus Epac1 floxed. Data are mean ± SEM (n = 7).
Figure 2.
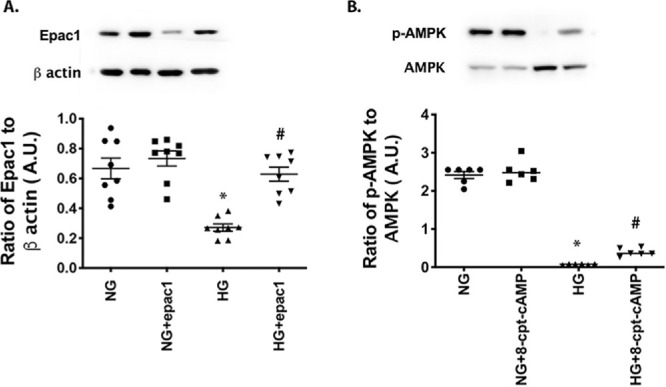
AMPK phosphorylation is regulated by Epac1 in human primary RECs. RECs were grown in normal glucose (NG) or high glucose (HG), and cells in each condition were treated with an Epac1 agonist (8-cpt-cAMP). (A) Control for the Epac1 agonist. (B) Results of western blotting comparing phosphorylated AMPK to total AMPK. *P < 0.05 versus NG; #P < 0.05 versus HG. Data are mean ± SEM (n = 7).
Figure 4.
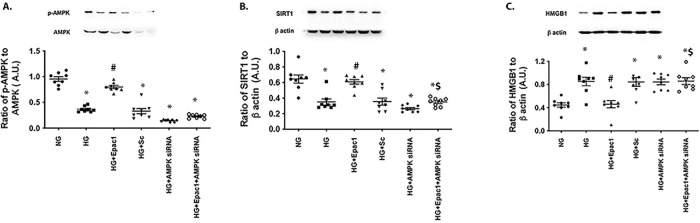
AMPK regulated SIRT1 and HMGB1 in RECs. RECs were grown in normal glucose (NG) or high glucose (HG), and cells in each condition were treated with an Epac1 agonist (8-cpt-cAMP), with AMPK siRNA and the Epac1 agonist, or with a scrambled siRNA (scsiRNA). (A) Results of western blotting comparing phosphorylated AMPK to total AMPK as a control for siRNA knockdown. (B) SIRT1 levels. (C) HMGB1 levels. *P < 0.05 versus NG; #P < 0.05 versus HG; $P < 0.05 versus 8-cpt-cAMP. Data are mean ± SEM (n = 7).
Results
AMPK Phosphorylation Is Reduced in cdh5/Epac1 Cre Mice
We have previously shown that Epac1 regulates HMGB1 in the retina and retinal endothelial cells.5,8 Because there is literature to support the observation that Epac1 can increase AMPK in other targets,15 we wanted to determine if Epac1 regulated AMPK phosphorylation in the retina. Figure 1 shows that a loss of Epac1 in the endothelial cells of the retina led to a significant decrease in the phosphorylation of AMPK, with an increase in total AMPK levels. Figure 1A illustrates the findings in normal Epac1 floxed and cdh5/Epac1 Cre mice, and Figure 1B illustrates the findings for diabetic Epac1 floxed and cdh5/Epac1 Cre mice. In both cases, a loss of Epac1 significantly reduced AMPK phosphorylation (t = 5.934 (8), P < 0.0001 and t = 6.525 (10), P < 0.001 for Figs. 1A and 1B, respectively).
Epac1 Regulates AMPK in Primary Human RECs
To confirm the in vivo data, we treated primary human RECs grown in normal (5-mM) or high (25-mM) glucose with an Epac1 agonist (8-CPT-2′-O-Me-cAMP) and measured Epac1 levels (Fig. 2A) and phosphorylation of AMPK (Fig. 2B). High-glucose culturing conditions reduced Epac1 levels (P < 0.001), which were increased by the Epac1 agonist, as we have reported previously, F(3, 28) = 16.83, P < 0.002 (Fig. 2A).7 Although high glucose reduced phosphorylation of AMPK in the RECs (P < 0.001), the Epac1 agonist was able to significantly increase AMPK phosphorylation compared to high glucose alone, F(3, 20) = 281, P < 0.03 versus high glucose only (Fig. 2B).
IGFBP-3 Regulates AMPK Phosphorylation
We have previously reported that Epac1 regulated HMGB1 through IGFBP-3 actions.8 In this study, we wanted to determine if AMPK actions were also modulated by IGFPB-3. We grew RECs in normal and high glucose and treated some cells with the Epac1 agonist or IGFBP-3 siRNA and the Epac1 agonist, followed by measurement of AMPK. Figure 3 shows that Epac1 did not increase AMPK phosphorylation when IGFBP-3 siRNA was used, F(5, 30) = 45.35, P < 0.001. This suggests that IGFBP-3 is upstream of AMPK actions in RECs grown in high glucose.
Figure 3.
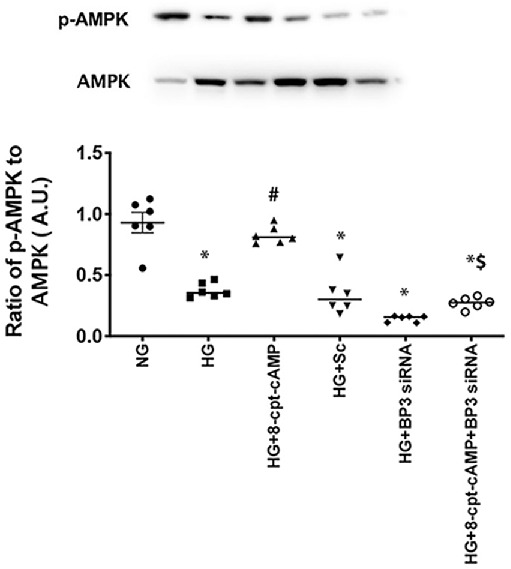
IGFBP-3 regulated AMPK. RECs were grown in normal glucose (NG) or high glucose (HG), and cells in each condition were treated with an Epac1 agonist (8-cpt-cAMP), with IGFBP-3 siRNA (BP3 siRNA) and the Epac1 agonist, or with a scrambled siRNA (scsiRNA). Results of western blotting comparing phosphorylated AMPK to total AMPK are shown. *P < 0.05 versus NG; #P < 0.05 versus HG; $P < 0.05 versus 8-cpt-cAMP. Data are mean ± SEM (n = 7).
AMPK Regulates SIRT1 and HMGB1 in RECs in High Glucose
Because we have shown that Epac1 regulates HMGB1 through increased IGFBP-3 and SIRT1 actions8 and the literature suggests that AMPK regulates SIRT1,10 we wanted to ascertain whether AMPK regulates SIRT1 and HMGB1 in our system. Figure 4 shows that Epac1 requires AMPK to regulate SIRT1 (Fig. 4B) and HMGB1 (Fig. 4C), as Epac1 did not increase SIRT1 or decrease HMGB1 when AMPK siRNA was used: F(5, 42) = 18.78, P = 0.5662 and F(5, 42) = 11.25, P > 0.999, for Figs. 4B and 4C, respectively. Figure 4A illustrates the control used to show the effectiveness of AMPK siRNA for knockdown: F(5, 42) = 103.7, P = 0.002 versus high glucose. Taken together, the data suggest that AMPK lies upstream of SIRT1 in the regulation of HMGB1 in RECs (Fig. 5).
Figure 5.

Schematic of Epac1 regulation of HMGB1 through AMPK.
Discussion
Rates of diabetes have been increasing worldwide. Although it has been suggested that a number of signaling cascades play a role in diabetic retinopathy, no therapeutics exist; thus, there remains a need to continue to seek additional targets for treatment. Several other studies have reported that inflammation is a key target for novel therapeutic regulation,1,17 and we have reported previously that Epac1 can reduce inflammatory mediators, including HMGB1.7,8 In the present study, we expanded our work to investigate whether AMPK actions are required for Epac1 regulation of HMGB1.
We chose to focus on AMPK because other studies have reported that AMPK blocks HMGB1 actions. In kidney inflammation, it has been shown that gastrodin increases AMPK actions while decreasing HMGB1.18 Similar to those on kidney inflammation, other studies have reported that free fatty acids inhibited AMPK actions and increased HMGB1 through reactive oxygen species in human umbilical vein endothelial cells (HUVECs). Inhibition of the free fatty acids in HUVECs was found to significantly increase AMPK actions, leading to a reduction in HMGB1.19 These findings are similar to what we observed in microvascular RECs. We have previously reported that SIRT1 deacetylated HMGB1, leaving it in the nucleus to reduce inflammation,8 so we sought to investigate whether AMPK was involved in this cellular signaling pathway. Studies support the suggestion that AMPK and SIRT1 work in concert to protect the vasculature in diabetes.20 Similar results have been found in vascular dementia.21 Further linking AMPK and our previous work on HMGB1 regulation, we found novel information regarding IGFBP-3 and AMPK in RECs. Our findings agree with studies involving 3T3 cells and mice on a high-fat diet that found that Lactobacillus plantarum (Ln4) could increase both IGFBP-3 levels and AMPK actions.22 Somewhat similarly, other studies have reported that a derivative of oleanolic acid increased both IGFBP-3 and AMPK phosphorylation in the liver of mice on a high-fat diet.23 To the best of our knowledge, we are the first to link IGFBP-3 to AMPK in the retinal vasculature.
Earlier studies have reported a link between AMPK and SIRT1 in diabetes and have demonstrated AMPK inhibition of HMGB1 in inflammation, but we were able to link Epac1 to AMPK in the diabetic retinal vasculature and in RECs cultured in high glucose. We found that Epac1 requires AMPK to increase SIRT1 levels and reduce HMGB1, thus adding to the current literature. Taken together, these findings suggest that Epac1 offers a novel therapeutic target for inhibition of HMGB1 through the actions of AMPK. Future studies are necessary to further investigate AMPK agonists used for other disorders.
Acknowledgments
This study was funded by grants from the National Eye Institute, National Institutes of Health (NEI R01EY028442 to JJS; P30EY04068 to Hazlett) and by an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute). The funders did not influence the design or execution of these studies.
Disclosure: Y. Jiang, None; J.J. Steinle, None
References
- 1. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu H, Chen Z, Xie J, Kang L-N, Wang L, Xu B. High mobility group box-1: a missing link between diabetes and its complications. Mediators Inflamm. 2016; 2016: 3896147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M, Abu El-Asrar AM. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp Eye Res. 2013; 107: 101–109. [DOI] [PubMed] [Google Scholar]
- 4. Liu L, Jiang Y, Steinle JJ. Glycyrrhizin protects the diabetic retina against permeability, neuronal, and vascular damage through anti-inflammatory mechanisms. J Clin Med. 2019; 8: 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu L, Jiang Y, Steinle JJ. Epac1 and glycyrrhizin both inhibit HMGB1 levels to reduce diabetes-induced neuronal and vascular damage in the mouse retina. J Clin Med. 2019; 8: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu L, Jiang Y, Steinle JJ. Inhibition of HMGB1 protects the retina from ischemia-reperfusion, as well as reduces insulin resistance proteins. PLoS One. 2017; 12: e0178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L, Jiang Y, Chahine A, Curtiss E, Steinle JJ. Epac1 agonist decreased inflammatory proteins in retinal endothelial cells, and loss of Epac1 increased inflammatory proteins in the retinal vasculature of mice. Mol Vis. 2017; 23: 1–7. [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang Y, Liu L, Steinle JJ. Epac1 deacetylates HMGB1 through increased IGFBP-3 and SIRT1 levels in the retinal vasculature. Mol Vis. 2018; 24: 727–732. [PMC free article] [PubMed] [Google Scholar]
- 9. Kubota S, Ozawa Y, Kurihara T, et al.. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011; 52: 9142–9148. [DOI] [PubMed] [Google Scholar]
- 10. Wang R, Xie Y, Qiu J, Chen J. The effects of dexmedetomidine in a rat model of sepsis-induced lung injury are mediated through the adenosine monophosphate-activated protein kinase (AMPK)/silent information regulator 1 (SIRT1) pathway. Med Sci Monit. 2020; 26: e919213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shati AA. Salidroside ameliorates diabetic nephropathy in rats by activating renal AMPK/SIRT1 signaling pathway. J Food Biochem. 2020; 44: e13158. [DOI] [PubMed] [Google Scholar]
- 12. de Moraes G, Layton CJ. Therapeutic targeting of diabetic retinal neuropathy as a strategy in preventing diabetic retinopathy. Clin Exp Ophthalmol. 2016; 44: 838–852. [DOI] [PubMed] [Google Scholar]
- 13. Roy Chowdhury SK, Smith DR, Saleh A, et al.. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012; 135: 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park SJ, Ahmad F, Philp A, et al.. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012; 148: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009; 21: 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang Y, Liu L, Curtiss E, Steinle JJ. Epac1 blocks NLRP3 inflammasome to reduce IL-1β in retinal endothelial cells and mouse retinal vasculature. Mediators Inflamm. 2017; 2017: 2860956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joussen AM, Poulaki V, Le ML, et al.. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18: 1450–1452. [DOI] [PubMed] [Google Scholar]
- 18. Ma JQ, Sun YZ, Ming QL, Tian Z-K, Zhang Y-J, Liu C-M. Effects of gastrodin against carbon tetrachloride induced kidney inflammation and fibrosis in mice associated with the AMPK/Nrf2/HMGB1 pathway. Food Funct. 2020; 11: 4615–4624. [DOI] [PubMed] [Google Scholar]
- 19. Qi Y, Du X, Yao X, Zhao Y. Vildagliptin inhibits high free fatty acid (FFA)-induced NLRP3 inflammasome activation in endothelial cells. Artif Cells Nanomed Biotechnol. 2019; 47: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 20. Li J, Yu S, Ying J, Shi T, Wang P. Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC-1α pathway. Oxid Med Cell Longev. 2017; 2017: 7584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Meng N, Guo X, et al.. Dl-3-n-butylphthalide promotes remyelination and suppresses inflammation by regulating AMPK/SIRT1 and STAT3/NF-κB signaling in chronic cerebral hypoperfusion. Front Aging Neurosci. 2020; 12: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee E, Jung S-R, Lee S-Y, Lee N-K, Paik H-D, Lim S-I. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients. 2018; 10: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ou-Yang Q, Xuan CX, Wang X, et al.. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018; 39: 1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]