Abstract
Small noncoding microRNAs (miRNAs) are important regulators of skeletal muscle size, and circulating miRNAs within extracellular vesicles (EVs) may contribute to atrophy and its associated systemic effects. The purpose of this study was to understand how muscle atrophy and regrowth alter in vivo serum EV miRNA content. We also associated changes in serum EV miRNA with protein synthesis, protein degradation, and miRNA within muscle, kidney, and liver. We subjected adult (10 mo) F344/BN rats to three conditions: weight bearing (WB), hindlimb suspension (HS) for 7 days to induce muscle atrophy, and HS for 7 days followed by 7 days of reloading (HSR). Microarray analysis of EV miRNA content showed that the overall changes in serum EV miRNA were predicted to target major anabolic, catabolic, and mechanosensitive pathways. MiR-203a-3p was the only miRNA demonstrating substantial differences in HS EVs compared with WB. There was a limited association of EV miRNA content to the corresponding miRNA content within the muscle, kidney, or liver. Stepwise linear regression demonstrated that EV miR-203a-3p was correlated with muscle mass and muscle protein synthesis and degradation across all conditions. Finally, EV miR-203a-3p expression was significantly decreased in human subjects who underwent unilateral lower limb suspension (ULLS) to induce muscle atrophy. Altogether, we show that serum EV miR-203a-3p expression is related to skeletal muscle protein turnover and atrophy. We suggest that serum EV miR-203a-3p content may be a useful biomarker and future work should investigate whether serum EV miR-203a-3p content is mechanistically linked to protein synthesis and degradation.
Keywords: atrophy, extracellular vesicles, miRNA, protein synthesis, skeletal muscle
INTRODUCTION
Skeletal muscle atrophy is a risk factor for all-cause morbidity and mortality in both aging and critical illness (38, 49, 50). As such, it has been suggested that skeletal muscle is an important organ for maintenance of overall health and homeostasis of other tissues via interorgan communication (28). That is, skeletal muscle releases factors that can be systemically delivered to other tissues/organs and impart biologically relevant adaptations or changes in organ function (26, 30, 43, 48, 51, 56). During times of muscle contraction or disuse, this communication paradigm may change and impact systemic function. Growing evidence suggests that extracellular vesicles (EVs) are an important component of this intercellular communication across a variety of circumstances. Despite a recent growth in the appreciation of EVs in mediating cross talk and the clinical importance for understanding muscle atrophy, there is a paucity of information about the role of EVs during muscle atrophy.
EVs are a heterogeneous population of small lipid membrane-bound vesicles that are released from virtually all cell types (6). The most commonly discussed EVs are exosomes (30–150 nm) and microvesicles (100 nm–1 μm), although apoptotic bodies (>1 μm) are now gaining support as vesicles that are not merely a by-product of apoptosis but can serve regulatory roles (5, 7). Exosomes are formed within the endosomal pathway and released from cells when multivesicular bodies (MVBs) merge with the plasma membrane and release the exosomes into the extracellular space (6). Microvesicles are formed from outward blebbing of the plasma membrane and subsequent scission and release of the plasma membrane bleb. There is still no consensus on specific markers of specific EV subtypes, however, so the classifications of vesicles for most studies are based primarily on physical characteristics such as size (i.e., <100 nm), density (i.e., low, middle, high), or biochemical composition (i.e., CD63+) (58). The overlap in the characteristics of the various subtypes of vesicles has led to the use of the term “extracellular vesicles” to collectively describe them (58). The release of EVs into the extracellular space facilitates intercellular/intertissue communication that is important for orchestrating systemic physiological outcomes. EVs are loaded with bioactive cargo such as proteins, lipids, RNA, and microRNA (miRNA) that impact the physiological function of cells to which they are delivered. A plethora of in vitro studies (reviewed in 54) have shown how EVs are involved in skeletal muscle cell function, but in vivo examinations are lacking, especially in the context of disuse atrophy.
Small noncoding microRNAs (miRNAs) are often carried through the systemic circulation in EVs and are an important component in the regulation of atrophy (24, 37). Circulating miRNAs (potentially within EVs) may be a mechanism contributing to skeletal muscle atrophy and its associated systemic effects (reviewed in 2). It has been shown that atrophying muscle cells lower internal miR-23a levels by selectively loading miR-23a into EVs, thus releasing the inhibition of expression of MAFbx and MuRF1, two critical ubiquitin ligases in muscle atrophy (21). In addition, there is evidence of muscle-kidney cross talk via EVs that export miR-23a, miR-27a, and miR-29a from the muscle to the kidneys, thereby modulating renal fibrosis in both a diabetic and a chronic kidney disease mouse model (62, 69). A muscle-liver communication paradigm has also been proposed via EVs that are liberated from muscle during exercise (65). Altogether, available data suggest that EV-mediated delivery of muscle-derived miRNAs may impact distant organ homeostasis under a variety of physiological conditions.
Finally, there is interest in finding noninvasive biomarkers that can provide diagnostic and prognostic value during pathophysiological states, such as during muscle disuse and atrophy. A study by Margolis et al. (34) demonstrated an inverse relationship between circulating muscle-specific miRNAs (myomiRs) and whole body rates of protein synthesis in human subjects. Numerous other studies have now shown that circulating miRNAs may indeed be useful biomarkers for understanding muscle atrophy diagnosis and muscle disease progression (23, 35, 36). The potential for investigating EVs as biomarkers with a mechanistic contribution to atrophy prompted the work presented here, with the aim of uncovering the relationship between serum EV miRNA and skeletal muscle atrophy in vivo. We hypothesized that there is a relationship between serum EV miRNA, muscle miRNA, and indexes of atrophy and regrowth in skeletal muscle.
METHODS
Animals and experimental procedures.
All procedures were approved by the University of Kentucky’s Institutional Animal Care and Use Committee. Male Brown Norway/F344 rats at 10 mo of age (National Institute on Aging, Bethesda, MD) were used in this study. Rats were randomly assigned into three groups: weight-bearing conditions (WB), hindlimb suspension for 7 days (HS), and hindlimb suspended for 7 days and subsequently reloaded and allowed to move around the cage freely for 7 days (HSR). Rats were allowed free access to food and water at all times and were housed on a 12:12-h light-dark cycle. Hindlimb suspension was performed as previously described (40). Briefly, a tail device containing a hook was attached with gauze and cyanoacrylate glue while the animals were anesthetized with isoflurane (2% by inhalation). After the animal regained consciousness, the tail device was connected via a thin cable to a pulley sliding on a vertically adjustable stainless steel bar running longitudinally above a high-sided cage. Rats were raised progressively over a 6-h period to allow them to adjust to the suspension. The system was designed in such a way that the rats could not rest their hindlimbs against any side of the cage but could move around the cage on their front limbs and could reach water and food easily. Cages were randomly placed in the room, and room temperature was 27°C. Rats in the HSR group were released from the tail suspension device after 7 days of unloading, and they were allowed to maintain normal ambulation for 7 days (reloading). The study timeline for the rats is depicted in Fig. 1.
Fig. 1.
Study timeline for rats. HS, hindlimb suspension; HSR, hindlimb suspension followed by reloading; WB, weight bearing.
Deuterium oxide labeling and determination of protein synthesis and degradation rates.
For determination of protein synthesis rates, rats received a bolus of (99%) deuterium oxide (D2O) (Sigma-Aldrich, St. Louis, MO) before the start of the experiment, equivalent to 5% of the body water pool, followed by drinking water enriched 8% with D2O for the remainder of the experimental period. Specifically, the HS rats received the bolus of D2O 2 days before the 7-day suspension period. The WB rats were euthanized 9 days after receiving the bolus and beginning D2O drinking water to match the labeling period of the HS group. Rats in the HSR group received the same bolus of D2O 2 days before the start of reloading followed by 8% D2O-enriched drinking water until euthanasia for a total of 9 days.
Protein synthesis was determined according to our established methods (27, 40, 41). Skeletal muscle tissue was homogenized 1:10 in isolation buffer (in mM: 100 KCl, 40 Tris·HCl, 10 Tris Base, 5 MgCl2, 1 EDTA, 1 ATP, pH = 7.5) with phosphatase and protease inhibitors (Halt; Thermo Fisher Scientific) with a bead homogenizer (Next Advance Inc., Averill Park, NY). After homogenization, subcellular fractions were isolated via differential centrifugation (9, 10, 39). Protein fractions were derivatized for analysis of deuterium enrichment in alanine with an Agilent 7890A GC coupled to an Agilent 5975C MS (9, 10, 39). To determine the precursor pool enrichment, serum samples were prepared for analysis on GC-MS (9, 10, 39).
Calculations.
Disuse atrophy, such as that caused by hindlimb suspension, results in a rapid loss of muscle mass as well as the protein pool of measurement, creating a non-steady-state condition. Therefore, we used our previously published equations that account for non-steady-state conditions (27, 40, 42). The mass of protein at time t, P(t), obeys the differential equation
| (1) |
where Ksyn is the synthesis rate, with dimensions of mass over time, and Kdeg is the degradation constant, with dimensions of inverse time. From this relationship:
| (2) |
Blood and tissue collection.
At the end of the experimental period, rats were anesthetized with pentobarbital sodium and blood was collected through a cardiac puncture; gastrocnemius muscles were then dissected, weighed, and frozen. A subset of rats (WB n = 5, HS n = 5, HSR n = 8) also had the liver and kidneys harvested for analysis. Muscles and organs were frozen in liquid nitrogen and stored at −80°C for later biochemical analyses. Serum was isolated by allowing blood to clot at room temperature for 30 min before centrifugation for 10 min at 2,000 g and 4°C. The serum supernatant was collected and stored at −80°C until analysis.
Mean fiber cross-sectional area.
Mean fiber cross-sectional area (CSA) was determined as previously described (40). Briefly, gastrocnemius cross sections (8 μm) were rehydrated in PBS and incubated in dystrophin antibody (1:50; Vector Laboratories, Burlingame, CA) for 1 h at room temperature and overnight at 4°C. Secondary antibody (1:200, directly conjugated Texas Red goat anti-mouse; Rockland Immunochemicals, Gilbertsville, PA) was applied, and sections were coverslipped. Images were captured with a Zeiss AxioImager MI upright fluorescent microscope (Zeiss, Gottingen, Germany). Mean fiber CSA was measured with the automated software program MyoVision (64).
Serum EV isolation and nanoparticle tracking analysis.
EVs were isolated from 500 µL of serum with ExoQuick Exosome Precipitation Solution [System Biosciences (SBI), Palo Alto, CA]. The serum was first centrifuged at 3,000 g for 15 min to remove cells and debris, and then the supernatant was collected and filtered through a 0.22-µm low-binding PVDF filter (Millex-GV; Millipore, Tullagreen, Ireland). Approximately 240 µL of ExoQuick was added to the sample and incubated at 4°C overnight. The ExoQuick-serum mixture was then centrifuged at 1,500 g for 30 min to pellet the EVs. The supernatant was removed, and the EV pellet was reconstituted in 300 µL of PBS. Validation of EV isolation from serum was made by measuring particle size with a ZetaView nanoparticle tracking analyzer (Particle Metrix, Grayslake, IL) and via Western blotting.
EV miRNA isolation and analysis.
Total RNA was isolated from EVs with the commercially available miRCURY RNA Isolation Kit (Exiqon, Woburn, MA) according to the manufacturer’s instructions. Sample miRNA abundance was quantified with a small RNA kit on an Agilent Bioanalyzer (Agilent, Santa Clara, CA). We submitted 50 ng of miRNA from WB EV miRNA (n = 3) and HS EV miRNA (n = 3) to the University of Kentucky Genomics Core Laboratory for miRNA microarray analysis. Samples were hybridized to the Thermofisher/Affymetrix miRNA 4.0 array. MetaboAnalyst 4.0 (66) was used to perform detailed statistical analyses of the microarray data. Predictions of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways altered by changes in serum EV miRNA expression during HS were made with DIANA miRpath v3.0 (61).
Reverse transcription of miRNA was performed with 10 ng of total RNA with the miRCURY LNA RT kit (Qiagen). RT-qPCR was performed with the miRCURY LNA SYBR Green PCR kit (Qiagen) combined with the appropriate miRCURY LNA primer sets for the miRNA of interest (Qiagen). The expression of each miRNA was normalized to the expression of UniSp6 with the −∆CT method (where CT is threshold cycle) (55). UniSp6 is an exogenous spike-in that resembles miRNAs in structure but lacks close sequence similarities to known miRNAs, provided by the miRCURY LNA RT kit (Qiagen).
EV protein isolation and Western blotting.
Protein was isolated from EVs with Pierce RIPA lysis buffer (Thermo Scientific, Rockford, IL) with a protease and phosphatase inhibitor cocktail (Halt protease inhibitor cocktail; Thermo Scientific, Rockford, IL) added. Protein concentration was determined with the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). Samples were then prepared in Laemmli buffer at a concentration of 1 µg/µL and boiled at 95°C for 5 min. After 5 µg of sample was loaded, proteins were separated by SDS-PAGE with 4–15% TGX gels (Criterion; Bio-Rad) by first stacking for 10 min at 100 V and then running for ~40 min at 200 V and room temperature. Samples were then transferred for 60 min at 100 V on ice onto a nitrocellulose membrane (Bio-Rad) in 20% methanol Tris-glycine buffer. The Revert Total Protein Stain Kit (Li-Cor Biosciences, Lincoln, NE) was used to stain total protein, and the membrane was imaged to verify transfer efficiency and loading. Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline-Tween (TBS-T, 0.1% Tween 20) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with primary antibody [anti-CD63, catalog no. EXOAB-CD63A-1; System Biosciences (SBI), Palo Alto, CA] at a 1:1,000 dilution in 5% nonfat dry milk in TBS-T. The membrane was then washed 3 × 5 min in TBS-T before a 1-h incubation with goat anti-rabbit secondary antibodies [1:10,000 dilution; System Biosciences (SBI), Palo Alto, CA]. Blots were developed with enhanced chemiluminescence (Clarity Western ECL substrate; Bio-Rad), imaged, and quantified with ImageJ (National Institutes of Health).
Human subjects and experimental procedures.
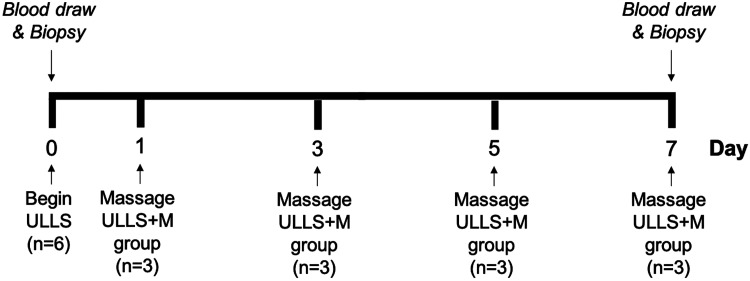
We recruited six healthy subjects (5 women, 1 man) between the ages of 18 and 30 yr with a body mass index (BMI) < 26 kg/m2. Exclusion criteria included subjects who had preexisting health conditions, current musculoskeletal injuries, previous surgeries involving lower extremities, and pregnancy. Subjects were randomly assigned to one of two study groups: unilateral lower limb suspension (ULLS; n = 3) or unilateral lower limb suspension and massage, an intervention used as a mechanotherapy (27, 40) (ULLS+M; n = 3) (Table 1). ULLS was used to induce skeletal muscle atrophy in a single leg as previously described (17). Briefly, a knee immobilizer was fitted and secured to the left leg, thereby immobilizing the lower limb. Crutches were fitted, and subjects ambulated with the crutches in a non-weight-bearing manner for the 7-day duration of the study. Subjects in the ULLS+M group had a controlled massage-like mechanotherapy applied to the immobilized vastus lateralis (VL) muscle for 30 min on the first, third, fifth, and final day of the intervention. Muscle biopsies of the VL were obtained before the intervention and on the final day of the intervention (day 7) with a Bergstrom needle as previously described (13). Briefly, ~100 mg of each biopsy was mounted with fibers perpendicular to a cork with tragacanth gum. The sample was then snap-frozen in liquid nitrogen-cooled isopentane and stored at –80°C until analysis of fiber CSA. A separate portion of the muscle biopsy was also snap-frozen and stored at –80°C until the analysis of miRNA. All study procedures were approved by the University of Kentucky’s Institutional Review Board, and written informed consent was obtained from all subjects before initiating participation in the study. The study timeline for the human subjects is depicted in Fig. 2.
Table 1.
Human subject characteristics
| Sex | Age, yr | BMI, kg/m2 | |
|---|---|---|---|
| ULLS | F | 19 | 24.1 |
| F | 23 | 22.5 | |
| F | 20 | 26.3 | |
| ULLS+M | F | 25 | 27.0 |
| M | 26 | 23.8 | |
| F | 33 | 26.6 |
BMI, body mass index; F, female; M, male; ULLS, unilateral lower limb suspension; ULLS+M, unilateral lower limb suspension and massage.
Fig. 2.
Study timeline for human subjects. ULLS, unilateral lower limb suspension; ULLS+M, unilateral lower limb suspension and massage.
Statistics.
Muscle atrophy outcome measures of CSA, mass, Ksyn, and Kdeg were visualized with histograms and Q-Q plots, and the Shapiro–Wilk normality test was used to determine whether they were normally distributed. All three variables were not normally distributed. In addition, the miRNA data were assessed for normality in a similar manner, and it was also determined that many of the miRNAs were not normally distributed. Since many of the outcome measures were not normally distributed, the nonparametric version of analysis of variance, Kruskal–Wallis, and independent t tests, Dunn, were used to determine differences across groups. The Bonferroni method corrected for multiple post hoc comparisons. Significance was assumed at P < 0.05.
A stepwise linear regression with stepwise selection was utilized to determine which miRNAs were associated with atrophy measures. To determine which miRNAs were included in the stepwise analysis, simple linear regressions were run for each miRNA and outcome measure. Only mRNAs with a P < 0.15 were selected for the stepwise analysis. Rats that were missing any miRNA were excluded, yielding 30 rats for the stepwise analysis. Nonparametric ANOVA, simple linear regression, and stepwise regression analyses were completed in R 3.6.1.
A dependent Student’s t test was used to examine changes in VL mean myofiber CSA and serum EV miR-203a-3p expression with ULLS across all six subjects. Significance was assumed at P < 0.05.
RESULTS
Muscle mass and protein turnover response to hindlimb suspension and reloading.
Hindlimb suspension caused significant muscle atrophy as exemplified by reduced gastrocnemius muscle mass and mean myofiber CSA in HS rats compared with WB (P = 0.00018 and P = 0.00009, respectively) (Fig. 3, A and B). In addition, myofibrillar protein synthesis rate (Ksyn, mg/day) was ~60% lower (P = 0.0018) and protein degradation (Kdeg, 1/t) was 100% higher (P = 0.0008) in HS muscle compared with WB (Fig. 3, C and D). Gastrocnemius mass in the HSR rats was significantly higher compared with HS (P = 0.013) but also significantly lower than WB (P = 0.009) (Fig. 3A). The mean myofiber CSA of HSR muscle was significantly higher than HS muscle (P = 0.0009) but still exhibited only a partial recovery of CSA, as it was significantly lower than WB muscle (P = 0.00014) (Fig. 3B). Myofibrillar protein synthesis was significantly higher and protein degradation was significantly lower in HSR compared with HS (Fig. 3, C and D) (P = 0.00014 and P = 0.0006, respectively) but recovered to levels not different from WB.
Fig. 3.
Response to hind limb suspension and reloading. A: gastrocnemius muscle mass. B: gastrocnemius mean fiber cross-sectional area (CSA). C: myofibrillar protein synthesis [synthesis rate (Ksyn)]. D: myofibrillar protein degradation [degradation constant (Kdeg)]. HS, hindlimb suspension; HSR, hindlimb suspension followed by reloading; WB, weight bearing. Data presented as means ± SE. *Significantly different compared with WB (P < 0.05); †significantly different compared with HS (P < 0.05).
Isolated serum EVs.
Nanoparticle tracking analysis (NTA; ZetaView) showed that the isolated serum EVs had a mode size of 92 ± 1 nm across all groups, verifying successful isolation of small EVs (Fig. 4A). Although these preparations were enriched for exosomes (30–150 nm), it is likely that small microvesicles (100–1,000 nm) were also captured in these preparations, which is why it is more appropriate to call these preparations EVs and not exosomes. This nomenclature follows the recommendations of the most recent position statement from the International Society for Extracellular Vesicles (58). Furthermore, Western blotting analysis demonstrated that although the ExoQuick preparation captured nonvesicular protein, which is a known drawback of this method (58), EV samples were enriched for CD63+ EVs (Fig. 4, B and C).
Fig. 4.
Characterization of extracellular vesicles (EVs). A: nanoparticle tracking analysis of EVs isolated from rat serum [n = 9 total; n = 3 from weight bearing (WB), hindlimb suspension (HS), and hindlimb suspension followed by reloading (HSR)] shows enrichment of small EVs with a peak of 92-nm diameter. B: representative Western blot image from serum and EV samples. C: quantification of CD63 blot shown in B normalized to the total protein abundance and displayed relative to serum values. a.u., Arbitrary unit.
EV miRNA.
Principal component analysis (PCA) of the miRNA microarray data demonstrated some overlap between WB and HS samples (Fig. 5A). There were 52 mature miRNAs and 20 pre-miRNAs that were significantly different (P < 0.05, unadjusted) in HS compared with WB (Fig. 5B and Table 2). As shown in Fig. 5B, there were a vast number of miRNAs that exhibited small but statistically significant changes. When false discovery rate (FDR) was applied to the data set, there were no significant changes in EV miRNA in HS compared with WB. Interestingly, miR-203a-3p and miR-30a-3p were the only mature miRNAs that exhibited a greater than twofold difference with HS (Fig. 5B and Table 2).
Fig. 5.
MicroRNA (miRNA) microarray analysis of serum extracellular vesicles (EVs) shows limited significant differences in hindlimb suspension (HS) compared with weight bearing (WB). A: principal component analysis of serum EV miRNA microarray results. PC, principal component. B: volcano plot of serum EV miRNA microarray results comparing HS with WB. Gray dots, not significantly different miRNA; purple dots, significantly different miRNA (P < 0.05); green dots, significantly different miRNA (P < 0.05) with a >2-fold difference when comparing HS with WB. FC, fold change.
Table 2.
Significantly altered serum EV miRNAs and pre-miRNAs with HS
| Transcript | Log2(FC) | P Value | Transcript | Log2(FC) | P Value |
|---|---|---|---|---|---|
| mir-215 | −0.29 | 0.0002 | miR-218a-5p | 0.46 | 0.0217 |
| miR-203a-3p | −1.66 | 0.0005 | mir-27b | 0.24 | 0.0223 |
| mir-203a | 0.21 | 0.0014 | miR-34c-5p | −0.43 | 0.0239 |
| miR-3558-5p | 0.41 | 0.0016 | mir-671 | 0.43 | 0.0249 |
| miR-328b-3p | −0.25 | 0.0018 | miR-22-3p | −0.22 | 0.0266 |
| miR-511-3p | −0.26 | 0.0026 | miR-582-3p | −0.56 | 0.0274 |
| miR-223-3p | −0.55 | 0.0029 | miR-361-5p | −0.18 | 0.0283 |
| mir-466c | −0.50 | 0.0034 | miR-150-3p | 0.27 | 0.0286 |
| miR-742-3p | −0.18 | 0.0036 | mir-6215 | −0.50 | 0.0287 |
| mir-3085 | 0.67 | 0.0050 | miR-500-5p | 0.23 | 0.0292 |
| miR-3562 | 0.12 | 0.0062 | mir-876 | 0.43 | 0.0305 |
| miR-877 | 0.28 | 0.0064 | miR-3550 | −0.48 | 0.0313 |
| mir-151 | −0.30 | 0.0077 | miR-20a-5p | −0.23 | 0.0342 |
| let-7a-1-3p | −0.45 | 0.0079 | miR-29a-3p | −0.25 | 0.0342 |
| let-7c-2-3p | −0.45 | 0.0079 | miR-152-3p | −0.28 | 0.0348 |
| mir-17-1 | −0.31 | 0.0079 | mir-16 | −0.18 | 0.0358 |
| miR-99a-3p | 0.44 | 0.0084 | miR-541-3p | −0.32 | 0.0378 |
| miR-192-5p | −0.26 | 0.0084 | miR-101b-3p | −0.78 | 0.0381 |
| miR-30a-3p | −1.21 | 0.0094 | miR-221-3p | −0.53 | 0.0387 |
| miR-652-3p | −0.45 | 0.0095 | miR-24-2-5p | −0.37 | 0.0388 |
| miR-375-5p | 0.29 | 0.0098 | miR-23b-5p | 0.39 | 0.0396 |
| mir-204 | −0.39 | 0.0105 | mir-383 | −0.33 | 0.0396 |
| miR-26a-5p | −0.22 | 0.0111 | mir-20b | −0.21 | 0.0396 |
| miR-34b-3p | 0.54 | 0.0123 | miR-802-3p | 0.22 | 0.0398 |
| miR-194-5p | −0.22 | 0.0125 | miR-378a-3p | −0.19 | 0.0403 |
| miR-181d-3p | 0.71 | 0.0130 | miR-31a-5p | −0.75 | 0.0404 |
| miR-675-5p | −0.75 | 0.0138 | mir-551b | −0.11 | 0.0424 |
| mir-219b | −0.46 | 0.0141 | miR-378b | −0.22 | 0.0428 |
| miR-325-5p | 0.35 | 0.0153 | miR-433-5p | 0.27 | 0.0432 |
| mir-134 | 0.34 | 0.0155 | miR-135b-5p | 0.35 | 0.0432 |
| miR-146a-5p | −0.37 | 0.0167 | mir-29b-1 | −0.22 | 0.0437 |
| miR-3577 | 0.49 | 0.0169 | miR-151-5p | −0.60 | 0.0461 |
| mir-361 | −0.28 | 0.0170 | miR-222-5p | 0.51 | 0.0464 |
| miR-27b-3p | −0.23 | 0.0190 | miR-92a-3p | −0.17 | 0.0470 |
| mir-181a-1 | 0.32 | 0.0193 | miR-18a-5p | −0.46 | 0.0481 |
| miR-3594-5p | 0.18 | 0.0199 | miR-203b-3p | 0.44 | 0.0493 |
MicroRNAs (miRNAs) in bold were significantly altered (P < 0.05) and greater than 2-fold difference compared with weight bearing (WB). EV, extracellular vesicle; FC, fold change; HS, hindlimb suspension.
To get a better understanding of how the differences in serum EV miRNA profiles may impact overall function, we uploaded the list of mature miRNAs that were significantly different (Table 2) to the DIANA miRpath v3.0 online software and generated KEGG pathways for the genes whose mRNA would be predicted to be targeted by the altered miRNAs (Table 3). There were multiple pathways related to muscle response to mechanical stimulus (11, 14), metabolic demand (25), and, ultimately, muscle growth (12, 18, 47) that were predicted to be affected by the differences in serum EV miRNA content between HS and WB.
Table 3.
Top KEGG pathways predicted to be targeted by altered miRNA with HS
| KEGG Pathway | P Value | No. of Genes | No. of miRNAs |
|---|---|---|---|
| Pathways in cancer | 0.00626 | 57 | 34 |
| MAPK signaling pathway | 0.00046 | 47 | 27 |
| Proteoglycans in cancer | 1.60E−05 | 42 | 26 |
| Focal adhesion | 0.00173 | 40 | 29 |
| Rap1 signaling pathway | 0.00156 | 37 | 29 |
| Transcriptional misregulation in cancer | 1.60E−05 | 35 | 26 |
| Endocytosis | 0.00626 | 34 | 23 |
| FoxO signaling pathway | 0.00127 | 31 | 23 |
| MicroRNAs in cancer | 0.0075 | 29 | 25 |
| Ubiquitin mediated proteolysis | 0.00727 | 28 | 24 |
| Estrogen signaling pathway | 0.00483 | 20 | 14 |
| Prostate cancer | 0.00996 | 19 | 21 |
| Renal cell carcinoma | 0.00011 | 18 | 20 |
| Gap junction | 0.00029 | 18 | 15 |
| TGF-β signaling pathway | 0.00271 | 18 | 13 |
| mTOR signaling pathway | 0.00447 | 16 | 15 |
| Pancreatic secretion | 0.00703 | 16 | 18 |
| ECM-receptor interaction | 1.60E−05 | 15 | 10 |
| Bile secretion | 0.00156 | 15 | 14 |
| Glioma | 0.00173 | 14 | 14 |
| Aldosterone-regulated sodium reabsorption | 0.00727 | 12 | 12 |
| Circadian rhythm | 0.00017 | 10 | 12 |
| 2-Oxocarboxylic acid metabolism | 1.60E−05 | 6 | 6 |
| Valine, leucine and isoleucine biosynthesis | 0.00078 | 2 | 3 |
ECM, extracellular matrix; HS, hindlimb suspension; KEGG, Kyoto Encyclopedia of Genes and Genomes; miRNA, microRNA; mTOR, mammalian target of rapamycin; TGF, transforming growth factor.
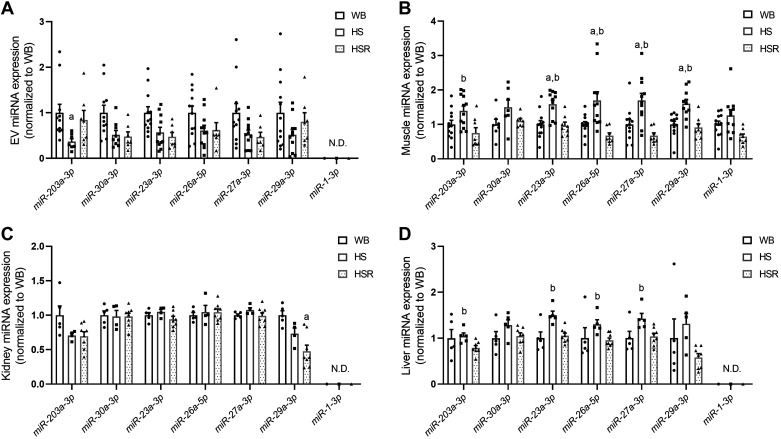
Follow-up RT-qPCR analysis was performed on miR-203a-3p and miR-30a-3p since these two miRNAs were significantly different (P < 0.05) and had a greater than twofold change in HS compared with WB. RT-qPCR confirmed lower EV miR-203a-3p in HS compared with WB (P = 0.003) and a trend for lower miR-203a-3p in HS compared with HSR (P = 0.066) (Fig. 6A). However, miR-30a-3p was only trending lower in HS compared with WB (P = 0.09), with no difference compared with HSR (Fig. 6A). We then examined miR-23, miR-26, miR-27, and miR-29 since they have been linked to skeletal muscle atrophy and hypertrophy (8, 20, 21, 29, 33, 62, 69) and exhibited changes in the miRNA microarray data set. RT-qPCR analysis demonstrated no significant differences in these miRNAs within EVs across groups, although the miRNAs tended to be lower in HS compared with WB (Fig. 6A). Interestingly, the muscle-specific miR-1 was not detectable in serum EVs under any of the conditions in rats.
Fig. 6.
RT-qPCR validation and follow-up of microRNA (miRNA) microarray: miR-203a-3p, miR-30a-3p, and selected atrophy-related miRNA measurements in serum extracellular vesicles (EVs; A), gastrocnemius muscle (B), kidney (C), and liver (D). HS, hindlimb suspension; HSR, hindlimb suspension followed by reloading; WB, weight bearing. miRNA expression values shown were normalized to UniSp6 with the −∆CT method (where CT is threshold cycle; 55) and then normalized to WB values. Data presented as means ± SE. aSignificantly different (P < 0.05) compared with WB; bsignificantly different (P < 0.05) compared with HSR. N.D., not detectable.
Muscle, kidney, and liver miRNA.
To better understand how changes in serum EV miRNA are related to muscle miRNA, we measured the same miRNAs within the gastrocnemius muscle. miR-203a-3p was not significantly different in HS muscle compared with WB but was significantly higher compared with HSR (Fig. 3B; P = 0.047). The miRNAs 23a-3p, 26a-3p, 27a-3p, and 29a-3p were elevated in HS muscle compared with both WB and HSR. There was no difference in muscle miR-30a-3p or miR-1-3p between WB, HS, and HSR (Fig. 6B).
Studies have established a potential cross talk between muscle and kidneys that is mediated by EVs (69, 70); thus we also measured the miRNAs in the kidney to see whether kidney miRNAs were altered or related to muscle levels (Fig. 6C). There were no differences in miR-203a-3p, miR-30a-3p, miR-26a-5p, and miR-27a-3p, while, as expected, muscle-specific miR-1 was undetectable. miR-29a-3p was significantly lower in HSR compared with WB (P = 0.019).
We measured the miRNAs in liver since there has been recent work claiming that exercise-induced release of EVs, which may be enriched for muscle-derived EVs, is preferentially delivered to the liver (65). Thus, under scenarios that induce muscle atrophy we postulated that there may be a relationship between the liver, muscle, and EVs. We found that miR-203a-3p, miR-23a-3p, miR-26a-5p, miR-27a-3p, and miR-29a-3p were higher in HS compared with HSR (Fig. 6D). There were no differences in miR-30a-3p, and, as expected, miR-1 was undetectable.
Relationship between EV, muscle, kidney, and liver miRNA.
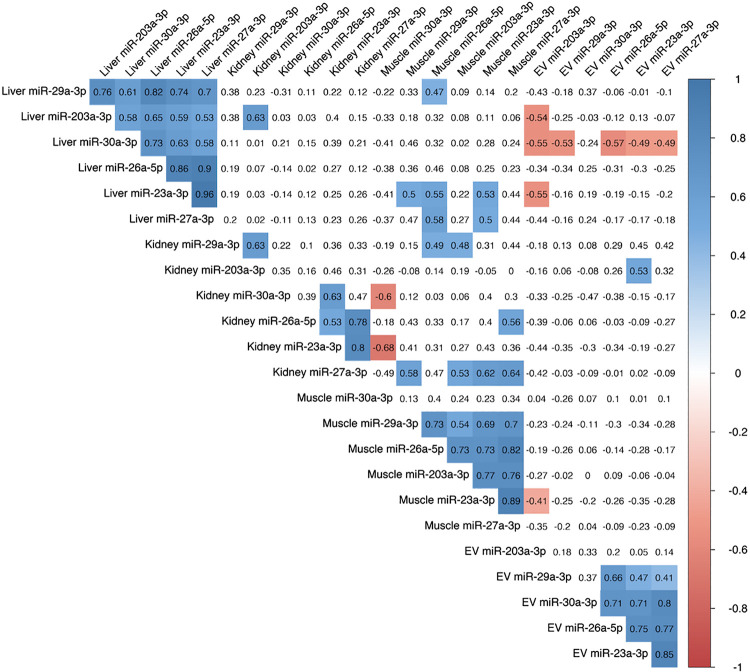
We further investigated the relationship between miRNA across EVs and organs via correlational analysis (Fig. 7). In general, the direction of change of the miRNAs that we measured was correlated within EVs, muscle, and liver across all rats. The direction of change of the miRNAs within the kidney was more moderately correlated but still showed patterns similar to each other. There was little relationship between serum EV miRNA and the corresponding miRNA within the muscle, kidney, and liver (i.e., EV miR-23a-3p vs. muscle miR-23a-3p). The only miRNA within serum EVs that showed a relationship to any organ was EV miR-203a-3p, which was negatively correlated with liver miR-203a-3p (R = −0.54, P < 0.05). Interestingly, there were significant correlations between muscle and kidney miRNAs 27a-3p (R = 0.64, P < 0.05) and 30a-3p (R = −0.60, P < 0.05). Muscle miR-23a-3p was also associated with liver miR-23a-3p (R = 0.53, P < 0.05).
Fig. 7.
Correlations of microRNA (miRNA) expression within serum extracellular vesicles (EVs), muscle, kidney, and liver. Values within the matrix represent correlation coefficients (R). Values that are highlighted represent significant (P < 0.05) correlations.
Relationship between muscle size, protein synthesis, protein degradation, and miRNA.
We examined the relationship of EV miRNA and muscle miRNA with muscle size and protein synthesis by stepwise regression analysis (Table 4). We found that miR-203a-3p was the only EV miRNA that associated with three of the outcomes examined, namely, gastrocnemius muscle mass, Ksyn, and Kdeg. Interestingly, muscle miR-29a-3p was inversely associated with muscle mass, mean myofiber CSA, Ksyn, and Kdeg.
Table 4.
Results from stepwise regression
| Mass | CSA | Ksyn | Kdeg | |
|---|---|---|---|---|
| Slope ± SE | ||||
| EV miR-203a-3p | 153.57 ± 70.42* | 8.40 ± 2.91** | −0.005 ± 0.002** | |
| Muscle miR-23a-3p | −1,252.33 ± 583.02* | |||
| Muscle miR-26a-5p | 0.004 ± 0.003 | |||
| Muscle miR-27a-3p | 1,091.52 ± 492.41* | −0.006 ± 0.004 | ||
| Muscle miR-29a-3p | −129.91 ± 93.69 | −918.99 ± 357.78* | −13.073 ± 3.87** | 0.012 ± 0.004** |
| Intercept | 1,835.51 ± 139.75*** | 4,335.85 ± 360.74*** | 38.18 ± 5.77*** | 0.013 ± 0.004** |
| Observations | 30 | 30 | 30 | 30 |
| R2 | 0.25 | 0.39 | 0.50 | 0.63 |
| Adjusted R2 | 0.19 | 0.32 | 0.46 | 0.57 |
| F statistic | 4.49* (df = 2; 27) | 5.62** (df = 3; 26) | 13.53*** (df = 2; 27) | 10.72*** (df = 4; 25) |
CSA, cross-sectional area; EV, extracellular vesicle; Kdeg, degradation constant; Ksyn, synthesis rate.
P < 0.05;
P < 0.01;
P < 0.001.
EV miR-203a-3p with muscle atrophy in humans.
ULLS caused a significant decrease in mean myofiber CSA of the vastus lateralis muscle (−16 ± 3%, P = 0.0023; Fig. 8A) in human subjects. Similar to the rats, there was a significant decrease in serum EV miR-203a-3p (−35 ± 10%, P = 0.0020; Fig. 8B) with muscle disuse atrophy in the humans.
Fig. 8.

Disuse atrophy in humans lowers serum extracellular vesicle (EV) miR-203-3p expression. A: mean myofiber cross-sectional area (CSA) in control leg and immobilized leg vastus lateralis after unilateral lower limb suspension (ULLS) for 7 days. B: serum EV miR-203a-3p expression before and after ULLS. Black dots represent ULLS subjects, and purple dots represent unilateral lower limb suspension and massage (ULLS+M) subjects. microRNA (miRNA) expression values shown were normalized to UniSp6 with the −∆CT method (where CT is threshold cycle; 55) and then normalized to preimmobilization values.
DISCUSSION
The overall goal of this study was to investigate the relationship between serum miRNAs and muscle atrophy. The main finding of our work is that serum EV miR-203a-3p expression is associated with protein synthesis, protein degradation, and muscle atrophy. This association is a novel finding and recognizes a previously unrecognized circulating marker for muscle protein turnover during disuse atrophy and regrowth. Our results further demonstrate that previously recognized muscle atrophy-related miRNAs may not be drastically altered in serum EVs during prolonged disuse atrophy. As such, changes in miRNAs 23a-3p, 26a-5p, and 27a-3p within the muscle may not contribute to, or be affected by, serum EV miRNAs. However, we did find an association between muscle miR-27a-3p and kidney miR-27a-3p suggesting a muscle-kidney cross talk and specific delivery of miR-27a-3p to the kidney as previously demonstrated by other laboratories (69).
The limited significant differences in serum EV miRNA with 7 days of HS were surprising and suggest either that we missed changes that occurred early in HS or that large shifts in serum EV miRNA expression do not occur with disuse atrophy. However, when we examined predicted pathways that may be altered by the differences in EV miRNAs with HS (miRNAs P < 0.05) we found multiple pathways related to anabolic and catabolic signaling (3, 4, 16, 57), response to mechanical stimulus (11, 14), and metabolic demand (25). In particular, KEGG pathways such as MAPK signaling, focal adhesion, FoxO signaling, ubiquitin-mediated proteolysis, transforming growth factor (TGF)-β signaling, and mammalian target of rapamycin (mTOR) signaling were predicted to be targeted by the differentially expressed EV miRNAs. This demonstrates that although there were few major changes in individual EV miRNAs, the general shifts in EV miRNA expression reflected the alterations in major anabolic, mechanosensitive, and catabolic pathways that typically occur with disuse atrophy.
Microarray analysis revealed that miRNAs 203a-3p and 30a-3p were the only two miRNAs to exhibit a greater than twofold change in serum EV expression with muscle disuse atrophy. However, qPCR validation revealed that only miR-203a-3p was significantly lower in EVs with HS, although there was a trend for lower miR-30a-3p. miR-203a-3p has been shown to promote proliferation of prostate cancer cells (67) and hepatocytes (71) while hindering proliferation of ovarian cancer cells (31), gastric cancer cells, and skeletal muscle cells (32). The impact on cellular proliferation and differentiation is a consequence of miR-203-3p targeting and suppression of IGF-1R (63), MEF2C (32), p63 (68), Rap1A (67), PTEN (71), and Akt/GSK-3β (31). Our data across both rats and humans suggest that EV miR-203a-3p is a good biomarker for representing the catabolic/anabolic state of skeletal muscle. It was the only EV miRNA associated with muscle mass, protein synthesis, and protein degradation. A previous study has shown that elevated circulating miR-203 levels are associated with muscle wasting (44), which is opposite to the positive association of EV miR-203 expression and muscle size shown in this study. Disparity between the studies may be due to the type of atrophy examined: our study was solely focused on disuse atrophy, whereas the study by Okugawa et al. (44) examined cancer-related muscle loss in colorectal cancer patients. It is possible that different relationships exist in circulating miR-203 depending on the paradigm in which muscle loss is induced. It is also important to note that we examined miR-203 in EVs whereas their studies encompassed all circulating miR-203.
miR-23, miR-26, miR-27, and miR-29 have been linked to skeletal muscle atrophy and hypertrophy (8, 20, 21, 29, 33, 62, 69) and exhibited differences with atrophy and regrowth in the muscle but showed no significant changes within EVs. Both miR-23a and miR-27a have been proposed to be modulated by EVs in a diabetic model of muscle wasting in mice (21, 69). Additionally, miR-29 has also been associated with muscle wasting in a model of chronic kidney disease and was also associated with exosome-driven regulation of kidney fibrosis (62). The work by Hudson et al. (21) suggested that miR-23a was reduced in muscle during dexamethasone-induced or diabetes-induced atrophy via expulsion of miR-23a from the muscle within exosomes. This finding contradicts the elevation we see in miR-23a in the gastrocnemius muscle with disuse atrophy and the lack of elevation in miR-23 in serum EVs. However, bed rest or limb immobilization in humans led to elevated miR-23a and miR-26a in skeletal muscle, which matches our results (53). Furthermore, miR-29 has been shown to attenuate atrophy in a mouse model of kidney disease (62), whereas other recent work by Li et al. (29) demonstrated that elevated miR-29 in muscle contributes to multiple types of atrophy. The study from Li et al. (29) agrees with our present data showing that elevated miR-29 in muscle is associated with atrophy. Resistance training-induced hypertrophy has also been associated with a reduction in the abundance of muscle miR-29a and miR-26a in muscle (8). Altogether, our data show no association between atrophy-associated miRNAs within EVs and those within muscle. It also clearly demonstrates that there is significant work left to understand how miRNA within muscle changes with atrophy and how miRNA loading into EVs plays a role.
There is no definitive evidence regarding the contribution of muscle EVs to the total pool of serum EVs. As mentioned above, miR-23a has been shown to be exported out of muscle cells via EVs with dexamethasone treatment and create an inverse relationship between EV and muscle miRNA miR-23a content (21). If we only consider the average differences in serum EV miRNA and muscle miRNA (Fig. 6) in our study, there is generally an inverse relationship between them, and it is tempting to speculate that these changes are related. However, when all data points from each rat are assessed via correlational analysis, we show that the serum EV expression of miR-203, miR-23, miR-26, miR-27, and miR-29 is not reflective of muscle expression values. We observed that the muscle-specific miR-1-3p was not readily detectable in serum EVs, which may also reflect a very small contribution of muscle-derived EVs to the serum or very little regulation of muscle miRNA via loading into EVs. It is also possible that there are abundant muscle-derived EVs circulating in the serum but they contain very small amounts of miR-1 relative to other miRNAs. Finally, it cannot be directly ascertained from our samples whether any serum miRNA changes are due to muscle or contributions from other organs since there are no reliable ways to track in vivo origin of serum EVs. There is much work needed in future studies to better understand these specific inquiries about EVs, their origin when in serum, and muscle atrophy.
Previous work has shown muscle-specific cross talk to the kidneys in two mouse models of muscle wasting: unilateral ureteral obstruction (UUO) [a model of chronic kidney disease (CKD)] (70) and a streptozotocin (STZ)-induced diabetic model (69). The muscle loss in the CKD model was associated with lower serum EV miR-26a-5p, which is similar to our study; however, their mice had lower expression of muscle miR-26a-5p with atrophy. Interestingly, when miR-26a-5p was overexpressed in the atrophied muscle via intramuscular injection of exosomes containing miR-26, it caused an increase in muscle and serum EV miR-26a-5p combined with an increase in kidney expression of miR-26a-5p. Our data support that muscle atrophy is associated with lower serum EV miR-26 but disuse atrophy in our rats caused an increase in muscle expression of miR-26. Our data did not show a relationship between muscle and kidney expression levels of miR-26 and suggest that the muscle and kidney expression of miR-26-5p are not related. Interestingly, the same research group showed evidence that muscle may also deliver miR-23 and miR-27 to the kidney to combat fibrosis in a STZ-induced diabetic mouse model (69). Specifically, when they elevated miR-23/27 via adeno-associated virus (AAV) injection into the muscle, they saw an increase in kidney miR-23/27 in the kidney via exosomal delivery. In our disuse atrophy model, we observed a significant positive relationship between muscle and kidney expression of miR-27a-3p, which supports the model of EV-mediated delivery of miR-27a-3p from the muscle to the kidney. Altogether, our data provide further support of a relationship between muscle and kidney miRNA cross talk, but further work is needed to verify the specific roles of EVs in this process.
Our data showing that miR-203a-3p may be a good biomarker for muscle atrophy and protein synthesis in rats are novel, so we further investigated whether this finding was clinically relevant in human subjects. The ULLS method had been used extensively to induce atrophy in human subjects (reviewed in 17), and our 7-day ULLS intervention caused significant atrophy of the vastus lateralis muscle in our subjects. Furthermore, the atrophy of a single limb was associated with a significant drop in serum EV miR-203a-3p expression similar to what we observed in the hindlimb suspension model with rats. The decrease in serum EV miR-203a-3p with the more subtle atrophy stimulus in humans suggests that serum EV miR-203a-3p is indeed a sensitive marker of muscle size status. Recently published work has highlighted a large disparity in EV small RNA profiles between rats and humans when EVs were isolated with ExoQuick methods (72). Despite this, we were able to find that miR-203a-3p content is a consistent marker of atrophy and protein turnover across species and lends confidence that our results in rats were not species specific or due to our methodology used for EV isolation.
Our data provide novel and useful information regarding changes in serum EV miRNA and their relationship to muscle atrophy and other organs; however, there are several limitations that must be recognized. First, the field of EV research is rapidly evolving, and techniques for the isolation of EVs have been a growing and debated topic. In particular, there has been a growing appreciation for the need to use labor-intensive density gradient ultracentrifugation and/or size exclusion chromatography techniques to isolate more pure EV samples since serum and plasma samples are saturated with an abundance of non-EV lipids, proteins, and nucleic acids (1, 45, 46). Because of the small sample volume availability in the present study, however, we opted to isolate EVs with the commercially available ExoQuick reagent. This reagent is extremely efficient at isolating a large yield of EVs from serum and plasma samples for miRNA analysis (19, 52, 72), but it is known that with these types of methods many other non-EV nucleic acids, proteins, and lipoproteins are precipitated and may have impacted some of the results (15, 22, 59, 60). Second, our results may not be transferable to other forms of atrophy as there are disparate results on miRNA changes with other forms of atrophy in the literature as discussed above. We believe, though, that the combination of our rat and human data provides adequate evidence to promote further investigation into miR-203a-3p and its role specifically in disuse atrophy. We also acknowledge that we may have missed divergent changes in miRNA occurring at earlier time points in disuse atrophy. Altogether, we believe that despite these limitations we have provided a novel glimpse into the relationship and changes between serum EV miRNA and muscle miRNA with disuse atrophy.
In conclusion, our novel findings demonstrate that serum EV miR-203a-3p expression is a sensitive measure of muscle protein status. Specifically, serum EV miR-203a-3p expression was associated with muscle mass, protein synthesis, and protein degradation across weight bearing, disuse, and reloading conditions. In general, we also show that although the overall changes in the profile of serum EV miRNAs are rather subtle in the hindlimb suspension model of atrophy and reloading, the changes generally reflect alterations in anabolic and catabolic signaling during atrophy and regrowth. Future studies should determine the mechanistic impact and specificity by which changes in serum EV miR-203a-3p expression alter function in both muscle and other organs.
GRANTS
This work was supported by NIH Grant AT-009268 (E.E.D.-V, T.A.B., and B.F.M.). The project described was supported by the NIH National Center for Advancing Translational Sciences through grant number UL1TR001998. The content is solely the responsibility if the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.W.V.P. and E.E.D.-V. conceived and designed research; D.W.V.P., I.J.V., M.M.L., and P.P. performed experiments; D.W.V.P., M.M.L., K.L.V.P., P.P., and B.F.M. analyzed data; D.W.V.P., I.J.V., K.L.V.P., B.F.M., T.A.B., and E.E.D.-V. interpreted results of experiments; D.W.V.P. and K.L.V.P. prepared figures; D.W.V.P. drafted manuscript; D.W.V.P., I.J.V., M.M.L., K.L.V.P., B.F.M., T.A.B., and E.E.D.-V. edited and revised manuscript; D.W.V.P., I.J.V., M.M.L., K.L.V.P., P.P., B.F.M., T.A.B., and E.E.D.-V. approved final version of manuscript.
REFERENCES
- 1.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1: 845–867, 2002. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Aoi W, Sakuma K. Does regulation of skeletal muscle function involve circulating microRNAs? Front Physiol 5: 39, 2014. doi: 10.3389/fphys.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay RD, Burd NA, Tyler C, Tillin NA, Mackenzie RW. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr 6: 146, 2019. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao PR, Kim HJ, Lecker SH. Ubiquitin-protein ligases in muscle wasting. Int J Biochem Cell Biol 37: 2088–2097, 2005. doi: 10.1016/j.biocel.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Caruso S, Poon IK. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 9: 1486, 2018. doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza-Schorey C, Schorey JS. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem 62: 125–133, 2018. doi: 10.1042/EBC20170078. [DOI] [PubMed] [Google Scholar]
- 8.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 110: 309–317, 2011. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 9.Drake JC, Bruns DR, Peelor FF 3rd, Biela LM, Miller RA, Hamilton KL, Miller BF. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab 307: E813–E821, 2014. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake JC, Peelor FF 3rd, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durieux AC, Desplanches D, Freyssenet D, Flück M. Mechanotransduction in striated muscle via focal adhesion kinase. Biochem Soc Trans 35: 1312–1313, 2007. doi: 10.1042/BST0351312. [DOI] [PubMed] [Google Scholar]
- 12.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49: 59–68, 2014. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJ, El Khouli RH, Johnson ZR, Westgate PM, Chen J, Morris AJ, Sullivan PG, Dupont-Versteegden EE, Kern PA. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 130: 2319–2331, 2020. doi: 10.1172/JCI134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi MV, Ruoss S, Valdivieso P, Mitchell KW, Smith K, Atherton PJ, Narici MV, Flück M. Regional regulation of focal adhesion kinase after concentric and eccentric loading is related to remodelling of human skeletal muscle. Acta Physiol (Oxf) 223: e13056, 2018. doi: 10.1111/apha.13056. [DOI] [PubMed] [Google Scholar]
- 15.Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borràs FE. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep 6: 33641, 2016. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev 43: 93–99, 2015. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackney KJ, Ploutz-Snyder LL. Unilateral lower limb suspension: integrative physiological knowledge from the past 20 years (1991-2011). Eur J Appl Physiol 112: 9–22, 2012. doi: 10.1007/s00421-011-1971-7. [DOI] [PubMed] [Google Scholar]
- 18.Haddad F, Adams GR. Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol (1985) 96: 203–210, 2004. doi: 10.1152/japplphysiol.00856.2003. [DOI] [PubMed] [Google Scholar]
- 19.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12: e0170628, 2017. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Chen X, Yu B, He J, Chen D. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem Biophys Res Commun 423: 265–269, 2012. doi: 10.1016/j.bbrc.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 21.Hudson MB, Woodworth-Hobbs ME, Zheng B, Rahnert JA, Blount MA, Gooch JL, Searles CD, Price SR. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol 306: C551–C558, 2014. doi: 10.1152/ajpcell.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim Biophys Acta Rev Cancer 1871: 109–116, 2019. doi: 10.1016/j.bbcan.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Jung HJ, Lee KP, Milholland B, Shin YJ, Kang JS, Kwon KS, Suh Y. Comprehensive miRNA profiling of skeletal muscle and serum in induced and normal mouse muscle atrophy during aging. J Gerontol A Biol Sci Med Sci 72: 1483–1491, 2017. doi: 10.1093/gerona/glx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby TJ, Chaillou T, McCarthy JJ. The role of microRNAs in skeletal muscle health and disease. Front Biosci 20: 37–77, 2015. doi: 10.2741/4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol (1985) 103: 388–395, 2007. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- 26.Laurens C, Bergouignan A, Moro C. Exercise-released myokines in the control of energy metabolism. Front Physiol 11: 91, 2020. doi: 10.3389/fphys.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence MM, Van Pelt DW, Confides AL, Hunt ER, Hettinger ZR, Laurin JL, Reid JJ, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE, Miller BF. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol (Oxf) 229: e13460, 2020. doi: 10.1111/apha.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legård GE, Pedersen BK. Muscle as an endocrine organ. In: Muscle and Exercise Physiology, edited by Zoladz JA. London: Academic Press, 2019, p. 285–307. [Google Scholar]
- 29.Li J, Chan MC, Yu Y, Bei Y, Chen P, Zhou Q, Cheng L, Chen L, Ziegler O, Rowe GC, Das S, Xiao J. miR-29b contributes to multiple types of muscle atrophy. Nat Commun 8: 15201, 2017. doi: 10.1038/ncomms15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little HC, Tan SY, Cali FM, Rodriguez S, Lei X, Wolfe A, Hug C, Wong GW. Multiplex quantification identifies novel exercise-regulated myokines/cytokines in plasma and in glycolytic and oxidative skeletal muscle. Mol Cell Proteomics 17: 1546–1563, 2018. doi: 10.1074/mcp.RA118.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HY, Zhang YY, Zhu BL, Feng FZ, Zhang HT, Yan H, Zhou B. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3β/Snail signaling pathway by targeting ATM. J Ovarian Res 12: 60, 2019. doi: 10.1186/s13048-019-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Luo W, Wu H, Ye Y, Li Z, Hao S, Kong L, Zheng X, Lin S, Nie Q, Zhang X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis 5: e1347, 2014. doi: 10.1038/cddis.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis LM, Lessard SJ, Ezzyat Y, Fielding RA, Rivas DA. Circulating microRNA are predictive of aging and acute adaptive response to resistance exercise in men. J Gerontol A Biol Sci Med Sci 72: 1319–1326, 2017. doi: 10.1093/gerona/glw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis LM, Rivas DA, Pasiakos SM, McClung JP, Ceglia L, Fielding RA. Upregulation of circulating myomiR following short-term energy restriction is inversely associated with whole body protein synthesis. Am J Physiol Regul Integr Comp Physiol 313: R298–R304, 2017. doi: 10.1152/ajpregu.00054.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinho R, Alcântara PS, Ottoch JP, Seelaender M. Role of exosomal microRNAs and myomiRs in the development of cancer cachexia-associated muscle wasting. Front Nutr 4: 69, 2018. doi: 10.3389/fnut.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzaka Y, Tanihata J, Komaki H, Ishiyama A, Oya Y, Rüegg U, Takeda SI, Hashido K. Characterization and functional analysis of extracellular vesicles and muscle-abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 myocytes and mdx mice. PLoS One 11: e0167811, 2016. doi: 10.1371/journal.pone.0167811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics 39: 219–226, 2009. doi: 10.1152/physiolgenomics.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002. doi: 10.1093/gerona/57.10.B359. [DOI] [PubMed] [Google Scholar]
- 39.Miller BF, Ehrlicher SE, Drake JC, Peelor FF 3rd, Biela LM, Pratt-Phillips S, Davis M, Hamilton KL. Assessment of protein synthesis in highly aerobic canine species at the onset and during exercise training. J Appl Physiol (1985) 118: 811–817, 2015. doi: 10.1152/japplphysiol.00982.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 596: 83–103, 2018. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller BF, Reid JJ, Price JC, Lin HL, Atherton PJ, Smith K. CORP: the use of deuterated water for the measurement of protein synthesis. J Appl Physiol (1985) 128: 1163–1176, 2020. doi: 10.1152/japplphysiol.00855.2019. [DOI] [PubMed] [Google Scholar]
- 42.Miller BF, Wolff CA, Peelor FF 3rd, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol (1985) 118: 655–661, 2015. doi: 10.1152/japplphysiol.00987.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab 24: 332–340, 2016. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okugawa Y, Toiyama Y, Hur K, Yamamoto A, Yin C, Ide S, Kitajima T, Fujikawa H, Yasuda H, Koike Y, Okita Y, Hiro J, Yoshiyama S, Araki T, Miki C, McMillan DC, Goel A, Kusunoki M. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J Cachexia Sarcopenia Muscle 10: 536–548, 2019. doi: 10.1002/jcsm.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onódi Z, Pelyhe C, Terézia Nagy C, Brenner GB, Almási L, Kittel Á, Manček-Keber M, Ferdinandy P, Buzás EI, Giricz Z. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol 9: 1479, 2018. doi: 10.3389/fphys.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otani K, Fujioka Y, Okada M, Yamawaki H. Optimal isolation method of small extracellular vesicles from rat plasma. Int J Mol Sci 20: 4780, 2019. doi: 10.3390/ijms20194780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99: 9213–9218, 2002. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 15: 383–392, 2019. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 49.Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil 80: 130–135, 1999. doi: 10.1016/S0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 50.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55: M168–M173, 2000. doi: 10.1093/gerona/55.3.M168. [DOI] [PubMed] [Google Scholar]
- 51.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291, 2014. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rekker K, Saare M, Roost AM, Kubo AL, Zarovni N, Chiesi A, Salumets A, Peters M. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem 47: 135–138, 2014. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Ringholm S, Biensø RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, Plomgaard P, Lundby C, Wojtaszewski JF, Calbet JA, Pilegaard H. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab 301: E649–E658, 2011. doi: 10.1152/ajpendo.00230.2011. [DOI] [PubMed] [Google Scholar]
- 54.Rome S, Forterre A, Mizgier ML, Bouzakri K. Skeletal muscle-released extracellular vesicles: state of the art. Front Physiol 10: 929, 2019. doi: 10.3389/fphys.2019.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 80: 115–125, 2015. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298: C38–C45, 2010. doi: 10.1152/ajpcell.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács AF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YX, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz AM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 3: 24858, 2014. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13: 423–433, 2011. [Erratum in Nat Cell Biol 17: 104, 2015). doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43: W460–W466, 2015. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Wang B, Zhang A, Hassounah F, Seow Y, Wood M, Ma F, Klein JD, Price SR, Wang XH. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther 27: 571–583, 2019. doi: 10.1016/j.ymthe.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Zhao Z, Yang Y, Luo M, Zhang M, Wang X, Liu L, Hou N, Guo Q, Song T, Guo B, Huang C. MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci Rep 8: 10119, 2018. doi: 10.1038/s41598-018-27583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JF, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc 6: 743–760, 2011. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- 67.Xiang J, Bian C, Wang H, Huang S, Wu D. MiR-203 down-regulates Rap1A and suppresses cell proliferation, adhesion and invasion in prostate cancer. J Exp Clin Cancer Res 34: 8, 2015. doi: 10.1186/s13046-015-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 452: 225–229, 2008. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang A, Li M, Wang B, Klein JD, Price SR, Wang XH. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle 9: 755–770, 2018. doi: 10.1002/jcsm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang A, Wang H, Wang B, Yuan Y, Klein JD, Wang XH. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J 33: 13590–13601, 2019. doi: 10.1096/fj.201900884R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Zheng Y, Zhu G. MiR-203a-3p targets PTEN to promote hepatocyte proliferation by regulating PI3K/Akt pathway in BRL-3A cells. Biosci Biotechnol Biochem 84: 725–733, 2020. doi: 10.1080/09168451.2019.1694860. [DOI] [PubMed] [Google Scholar]
- 72.Zhao F, Cheng L, Shao Q, Chen Z, Lv X, Li J, He L, Sun Y, Ji Q, Lu P, Ji Y, Ji J. Characterization of serum small extracellular vesicles and their small RNA contents across humans, rats, and mice. Sci Rep 10: 4197, 2020. doi: 10.1038/s41598-020-61098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]