Abstract
Severe burn injury induces a myriad of deleterious effects to skeletal muscle, resulting in impaired function and delayed recovery. Following burn, catabolic signaling and myofiber atrophy are key fiber-intrinsic determinants of weakness; less well understood are alterations in the interstitial environment surrounding myofibers. Muscle quality, specifically alterations in the extracellular matrix (ECM), modulates force transmission and strength. We sought to determine the impact of severe thermal injury on adaptation to the muscle ECM and quantify muscle fibrotic burden. After a 30% total body surface area dorsal burn, spinotrapezius muscle was harvested from mice at 7 (7d, n = 5), 14 (14d, n = 4), and 21 days (21d, n = 4), and a sham control group was also examined (Sham, n = 4). Expression of transforming growth factor-β (TGFβ), myostatin, and downstream effectors and proteases involved in fibrosis and collagen remodeling were measured by immunoblotting, and immunohistochemical and biochemical analyses assessed fibrogenic cell abundance and collagen deposition. Myostatin signaling increased progressively through 21 days postburn alongside fibrogenic/adipogenic progenitor cell expansion, with abundance peaking at 14 days postburn. Postburn, elevated expression of tissue inhibitor of matrix metalloproteinase 1 supported collagen remodeling resulting in a net accumulation of muscle collagen content. Collagen accumulation peaked at 14 days postburn but remained elevated through 21 days postburn, demonstrating minimal resolution of burn-induced fibrosis. These findings highlight a progressive upregulation of fibrogenic processes following burn injury, eliciting a fibrotic muscle phenotype that hinders regenerative capacity and is not resolved with 21 days of recovery.
Keywords: burn, extracellular matrix, fibrogenic/adipogenic, muscle fibrosis, myostatin, progenitor cell
INTRODUCTION
Five percent of pediatric injury-related mortalities are caused by burns (33). Additionally, accidental burn injuries are among the top 10 most prevalent nonfatal injuries for children 0 to 4 yr of age treated in emergency departments in the United States, with the majority of pediatric burn injuries being scald burns (33). Treatment of these burn injuries costs 7.5 billion dollars annually in the United States alone (32), underscoring pediatric burn care as an issue of significant prevalence with both clinical and financial relevance.
Burn injuries induce deleterious effects in skeletal muscle both local and distal to the site of injury (10, 24, 36, 44). Significant skeletal muscle atrophy and subsequent weakness follow a severe burn [greater than 20% total body surface area (TBSA)] and complicate systemic recovery (28). Our laboratory has recently shown in a murine model of severe burn injury that myofiber atrophy peaks 14 days after severe burn injury with a concurrent peak in muscle stem cell (satellite cell; muSC) activity (10). With upregulated muSC activity supporting muscle regeneration, we have shown myofiber atrophy is resolved at 21 days postburn; however, other components of muscle quality that impact function, such as the extracellular matrix (ECM), have been less explored.
Transforming growth factor-β (TGFβ) superfamily signaling, including expression of myostatin, a well-characterized negative regulator of skeletal muscle size, increases dramatically following a severe burn (19). Myostatin positively regulates its own expression through activation of the SMAD family of transcription factors (14), facilitating persistent overexpression in the muscle, which can impede rehabilitation and recovery. Importantly, myostatin promotes excessive collagen and ECM accumulation within muscle by binding to the activin receptor-type 2B on fibrogenic cells, stimulating cell proliferation and subsequent synthesis of ECM components mediated through SMAD transcription factors (21). Case reports and studies in rodents have shown evidence of burn-induced adaptations to the muscle ECM, with accumulation of collagenous tissue between muscle fibers indicated by hematoxylin and eosin staining associated with impaired muscle function in electrophysiology studies (4, 13, 29). These reports highlight a need for further study of ECM remodeling following burn injury, with an emphasis on upstream signaling, metalloproteases, and multiple cell types contributing to ECM remodeling.
To address this need, we investigated a time course (up to 21 days postburn) of TGFβ superfamily signaling in skeletal muscle following scald burn injury, concurrently with fibrogenic cell abundance and collagen content and turnover. We hypothesized that expression of TGFβ and myostatin would be upregulated along with activation of downstream effectors after burn injury, supporting expansion of fibrogenic cells, increased collagen turnover, and excessive ECM content to produce a fibrotic phenotype that would be resolved 21 days after burn.
MATERIALS AND METHODS
Mice.
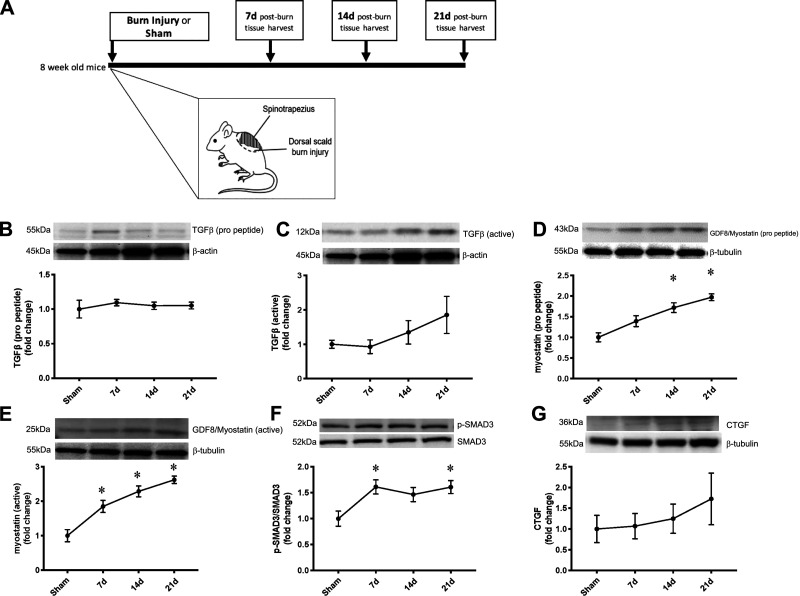
Mice were a mixed C57BL/6-129 background strain. All mice were housed in a temperature- and humidity-controlled vivarium on a 12:12-h light-dark cycle. At 8 wk of age, mice were randomized to sham or dorsal scald burn injury groups. Mice randomized to receive burn injury were further randomized for tissue harvest at 7, 14, or 21 days postburn injury. Randomization resulted in the following groups: sham injury (Sham; n = 4), 7 days postburn (7d; n = 5), 14 days postburn (14d; n = 4), and 21 days postburn (21d; n = 4). All experiments were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee and were conducted according to the National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Mice included in the current study represent a small cohort from a previously published study (10). Figure 1A denotes the study design, including a diagram of the scald burn injury.
Fig. 1.
Transforming growth factor-β (TGFβ) superfamily signaling is progressively upregulated in skeletal muscle after severe burn injury. A: study design. Eight-week-old mice were randomized to receive sham injury (Sham; n = 4) or dorsal scald burn after which tissue was harvested at 7 days postburn (7d; n = 5), 14 days postburn (14d; n = 4), and 21 days postburn (21d; n = 4). B: TGFβ (propeptide) protein expression in spinotrapezius. C: TGFβ (active) protein expression in spinotrapezius. D: myostatin (propeptide) protein expression in spinotrapezius. E: myostatin (active dimer) protein expression in spinotrapezius. F: phosphorylation of SMAD3 relative to total SMAD3 protein level in spinotrapezius. G: cytokine connective tissue growth factor (CTGF) protein expression in spinotrapezius. No differences in β-actin or β-tubulin shown as loading controls. Data are presented as mean fold change compared with Sham ± SE. One-way ANOVA with Tukey’s post hoc test. *P < 0.05 compared with sham. n = 4 mice/group.
Dorsal scald burn injury and tissue harvest.
For mice randomized to thermal injury groups, a full-thickness dorsal scald burn was induced as previously described (37). Briefly, buprenorphine was administered subcutaneously 30 min before the burn procedure for pain (15). Following anesthetization with 2.5% isoflurane, the dorsal surface of the mice was shaved and 1 mL of saline was injected subcutaneously to protect the spinal cord. While still under deep anesthesia, mice were placed in a preformed mold exposing 30% TBSA along the dorsal side. Mice inside the mold were lowered into a water bath at 98° for 10 s. Mice were resuscitated by 2 mL of lactated Ringer’s solution administered intraperitoneally. After scald burn injury, mice were monitored twice daily for indication of pain and/or stress using a rodent health score for objective assessment. Administration of additional medication for pain or inflammation was not indicated for any mice after the dorsal scald burn, and all mice displayed normal ambulatory patterns.
Following burn injury, mice were singly housed in the institutional vivarium for 7, 14, or 21 days. All mice were ambulatory for the duration of the study. Excluding burn administration, mice randomized to the Sham group received identical treatment and care. After the corresponding study period, all mice were anesthetized with 2.5% isoflurane and euthanized by cervical dislocation. Mice in the Sham group were euthanized at the same time point as mice in the 21d group, resulting in an age range of 9–11 wk with the following ages for each group: Sham 11 wk, 7d 9 wk, 14d 10 wk, and 21d 11 wk. The spinotrapezius muscle, a dorsal skeletal muscle proximal to the scald burn injury, was harvested for analysis. Additionally, the tibialis anterior muscle from the hind limb was also collected.
Western blotting for protein expression of the spinotrapezius muscle.
The spinotrapezius muscle was harvested and quickly cut in half. One-half was prepared for immunohistochemical analysis as described below; the other half was immediately flash frozen in liquid nitrogen for Western blot analysis of protein expression. Western blot experiments were performed as previously described (8). Spinotrapezius muscle samples were homogenized by bead milling with 0.5-mm zinc oxide beads (Bullet Blender; Next Advance) in ice-cold buffer with phosphatase and protease inhibitor tablets. The Bradford method was used to quantitate total protein concentration, and 50 μg total protein were loaded for each sample. For each electrophoresis run, all samples were loaded on a single precast SDS polyacrylamide gel (4–20%, Bio-Rad) and separated for 90 min at 150 V, followed by transfer of protein to a PVDF membrane at 50 V for 1 h at 4°C. Membranes were blocked for 1 h at room temperature in 2% bovine serum albumin (BSA). The following rabbit IgG primary antibodies were used at 1:1,000 in 2% bovine serum albumin (BSA): anti-GDF8/myostatin (no. ab71808; Abcam), anti-phosphorylated SMAD3 (Ser423/425) (no. 9520; Cell Signaling), anti-SMAD3 (no. 56783; Cell Signaling), anti-TGFβ (no. 3709, Cell Signaling), anti-cytokine connective tissue growth factor (anti-CTGF; no. ab6992, Abcam), anti-matrix metalloproteinase 2 (anti-MMP2; no. 87809; Cell Signaling), anti-MMP9 (no. 13667; Cell Signaling), anti-tissue inhibitor of metalloproteinases 1 (anti-TIMP1; no. 8946; Cell Signaling), and anti-lysyl oxidase (anti-LOX; no. 58135; Cell Signaling). Membranes were stripped and subsequently reprobed for multiple proteins as appropriate per molecular weight, and anti-β-tubulin (no. 2146; Cell Signaling), anti-β-actin (no. 4970, Cell Signaling), or anti-GAPDH (no. 2118, Cell Signaling) was used as a loading control. A horseradish peroxidase (HRP)-linked donkey anti-rabbit IgG secondary antibody was used followed by ECL detection. Optical density was measured with a phosphoimager (Bio-Rad), and densitometric analysis was completed with Quantity One software (v 4.5.2, Bio-Rad). Background density was subtracted from each respective band to account for any density gradient across the membrane. For quantification of LOX, a fluorophore conjugated goat anti-rabbit IgG secondary (no. 12005870, Bio-Rad) was used with fluorescent detection (ChemiDoc MP, Bio-Rad) analyzed by ImageLab software (v 6.0.1, Bio-Rad). Densitometry values for burn mice are expressed as a fold change of the mean Sham value, with the mean Sham value set at 1. Individual sham values are presented as a fold change relative to the mean of the Sham group.
Immunohistochemistry for morphology of the spinotrapezius muscle.
Upon removal and splitting as described above, one-half of the spinotrapezius muscle was covered in Tissue Tek (O.C.T. Compound, Sakura Finetek, Torrance, CA) at resting length on a foil-covered cork and frozen in liquid nitrogen-cooled 2-methylbutane. All samples were stored at −80°C until analysis. Seven-micrometer-thick sections were cut using a cryostat (HM525-NX, Thermo Fisher Scientific, Waltham, MA) and allowed to air dry for 1 h. Sections on slides were then stored at −20°C until immunohistochemical staining was performed.
For assessment of myofibroblast abundance via Tcf4/Tcf7L2 staining, sections were fixed in 4% paraformaldehyde followed by epitope retrieval with sodium citrate (10 mM, pH 6.5) at 92°C. Three percent hydrogen peroxide in phosphate-buffered saline (PBS) was used to block endogenous peroxidase activity. Sections were blocked for 1 h in 1% blocking reagent included in a commercially available tyramide signal amplification kit (TSA, Life Technologies/Thermo Fisher Scientific, Waltham, MA) followed by overnight incubation at 4°C in rabbit monoclonal IgG primary antibody against Tcf4/Tcf7L2 (Cell Signaling, no. 2569) at 1:100 in the 1% blocking reagent. The next day, slides were washed in PBS and sections were incubated for 85 min in a goat anti-rabbit biotinylated IgG secondary antibody (Jackson Immuno Research, no. 111-065-003) at 1:1,000 and wheat germ agglutinin (WGA; Invitrogen, no. W834) at 1:50 in 1% blocking reagent. WGA was used to identify the myofiber border. Slides were then washed in PBS and incubated for 1 h in streptavidin-horseradish peroxidase included in a commercially available TSA kit (SA-HRP, Life Technologies/Thermo Fisher Scientific). TSA-Alexa Fluor 488 was used to amplify Tcf4/Tcf7L2. Lastly, sections were incubated for 10 min in 4′,6-diamidino-2-phenylindole (DAPI; 10 nM, LifeTechnologies/Thermo Fisher Scientific), washed in PBS, and mounted using Vectashield fluorescence mounting media (Vector Laboratories, Burlingame, CA).
For platelet-derived growth factor receptor-α (PDGFRα)-laminin staining to denote fibrogenic adipogenic progenitor cells (FAPs), sections were fixed in 4% paraformaldehyde for 7 min, washed in PBS, and blocked in 1% blocking reagent included in a commercially available TSA kit (Life Technologies/Thermo Fisher Scientific) for 1 h. Sections were incubated overnight at 4°C in goat IgG primary antibody against PDGFRα at 1:100 and rabbit IgG primary antibody against laminin at 1:200 in 1% blocking reagent. The following day, sections were washed in PBS and incubated for 1 h in rabbit anti-goat IgG secondary antibody conjugated to Alexa Fluor 555 (Invitrogen, no. A-21431) at 1:250 in PBS to visualize PDGFRα. Sections were washed and incubated for 1 h in goat anti-rabbit IgG secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, no. A-11034) at 1:500 in PBS to visualize the laminin myofiber border. Finally, sections were incubated in DAPI (10 nM, LifeTechnologies/Thermo Fisher Scientific) for 10 min, washed in PBS, and mounted using Vectashield fluorescence mounting media (Vector Laboratories).
For immunofluorescent detection of collagen 3, sections were fixed in acetone at −20°C for 10 min. Sections were washed in PBS, blocked in 2.5% normal horse serum (NHS; Vector Laboratories, no. S-2012) for 1 h, and incubated overnight at 4°C in rabbit IgG primary antibody against collagen 3 (Abcam, no. ab7778) at 1:100 in 2.5% NHS. Slides were then washed in PBS and incubated 1 h in goat anti-rabbit IgG secondary antibody conjugated to Alexa Fluor 555 (LifeTechnologies/Thermo Fisher Scientific, no. A-21428) at 1:500 in PBS to visualize collagen 3. Sections were then incubated for 10 min in DAPI (10 nM, LifeTechnologies/Thermo Fisher Scientific), washed, and mounted using Vectashield fluorescence mounting media (Vector Laboratories).
Collagen 4 was detected by first fixing sections in acetone at −20°C for 10 min. Sections were then blocked in 1% blocking reagent included in a commercially available TSA kit (Life Technologies/Thermo Fisher Scientific) for 1 h, followed by overnight incubation in rabbit polyclonal primary antibody against collagen 4 (Abcam, no. ab6586). The following day, slides were washed in PBS and incubated in goat anti-rabbit secondary antibody conjugated to Alexa Fluor 555. Sections were then incubated for 10 min in DAPI (10 nM, LifeTechnologies/Thermo Fisher Scientific), washed, and mounted using Vectashield fluorescence mounting media (Vector Laboratories).
We recently published a similar method of collagen hybridizing peptide (CHP) detection in human skeletal muscle (1), and we have modified this method for mouse muscle tissue. For collagen 1 and CHP detection, sections were fixed in acetone for 10 min at −20°C and then blocked for 1 h in 2.5% NHS (Vector Laboratories, no. S-2012). Sections were then washed in PBS. For CHP detection, 3Helix-5-FAM conjugate (3Helix, no. FLU300) was diluted to a working solution of 20 μM in PBS and placed on the heating block at 80°C for 5 min to denature 3Helix trimers, breaking apart triple helices to allow binding with synthesizing and degrading collagen. The 20 μM working solution was removed from the heating block and cooled on wet ice for 2 min to avoid heat-damaging muscle sections. Immediately after cooling, rabbit IgG primary antibody against collagen 1 (Abcam, no. ab34710) was added to the 3Helix-5-FAM 20 μM working solution at 1:200 followed by overnight incubation at 4°C. The following morning, slides were washed in PBS and incubated for 1 h at room temperature in goat anti-rabbit IgG secondary conjugated to Alexa Fluor 647 (Life Technologies/Thermo Fisher Scientific, no. A-21244) to detect collagen 1. Slides were then incubated for 10 min in DAPI (10 nM, LifeTechnologies/Thermo Fisher Scientific), washed, and mounted using Vectashield fluorescence mounting media (Vector Laboratories).
For detection of macrophages, sections were fixed in acetone for 10 min at −20°C. Slides were then washed in PBS, and endogenous peroxidases were blocked with 3% hydrogen peroxide. Sections were blocked in mouse-on-mouse IgG blocking reagent (Vector Laboratories, no. MKB-2213) for 1 h, followed by a second blocking step in 2.5% normal horse serum (Vector Laboratories, no. S-2012). Slides were incubated overnight in primary antibodies against the macrophage marker F4/80 (AbD Serotec, no. MCA497GA), M2 macrophage marker CD163 (SCBT, no. sc-33560), and dystrophin (Vector Laboratories, no. VP D505) in 2.5% NHS (Vector Laboratories, no. S-2012) at 4°C. The following morning, slides were washed in PBS and incubated for 1 h at room temperature in biotinylated goat anti-rat IgG secondary antibody (Invitrogen, no. 31830), goat anti-rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen, no. A11034) to detect CD163, and goat anti-mouse IgG1 conjugated to Alexa Fluor 647 (Invitrogen, no. A-21240) for detection of dystrophin. Sections were washed and incubated for 1 h in SA-HRP included in a commercially available TSA kit (Life Technologies/Thermo Fisher Scientific). TSA-Alexa Fluor 555 was used to amplify F4/80. Sections were then incubated in DAPI (10 nM, LifeTechnologies/Thermo Fisher Scientific) for 10 min, washed in PBS, and mounted using Vectashield fluorescence mounting media (Vector Laboratories).
Image acquisition and analysis.
Images were captured at ×100–400 magnification at room temperature using a Zeiss upright microscope (AxioImager M1; Zeiss, Oberkochen, Germany); analysis was performed with AxioVision Rel softward (v4.9). All image analysis was conducted in a blinded manner to group. Myofibroblast and FAPs were identified as previously described (11). Briefly, myofibroblast abundance was determined by costaining of Tcf4/Tcf7L2 and DAPI, using WGA to denote the myofiber border. Tcf4+/Tcf7L2+/DAPI+ cells in the interstitial space were counted as myofibroblasts and normalized to myofiber number and area. FAP abundance was assessed by costaining of PDGFRα and DAPI using laminin to mark myofiber border. PDGFRα+/DAPI+ cells in the interstitial space were counted as FAPs and normalized to myofiber number and area. Collagen isoforms and CHP were quantified as previously described (1). Collagen 1, 3, and 4 were quantified using the thresholding feature of Zeiss AxioVision software (v4.9), and the area of each collagen isoform was expressed relative to the total muscle area (mm2). CHP quantification was conducted similarly to assess area of collagen remodeling. The area of the CHP was measured by the thresholding feature of AxioVision and normalized to the total muscle area (mm2). Muscle macrophage abundance was determined by CD163- F4/80+ DAPI+ cells (classified as M1 macrophages) and CD163+ F4/80+ DAPI+ (classified as M2 macrophages) in the interstitial space normalized to myofiber number and total muscle cross-sectional area (mm2). All immunohistochemical images were analyzed by a single assessor (C. R. Brightwell and M. E. Hanson) in a blinded manner.
Hydroxyproline biochemical assay for total collagen content of spinotrapezius pellet.
To determine total collagen content, hydroxyproline (HOP) in the muscle pellet was measured with a modified protocol using a commercially available Hydroxyproline Assay Kit (MAK008, Millipore Sigma, Darmstadt, Germany). Spinotrapezius muscle samples were homogenized by bead milling with 0.5-mm zinc oxide beads (Bullet Blender; Next Advance) in ice-cold buffer with phosphatase and protease inhibitor tablets. Following centrifugation, the supernatant was removed for Western blot analysis (described above), and the pellet containing the collagen fraction was weighed and stored at −80°C. The pellet was homogenized in double-distilled water (volume equal to 10× pellet weight; i.e., 100 μL double-distilled water for 10 mg pellet weight). Following homogenization, the sample was vortexed and a volume of 12M HCl equal to the water was added to hydrolyze the sample overnight at 105°C. The next day after hydrolysis, the sample/hydrolysate was vortexed and 40 μL were loaded in duplicate onto a 96-well plate along with hydroxyproline standard included in the Hydroxyproline Assay Kit; samples and standards were dried overnight in an oven at 60°C. Chloramine T/Oxidation Buffer included in the Hydroxyproline Assay Kit was added to each well followed by incubation at room temperature for 5 min. Diluted p-dimethylaminobenzaldehyde (DMAB) reagent was then added to each well, and the microplate was incubated at 60°C for 90 min. Absorbance was measured at 595 nm, and hydroxyproline content was calculated using a standard curve and normalized to the loaded sample volume (40 μL). Hydroxyproline content was also measured identically in the tibialis anterior hindlimb muscle to assess a potential systemic-mediated fibrotic impact of burn injury on skeletal muscle.
Pyridinium crosslink assay for collagen crosslinking of spinotrapezius.
To determine collagen crosslinking in the spinotrapezius, the muscle pellet hydrolysate remaining after hydroxyproline content analysis was used. NaOH (6 N) and Tris (1 M) were added in a 1:1 ratio to the same volume of hydrolysate. The total volume of hydrolysate was dependent on the volumes of water and 12 N HCl previously added to the pellet, proportional to pellet weight, as described above. Pyridinium (PYD) crosslink (pyridinoline and deoxypyridinoline) concentration was determined by enzyme-linked immunoassay Metra PYD EIA kit (Quidel Corporation, San Diego, CA), as previously described using skeletal muscle hydrolysate (9, 41). All standards and samples were analyzed in duplicate, and a four-parameter logistic curve was used to calculate sample PYD concentration. Data are expressed as nmol pyridinoline (PYD)/L and relative to total collagen measured by hydroxyproline content [PYD (nmol/L)/HOP (μg/μL)].
Statistical analysis.
All statistical analyses were performed with GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA). One-way ANOVA was used to assess group differences: Sham, 7 days postburn (7d), 14 days postburn (14d), and 21 days postburn (21d). Tukey’s post hoc comparisons were conducted when a main effect was detected. When the equal variance assumption for ANOVA was not met, the Kruskal-Wallis test was used to detect group differences with Dunn’s multiple comparisons test. Data are reported as means ± SE with P < 0.05 considered statistically significant. Pearson correlation was used to assess relationships between fibrogenic cell abundance and collagen content, inclusive of all experimental groups; Pearson r and P values are reported.
RESULTS
TGFβ superfamily signaling is upregulated in skeletal muscle after severe burn injury.
Expression of the uncleaved TGFβ1 propeptide remained unchanged after burn (Fig. 1B); protein levels of the cleaved, active form of TGFβ1 demonstrated a gradual, nearly twofold increase at 21 days postburn injury (Fig. 1C). However, TGFβ1 expression was not significantly different at any point postburn. Myostatin propeptide expression was elevated after burn (main effect of burn: P = 0.0003, Sham vs. 7d: P = 0.11, Sham vs. 14d: P = 0.0032, Sham vs. 21d: P = 0.0003), revealing a stepwise increase each week after burn injury peaking at nearly twofold greater expression at 21 days postburn injury (Fig. 1D). Expression of the active myostatin dimer was also progressively elevated following burn injury (main effect of burn: P < 0.0001, Sham vs. 7d: P = 0.01, Sham vs. 14d: P < 0.0004, Sham vs. 21d: P < 0.0001), with nearly threefold greater expression at 21 days postburn compared with Sham (Fig. 1E). SMAD3, a downstream effector of TGFβ and myostatin, phosphorylation relative to total SMAD3 was elevated after burn (main effect of burn: P = 0.02, Sham vs. 7d: P = 0.04, Sham vs. 14d: P = 0.13, Sham vs. 21d: P = 0.04) (Fig. 1F). Expression of the profibrotic cytokine connective tissue growth factor (CTGF) was 72% higher at 21 days postburn (Fig. 1G); however, there was no statistically significant change in CTGF expression after burn. The expression of loading controls β-actin and β-tubulin did not differ between groups.
Fibrogenic cell abundance is elevated in skeletal muscle after severe burn injury.
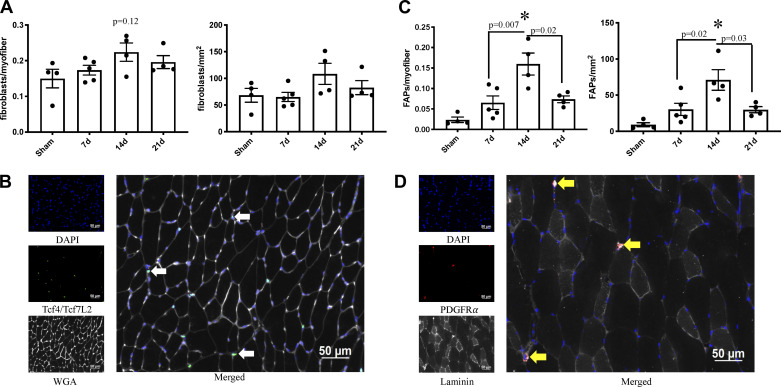
Tcf4/Tcf7L2+ myofibroblast abundance showed a numerical, but not statistically significant, elevation at 14 days postburn (normalized to myofiber number: P = 0.13, Sham vs. 14d: P = 0.12; normalized to muscle area: P = 0.15, Sham vs. 14d: P = 0.23, 7d vs. 14d: P = 0.15) (Fig. 2A). PDGFR-α+ fibrogenic adipogenic progenitors (FAPs) showed greater differences in burned mice than myofibroblasts, with FAP abundance peaking at 14 days postburn (normalized to myofiber number (main effect of burn): P = 0.0007, Sham vs. 14d: P = 0.0005, 7d vs. 14d: P = 0.007, 14d vs. 21d: P = 0.02; normalized to spinotrapezius area (main effect of burn): P = 0.002, Sham vs. 14d: P = 0.002, 7d vs. 14d: P = 0.02, 14d vs. 21d: P = 0.03) (Fig. 2C). Representative images of Tcf4/Tcf7L2+ myofibroblasts and PDGFR-α+ FAPs are shown in Fig. 2, B and D, respectively.
Fig. 2.
Fibrogenic cell abundance is elevated in skeletal muscle after severe burn injury. A: fibroblast abundance relative to myofiber number and area of spinotrapezius cross section. B: representative images of fibroblast abundance in spinotrapezius cross section. Tcf4/Tcf7L2 and DAPI costaining denote myofibroblasts (white arrow). C: fibrogenic adipogenic progenitor cell (FAP) abundance relative to myofiber number and area of spinotrapezius cross section. D: representative images of FAP abundance in spinotrapezius cross section. Platelet-derived growth factor receptor-α (PDGFRα) and DAPI costaining denote FAPs (yellow arrow). Data are presented as means ± SE. One-way ANOVA with Tukey’s post hoc test. *P < 0.05 compared with sham; n = 4–5 mice/group.
Collagen turnover and crosslinking-associated signaling proteins are altered in skeletal muscle after severe burn injury.
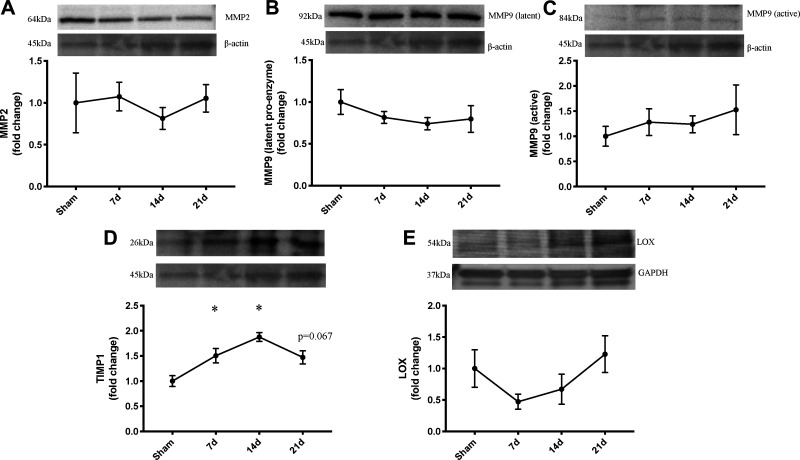
Our data indicate no change in matrix metalloproteinase 2 (MMP2) expression after severe burn injury, as well as no change in the latent proenzyme or cleaved active form of matrix metalloproteinase 9 (MMP9) (Fig. 3, A–C). Tissue inhibitor of metalloproteinase 1 (TIMP1) expression was elevated in all groups postburn (main effect of burn: P = 0.002, Sham vs. 7d: P = 0.048, Sham vs. 14d: P = 0.001, Sham vs. 21d: P = 0.067, 14d vs. 21d: P = 0.13) (Fig. 3D). Expression of lysyl oxidase (LOX), a catalyst of collagen fibril crosslink formation (17), was 50% lower 7d after burn injury, but heterogeneity within LOX expression precluded the identification of statistically significant differences in expression (main effect of burn: P = 0.19) (Fig. 3E). β-actin expression did not differ between groups.
Fig. 3.
Collagen turnover and crosslinking-associated signaling proteins are altered in skeletal muscle after severe burn injury. A: matrix metalloproteinase 2 (MMP2) (active) protein expression in spintrapezius. B: MMP9 (latent proenzyme) protein expression in spinotrapezius. C: MMP9 (active) protein expression in spinotrapezius. D: tissue inhibitor of metalloproteinase 1 (TIMP1) protein expression in spinotrapezius. E: lysyl oxidase (LOX) protein expression in spinotrapezius. No difference in β-actin shown as loading control. Data are presented as mean fold change compared with Sham ± SE. One-way ANOVA with Tukey’s post hoc test (A–D). Kruskall-Wallis with Dunn’s multiple comparison test (E). *P < 0.05 compared with sham; n = 4 mice/group.
Collagen content is elevated with reduced crosslinking in skeletal muscle after severe burn injury.
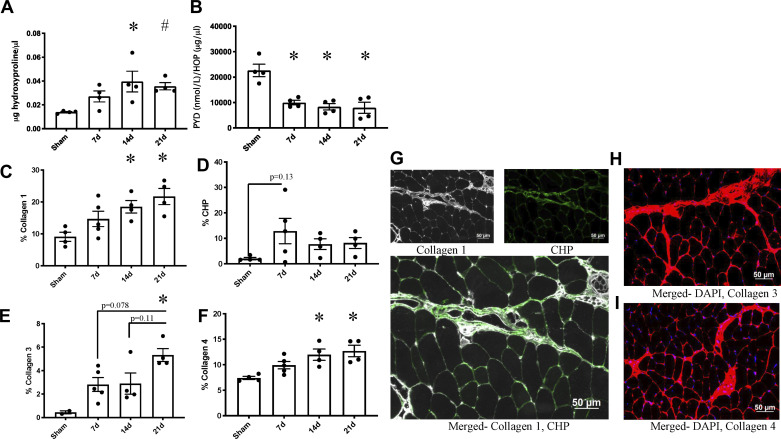
We observed greater total collagen content, measured by hydroxyproline (Fig. 4A). Total collagen was significantly elevated at 14 and 21 days postburn (main effect of burn: P = 0.005, Sham vs. 7d: P = 0.003, Sham vs. 14d: P = 0.02, Sham vs. 21d: P = 0.05, 14d vs. 21d: P = 0.95) (Fig. 4A). Collagen crosslinking as measured by PYD concentration relative to hydroxyproline was lower at each time point after burn injury (main effect of burn: P = 0.0002, Sham vs. 7d: P = 0.002, Sham vs. 14d: P = 0.001, Sham vs. 21d: P = 0.001) (Fig. 4B). Absolute levels of PYD were not changed as a result of the burn injury (Sham: 318.10 ± 41.55 nmol/L, 7d: 262.60 ± 37.22 nmol/L, 14d: 303.60 ± 35.21 nmol/L, 21d: 274.90 ± 68.67 nmol/L; P = 0.834, P > 0.05).
Fig. 4.
Collagen deposition is elevated in skeletal muscle after severe burn injury and not resolved by 21 days postburn. A: total collagen content in spinotrapezius as measured by hydroxyproline content in muscle pellet. B: pyridinium crosslinks relative to total collagen content measured by hydroxyproline in spintrapezius muscle pellet. HOP, hydroxyproline. C: collagen 1 content relative to area of spinotrapezius cross section. D: collagen turnover measured by collagen hybridizing peptide (CHP) content relative to area of spinotrapezius cross section. E: collagen 3 content relative to area of spinotrapezius cross section. F: collagen 4 content relative to area of spinotrapezius cross section. G: representative image of collagen 4 content in spinotrapezius cross section after burn injury. H: representative image of collagen 3 content in spinotrapezius cross section after burn injury. I: representative images of collagen 1 and CHP content in spinotrapezius cross section after burn injury. Data are presented as means ± SE. One-way ANOVA with Tukey’s post hoc test. *P < 0.05 compared with sham. #P = 0.051 compared with sham; n = 4–5 mice/group.
Collagen isoform content relative to spinotrapezius cross-sectional area was measured by immunofluorescence. Collagen 1 content demonstrated a similar pattern to total collagen measured by hydroxyproline content with a step-wise elevation at 14 days postburn that was maintained at 21 days postburn (main effect of burn: P = 0.01, Sham vs. 7d: P = 0.30, Sham vs. 14d: P = 0.049, Sham vs. 21d: P = 0.008) (Fig. 4C). Collagen hybridizing peptide (CHP) content, indicative of collagen turnover, showed no significant differences postburn (Fig. 4D). Collagen 3 content was elevated postburn, with greatest abundance at 21 days postburn (main effect of burn: P = 0.01, Sham vs. 7d: P = 0.22, Sham vs. 14d: P = 0.22, Sham vs. 21d: P = 0.008) (Fig. 4E). Collagen 4 mirrored collagen 1 accumulation (main effect of burn: P = 0.0005, Sham vs. 7d: P = 0.21, Sham vs. 14d: P = 0.01, Sham vs. 21d: P = 0.005, 14d vs. 21d: P = 0.94) (Fig. 4F). Representative images of collagen 1 and CHP, collagen 3, and collagen 4 content are shown in Fig. 4, G, H, and I, respectively. To investigate potential systemic muscle fibrogenesis, total collagen content (via hydroxyproline) in the tibialis anterior was assessed and demonstrated no difference in burn injured mice (main effect of burn: P = 0.25, Sham: 0.064 ± 0.008 µg/µl, 7d: 0.076 ± 0.019 µg/µl, 14d: 0.047 ± 0.006 µg/µl, 21d: 0.098 ± 0.023 µg/µl).
Fibrogenic cell abundance is related to skeletal muscle collagen content after severe burn injury.
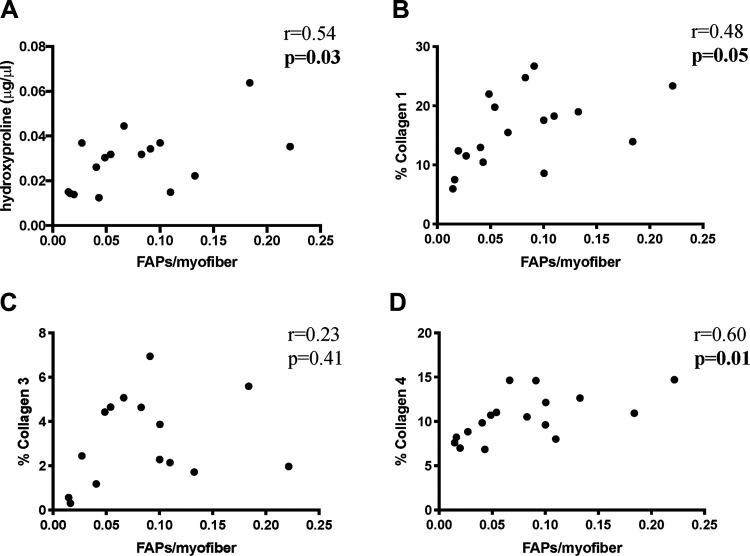
Tcf4+/Tcf7L2+ myofibroblast abundance was not significantly correlated to collagen content, although there was a trend toward a statistically significant correlation with collagen 1 content (total collagen measured by hydroxyproline: r = 0.32, P = 0.22; collagen 1: r = 0.45, P = 0.07; collagen 3: r = 0.33, P = 0.23; collagen 4: r = 0.22, P = 0.40). PDGFR-α+ FAP abundance was significantly correlated to total collagen measured by hydroxyproline (r = 0.54, P = 0.03) (Fig. 5A), collagen 1 content (r = 0.48, P = 0.05) (Fig. 5B), and collagen 4 content (r = 0.60, P = 0.01) (Fig. 5D) but not to collagen 3 content (r = 0.23, P = 0.41), inclusive of all experimental groups.
Fig. 5.
Fibrogenic cell abundance is related to skeletal muscle collagen content after severe burn injury. Correlation of fibrogenic adipogenic progenitor cell (FAP) abundance relative to myofiber number with total collagen in the muscle pellet as measured by hydroxyproline content of spinotrapezius (A); collagen 1 content relative to area of spinotrapezius cross section (B); collagen 3 content relative to area of spinotrapezius cross section (C); and collagen 4 content relative to area of spinotrapezius cross section (D). Pearson correlation. n = 4–5 mice/group.
Skeletal muscle macrophage abundance after severe burn injury.
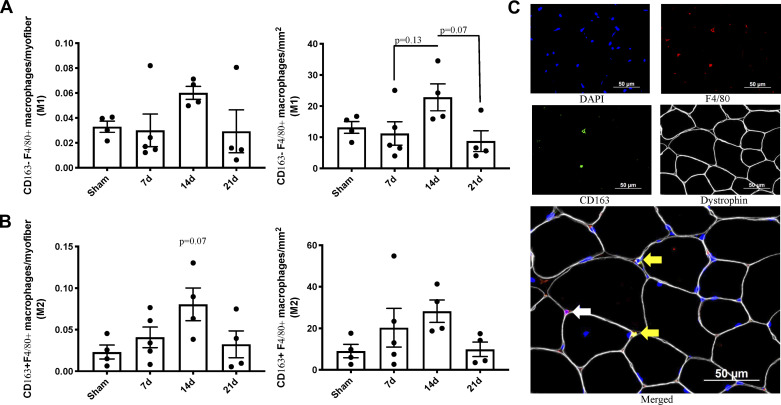
CD163- F4/80+ M1 macrophage abundance was nonsignificantly greater at 14 days after burn injury (main effect of burn: P = 0.07, Sham vs. 14d: P = 0.28, 7d vs. 14d: P = 0.13, 14d vs. 21d: P = 0.07) (Fig. 6A). A similar pattern with a trend toward a statistically significant peak at 14 days postburn occurred in CD163+ F4/80+ M2 macrophages (main effect of burn: P = 0.08, Sham vs. 14d: P = 0.07) (Fig. 6B). Representative images of CD163- F4/80+ M1 macrophages and CD163+ F4/80+ M2 macrophages are shown Fig. 6C.
Fig. 6.
Macrophage abundance in skeletal muscle after severe burn injury. A: M1 (CD163- F4/80+) macrophage abundance relative to myofiber number and area of spinotrapezius cross section. B: M2 (CD163+ F4/80+) macrophage abundance relative to myofiber number and area of spinotrapezius cross section. C: representative images of M1 and M2 macrophages in spinotrapezius cross section after burn injury. F4/80 and DAPI costaining denote M1 macrophages (white arrow). CD163, F4/80, and DAPI costaining denote M2 macrophages. Data are presented as means ± SE. One-way ANOVA with Tukey’s post hoc test; n = 4–5 mice/group.
DISCUSSION
Our current work provides novel insight into the pathogenesis of skeletal muscle TGFβ ligand signaling and subsequent fibrogenesis after a severe burn, indicating that, unlike myofiber atrophy (10) and contrary to our original hypothesis, the fibrotic phenotype is not resolved with 21 days of recovery after burn. We and others have shown upregulation of TGFβ superfamily signaling and indices of ECM remodeling acutely following burn injury (13, 19), but the results of our current study underpin greater magnitude and prolonged duration of a fibrogenic program in skeletal muscle that likely compromises burn recovery.
TGFβ superfamily signaling is well-established to stimulate synthesis of ECM components, which can progress to pathological fibrosis, often mediated by fibroblasts (25). The expression of myostatin, a TGFβ superfamily member, after burn was significantly and progressively elevated, underscoring its utility as a therapeutic target to mitigate muscle fibrosis. We observed stable levels of the uncleaved TGFβ1 propeptide after burn; however, cleaved/active TGFβ expression showed a nonsignificant, nearly twofold greater expression at 21 days postburn. Despite the lack of statistically significant differences in TGFβ signaling after burn injury, our data suggest that further exploration of this pathway is needed. Other groups have shown upregulated transcription of members of the TGFβ superfamily in skeletal muscle after burn but only investigated 24 h following the injury (19). The current study indicates that pathological expression of TGFβ ligands (particularly myostatin) is not resolved 3 wk after a severe burn. Recently, clinical support for our findings shows increased myostatin expression in myoblast cultures treated with serum from burn patients up to one year after severe burn injury (38). This progressive and sustained expression pattern aligns with previous findings that myostatin regulates its own expression through activation of downstream effectors in the SMAD family of transcription factors (3), as we also observed greater SMAD3 phosphorylation following the burn injury. In addition to driving sustained myostatin overexpression, activation of SMAD3 supports the fibrotic phenotype we observed after burn as collagen isoforms are well-established targets of the SMAD transcription factors. Myostatin signaling through SMAD3 has been determined to promote fibrogenesis in skeletal muscle (21); however, to our knowledge, our work is the first to demonstrate this finding after severe burn injury. Intriguingly, our previous work showed resolution of atrophy by 21 days postburn (10), when our current results show greatest myostatin expression levels. While speculative at this time, our results suggest that myostatin may have a greater effect on fibrogenic signaling compared with muscle fiber size following burn.
Fibroblasts and FAPs are primary fibrogenic cell types in skeletal muscle, and our data indicate expansion of both populations after severe burn injury. TGFβ and myostatin signaling stimulate fibroblast expansion and collagen production within the interstitial space of skeletal muscle (27, 39). We and others have shown FAP expansion and contribution to muscle pathophysiology in various chronic conditions, such as chronic kidney disease (1, 7) and anterior cruciate ligament tear (27). In the current study, we show that FAPs likely contribute to thermal injury-induced skeletal muscle fibrogenesis. Abundance of PDGFRα+ FAPs peaked 14 days after burn, and FAP abundance was positively correlated with several indexes of collagen abundance.
Macrophages and FAPs interact to regulate injury-induced muscle ECM remodeling (20), and we show macrophage abundance follows a similar pattern to that of FAPs. Macrophages exist on a continuum of pro- to anti-inflammatory with relatively polarized gene expression, referred to as M1 and M2, respectively. M1 macrophages are located proximally to myofibroblasts and FAPs and contribute to fibrosis via secretion of cytokines, including TGFβ superfamily ligands, and chemokines involved in recruitment and stimulation of fibrogenic cells (35, 43). M1 macrophage abundance peaked at 14 days postburn, albeit the increase did not reach statistical significance, likely supporting the concurrent increase in fibrogenic cell abundance. M2 macrophages promote muSC activity (5, 40), and our current results show nonsignificant expansion of M2 macrophages peaking at 14 days postburn. This time point coincides with previous data from our group demonstrating greatest activation of muSC postburn (10). muSCs also regulate ECM remodeling (12), helping to support integrated ECM remodeling through the coordinated activities of FAPs, fibroblasts, muSCs (10), and macrophages postburn.
In addition to synthesizing ECM proteins, fibrogenic cells secrete other products necessary for collagen turnover and remodeling, including MMPs and TIMPs (34). Collagen turnover is a dynamic process; in healthy muscle not undergoing extensive remodeling, collagen is degraded and proportionally synthesized to maintain tissue homeostasis. Collagen fibrils are then crosslinked via condensation of lysine or hydroxylysine residues to enhance tensile strength of the ECM. However, in excess, collagen synthesis and crosslinking may become pathogenic, producing a rigid fibrotic phenotype (23). MMP catalytic activity accounts for most collagen degradation in skeletal muscle, with different MMPs exhibiting enzymatic activity in a preferential manner for specific collagen isoforms (6, 30). We show no augmentation of MMP2 and MMP9 expression after burn, but interestingly, we observed altered expression of TIMP1. TIMPs serve various biological roles, including modulation of MMP-mediated ECM degradation (2). Balanced expression of MMPs and TIMPs is known to be dysregulated in chronic conditions exhibiting a fibrotic phenotype, such as muscular dystrophies (2), contributing to impaired ECM turnover. After burn, we observe a dynamic state in skeletal muscle in which the primary enzymes responsible for ECM degradation (MMPs) remain relatively stable with concurrent upregulation of their inhibitors (TIMPs), likely supporting collagen accumulation in the ECM. LOX, an enzyme vital to collagen fibril linking (17), expression was not significantly 50% lower at 7d postburn. PYD, a measure of collagen crosslinks (9, 41) formed by enzymatic action of LOX on lysine (31), was markedly and significantly lower following burn injury when normalized to hydroxyproline content. These data support a lower degree of collagen crosslinking after burn despite greater collagen abundance. Altered collagen crosslinking may facilitate proliferation and migration of interstitial cells (16), and we observed elevated abundance of fibrogenic cells after burn in the current study. Additionally, we previously reported progressively upregulated satellite cell proliferation in spinotrapezius after burn injury (10), further supporting a role for enhanced cell migration and expansion with reduced collagen crosslinking.
Our laboratory has previously shown that ECM components accumulate during the flow phase following burn in pediatric patients (13). Others have demonstrated ECM alterations acutely after severe burn injury (4, 29), but the duration of ECM remodeling and the longer term consequence of the acute fibrotic phenotype is not known. We show elevated muscle collagen that is not resolved through 21 days postburn. Total collagen, in addition to expression of collagens 1, 3 and 4, showed a similar pattern after thermal injury, indicative of a progressive fibrotic phenotype.
Tcf4 is expressed by multiple mononuclear cell types in skeletal muscle, including fibroblasts, FAPS, satellite cells, immune cells, and tenocyte-like cells (42); however, Tcf4/Tcf7L2 is a fibroblast-specific marker (22, 26). Tcf4/Tcf7L2+ fibroblasts have previously been reported to be the primary source of collagen 4 synthesis contributing to fibrosis in muscular dystrophies (45). Interestingly, fibroblast abundance did not correlate with collagen 3 or 4 content, nor total collagen content, and exhibited only a trend for a positive association with collagen 1 content. We found much stronger associations of FAPs with total collagen content as well as collagen isoforms 1 and 4, providing support that FAPs may be more important contributors to collagen remodeling in skeletal muscle after burn. The FAP population expanded more dramatically after burn injury compared with fibroblasts, providing further support for a greater role in postburn muscle fibrogenesis.
The mice in our current study were aged 9–11 wk at the time tissue was harvested, a time when skeletal muscle is still developing and growing. The observed fibrogenesis in immature skeletal muscle after severe burn may present dire long-term consequences if normal muscle development is impeded. Additionally, the young mice in our study are representative of pediatric patients, a group in which burn injury is particularly prevalent and negatively impacts developing skeletal muscle and physical function long after the acute injury (18, 33). Our findings suggest that ECM remodeling and unresolved skeletal muscle fibrosis after burn injury, at a time at which myofiber atrophy has demonstrated resolution (10), likely contribute to the impaired recovery of physical function after burn injury in pediatric patients.
Conclusion and future perspectives.
In conclusion, TGFβ superfamily signaling is upregulated in a progressive manner through 21 days after severe burn injury, translating into expansion of fibrogenic cells and elevated collagen turnover with a net accumulation of collagen. Excessive collagen deposition peaks at 14 days after burn; however, the fibrotic phenotype is maintained at 21 days after burn. Future studies should examine additional time points beyond 21 days postburn to determine resolution of fibrosis and quantify negative implications on muscle function. Therapies to mitigate the sustained TGFβ superfamily signaling, such as myostatin inhibitors, should be explored to control the highly dynamic skeletal muscle environment after burn injury, potentially mitigating aberrant burn-induced muscle fibrosis and partially preserving muscle function to enhance rehabilitation.
GRANTS
C.R.B. was supported by the National Institutes of Health Training Grant T32AG000270. This work was supported by Shriners Hospitals for Children Grant 85116 to C.S.F. and by the National Institutes of Health Grants R01 GM112936 to C.C.F. and R01 AR072061 to C.S.F.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C.F. and C.S.F. conceived and designed research; C.R.B., M.E.H., A.E.A., A.P., Y.W., and C.S.F. performed experiments; C.R.B. and M.E.H. analyzed data; C.R.B. and C.S.F. interpreted results of experiments; C.R.B. prepared figures; C.R.B. drafted manuscript; C.R.B., M.E.H., A.E.A., A.P., Y.W., C.C.F., and C.S.F. edited and revised manuscript; C.R.B., M.E.H., A.E.A., A.P., Y.W., C.C.F., and C.S.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Daniel Bechet for sharing a protocol to measure pyridinium crosslinks in muscle hydrolysate and Dr. Thomas Chaillou for French to English translation of the protocol.
REFERENCES
- 1.Abramowitz MK, Paredes W, Zhang K, Brightwell CR, Newsom JN, Kwon HJ, Custodio M, Buttar RS, Farooq H, Zaidi B, Pai R, Pessin JE, Hawkins M, Fry CS. Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am J Physiol Renal Physiol 315: F1658–F1669, 2018. doi: 10.1152/ajprenal.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alameddine HS, Morgan JE. Matrix metalloproteinases and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles. J Neuromuscul Dis 3: 455–473, 2016. doi: 10.3233/JND-160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292: C188–C199, 2007. doi: 10.1152/ajpcell.00542.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya V, Purwar S, Joshi D, Kumar M, Mandal S, Chaudhuri GR, Bhattacharya S. Electrophysiological and histological changes in extrinsic muscles proximal to post burn contractures of hand. Burns 37: 692–697, 2011. doi: 10.1016/j.burns.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Cantini M, Massimino ML, Bruson A, Catani C, Dalla Libera L, Carraro U. Macrophages regulate proliferation and differentiation of satellite cells. Biochem Biophys Res Commun 202: 1688–1696, 1994. doi: 10.1006/bbrc.1994.2129. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhes Migr 3: 337–341, 2009. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int 91: 119–128, 2017. doi: 10.1016/j.kint.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 111: 135–142, 2011. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubost A, Micol D, Meunier B, Lethias C, Listrat A. Relationships between structural characteristics of bovine intramuscular connective tissue assessed by image analysis and collagen and proteoglycan content. Meat Sci 93: 378–386, 2013. doi: 10.1016/j.meatsci.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Finnerty CC, McKenna CF, Cambias LA, Brightwell CR, Prasai A, Wang Y, El Ayadi A, Herndon DN, Suman OE, Fry CS. Inducible satellite cell depletion attenuates skeletal muscle regrowth following a scald-burn injury. J Physiol 595: 6687–6701, 2017. doi: 10.1113/JP274841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res 35: 1876–1885, 2017. doi: 10.1002/jor.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry CS, Porter C, Sidossis LS, Nieten C, Reidy PT, Hundeshagen G, Mlcak R, Rasmussen BB, Lee JO, Suman OE, Herndon DN, Finnerty CC. Satellite cell activation and apoptosis in skeletal muscle from severely burned children. J Physiol 594: 5223–5236, 2016. doi: 10.1113/JP272520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297: E157–E164, 2009. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- 15.Guillory AN, Clayton RP, Prasai A, Jay JW, Wetzel M, El Ayadi A, Herndon DN, Finnerty CC. Buprenorphine-sustained release alters hemodynamic parameters in a rat burn model. J Surg Res 232: 154–159, 2018. doi: 10.1016/j.jss.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haugh MG, Murphy CM, McKiernan RC, Altenbuchner C, O’Brien FJ. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng Part A 17: 1201–1208, 2011. doi: 10.1089/ten.tea.2010.0590. [DOI] [PubMed] [Google Scholar]
- 17.Herchenhan A, Uhlenbrock F, Eliasson P, Weis M, Eyre D, Kadler KE, Magnusson SP, Kjaer M. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem 290: 16440–16450, 2015. doi: 10.1074/jbc.M115.641670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS One 6: e21245, 2011. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang CH, Silvis C, Nystrom G, Frost RA. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J 15: 1807–1809, 2001. doi: 10.1096/fj.00-0849fje. [DOI] [PubMed] [Google Scholar]
- 20.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 21.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371–19378, 2008. doi: 10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138: 371–384, 2011. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick RJ. The flexibility of the collagen compartment of muscle. Meat Sci 36: 79–91, 1994. doi: 10.1016/0309-1740(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 24.Merritt EK, Thalacker-Mercer A, Cross JM, Windham ST, Thomas SJ, Bamman MM. Increased expression of atrogenes and TWEAK family members after severe burn injury in nonburned human skeletal muscle. J Burn Care Res 34: e297–e304, 2013. doi: 10.1097/BCR.0b013e31827a2a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 85: 4894–4897, 1988. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peck BD, Brightwell CR, Johnson DL, Ireland ML, Noehren B, Fry CS. Anterior cruciate ligament tear promotes skeletal muscle myostatin expression, fibrogenic cell expansion, and a decline in muscle quality. Am J Sports Med 47: 1385–1395, 2019. doi: 10.1177/0363546519832864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol 37: 1948–1961, 2005. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Quintana HT, Bortolin JA, da Silva NT, Ribeiro FA, Liberti EA, Ribeiro DA, de Oliveira F. Temporal study following burn injury in young rats is associated with skeletal muscle atrophy, inflammation and altered myogenic regulatory factors. Inflamm Res 64: 53–62, 2015. doi: 10.1007/s00011-014-0783-8. [DOI] [PubMed] [Google Scholar]
- 30.Sassoli C, Nosi D, Tani A, Chellini F, Mazzanti B, Quercioli F, Zecchi-Orlandini S, Formigli L. Defining the role of mesenchymal stromal cells on the regulation of matrix metalloproteinases in skeletal muscle cells. Exp Cell Res 323: 297–313, 2014. doi: 10.1016/j.yexcr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Seibel MJ, Robins SP, Bilezikian JP. Urinary pyridinium crosslinks of collagen: specific markers of bone resorption in metabolic bone disease. Trends Endocrinol Metab 3: 263–270, 1992. doi: 10.1016/1043-2760(92)90129-O. [DOI] [PubMed] [Google Scholar]
- 32.Seifert J. Incidence and economic burden of injuries in the United States. J Epidemiol Community Health 61: 926, 2007. doi: 10.1136/jech.2007.059717. [DOI] [Google Scholar]
- 33.Shah A, Suresh S, Thomas R, Smith S. Epidemiology and profile of pediatric burns in a large referral center. Clin Pediatr (Phila) 50: 391–395, 2011. doi: 10.1177/0009922810390677. [DOI] [PubMed] [Google Scholar]
- 34.Tandara AA, Mustoe TA. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts–impact of coculture and hydration. J Plast Reconstr Aesthet Surg 64: 108–116, 2011. doi: 10.1016/j.bjps.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson RW, Pesce JT, Ramalingam T, Wilson MS, White S, Cheever AW, Ricklefs SM, Porcella SF, Li L, Ellies LG, Wynn TA. Cationic amino acid transporter-2 regulates immunity by modulating arginase activity. PLoS Pathog 4: e1000023, 2008. doi: 10.1371/journal.ppat.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toader-Radu M. Dynamics of regeneration in skeletal muscle following localized heat injury. Morphol Embryol (Bucur) 24: 69–73, 1978. [PubMed] [Google Scholar]
- 37.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol 174: 404–410, 2005. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 38.Wallner C, Huber J, Drysch M, Schmidt SV, Wagner JM, Dadras M, Lehnhardt M, Behr B. Myostatin upregulation in patients in the chronic phase of severe burn injury leads to muscle cell catabolism. Eur Surg Res 60: 86–96, 2019. doi: 10.1159/000500760. [DOI] [PubMed] [Google Scholar]
- 39.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 8: 461, 2017. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, Finlin BS, Zhu B, Kern PA, Peterson CA. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep 9: 969, 2019. doi: 10.1038/s41598-018-37187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Listrat A, Meunier B, Gueugneau M, Coudy-Gandilhon C, Combaret L, Taillandier D, Polge C, Attaix D, Lethias C, Lee K, Goh KL, Béchet D. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell 13: 254–262, 2014. doi: 10.1111/acel.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Reports 27: 2029–2035.e5, 2019. doi: 10.1016/j.celrep.2019.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30: 245–257, 2010. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M, Fujita T, Martyn JA. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab 279: E1114–E1121, 2000. doi: 10.1152/ajpendo.2000.279.5.E1114. [DOI] [PubMed] [Google Scholar]
- 45.Zou Y, Zhang RZ, Sabatelli P, Chu ML, Bönnemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol 67: 144–154, 2008. doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]