Abstract
Combined checkpoint inhibition therapy targeting the programmed cell death 1 (PD-L1) and cytotoxic T-lymphocyte associated protein 4 pathways has been a successful approach in the treatment of metastatic melanoma, leading to its investigation in the treatment of head and neck squamous cell carcinoma (HNSCC) with PD-L1 expression. Despite the potential for excellent responses, an increased rate of autoimmune neurological toxicity and paraneoplastic conditions has been observed when using these treatment modalities. We present the case of a patient with metastatic HNSCC treated with combination ipilimumab/nivolumab who experienced severe cerebellar ataxia with a positive screen for the anti-Zic4 antibody. This is the first case, to our knowledge, of anti-Zic4 antibody-mediated cerebellar toxicity reported in association with HNSCC. Although the patient experienced an impressive partial response with dual checkpoint inhibition, he suffered grade 4 neurotoxicity. Despite exciting advances in cancer immunotherapy, clinicians must be aware of the rare, debilitating and possibly previously undescribed paraneoplastic and autoimmune toxicities that may occur.
Keywords: oncology, neurological injury, cancer intervention, immunology, head and neck cancer
Background
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common malignancy worldwide,1 and often presents with locoregionally advanced disease due to its propensity for lymphatogenous spread.2 In patients with metastatic, recurrent disease refractory to platinum-based chemotherapy, prognosis is poor and further treatment options have historically been very limited. Given the success of immune checkpoint inhibitors in other malignancies, most notably metastatic melanoma and non-small cell lung cancer (NSCLC), some select patients with metastatic HNSCC are currently being treated with dual checkpoint inhibition with nivolumab and ipilimumab as first-line therapy and are being compared with patients receiving the standard of care chemoimmunotherapy regimen.3 Alongside impressive responses, several immune-related adverse effects (irAEs) have been noted with varying degrees of frequency and severity, and in some cases can be life-threatening or fatal.4 We present the case of a patient with metastatic p16-positive HNSCC treated with dual checkpoint inhibition with ipilimumab and nivolumab who experienced severe cerebellar ataxia with a positive screen for the anti-Zic4 antibody, which has been associated with cerebellar degeneration in small cell lung cancer (SCLC) and has thus far never been reported in association with HNSCC.5
Case presentation
A 37-year-old Caucasian man of Cuban descent with a medical history significant only for well-controlled hypertension and absent of any previous tobacco use sought medical care for oropharyngeal bleeding, and was diagnosed with p16-positive HNSCC in October 2016. He initially presented with stage II (cT2N0M0) disease which was treated with radiation therapy consisting of 69.96 Grey in 33 fractions with no concurrent chemotherapy, completed by January 2017. Up until this point in time, the patient’s diagnosis and treatment occurred at outside institutions and not at our own. Follow-up positive emission tomography scan in April 2017 at our institution showed complete response with no evidence of residual or recurrent disease. In October 2018, he developed chest wall pain, and subsequent CT at an outside institution showed a 4.2 cm left lower lobe pulmonary mass suspicious for malignancy. At this juncture, he was referred to our centre for pulmonary evaluation. Bronchoscopy revealed that the left lower lobe basilar segment was completely occluded by tumour, and under endobronchial ultrasound enlarged subcarinal and left hilar lymph nodes were noted. Biopsies were taken from the left lower lobe and the enlarged subcarinal lymph node. Pathology for both biopsies returned positive for squamous cell carcinoma positive for p16 by immunohistochemistry, with programmed cell death 1 (PD-L1) Tumor Proportion Score (TPS) of 70%.
Due to a personal preference to avoid chemotherapy, he received 30 Grey of radiation to the dominant left lower lobe lesion in December 2018. Prior to the completion of radiation therapy, however, we performed apositron emission tomography (PET) scan which revealed a more extensive and multifocal metastatic burden than previously realised, with disease present in both lungs, mediastinum and the thoracic spine. He did not have any pain or neurological deficits from his thoracic spine lesion. Given his PD-L1 TPS of 70% and desire for the most aggressive therapy available without the use of any chemotherapeutic agents, we explored the option of immune checkpoint inhibitor therapy. The use of combination checkpoint inhibitor therapy with the anti-PD-L1 monoclonal antibody nivolumab and the anti-cytotoxic T-lymphocyte associated protein 4 (anti-CTLA4) monoclonal antibody ipilimumab in the treatment of recurrent or metastatic HNSCC was being investigated in the CheckMate 651 trial, which studied this combination compared with the standard first-line chemotherapy regimen, and had garnered significant interest for its clinical responses; however, data from the trial have not yet been released and this combination had not received approval by the Food and Drug Administration (FDA) for this indication.3 Nevertheless, after a thorough discussion of the possible adverse effects of first-line combined chemotherapy versus those of dual checkpoint inhibition, the patient opted for dual immunotherapy. In February 2019, he was initiated on off-label dual immune checkpoint inhibition with the anti-PD-L1 monoclonal antibody nivolumab at a dose of 3 mg/kg administered intravenously every 2 weeks with the anti-CTLA4 monoclonal antibody ipilimumab at the low dose of 1 mg/kg administered intravenously every 6 weeks. Subsequent to having received four infusions of nivolumab and two infusions of ipilimumab by March 2019, he noted sudden-onset moderately blurred vision. After 9 days of blurred vision without improvement, he presented to the eye hospital at our centre in April 2019. Ophthalmic examination was unremarkable, and he was referred to our hospital for workup. Autoimmune toxicity was suspected and the patient was admitted. Within 2 days of admission, the patient developed severe dysarthria and ataxia without any alteration in mental status. He was unable to ambulate and was largely bed-bound and wheelchair-bound due to fall risk.
Investigations
Initial brain imaging with CT and MRI and routine serum testing were unremarkable. Lumbar puncture was performed. Cytology of the cerebrospinal fluid returned negative for malignant cells. The serum and cerebrospinal fluid paraneoplastic panels returned negative for common autoimmune culprits in cerebellar encephalopathy including anti-Hu, anti-Yo, anti-NMDAR and anti-GAD65 antibodies; however, the anti-Zic4 antibody was detected in the serum. Repeat MRI in May 2019 revealed a new fluid-attenuated inversion recovery (FLAIR) hyperintense lesion in the left cerebellar hemisphere. FLAIR hyperintensity is a non-specific finding; as this lesion did not enhance with gadolinium contrast nor did it display any surrounding oedema or mass effect, we feel that this lesion represented an inflammatory focus.
Treatment
For the treatment of grade 4 immune-related encephalitis/leucoencephalopathy associated with immune checkpoint inhibitors, the American Society of Clinical Oncology recommends a trial of intravenous methylprednisolone 1–2 g daily for 3–5 days, potentially with intravenous Ig, and to consider rituximab or plasmapheresis of an antibody is detected or if there is insufficient clinical response.6 Similarly, the Society for Immunotherapy of Cancer recommends treatment with 1–2 g per day of intravenous methylprednisolone or the equivalent, and consideration of plasmapheresis if no improvement in 3 days.7
While awaiting the results of complex autoimmune and paraneoplastic syndrome testing of the cerebrospinal fluid and blood, the patient was treated with multiple modalities in an escalating fashion with minimal improvement, starting on hospital day 3 with pulse-dose intravenous corticosteroids (methylprednisolone 1 g daily for 3 days), and followed by intravenous Ig at a dose of 0.4 mg/kg for 5 days and subsequently with plasmapheresis. After the anti-Zic4 antibody was detected and repeat MRI revealed a hyperintense cerebellar lesion (figure 1), rituximab was initiated inpatient, and the patient experienced stabilisation and mild improvement in symptoms. He was subsequently discharged home.
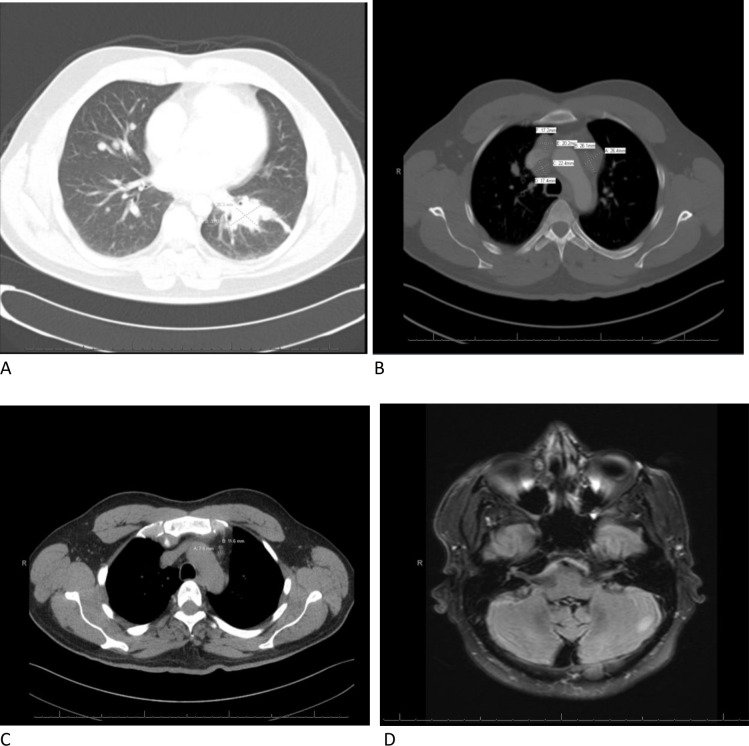
Figure 1.
(A) Contrast-enhancing dominant left lower lobe pulmonary lesion on CT, preimmunotherapy in December 2018. (B) Mediastinal adenopathy on CT, preimmunotherapy in February 2019. (C) Restaging CT postimmunotherapy in April 2019 with near resolution of intrathoracic disease. (D) Hyperintense left cerebellar lesion on T2-weighted fluid-attenuated inversion recovery MRI, not present on previous examinations, postimmunotherapy in May 2019.
Outcome and follow-up
As of August 2020, the patient is alive and continues to be wheelchair-bound. Postimmunotherapy restaging CT scans obtained during his hospitalisation in April 2019 showed an impressive response with resolution of previously extensive metastatic disease in the thorax noted 2 months prior before the initiation of immunotherapy; however, there was no improvement in the osseous lesions (figure 1). The patient received two additional infusions of rituximab outpatient, and his Eastern Cooperative Oncology Group (ECOG) performance status improved from 3 to 2.8 Meanwhile, however, his metastatic disease progressed. He is currently receiving systemic chemotherapy with carboplatin and paclitaxel and remains at ECOG performance status 3.
Discussion
HNSCC is a group of primary malignancies that originate from the oral and nasal cavities, paranasal sinuses, hypopharynx, oropharynx or larynx, and is the seventh most common malignancy worldwide.1 Approximately 600 000 new cases of HNSCC are diagnosed each year, including about 50 000 in the USA alone.9 Historically, HNSCC was a disease that affected older individuals with a history of tobacco use and heavy alcohol consumption; in more recent years, however, infection with high-risk strains of the human papillomavirus (HPV), especially serotype 16, have skewed this trend to include younger individuals.10 11
These tumours penetrate the basement membrane of the squamous epithelium and patients often initially present with locoregionally advanced disease. Nodal metastasis is often rapidly attained through the region’s rich lymphatic network.2 Despite a multidisciplinary arsenal of diagnostics and treatment modalities, the 5-year progression-free survival of those patients with locally advanced disease is approximately 40%–50%, and survival rates for recurrent or metastatic disease is grave, with a median survival of 10 months.11 12 In patients with metastatic, recurrent disease refractory to platinum-based chemotherapy, prognosis is poor and further treatment options have historically been very limited.
The ability of immunotherapy to ameliorate tumour burden and facilitate regression has been shown in a variety of malignancies. In the treatment of metastatic melanoma, combined checkpoint inhibition therapy targeting the PD-L1 and CTLA4 pathways has proven to be a successful approach. The Checkmate 069 trial conducted in 2014 showed significant improvements in objective response and progression-free survival with the combined use of the anti-PD-L1 antibody nivolumab and the anti-CTLA4 antibody ipilimumab versus ipilimumab alone.13 In fact, this trial also demonstrated that there was a median reduction in tumour burden with combination therapy and a small increase in tumour with ipilimumab alone.13 These advances lead to the investigation of immune checkpoint inhibitors in the treatment of HNSCC and in 2016 two anti-PD-L1 antibodies, nivolumab and pembrolizumab, were granted FDA-approval for the treatment of recurrent HNSCC. The Checkmate 141 trial, conducted in 2014, demonstrated that in those patients with recurrent HNSCC who had disease progression after platinum-based chemotherapy, therapy with nivolumab resulted in significantly longer survival than treatment with standard therapy (defined as methotrexate, docetaxel, and cetuximab).14 Further, Keynote 048 compared pembrolizumab plus chemotherapy versus cetuximab plus chemotherapy in the treatment of recurrent HNSCC, and found the former significantly increased overall survival compared with the latter.15
As with the treatment of metastatic melanoma and NSCLC,16 17 some select patients with metastatic HNSCC are currently being treated with dual checkpoint inhibition with nivolumab and ipilimumab as first-line therapy and are being compared with patients receiving the standard of care chemoimmunotherapy regimen in the CheckMate 651 trial.3 As the use of checkpoint inhibitor mono and combination therapy expands, so is the already heterogenous and complex list of treatment-associated irAEs in the literature. In the recent Checkmate 227 trial published in November 2019 which showed improved overall survival in treatment of NSCLC with nivolumab and ipilimumab versus standard of care chemotherapy, grade 3–4 treatment-related adverse effects were more frequent in the chemotherapy arm; however, treatment-related death was more frequent in the immunotherapy arm, in which fatal immune-mediated toxicity manifested as pneumonitis, myocarditis, acute tubular necrosis and cardiac tamponade.16 In the Checkmate 067 trial, it was shown that treatment related adverse events of any grade, including grades 3–4, were more common in the treatment of untreated melanoma with the combined use of nivolumab and ipilimumab than with either agent alone.17 After years of the increasing utilisation of checkpoint inhibitors, their more potentially life-threatening irAEs such as fulminant myocarditis are becoming increasingly more well described in the literature and better known in clinical practice.18
Many reviews have been published in recent years investigating the occurrence of immune-related toxicity, including neurotoxicity specifically, associated with checkpoint inhibitor therapy.19In a 2017 review published in The Oncologist, a database search that included 3763 patients with advanced melanoma among 12 clinical trials treated with either nivolumab alone or nivolumab in combination with ipilimumab identified an approximately 1% incidence of treatment‐related serious neurologic irAEs across 12 clinical trials; 0.2% of these cases constituted immune-mediated encephalitis. As noted, high-grade immune-related encephalitis is an exceedingly rare adverse effect of checkpoint inhibition.19
It is notable that our patient experienced severe neurotoxicity despite having received relatively low-dose ipilimumab. The CheckMate 511 trial showed that in the treatment of metastatic melanoma, a lower dosing of ipilimumab plus nivolumab (low-dose ipilimumab 1 mg/kg plus nivolumab 3 mg/kg, versus ipilimumab 3 mg/kg plus nivolumab 1 mg/kg, both every 3 weeks for four doses followed by nivolumab 480 mg every 4 weeks) had an improved safety profile without compromising efficacy.20 The FDA-approved dose of this drug combination for metastatic NSCLC is nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks, a lower dose of ipilimumab than either melanoma dose used in CheckMate 511, based on the Checkmate 227 trial.16 Our patient received low-dose ipilimumab 1 mg/kg every 6 weeks plus nivolumab 3 mg/kg every 2 weeks, which was the dose used in the afore-mentioned CheckMate 511 trial for NSCLC as well as the CheckMate 651 and CheckMate 714 trials in the treatment of recurrent/metastatic HNSCC and lower than either of the doses used to treat metastatic melanoma in CheckMate 511. Unfortunately, despite receiving low-dose ipilimumab, he still experienced severe immune-related toxicity.
Despite the potential for excellent responses in metastatic HNSCC to immune checkpoint inhibition, and possibly with increased efficacy with the use of dual checkpoint inhibition based on the afore-mentioned trials in metastatic melanoma and NSCLC, an increased rate of autoimmune neurological toxicity and paraneoplastic conditions has been observed with the use of this treatment modality.4 Our report highlights the case of a patient with metastatic HPV-induced HNSCC treated with ipilimumab/nivolumab who experienced severe cerebellar ataxia with a positive screen for the anti-Zic4 antibody, which has been associated with cerebellar degeneration in SCLC, and has thus far never been reported in HNSCC.5 The clinician is recommended to maintain a high level of vigilance for irAEs in patients who have received checkpoint inhibition, especially with dual checkpoint inhibitors targeting multiple pathways, as autoimmune toxicity without any previously described association with the patient’s malignancy has the potential to occur.
Learning points.
Immune checkpoint inhibition may induce impressive responses in the treatment of certain cancers; however, severe immune-related adverse events may occur, especially with the use of dual checkpoint inhibition.
Prompt recognition and empiric treatment of potential autoimmune toxicity and may help save the lives and function of these patients.
Broad testing and the use of comprehensive autoimmune/paraneoplastic panels are recommended, as the patient may present with a rare syndrome or with a previously undescribed association.
Footnotes
SGI and NSK contributed equally.
Contributors: SI was the primary author on this manuscript. CP was the patient’s primary medical oncologist. NSK and GA were the contributing authors on this manuscript. All authors were directly involved in this patient’s medical care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Elkashty OA, Ashry R, Tran SD. Head and neck cancer management and cancer stem cells implication. Saudi Dent J 2019;31:395–416. 10.1016/j.sdentj.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haines J.Radosevich J, Pathology of head and neck cancer I: epithelial and related tumors. Chicago: Springer, 2013: 259–67. [Google Scholar]
- 3.Samra B, Tam E, Baseri B, et al. Checkpoint inhibitors in head and neck cancer: current knowledge and perspectives. J Investig Med 2018;66:1023–30. 10.1136/jim-2018-000743 [DOI] [PubMed] [Google Scholar]
- 4.Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250–5. 10.1093/annonc/mdx642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataller L, Wade DF, Graus F, et al. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology 2004;62:778–82. 10.1212/01.WNL.0000113749.77217.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol 1982;5:649–56. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Genetics Home Reference Head and neck squamous cell carcinoma, 2018. Available: https://ghr.nlm.nih.gov/condition/head-and-neck-squamous-cell-carcinoma#definition
- 10.Lo Nigro C, Denaro N, Merlotti A, et al. Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res 2017;9:363–71. 10.2147/CMAR.S115761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jou A, Hess J. Epidemiology and molecular biology of head and neck cancer. Oncol Res Treat 2017;40:328–32. 10.1159/000477127 [DOI] [PubMed] [Google Scholar]
- 12.Economopoulou P, de Bree R, Kotsantis I, et al. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front Oncol 2019;9:1–13. 10.3389/fonc.2019.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–68. 10.1016/S1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Licitra L, Fayette J, et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use. Clin Cancer Res 2019;25:5221–30. 10.1158/1078-0432.CCR-18-3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 16.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J, Chmielowski B, Lao CD, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist 2017;22:709–18. 10.1634/theoncologist.2016-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebbé C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019;37:867–75. 10.1200/JCO.18.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]