Abstract
SLC4A11 is the only member of the SLC4 family that transports protons rather than bicarbonate. SLC4A11 is expressed in corneal endothelial cells, and its mutation causes corneal endothelial dystrophy, although the mechanism of pathogenesis is unknown. We previously demonstrated that the magnitude of the H+ conductance (Gm) mediated by SLC4A11 is increased by rises in intracellular as well as extracellular pH (pHi and pHe). To better understand this feature and whether it is altered in disease, we studied the pH dependence of wild-type and mutant mouse Slc4a11 expressed in Xenopus oocytes. Using voltage-clamp circuitry in conjunction with a H+-selective microelectrode and a microinjector loaded with NaHCO3, we caused incremental rises in oocyte pHi and measured the effect on Gm. We find that the rise of Gm has a steeper pHi dependence at pHe =8.50 than at pHe =7.50. Data gathered at pHe =8.50 can be fit to the Hill equation enabling the calculation of a pK value that reports pHi dependence. We find that mutation of lysine residues that are close to the first transmembrane span (TM1) causes an alkaline shift in pK. Furthermore, two corneal-dystrophy-causing mutations close to the extracellular end of TM1, E399K and T401K (E368K and T370K in mouse), cause an acidic shift in pK, while a third mutation in the fourth intracellular loop, R804H (R774H in mouse), causes an alkaline shift in pK. This is the first description of determinants of SLC4A11 pH dependence and the first indication that a shift in pH dependence could modify disease expressivity in some cases of corneal dystrophy.
Keywords: acid-base, BTR1, cornea, fluid transport, proton
INTRODUCTION
SLC4A11 is a H+ (or OH–)-transporting membrane protein and is the only member of the SLC4 family of acid-base transporters that does not transport (20, 26). SLC4A11 is predominantly expressed in corneal endothelial cells and cochlear fibroblasts (18, 29). Mutations in SLC4A11 cause autosomal-dominant inheritance of progressive vision loss [Fuchs endothelial corneal dystrophy (FECD)], autosomal-recessive inheritance of congenital vision loss [congenital hereditary endothelial dystrophy (CHED)], and autosomal-recessive inheritance of congenital vision loss with progressive hearing loss (Harboyan Syndrome) (6, 30, 31). Slc4a11-null mice exhibit congenital corneal edema and accelerated hearing loss (8, 9, 18). The genotype-phenotype correlations are not well understood. One of the major challenges to understanding how mutations in SLC4A11 result in disease is our incomplete understanding of the physiological role of the protein. Numerous actions have been assigned to SLC4A11. Several early hypotheses such as sodium-borate cotransport (25), Na+-coupled pH regulation (10, 24), and ammonium transport (34), have since been revisited and refined. Thus the current hypotheses regarding the multiple actions that could alone, or in combination, explain the physiological importance of SLC4A11 now only include pH-sensitive H+ (or OH–) transport with or without cotransport of ammonia (12, 13, 20, 25, 34), water transport (29), and extracellular matrix binding (19).
Corneal dystrophy involves the loss of endothelial cell function, sometimes even loss of endothelial cells themselves, and ultimately corneal swelling (edema) and loss of transparency (33). Several studies have focused on the role of SLC4A11 in maintaining the fluid-pumping function of the endothelial cells, an action that resists edema formation. The majority of the thickness of the cornea is accounted for by a proteoglycan layer known as the stroma. The endothelial cells are located on the posterior surface of the stroma facing the aqueous humor. The endothelial-cell layer is leaky, so the stromal matrix has a natural tendency to absorb water from the aqueous humor: an action that, if unopposed, would distend the matrix causing it to scatter light and become opaque. Endothelial cells pump osmolytes such as and lactate (and consequently fluid) from the stroma back into the aqueous humor and thereby maintain corneal clarity (10, 29). The driving force for the fluid pump is established by the coordinated action of a number of basolateral membrane transport proteins including the Na+- cotransporter NBCe1-B and the H+-lactate cotransporter MCT1 (3). SLC4A11 is located in this same membrane (29). With observations of water flux mediated by SLC4A11, it has been proposed that the protein is directly responsible for the movement of water (17, 29). On the other hand, the pH-sensitive H+ transport function could, if it responded appropriately to challenges in intracellular pH (pHi), allow the pump to function without disturbing pHi, even in the absence of tight functional coupling between NBCe1-B and MCT1 (20, 22). That is to say that, if the activity of one outpaced the activity of the other, SLC4A11 could act to counter a disturbance of pH.
In a previous study we showed that the magnitude of SLC4A11-mediated H+ conductance is increased by raising extracellular pH (pHe) at constant pHi or by raising pHi at constant pHe but we did not parameterize these observations (20). [We also found that the application of NH3/ enhanced H+ conductance but, as the elicited conductance persists after NH3/ removal, concluded that this effect reflects an indirect effect upon SLC4A11 mediated by the influence of NH3/ on cell pH and membrane potential (20).] In the present study we aim to further our understanding of the importance of the pH dependence of SLC4A11-mediated H+ transport in health and disease by determining, for the first time, values for its intracellular pH dependence (pKi) and pH sensitivity (Napp). We use these values as a benchmark for identifying determinants of pH dependence in wild-type Slc4a11 and for assessing disturbances of pH dependence in disease-linked mutants.
METHODS
Constructs.
The construction of mouse Slc4a11 cDNA in the Xenopus expression vector pGH19 and the mutant “R99H” (equivalent to human CHED mutant “R125H”) has been previously reported (20). The other mutants used in this study were generated using either the QuikChange II mutagenesis kit (Agilent Technologies, Santa Clara, CA) in the case of the K343Q mutant or the Phusion site-directed mutagenesis kit (Thermo Fisher Scientific, Waltham, MA) for all other mutants, using the primers shown in Table 1 according to manufacturers’ recommendations.
Table 1.
Primers and templates used in the generation of the mouse Slc4a11 mutants used in this study
| Sequence | |
|---|---|
| K343Q (equivalent to human K374Q): template: Slc4a11.pGH19 | |
| FWD | 5′-GCAAGAGCGTGGGCCAATATGTCACCACC-3′ |
| RVS | 5′-GGTGGTGACATATTGGCCCACGCTCTTGC-3′ |
| SSQ (K337S, K339S, K343Q: equivalent to human K368S, K370S, K374Q): template: Slc4a11/K343Q.pGH19 | |
| FWD | 5′-CATCATTGGGAGTAGCAGTAGCGTGGGCCA-3′ |
| RVS | 5′-CCATCAGTGAAGTCCATTGGGTACACAGGA-3′ |
| Pre-TM1-del (Δ337–342, K343Q: equivalent to human Δ368–373, K374Q): template: Slc4a11/K343Q.pGH19 | |
| FWD | 5′-GGCCAATATGTCACCACCACAC-3′ |
| RVS | 5′-AATGATGCCATCAGTGAAGTCC-3′ |
| E368K (equivalent to human FECD mutant E399K): template: Slc4a11.pGH19 | |
| FWD | 5′-CCTCAACGATAAGAACACAAACGGGGC-3′ |
| RVS | 5′-GATCCAAAAGCAATGGTCGGTAGGAG-3′ |
| T370K (equivalent to human CHED mutant T401K): template: Slc4a11.pGH19 | |
| FWD | 5′-CAACGATGAGAACAAAAACGGGGCC-3′ |
| RVS | 5′-AGGGATCCAAAAGCAATGGTCGGTAG-3′ |
Mutant codons introduced by the primers are underlined. FWD, forward; RVS, reverse.
Oocyte preparation.
Ovaries from Xenopus laevis (Xenopus Express Inc., Brooksville, FL) were extracted in accordance with Institutional Animal Care and Use Committee protocols approved at the University at Buffalo. Frogs were anesthetized with 0.2% tricaine solution until unresponsive to toe pinch. The ovary was harvested and the frog was euthanized by exsanguination. Ovaries were cut into 1-cm fragments and washed with filter-sterilized calcium-free normal Ringer solution (Ca-free NRS: 82 mM NaCl, 20 mM MgCl2, 5 mM HEPES, and 2 mM KCl) for 3 × 5 min. To extract the oocytes from the ovarian connective tissue, the fragments were agitated in Ca-free NRS solution containing 2 mg/ml type-1A collagenase (Sigma-Aldrich, St. Louis, MO) until the follicles began to dissociate. Oocytes were washed with Ca-free NRS for 3 × 10 min, ND96 for 1 × 10 min, and finally in OR3 medium for 1 × 10 min (OR3: 14 g Leibovitz’s L-15 medium powder; Thermo Fisher Scientific), 5 mM HEPES, 20 mL 100× penicillin-streptomycin solution (Corning Inc., Corning, NY), pH 7.50, and 200 mosmol/kgH2O. Cells were stored in fresh OR3 at 18°C.
cRNA preparation and injection.
Plasmids were linearized with NotI enzyme and purified with the MinElute PCR Purification kit (Qiagen). The linearized DNA was converted into capped cRNA using the T7 mMessage mMachine Transcription kit (Invitrogen, Carlsbad, CA) and purified using with the RNeasy MinElute Cleanup kit (Qiagen). RNA was quantified using a Nanodrop 2000 (Thermo Fisher Scientific). cRNA was injected into oocytes using a Nanoject III programmable injector (Drummond Scientific Co., Broomhall, PA).
Electrophysiology.
Xenopus oocytes were placed into a small chamber (no. RC-3Z, Warner Instruments, Hamden, CT) on an antivibration worktable (Vision IsoStation, Newport Corp., Irvine, CA). Oocytes were superfused with solution fed from syringe pumps (Harvard Apparatus, Holliston, MA) at a rate of 2 mL/min. Glass microelectrodes were pulled from borosilicate glass (no. BF200-156-10, Sutter Instrument, Novato, CA) using a model P-1000 micropipette puller (Sutter Instrument) to achieve a tip resistance, when filled with saturated KCl solution, of 0.1–2 MΩ. The oocyte was impaled with two such microelectrodes (current passing and voltage sensing) connected to an OC275 oocyte clamp (Warner Instruments, Hamden, CT) while an oocyte bath clamp (no. 725I, Warner Instruments) was used to clamp the bath potential to 0 mV. Current-voltage (I-V) relationships were obtained by clamping the cell membrane potential (Vm) at its spontaneous value (resting potential) and then from –160 mV to +20 mV in 20-mV steps for 100 ms, returning to resting potential for 100 ms in between each step. A third, H+-selective microelectrode, was introduced into the opposite side of the oocyte to the I-V microelectrodes and connected to a HiZ-223 dual channel electrometer (Warner Instruments) to measure intracellular pH (pHi) as previously described (15, 20). I-V data were digitized and acquired using a Digidata 1550 with Clampex 10.4 software (Molecular Devices LLC, San Jose, CA). Continuous recordings of pHi and Vm were acquired using custom software (written by Dale Huffman for Walter Boron’s laboratory at Case Western Reserve University, Cleveland, OH). To raise oocyte pHi in H2O-injected cells, or in Slc4a11-expressing cells that are not expressing robust Slc4a11 activity (i.e., Any Slc4a11 clone when pHe =7.50, plus R99H when pHe =8.50), the needle of a Nanoject II microinjector (Drummond Scientific Co.), primed with 1.2 M solution of sodium bicarbonate, was introduced into the oocyte before the start of the experiment. Oocytes were subject to repeated injections of 2.3 nL to effect a gradual rise in pHi until the injections had no further effect on pHi. During this time and between injections, when pHi was not rapidly changing, I-V measurements were taken. Thus, for most experiments, the oocyte was impaled with a total of four glass capillaries. However, in cells expressing any Slc4a11 construct (except R99H) when pHe =8.50, there is sufficient driving force and H+ permeability (due to Slc4a11 activity) to permit spontaneous Slc4a11-mediated alkalinization of oocyte pHi. Thus NaHCO3 injection was not required to raise pHi in Slc4a11-expressing cells under these conditions; we merely expedited the process by voltage-clamping Vm at a value (–30 mV) more positive than the reversal potential for H+ (EH) to bolster the driving force for H+ extrusion (according to the Nernst equation, EH is always more negative than –58 mV considering the >1 pH unit gradient across the plasma membrane).
Electrophysiology solutions.
The basic recipe for ND96 bath solution used in electrophysiological experiments is 93.5 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM of buffer. For pH 7.50 solution (pHe =7.50), the buffer was HEPES, while for pH 8.50 solution (pHe =8.50) the buffer was Bicine.
Data analysis.
Conductance (Gm) was calculated as the slope of I-V plots from –120 mV to +20 mV using Microsoft Excel. The lowest voltages (–160 mV and –140 mV) were omitted from slope calculation due to a noticeable rectification in this range. We fit plots of pHi versus Gm to the Hill equation (as defined in Ref. 23) using the solver function of Microsoft Excel.
Hill is normally used for ligand or agonist assays in which EC50 is the concentration of agonist that elicits a half-maximal activity of the protein. In our case, we consider OH– as our agonist (as it rises with pH). Our measure of pH dependence is pKi = pHi at 50% Gm,max = 14 – log(EC50). Napp is the apparent Hill coefficient, which in simple reactions can indicate the number of binding sites for the agonist on the protein. However, the interpretation of the Hill coefficient is most precise in systems where cooperativity of binding increases with each subsequent binding event. In more complex reactions, for example, proteins with multiple binding sites or in our case dimeric membrane proteins with many charged residues with unknown importance in pH sensing, we may only conclude that Napp represents a minimum number of titratable residues (32) and note that this value can also be influenced by the strength of their cooperativity (27). Statistical analyses were performed either by t test with Bonferroni correction for multiple comparisons, ANOVA, or general linear model as appropriate using MiniTab 19 software.
RESULTS
Investigating the pHi dependence of Slc4a11 activity at pHe = 7.50.
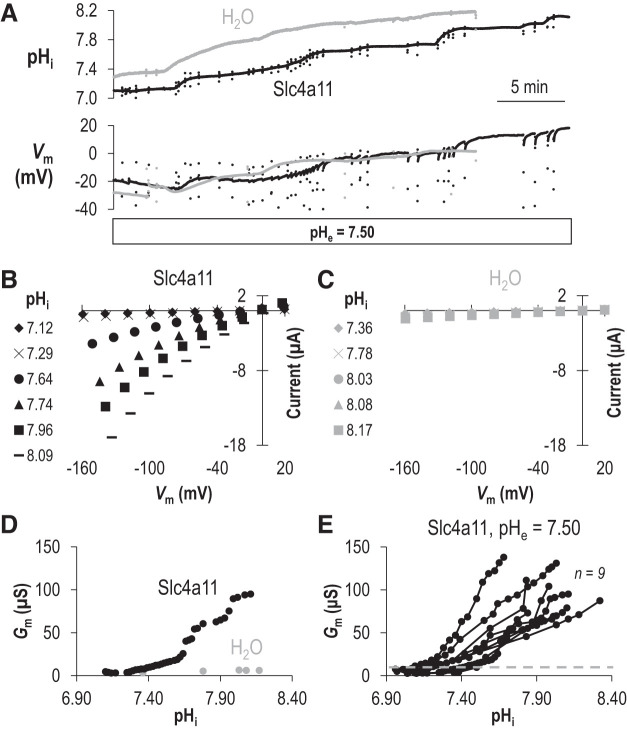
We superfused Slc4a11-expressing or H2O-injected-control oocytes with ND96 solution (pHe =7.50) and gathered multiple I-V relationships from these cells by voltage clamp as we caused pHi to increase by injecting 1.2 M NaHCO3 solution. Figure 1A shows representative pHi and Vm traces gathered during such an experiment. The black traces indicate data from an Slc4a11-expressing oocyte, while the gray traces indicate data from a H2O-injected oocyte. Initially, we see that the Slc4a11-expressing oocyte has a more acidic pHi and a more depolarized Vm than the H2O-injected oocyte, consistent with our previous observations of Slc4a11-expressing cells (20). NaHCO3 injections cause both cells to depolarize and alkalinize. Samples of the multiple I-V relationships gathered from the cells during the experiments shown in Fig. 1A are shown in Fig. 1B (the representative mSlc4a11-expressing cell) and Fig. 1C (the representative H2O-injected cell). At their starting (i.e., most acidic) pHi, the I-V plots for each cell look similar. However, as pHi rises, the slope of the I-V relationship (i.e., the conductance, Gm) of the mSlc4a11-expressing cell greatly increases (Fig. 1B), as shown by the data plotted in Fig. 1D for all of the I-V plots gathered in this experiment (black circles). On the other hand, the conductance of the H2O-injected cell does not greatly increase despite a comparable rise in pHi (Fig. 1, C and D, gray circles). Figure 1E shows the pHi versus Gm relationships for nine mSlc4a11-expressing cells (black circles). The gray dashed line represents the maximum value for Gm observed in any of the six H2O-injected cells at their most alkaline (data points are omitted here for clarity but are presented on an expanded axis in Supplemental Fig. S1A; see https://doi.org/10.6084/m9.figshare.12400946). It is not clear, from any of the pHi/Gm relationships gathered from individual Slc4a11-expressing cells, whether a maximum conductance (Gm,max) has been reached. However, estimating a minimum pKi value for each cell (pHi at which Gm is half of maximum observed Gm in each experiment) reports a range of 7.44–7.82. This indicates that pKi for Slc4a11 at pHe =7.50 is unlikely to be lower than 7.44.
Fig. 1.
The intracellular pH (pHi) dependence of Slc4a11 activity when extracellular pH (pHe) = 7.50. A: representative recording of pHi and membrane potential (Vm) versus time as an oocyte expressing Slc4a11 (black trace) or a control H2O-injected oocyte (gray trace) is injected with NaHCO3 to raise pHi. The scattered dots represent the excursions at which current-voltage (I-V) plots were taken (which was faster than our sampling rate) B: a sampling of current-voltage relationships (I-V plots) gathered from the Slc4a11-expressing cell during the experiment shown in A. C: a sampling of I-V plots gathered from the H2O-injected cell during the experiment shown in A. D: the relationship between pHi and membrane conductance (Gm) for the Slc4a11-expressing (black) and water-control oocyte (gray) calculated from all of the I-V plots gathered during the experiment shown in A. E: data similar to that shown in D for a greater number of Slc4a11-expressing oocytes. Gray dashed line shows maximum Gm calculated from a greater number of H2O-injected oocytes (see Supplemental Fig. 1A for data points; https://doi.org/10.6084/m9.figshare.12400946).
Revisiting the pHi dependence of Slc4a11 activity at pHe 8.50.
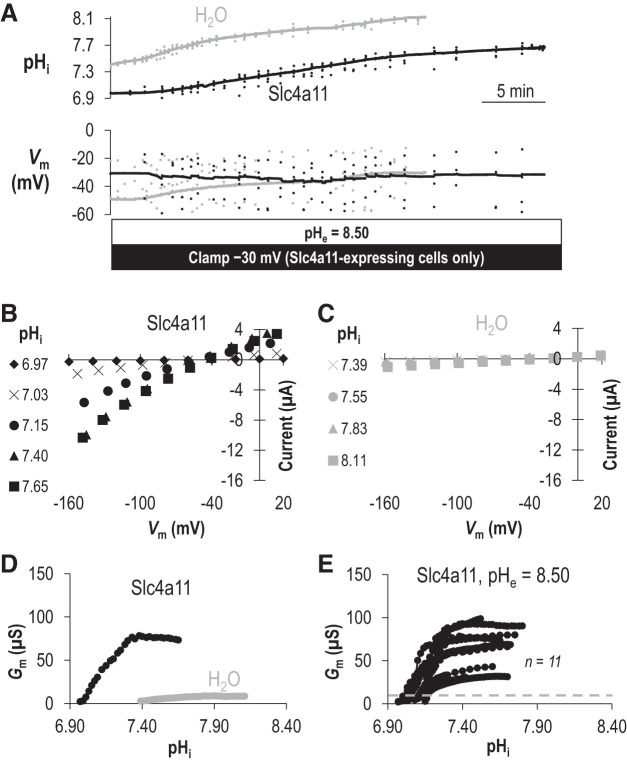
In a previous study, we determined that the H+ transport activity of Slc4a11 was substantially more robust at pHe =8.50 than at pHe =7.50 (20), a property that we decided to exploit for the present study to observe Slc4a11 operating at Gm,max and calculate a definite value of pKi for Slc4a11. Figure 2A shows data equivalent to that in Fig. 1A but gathered at pHe =8.50. Figure 2B shows a sample of the I-V relationships collected during the experiment in Fig. 2A for the Slc4a11-expressing cell while it is clamped at –30 mV. Figure 2C shows a sample of the I-V relationships for the H2O-injected cell while it was injected with NaHCO3. As with the equivalent data gathered at pHe =7.50, when pHi is at its most acidic in each cell type, I-V plots are similar between Slc4a11-expressing and H2O-injected cells but only in Slc4a11-expressing cells is Gm steeply dependent on pHi (Fig. 2D). In contrast to data gathered at pHe = 7.50, at pHe = 8.50 Slc4a11 activity reached a plateau within our measured pHi range (Fig. 2D, black circles), allowing us to define Gm,max. Although Gm for the H2O-injected cell also increased gradually (Fig. 2D, gray circles), it did not rise above 9 μS. Figure 2E shows data similar to that shown in Fig. 2D but for a greater number of cells. The gray line indicates the maximum value of Gm seen in any of the six H2O-injected oocytes tested at any pHi value in our experiments at pHe 8.50 (omitted data points are presented on an expanded axis in Supplemental Figure S1B; see https://doi.org/10.6084/m9.figshare.12400946). Figure 3A shows each Slc4a11 data set from Fig. 2D normalized to its own Gm,max. Figure 3B shows the lines of best-fit to the Hill equation for each data set (sum of squares = 0.07 ± 0.01). From these data, we calculate that the pHi dependence of Slc4a11 at pHe 8.50 is described by an average pKi of 7.16 ± 0.01 and the pHi sensitivity is described by an average Napp of 9 ± 1.
Fig. 2.
The intracellular pH (pHi) dependence of Slc4a11 activity when extracellular pH (pHe) is 8.50. A: representative recording of pHi and membrane potential (Vm) versus time as an oocyte-expressing Slc4a11 (black trace) is clamped at −30 mV or a control H2O-injected oocyte (gray trace) is injected with NaHCO3 to raise pHi. B: a sampling of current-voltage (I-V) plots gathered from the Slc4a11-expressing cell during the experiment shown in A. C: a sampling of I-V plots gathered from the H2O-injected cell during the experiment shown in A. D: the relationship between pHi and membrane conductance (Gm) for the Slc4a11-expressing (black) and water-control oocyte (gray) calculated from all of the I-V plots gathered during the experiment shown in A. E: data similar to that shown in D for a greater number of Slc4a11-expressing oocytes. Gray dashed line shows maximum Gm calculated from any of a greater number of water-control oocytes (see Supplemental Fig. 1B for data points; https://doi.org/10.6084/m9.figshare.12400946).
Fig. 3.
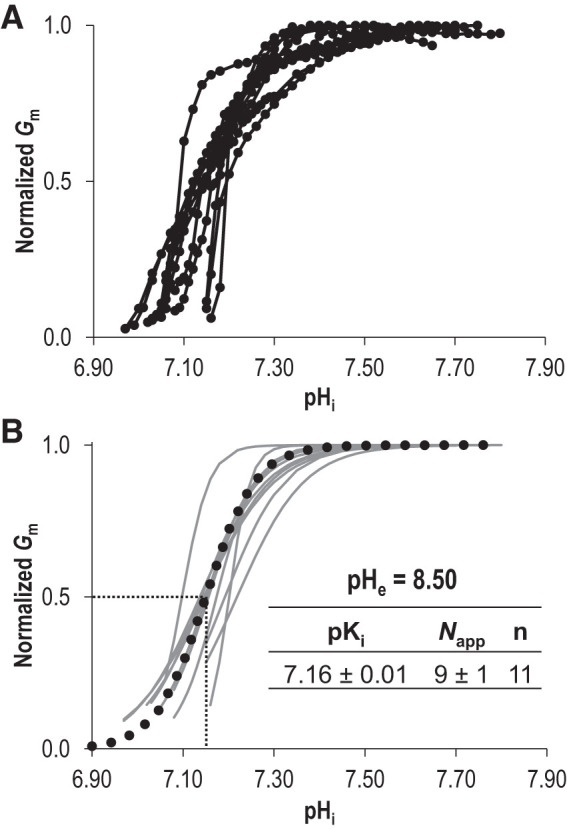
Hill equation fits of Slc4a11 activity at extracellular pH (pHe) 8.50. A: each Slc4a11 trace from Fig. 2E is shown here normalized to its own maximum conductance (Gm). B: gray lines represent best fit to the Hill equation for each trace in A. The average values for intracellular pH dependence (pKi) and apparent Hill coefficient (Napp) were used to generate the averaged best-fit trace shown as a black dotted line. pHi, intracellular pH.
The pHe dependence of Slc4a11 activity.
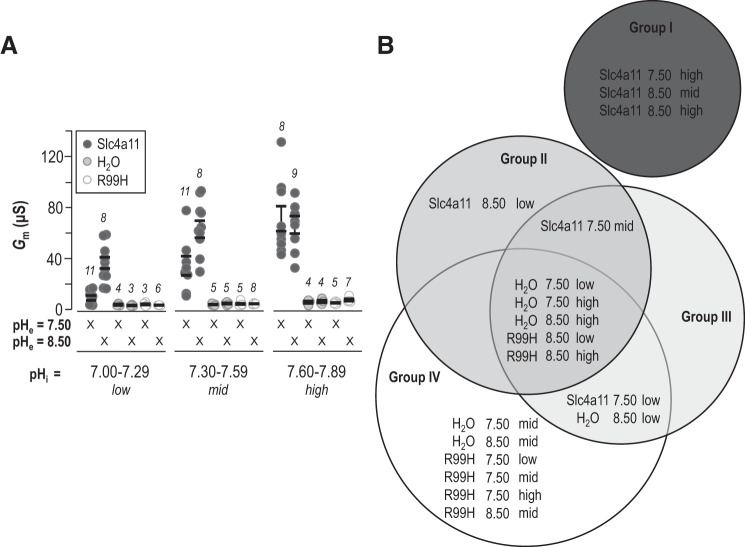
To facilitate direct comparison of the behavior of Slc4a11- versus H2O-injected cells at pHe =7.50 and 8.50, and probe the activities of Slc4a11 at fixed pHi but varying pHe, we binned pHi versus Gm data for each cell from Fig. 1E and Fig. 2E into three pHi ranges: 7.00–7.29 (low), 7.30–7.59 (mid), and 7.60–7.89 (high). These data are shown in Fig. 4A alongside data gathered from H2O-injected cells and, for the first time in this manuscript, equivalent data gathered from the inactive Slc4a11 mutant R99H (extracted from data shown in Supplemental Fig. S2A and S2B; see https://doi.org/10.6084/m9.figshare.12400946). The results of statistical analysis of these data by general linear model are illustrated in the form of a Venn diagram in Fig. 4B in which data sets that do not share a group are significantly different (P < 0.05). From this conservative analysis, we note the following:
Fig. 4.
Comparison of intracellular pH (pHi) dependence of Slc4a11 at extracellular pH (pHe) =7.50 and 8.50. A: for each cell which has a data set shown in Fig. 1E or Fig. 2E, we binned conductance (Gm) values into three pHi ranges such that each cell could be attributed a single average Gm value corresponding to a specific value of pHe in each of the three pHi ranges. These data are plotted alongside equivalent data gathered from the inactive Slc4a11 mutant R99H (see Supplemental Fig. 2 for data points; https://doi.org/10.6084/m9.figshare.12400946). Each data point represents an individual cell and is the average of between 1 and 25 Gm measurements that were incidentally gathered within that pHi range. B: statistical analysis of data shown in A illustrated as a Venn diagram of the groups (I, II, III, and IV) identified by general linear ANOVA model. Data sets that do not share a common group are significantly different from one another.
-
•
All data sets gathered from H2O-injected cells or R99H-injected cells (together: “control cells”) fall within group IV and are thus not significantly different from each other.
-
•
When pHe = 7.50, Gm values gathered from Slc4a11-injected cells and from control cells are significantly different from each other but only when pHi = “mid” or “high.”
-
•
When pHe = 8.50, Gm values gathered from Slc4a11-injected cells and from control cells were significantly different from each other for any given category of pHi.
-
•
Gm values gathered from Slc4a11-expressing cells at pHe =7.50 are significantly greater when pHi = “high” than when pHi = “low” or “mid.”
-
•
Gm values gathered from Slc4a11-expressing cells at pHe =8.50 are significantly greater when pHi is “mid” or “high” than when pHi is “low.”
-
•
When pHi = “low” or “mid,” Gm values gathered from Slc4a11-expressing cells at pHe = 8.50 are significantly greater than equivalent values gathered at pHe =7.50.
-
•
When pHi = “high,” Gm values gathered from Slc4a11-expressing cells at pHe = 8.50 are not significantly different from equivalent values gathered at pHe =7.50.
Thus, in summary, the Gm increases observed in Slc4a11-expressing cells are significantly greater than those observed in H2O-injected cells, increase significantly with pHi at either value of pHe, and rise over a significantly lower pHi range when pHe is 8.50 compared with when is pHe =7.50. However, whether pHe is 7.50 or 8.50, with a high enough pHi, Gm is ultimately the same, as if pHe exerts its influence by altering pKi rather than Gm,max.
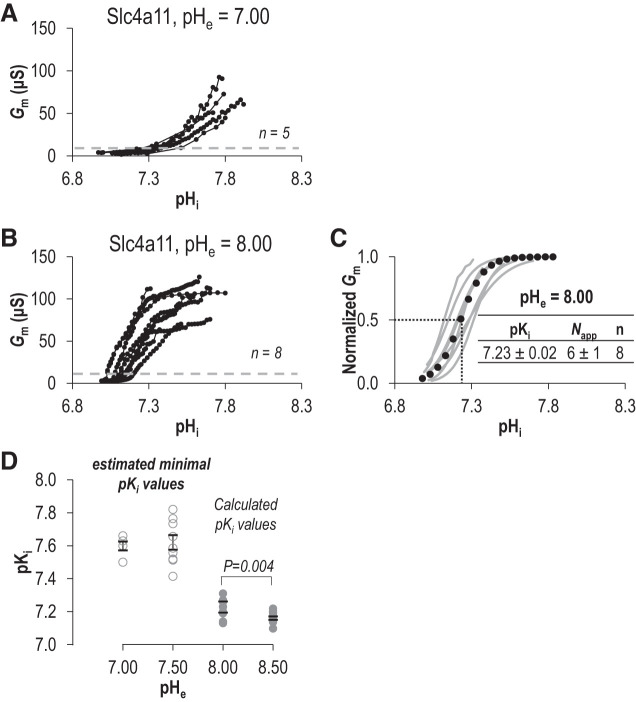
To further investigate this phenomenon, we performed a series of Fig. 1-like experiments in which we probed the pHi/Gm relationship at pHe =7.00 (Fig. 5A) and a series of Fig. 2-like experiments in which we probed the pHi/Gm relationship of pHe 8.00 (Fig. 5B). As with data gathered at pHe =7.50, when pHe = 7.00 the relationship between pHi and Gm did not reach a plateau and thus we can only estimate that the minimal pKi at pHe =7.00 is likely to be no lower than 7.50. On the other hand, when pHe = 8.00, we could gather sufficient data for a fit to the Hill equation (Fig. 5C, sum of squares = 0.11 ± 0.03). When pHe was 8.00, we calculated that pKi = 7.23 ± 0.02 and Napp = 6 ± 1. Figure 5D shows the relationship between pHe and pKi (or our proxy: estimated minimum pKi). pKi at pHe = 8.00 is significantly more alkaline than at pHe 8.50 (P < 0.05, one-tailed unpaired t test), and Napp is reduced (P < 0.05, one-tailed unpaired t test). Overall, there is a general trend for our measures of pKi to decrease as pHe rises from neutral to alkaline.
Fig. 5.
Comparison of the intracellular pH (pHi) dependence (pKi) of Slc4a11 at extracellular pH (pHe) 7.00 and 8.00. A and B: the relationship between pHi and conductance (Gm) for Slc4a11-expressing oocytes at pHe 7.00 (A) or pHe 8.00 (B). C: gray lines represent best fit to the Hill equation for each trace in B. The average value for pKi and apparent Hill coefficient (Napp) were used to generate the averaged best-fit trace shown as a black dotted-line. D: the range of pKi values at each tested value of pHe. pKi values are calculated from Hill plots fits for pHe 8.00 and 8.50 (solid circles), or estimated minimal pKi values from pHi at 50% of maximum observed Gm (open circles).
The influence of an intracellular lysine cluster on the pH dependence of Slc4a11.
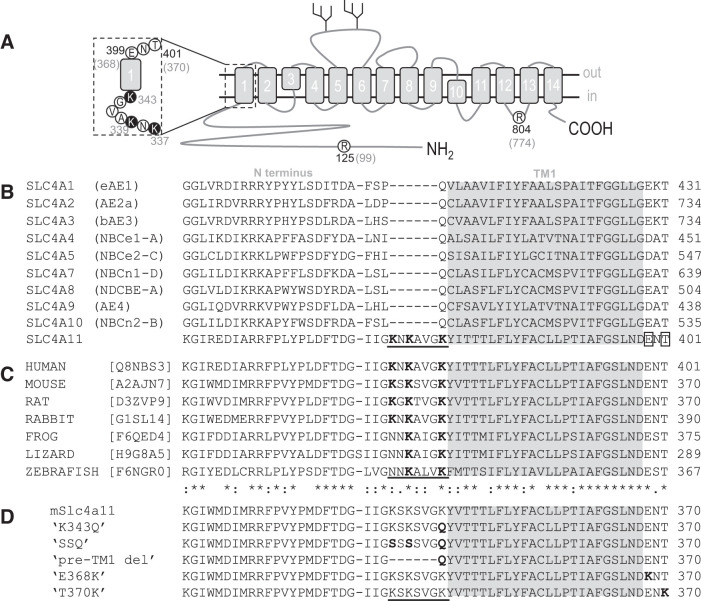
Analysis of the SLC4A11 protein sequence reveals a previously unremarked cluster of lysine residues “K-x-K-x-x-x-K” at the cytosolic end of transmembrane span 1 (TM1) that is unique among SLC4 paralogs (Fig. 6A and underlined in Fig. 6B) and conserved among mammalian Slc4a11 orthologs (Fig. 6C). To assess the contribution of these residues to the pH dependence of Slc4a11, we created a series of mutant clones (Fig. 6D):
Fig. 6.
Slc4a11 residues mutated in this study. A: topological cartoon of Slc4a11 showing the 14 transmembrane spanning regions. Amino-acid residues pertinent to this study are circled and numbered according to their location in the human (black numbers) or mouse (gray numbers) polypeptide chain. The region that corresponds to the sequence alignments in B–D is emphasized. B: alignment of all human SLC4 family protein sequences showing a unique lysine-rich region in SLC4A11 (underlined) and 2 nearby residues mutated in corneal dystrophy (E399K and T401K, boxed). Gray region shows likely limits of the first transmembrane span C: alignment of a selection of vertebrate Slc4a11 sequences showing conservation of the sequence features highlighted in Fig. 5B. D: sequence of the mutants used in this study (altered regions are shown in bold).
-
•
“K343Q” in which we replaced only the lysine closest to TM1 with the glutamine that is found at the equivalent location in other SLC4 proteins.
-
•
“SSQ” in which we replaced all three lysines: the first two lysine residues (K337 and K339) with serine residues and the third with glutamine (per K343Q).
-
•
“pre-TM1-del” in which we deleted the cluster, replacing it with a juxtamembrane glutamine to mimic the pre-TM1 primary structure of other SLC4 proteins.
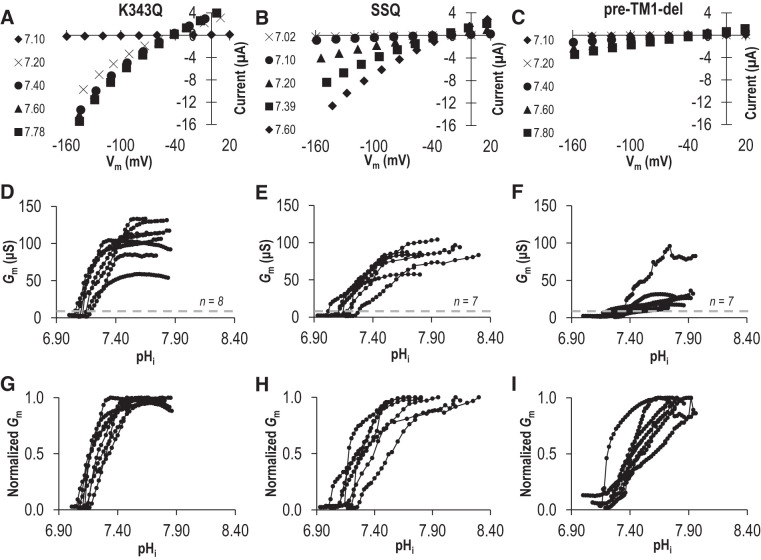
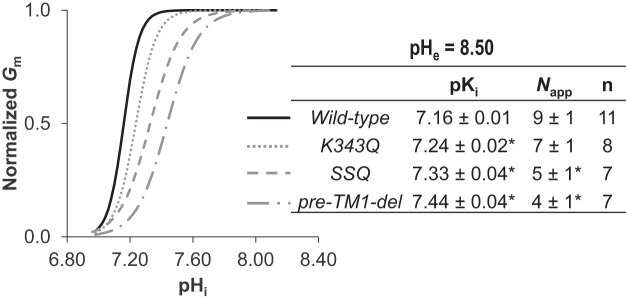
Due to the challenge associated with defining the parameters of pHi dependence and sensitivity at pHe =7.50, we performed our characterization of these mutants at pHe 8.50. From data similar to those in Fig. 2A (not shown), we obtained series of I-V plots for each mutant as pHi increased, a selection of which, from representative cells expressing either K343Q, SSQ, or pre-TM1-del, are shown in Fig. 7, A–C. The pHi/Gm relationships for a larger number of cells from each group are shown in Fig. 7, D–F. Those data, normalized to the Gm,max for each cell, are shown in Fig. 7, G–I. When we fit these data to the Hill equation (Fig. 8: average sum of squares = 0.09 ± 0.01, 0.06 ± 0.02, and 0.09 ± 0.02 respectively), we observe that all mutants exhibit a significantly more alkaline pKi compared with wild-type Slc4a11, but only SSQ and pre-TM1-del exhibit a significantly lower Napp (P < 0.016, i.e., P < 0.05/3 comparisons, one-tailed unpaired t test with Bonferroni correction). Supplemental Fig. S3, A–C (see https://doi.org/10.6084/m9.figshare.12400946), shows data equivalent to that shown in Fig. 7, D–F, but gathered at pHe =7.50. These data do not allow us to determine a value for pKi at pHe =7.50 but, mirroring the situation at pHe 8.50, we find that the estimated minimum pKi values for these mutants at pHe 7.50 tend to be more alkaline than the estimated minimum pKi values for wild-type Slc4a11 at pHe =7.50 (see Supplemental Fig. S3D; https://doi.org/10.6084/m9.figshare.12400946).
Fig. 7.
The influence of an intracellular lysine cluster on the intracellular pH (pHi) dependence of Slc4a11 at extracellular pH (pHe) 8.50. Three Slc4a11 mutants K343Q, SSQ, and pre-TM1-del (per Fig. 6D) were tested for their response to changes in intracellular pH (pHi). A–C: a sampling of current-voltage (I-V) plots gathered from a representative mutant-Slc4a11-expressing cell during the experiments equivalent to that shown in Fig. 2A. D–F: the relationship between pHi and membrane conductance (Gm) for a greater number of mutant-Slc4a11-expressing oocyte calculated from all of the I-V plots gathered for each cell. G–I: each Slc4a11 trace from Fig. 6, D–F, is shown here normalized to its own maximum conductance. Vm, membrane potential.
Fig. 8.
Hill equation fits of intracellular lysine mutants of Slc4a11 activity at extracellular pH (pHe) 8.50. The best fit to the Hill equation for each of the traces in Fig. 7, G–I, was used to determine a range of values for intracellular pH (pHi) dependence (pKi) and apparent Hill coefficient (Napp) for each mutant. The average value for these constants was used to generate the averaged best-fit traces shown in the graph. *Significant difference from wild-type values (P < 0.016, one-tailed, unpaired t test with Bonferroni correction).
The influence of disease-causing mutants on the pH dependence of Slc4a11.
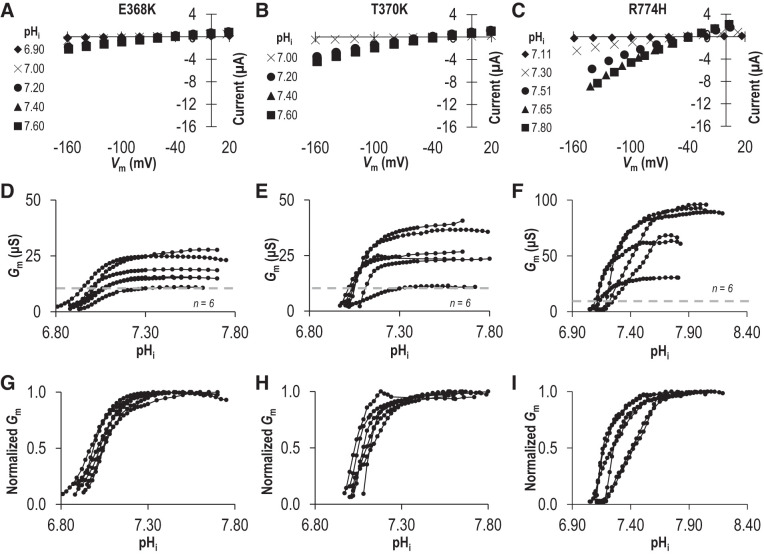
While the lysine-deficient mutants K343Q, SSQ, and pre-TM1-del are artificial constructs that have never been observed in a living subject, there are two disease-causing mutations on the extracellular side of TM1 that result in the introduction of an additional lysine residue: E399K (a dominant mutation linked to FECD) and T401K (a recessive mutation linked to CHED). The mutated residues (Fig. 6A and boxed in sequence alignment in Fig. 6B) are conserved among SLC4 paralogs (“D/E-x-T”) and among Slc4a11 orthologs (Fig. 6, B and C). We recreated these mutants in our mouse clone (E368K and T370K in Fig. 6D) and examined the transport properties of the mutant transporters at pHe = 8.50. From data similar to those in Fig. 2A (not shown), we obtained series of I-V plots for each mutant as pHi increased, a selection of which, from representative cells expressing either E368K or T370K, is shown in Fig. 9, A and B. The pHi/Gm relationships for a larger number of cells from each group are shown in Fig. 9, D and E. Finally, those data, normalized to the Gm,max for each cell, are shown in Fig. 9, G and H. A third mutation R804H (recessive, CHED-linked: equivalent to mouse R774H) caught our attention due to its alteration of a titratable residue in an intracellular juxtamembrane loop (Fig. 6A). In Fig. 9, C, F, and I (note the change of scale in Fig. 9F), we show the relationship between pHi and Gm for R774H at pHe 8.50. When we fit the data for all three mutants to the Hill equation (Fig. 10: average sum of squares = 0.02 ± 0.01, 0.07 ± 0.02, and 0.06 ± 0.02, respectively), we find that E368K and T370K have a significantly more acidic pKi compared with wild-type Slc4a11, while R774H has a significantly more alkaline pKi compared with wild-type Slc4a11 (P < 0.016, one-tailed unpaired t test with Bonferroni correction) with no significant alteration in the Napp in any case (P > 0.016, one-tailed unpaired t test with Bonferroni correction). Although the low Gm,max of E368K and T370K precluded their study at pHe =7.50, the Gm,max for R774H was not different from that of wild-type (P = 0.44, two-tailed unpaired t test) and thus we were able to gather data from R774H-expressing oocytes at pHe =7.50 (Fig. 11, A and B). Mirroring the situation at pHe 8.50, we find that the estimated minimum pKi value for R774H tends to be more alkaline than the estimated minimum pKi values for wild-type Slc4a11 at pHe =7.50 (Fig. 11C).
Fig. 9.
The influence of three dystrophy-causing mutations on the intracellular pH (pHi) dependence of Slc4a11 at extracellular pH (pHe) 8.50. Three mutants (E369K, T370K, and R774H: equivalent to human dystrophy-causing mutants E399K, T401K, and R804H) were tested for their response to changes in pHi. A–C: a sampling of current-voltage (I-V) plots gathered from representative mutant-Slc4a11-expressing cells during experiments equivalent to those shown in Fig. 2, A, D, E, and F: The relationship between pHi and membrane conductance (Gm) for a greater number of mutant-Slc4a11-expressing oocytes drawn from all of the I-V plots gathered for each cell. Gray dashed line shows maximum Gm calculated from any of a greater number of water-control oocytes (from Supplemental Fig. 1B: https://doi.org/10.6084/m9.figshare.12400946). G–I: each Slc4a11 trace from D–F is shown here normalized to its own maximum conductance. Vm, membrane potential.
Fig. 10.
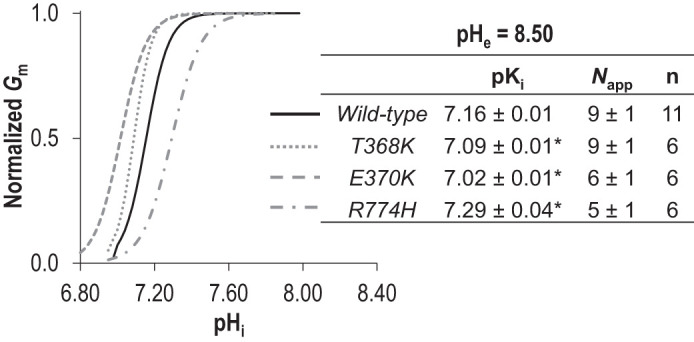
Hill equation fits of three dystrophy-linked mutants of Slc4a11 activity at extracellular pH (pHe) 8.50. The best-fit to the Hill equation for each of the traces in Fig. 9, G–I was used to determine a range of values for intracellular pH (pHi) dependence (pKi) and apparent Hill coefficient (Napp) for each mutant. The average value for these constants was used to generate the averaged best-fit traces shown in the graph. *Significant difference from wild-type values (P < 0.016, one-tailed, unpaired t test with Bonferroni correction).
Fig. 11.
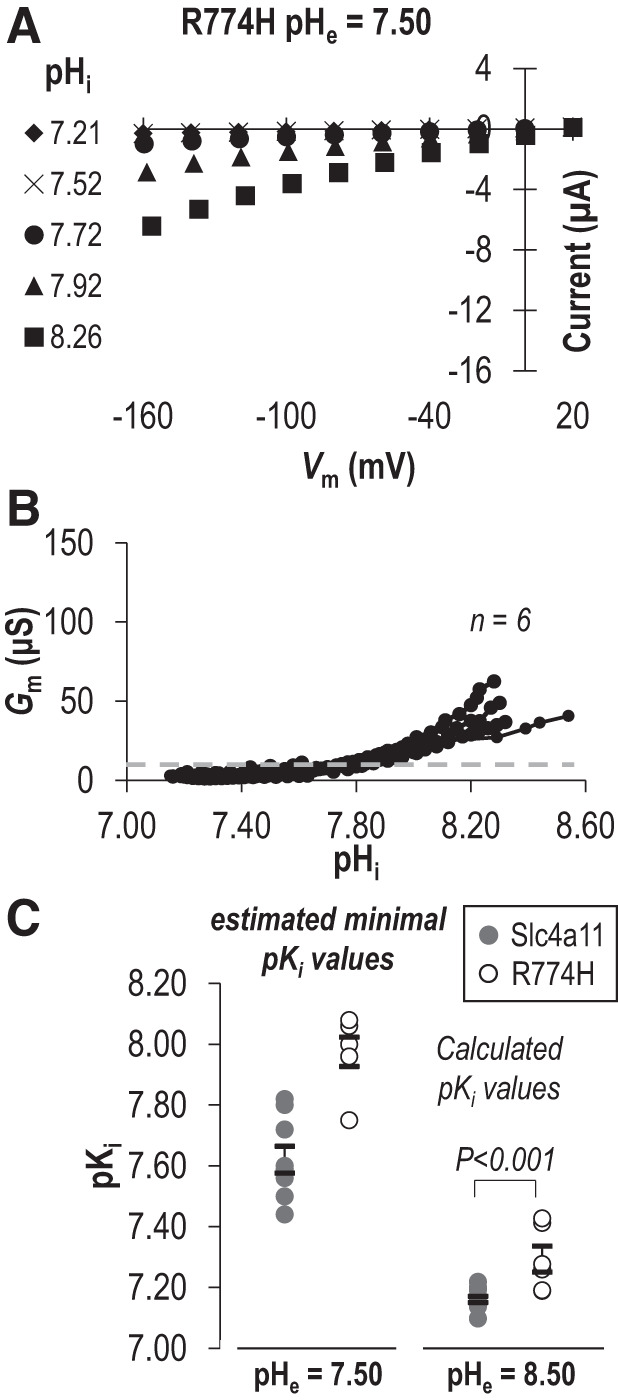
The influence of R774H on the intracellular pH (pHi) dependence (pKi) of Slc4a11 at extracellular pH (pHe) =7.50. A: a sampling of current-voltage (I-V) plots gathered from representative R774H-expressing cells during experiments equivalent to that shown in Fig. 1A. B: the relationship between pHi and membrane conductance (Gm) for a greater number of R774H-expressing oocytes drawn from all of the I-V plots gathered for each cell. Gray dashed line shows maximum Gm calculated from any of a greater number of water-control oocytes (from Supplemental Fig. 1A: https://doi.org/10.6084/m9.figshare.12400946). C: the range of pKi values for wild-type and R774H at each tested value of pHe. pKi values are calculated from Hill plots fits (pHe 8.50) or estimated minimal pKi values from pHi at 50% of maximum observed Gm (pHe =7.50). Vm, membrane potential.
DISCUSSION
The pH dependence of wild-type Slc4a11.
Our data are consistent with a scheme in which the pHi dependence of the Slc4a11 H+ conductance is governed by a single pKi, the value of which is dependent on pHe. The more alkaline pHi becomes, the greater Gm becomes (e.g., Fig. 1E) but the more acidic pHe becomes, the more alkaline pKi becomes (Fig. 5D) and the lower Gm becomes. For cells expressing Slc4a11, there are obvious advantages to increasing Gm as pHi increases; this would tend to promote H+ influx to counter local rises in pHi. We have previously postulated that the role of Slc4a11 is to counter rises in pHi caused by NBCe1 action, maintaining the transcellular passage of with minimal challenge to local pHi at the basolateral membrane. However, what of the dependence on pHe? Any acidic shift in pHe might tend to cause a slight but counterintuitive dampening of Gm. However, this may be a minor drawback for the broader benefit of pHe dependence as this also means that Gm is lowered in the face of pathological extracellular acidosis (while pHi is normal) protecting pHi from the extracellular insult. In this way, Slc4a11 could respond acutely to counteract a rise in pHi while resisting the tendency to cause intracellular acidosis in the presence of an inwardly directed H+ gradient. This would be consistent with its hypothesized role in pHi regulation and would be another point of differentiation from H+ channels such as Hv1, which do sense H+ gradients but have a pH-gradient- and voltage-dependent directionality that favors H+ extrusion (4, 5). The pHe dependence of Slc4a11 might also help to keep the H+ conductance quiescent as the protein progresses through the secretory pathway, the lumen of which is acidic and equivalent to the extracellular space.
We encountered some unexpected technical issues during our study that precluded us from defining a value for pKi at pHe = 7.50. Under this condition, pKi is so alkaline that we could not raise oocyte pHi high enough to establish the requisite pHi/Gm plateau that would allow us to fit data to the Hill equation. That is not to say that Slc4a11 is inactive, or operating in a different mode, under these conditions (see Supplemental Fig. S4 and S5; see https://doi.org/10.6084/m9.figshare.12400946), but it is likely to be only operating at a fraction (we estimate ~10%) of its maximum capacity at rest due to the substantially more alkaline pKi. It is for this technical reason that we can only perform statistical comparisons among values for pKi and Napp that we gathered at pHe 8.50. We can, however, use data gathered at pHe =7.50 to read off a minimal value of pKi based on the highest observed value of Gm in each experiment. We are wary of using these values in statistical comparisons as the sets necessarily include a greater variability than the values gathered at pHe 8.50 because they are influenced by the expression level of Slc4a11 in each cell and do not have any precise physiological meaning. However, these data are still valuable as they show that the statistically significant differences in pKi observed at pHe 8.50 are preserved, at least in trend (with the sole exception of “pre-TM1-del”), at the physiological value of pHe =7.50. For these same technical reasons, we are also unable to ascertain the nature of the mathematical relationship between pHe and pKi, beyond the general trend (Fig. 5D).
Determinants of pH dependence.
The Hill coefficient for Slc4a11 indicates that the pHi dependence of H+ conductance is governed by more than one titratable residue. Indeed, structure-function studies of a related acid-loading protein the Cl−/ exchanger AE2 (SLC4A2), which is also activated by rises in pHi and pHe (14), show that the pHi dependence of Slc4 proteins can be complex and involve interactions between numerous pH-sensitive regions, both intracellular and extracellular (28, 35). However, Slc4a11 bears no more similarity to AE2 than it does to any other, less pH-sensitive Slc4 ortholog. Instead, sequence alignments of Slc4 sequences drew our attention to a unique cluster of lysine residues in Slc4a11 near to TM1 which is reminiscent of a critical lysine reside that is a located at the intracellular end of TM1 of the pH-dependent renal outer medullary K+-channel (ROMK, Kir 1.1) (7). ROMK is activated by acidic pH with a pK of ~6.8, but when the lysine is mutated to methionine, the result is such a pronounced acidic shift in pK (pK ~5.3) that it effectively renders ROMK constitutively active. Moreover, the mutation causes the Hill coefficient to drop from 3.9 to 0.5 (7). In the case of Slc4a11, the effect of removing lysines from this region is a significant alkaline shift in pKi, but the change is less dramatic than with ROMK, presumably because Slc4a11 includes numerous other critical pH-sensitive determinants of transport that have yet to be identified. Thus the unique juxta-TM1 lysine residues influence the pH dependence of Slc4a11 but are not required for the unique H+ conducting action of Slc4a11. Finally, in regard to why lysine replacement in Slc4a11 might cause an alkaline shift in pKi rather than the acidic shift observed for ROMK, we believe that this reflects the complex cooperativity among the multiple determinants of pH dependence that are likely to be different between the two proteins. The effect upon Slc4a11 pKi of other amino-acid substitutions at this location has not been tested but could reveal the extent of the influence of K343, and other lysine residues in this region, upon pKi. However, it is possible that not all substitutions will be well tolerated; for example, in the related transporter AE1 (SLC4A1), introducing an alanine at the K343-equivalent position (Q404) compromises cell surface presentation (11). Finally we note that, although our study was not powered to assess changes in ion selectivity of the mutants, our data are not suggestive of such a phenomenon (see Supplemental Figures S5; see https://doi.org/10.6084/m9.figshare.12400946).
The contribution of altered pH dependence to disease.
The potential for loss of function under physiological conditions that could be caused by an alkaline shift in pKi led us to examine a few select disease-causing mutants for alterations in pKi. E399K (E368K in our mouse clone) and T401K (T370K in our mouse clone) were of interest because they represent the extracellular, juxtamembrane introduction of lysine residues, providing a counterpoint to our intracellular lysine replacement experiments at the intracellular end of TM1. Both E386K and T370K exhibit a significant acidic shift in pKi, demonstrating the principal that disease-causing mutations can influence pH dependence. Although a pKi shift in either direction could disrupt a finely balanced regulatory mechanism, it is unlikely that the pK is a major etiological factor in these examples; a greater issue is that both mutants are significantly retained in the endoplasmic reticulum (ER) when expressed in mammalian cells (1). However, this shift in pK does raise a couple of interesting speculative considerations. 1) If some Slc4a11 mutants are effectively hyperactive at physiological pH, small molecule chaperones that increase plasma membrane expression (2) may restore adequate H+ conductance with less rescued protein. (It would also be interesting to learn whether such chaperones, in aiding protein folding, normalize pKi.) 2) An ER-retained mutant with sufficiently acid-shifted pKi might allow H+ leak from the ER as the alkaline shift imposed up pKi by the relative acidity of the lumen (~7.1, equivalent to pHe) may no longer be sufficient to move pKi out of physiological pHi range and hold Slc4a11 inactive; a gain of function that could contribute to the dominance of disease in some cases. However, to be clear, we have no data that speak to the activity of either mutant at pHe <8.50.
R804H (R774H in our mouse clone) caught our attention as a candidate mutation that might raise pKi as, like our lysine-substituted constructs, it represents the replacement of a basic amino acid residue in an intracellular, juxtamembrane region. We note however, that three-dimensional structural models of SLC4 proteins (e.g., PDB ID: 6CAA, 4YZF) indicate that this arginine is not located in the immediate vicinity of the pre-TM1 lysines. In the case of R774H, we observed an alkaline shift in pKi that would likely cause a greater than 50% decrease in Gm under physiological conditions, an alteration that could contribute to disease etiology. Gm,max for R774H is the same as for wild-type Slc4a11 in our experiments, but whether this altered pK is the only functional defect with R774H is unknown. We are unaware of any assessment of H2O transport by this mutant, and it is unclear whether the mutant protein is substantially withheld from the plasma membrane in mammalian cells. This latter issue has been preliminarily assessed in mammalian cells by two methods as part of a wider screen of 54 SLC4A11 mutants; the data regarding R804H were not altogether consistent and will require further verification (1). One method (a bioluminescence resonance energy transfer assay that reports colocalization with plasma membrane expressed K-Ras) suggested normal plasma membrane abundance, while a second method (quantification of the fraction of SLC4A11 with mature glycosylation, considered by those authors to be the more reliable of the two assays) suggested a 50% decrease in plasma membrane abundance. Reduced plasma-membrane expression or not, a substantial reduction in Gm would be anticipated to impact the expressivity of disease and also compromise the usefulness of therapeutic strategies to enhance plasma membrane expression for this mutant. R804H is one of several arginine to histidine mutations that have been reported in Slc4a11. Many of these mutants are intracellularly retained, but there is one particularly notable exception, R125H (R99H in mice, see Fig. 6A) that has been used as a control in many experiments, including in the present study, due to its lack of H2O or H+ transport activity despite a normal plasma membrane abundance. The reason behind the functional importance of R125 is unknown, but our study raises the possibility that R125/R99 is a major determinant of pKi and that the pKi of R125H/R99H is so alkaline shifted as to render the mutant protein inactive under experimental conditions. Three pieces of evidence speak to this possibility: 1) we have demonstrated that a similar intracellular arginine to histidine substitution R774H causes an alkaline shift in pKi; 2) in some experiments performed at pHe 8.50 (when pKi would be at its most acidic), the trend of the pHi versus Gm relationship for R99H suggests that its activity could exceed that of H2O-injected cells if pHi was sufficiently alkaline (see Supplemental Figure S2B: see https://doi.org/10.6084/m9.figshare.12400946); and 3) H+ transport by R125H (R109H in that study) is partially rescued by preincubation with DIDS (12), a compound that derivatizes extracellular lysine residues. This phenomenon that could be explained if the reaction disrupts the relationship between pHe and pKi, causing a normalizing of pKi.
Ultimately, the pathogenesis of endothelial dystrophy is complex and involves interplay among multiple stresses (e.g., oxidative stress, unfolded protein response, and mitophagy) (21). There may even be numerous mechanisms by which Slc4a11 mutation can lead to disease such as loss of function (16, 20, 29), the accumulation of misfolded SLC4A11 in the endoplasmic reticulum leading to unfolded protein response (30), or loss of extracellular matrix-interacting determinants (19). Our study has suggested a mechanism by which some mutations can result in loss of function, raises some considerations for personalization of therapeutic intervention, and increases our understanding of the structure-function relationships in this unusual Slc4 protein.
GRANTS
This work was supported by National Eye Institute R01 award EY028580 (to M.D.P).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.N.Q. and M.D.P. conceived and designed research; B.N.Q. and A.M. performed experiments; B.N.Q. and M.D.P. analyzed data; B.N.Q. and M.D.P. interpreted results of experiments; B.N.Q. and M.D.P. prepared figures; B.N.Q. and M.D.P. drafted manuscript; B.N.Q. and M.D.P. edited and revised manuscript; B.N.Q., A.M., and M.D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Evan J. Myers for performing preliminary experiments.
REFERENCES
- 1.Alka K, Casey JR. Molecular phenotype of SLC4A11 missense mutants: Setting the stage for personalized medicine in corneal dystrophies. Hum Mutat 39: 676–690, 2018. doi: 10.1002/humu.23401. [DOI] [PubMed] [Google Scholar]
- 2.Alka K, Casey JR. Ophthalmic nonsteroidal anti-inflammatory drugs as a therapy for corneal dystrophies caused by SLC4A11 mutation. Invest Ophthalmol Vis Sci 59: 4258–4267, 2018. doi: 10.1167/iovs.18-24301. [DOI] [PubMed] [Google Scholar]
- 3.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2012. doi: 10.1016/j.exer.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decoursey TE. Voltage-gated proton channels. Compr Physiol 2: 1355–1385, 2012. doi: 10.1002/cphy.c100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCoursey TE. Voltage and pH sensing by the voltage-gated proton channel, HV1. J R Soc Interface 15: 20180108, 2018. doi: 10.1098/rsif.2018.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet 44: 322–326, 2007. doi: 10.1136/jmg.2006.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J 15: 4093–4099, 1996. doi: 10.1002/j.1460-2075.1996.tb00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gröger N, Fröhlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem 285: 14467–14474, 2010. doi: 10.1074/jbc.M109.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SB, Ang HP, Poh R, Chaurasia SS, Peh G, Liu J, Tan DT, Vithana EN, Mehta JS. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci 54: 6179–6189, 2013. doi: 10.1167/iovs.13-12089. [DOI] [PubMed] [Google Scholar]
- 10.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013. doi: 10.1167/iovs.13-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanki T, Young MT, Sakaguchi M, Hamasaki N, Tanner MJ. The N-terminal region of the transmembrane domain of human erythrocyte band 3. Residues critical for membrane insertion and transport activity. J Biol Chem 278: 5564–5573, 2003. doi: 10.1074/jbc.M211662200. [DOI] [PubMed] [Google Scholar]
- 12.Kao L, Azimov R, Abuladze N, Newman D, Kurtz I. Human SLC4A11-C functions as a DIDS-stimulatable H+(OH−) permeation pathway: partial correction of R109H mutant transport. Am J Physiol Cell Physiol 308: C176–C188, 2015. doi: 10.1152/ajpcell.00271.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao L, Azimov R, Shao XM, Abuladze N, Newman D, Zhekova H, Noskov S, Pushkin A, Kurtz I. SLC4A11 function: evidence for H+(OH−) and NH3-H+ transport. Am J Physiol Cell Physiol 318: C392–C405, 2020. doi: 10.1152/ajpcell.00425.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem 281: 1885–1896, 2006. doi: 10.1074/jbc.M509734200. [DOI] [PubMed] [Google Scholar]
- 15.Lee SK, Boron WF, Parker MD. Monitoring ion activities in and around cells using ion-selective liquid-membrane microelectrodes. Sensors (Basel) 13: 984–1003, 2013. doi: 10.3390/s130100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Hundal KS, Chen X, Choi M, Ogando DG, Obukhov AG, Bonanno JA. R125H, W240S, C386R, and V507I SLC4A11 mutations associated with corneal endothelial dystrophy affect the transporter function but not trafficking in PS120 cells. Exp Eye Res 180: 86–91, 2019. doi: 10.1016/j.exer.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loganathan SK, Schneider HP, Morgan PE, Deitmer JW, Casey JR. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am J Physiol Cell Physiol 311: C735–C748, 2016. doi: 10.1152/ajpcell.00078.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem 284: 26882–26896, 2009. doi: 10.1074/jbc.M109.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra D, Jung M, Fecher-Trost C, Lovatt M, Peh GS, Noskov S, Mehta JS, Zimmermann R, Casey JR. Defective cell adhesion function of solute transporter, SLC4A11, in endothelial corneal dystrophies. Hum Mol Genet 29: 97–116, 2020. doi: 10.1093/hmg/ddz259. [DOI] [PubMed] [Google Scholar]
- 20.Myers EJ, Marshall A, Jennings ML, Parker MD. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am J Physiol Cell Physiol 311: C945–C959, 2016. doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanda GG, Alone DP. REVIEW: Current understanding of the pathogenesis of Fuchs’ endothelial corneal dystrophy. Mol Vis 25: 295–310, 2019. [PMC free article] [PubMed] [Google Scholar]
- 22.Nehrke K. H(OH), H(OH), H(OH): a holiday perspective. Focus on “Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH− conductance pathway that is stimulated by rises in intracellular and extracellular pH”. Am J Physiol Cell Physiol 311: C942–C944, 2016. doi: 10.1152/ajpcell.00309.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neubig RR, Spedding M, Kenakin T, Christopoulos A; International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification . International union of pharmacology committee on receptor nomenclature and drug classification. XXXVIII. update on terms and symbols in quantitative pharmacology. Pharmacol Rev 55: 597–606, 2003. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 24.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator. Am J Physiol Cell Physiol 305: C716–C727, 2013. doi: 10.1152/ajpcell.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+ -coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segel LA. Mathematical Models in Molecular Cellular Biology. New York: Cambridge University Press, 1980. [Google Scholar]
- 28.Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH(2)-terminal cytoplasmic domain. Am J Physiol Cell Physiol 281: C1344–C1354, 2001. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- 29.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 31.Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet 17: 656–666, 2008. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- 32.Weiss JN. The Hill equation revisited: uses and misuses. FASEB J 11: 835–841, 1997. doi: 10.1096/fasebj.11.11.9285481. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JS, Møller HU, Aldave AJ, Seitz B, Bredrup C, Kivelä T, Munier FL, Rapuano CJ, Nischal KK, Kim EK, Sutphin J, Busin M, Labbé A, Kenyon KR, Kinoshita S, Lisch W. IC3D classification of corneal dystrophies–edition 2. Cornea 34: 117–159, 2015. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Ogando DG, Bonanno JA, Obukhov AG. Human SLC4A11 is a novel NH3/H+ co-transporter. J Biol Chem 290: 16894–16905, 2015. doi: 10.1074/jbc.M114.627455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. J Biol Chem 271: 5741–5749, 1996. doi: 10.1074/jbc.271.10.5741. [DOI] [PubMed] [Google Scholar]